Abstract
Individuals with hemophilia are at risk for bleeding episodes, which range from mild mucosal/soft-tissue bleeding to life-threatening hemorrhages. This report describes the dental/medical management provided to a 23-year-old patient suffering from uncontrolled bleeding after an electrosurgical procedure (operculectomy) in relation to the mandibular right third molar, in which hemophilia was a true accidental finding. Various safety measures that need to be considered during the dental surgical management of hemophilic patients are discussed.
Key words: Bleeding, cautery, hemophilia, pericoronitis
INTRODUCTION
Bleeding disorders are classified as deficiencies in coagulation factors, platelet function disorders, fibrinolytic defects, and vascular disorders.[1] Hemophilia is an X-linked recessive disorder caused by insufficiency in coagulation factors, clinically characterized by prolonged clotting time.[2]
Hemophilia A, which is known as classical hemophilia, is a hereditary hemorrhagic disorder that results due to scarcity or congenital deficit of factor VIII (FVIII), which manifests as unusual and excessive bleeding either spontaneously or secondary to trauma. About 50% of male offsprings inherit the disorder due to female hemophilia A gene carriers. Female hemophilia gene carriers have lower quantities of FVIII compared to healthy females, but they do not manifest symptoms of hemophilia A. The gene with the information for building factor is found only on the sex chromosome labeled X (dominant). This is the reason why only female offsprings will be carriers to the male hemophilia A patients.[3]
Hemophilia A, which occurs in one out of 5000 males which accounts for 80% of hemophilia cases, is considered the most common hereditary disorder of hemostasis. More than 400,000 males are affected by hemophilia A, many of whom remain undiagnosed in developing countries. Severe hemophilia often manifests in the first months of life, whereas mild or moderate hemophilia will present later in childhood or adolescence often incidentally or following trauma.[4]
Pericoronitis is defined as inflammation in the soft-tissues encircling the crown of a partially erupted tooth or sometimes fully erupted tooth. Mostly, it is seen in teeth that erupt very slowly or partially erupted tooth and more frequently affects the lower 3rd molar. Patients with chronic pericoronitis complain of a dull pain or mild discomfort lasting a day or two, with remission lasting many months.[5] The highest incidence of pericoronitis was found in the 20–29 years age group. The condition was rarely seen before 20 or after 40 years of age.[6]
The purpose of the current report is to outline and discuss the diagnostic and therapeutic actions provided to an adult patient suffering from pericoronitis in relation to the mandibular right 3rd molar, in which hemophilia was a true incidental finding.
CASE REPORT
A 23-year-old male patient, warden of a private boys hostel, presented to the Department of Periodontology, (Narayana Dental College and Hospital) with a complaint of food lodgment and intermittent pain in relation to the mandibular right wisdom tooth for few weeks. The patient's general health status was good with no past significant bleeding events or exposure to surgical interventions. On clinical examination, there was an inflamed soft-tissue covering the distobuccal and distoocclusal surface of the mandibular right 3rd molar (tooth number-48). Clinically, the tooth was fully erupted and in the line of occlusion with 18 (maxillary right 3rd molar) [Figure 1a]. An intraoral periapical radiograph was taken to examine the surrounding hard tissue. Based on clinical and radiographic findings, it was diagnosed as chronic pericoronitis in relation to fully erupted 48. Basic blood investigations were done, and the results are in the normal range (bleeding time: 3 min 30 s and clotting time: 6 min 43 s).
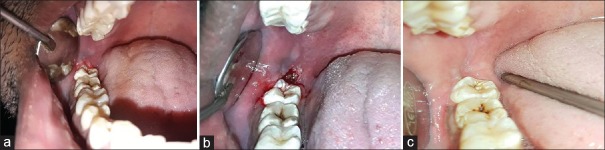
Figure 1.
(a) Pericoronitis in relation to 48; (b) immediate postoperative; (c) postoperative healing after 10 days
Full-mouth scaling was performed, and acute pericoronitis was treated by gently flushing the area with sterile saline to remove debris and exudate and swabbing with antiseptic after elevating the flap gently from the tooth with a curette. After taking written informed consent, operculectomy was planned on the same day, as there were no systemic signs, swelling, or lymphadenopathy, and there was available space for the 3rd molar with proper alignment in the arch. Under local anesthesia, the pericoronal flap was surgically excised using electrocautery (ART – Electorsurge – Unicorn). Electrocautery instrument was prepared and a bracelet was put on the patient's arm, connecting it with the main electrosurgical equipment. The surgical site and the surrounding tissue were prepared by swabbing with povidone-iodine. After topical application of anesthesia, the area was infiltrated with lidocaine HCL 2% and epinephrine 1:100,000. The pericoronal flap was then surgically excised using a loop and straight electrode, while hemostasis was achieved using a ball electrode [Figure 1b]. The operated area was irrigated with sterile saline and povidone-iodine. The patient was instructed to avoid chewing and brushing on that side for first 2 days and prescribed analgesics (Zerodol P: a combination of aceclofenac 100 mg and paracetamol 500 mg) for 2 days.
The patient reported back to the department the next day morning with a complaint of bleeding from the operated area for 4 h. Bleeding could not be controlled with the pressure pack. Under local anesthesia (infiltration), hemostasis was achieved using a ball electrode. Postoperative instructions were reinforced. On the 4th day, the patient returned with a complaint of uncontrolled bleeding for few hours. On examination, mild continuous bleeding was observed and hemostasis was achieved with pressure pack. Patient was advised for a complete haemogram and all the parameters were in the normal range, except for activated partial thromboplastin time which is 48.7 s. The same parameter was repeated again in a different laboratory, which showed 54 s. Then, the patient was referred to a general physician and administered a subcutaneous injection of Vitamin K1 (Phytonadione Injectable Emulsion, USP) once daily for 3 days. The patient was closely monitored in an ambulatory approach. Close control visits were programmed for 7 days, and at the 10th day, there were no bleeding or other complications at the operated site [Figure 1c]. Then, the patient was referred to a higher center (Department of Hematology, Christian Medical College, Vellore, Tamil Nadu, India) for further investigations and management. In the coagulation workup report, they found all the parameters were in normal range except for FVIII, which is 7.1% (reference range: 50%–150%). With clinical and laboratory findings, the patient was diagnosed as having mild hemophilia A. All India Haemophilic society has various branches at district level, where this patient got registered under hemophilia federation (India), Kadapa Chapter.
DISCUSSION
Hemophilia A is characterized by a deficiency of FVIII, whereas hemophilia B is caused by a deficiency of FIX. Women tend to be asymptomatic and men typically express diseases such as hemophilia which is X-linked. It is common in dental practice to encounter patients with bleeding disorders in daily practice; therefore, it is essential to be able to identify such patients and safely manage their dental treatment.[7]
Based on the total amount of clotting factors in a human blood, hemophilia is classified into mild, moderate, and severe. The normal range of FVIII and FIX is between 50% and 150%. Patients suffering from severe form of hemophilia (<1% factor activity) account for 60% of total hemophilia patients who may experience spontaneous bleeding into joints and muscles. Moderate hemophilia (1%–5% factor activity) patients account for about 15% of hemophilia population and exhibit bleeding after minor injuries and occasional episodes of spontaneous bleeding. Bleeding generally occurs only after trauma or surgery in mild hemophilic individuals (6%–49% factor activity).[8] Individuals with mild hemophilia accounts for about 25% who may have very few symptoms otherwise.
The incidence of hemophilia A is approximately 1 in every 10,000 persons. However, 30% of cases are caused by new mutations and hence may not be associated with a family history.[9,10] According to a study conducted by the World Federation of Hemophilia, almost 50% of the world's hemophilia population lives in India, and over 70% of people with hemophilia do not have adequate knowledge or access to treatment.[11]
CONCLUSION
General dentists should always be aware of the potential risks of bleeding disorders and should give special attention; hemophilia is one of the most common clotting disorders worldwide, and in many cases, it was undiagnosed in developing countries such as India. These disorders represent a serious challenge during the clinical practice since routine dental treatment can produce life-threatening conditions. In the case reported by the authors, using electrocautery helped the patient to some extent in minimizing the unnecessary postsurgical complication.
The involvement of specialist and the experienced multidisciplinary team at hemophilia treatment center is very important for effective planning and undertaking elective surgery. For a successful elective surgery in hemophilia patients, careful preoperative planning that includes dental examination, physical assessment and prehabilitation, laboratory testing, development of hemostasis, and pain management plans is very important. Care should be taken by hemophilic patients to maintain good oral hygiene to prevent periodontal and other dental diseases. It is the responsibility of a dentist to assess the oral hygiene and oral source of infection as it takes several weeks to resolve.[12] Newer tools, dental bleeding risk assessment and treatment tool, which facilitate a comprehensive evaluation of bleeding risk with the potential to minimize unnecessary treatment and aid interdisciplinary communication among different clinical teams should be used for successful outcomes in dental management of patients with inherited bleeding disorders.[13]
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Martínez-Rider R, Garrocho-Rangel A, Márquez-Preciado R, Bolaños-Carmona MV, Islas-Ruiz S, Pozos-Guillén A, et al. Dental management of a child with incidentally detected hemophilia: Report of a clinical case. Case Rep Dent. 2017;2017:7429738. doi: 10.1155/2017/7429738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fijnvandraat K, Cnossen MH, Leebeek FW, Peters M. Diagnosis and management of haemophilia. BMJ. 2012;344:e2707. doi: 10.1136/bmj.e2707. [DOI] [PubMed] [Google Scholar]
- 3.Salen P, Babiker HM, Hemophilia A. StatPearls. Treasure Island (FL): StatPearls Publishing; 2018. [Google Scholar]
- 4.Arruda VR, Doshi BS, Samelson-Jones BJ. Novel approaches to hemophilia therapy: Successes and challenges. Blood. 2017;130:2251–6. doi: 10.1182/blood-2017-08-742312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moloney J, Stassen LF. Pericoronitis: Treatment and a clinical dilemma. J Ir Dent Assoc. 2009;55:190–2. [PubMed] [Google Scholar]
- 6.Nitzan DW, Tal O, Sela MN, Shteyer A. Pericoronitis: A reappraisal of its clinical and microbiologic aspects. J Oral Maxillofac Surg. 1985;43:510–6. doi: 10.1016/s0278-2391(85)80029-x. [DOI] [PubMed] [Google Scholar]
- 7.Shastry SP, Kaul R, Baroudi K, Umar D. Hemophilia A: Dental considerations and management. J Int Soc Prev Community Dent. 2014;4:S147–52. doi: 10.4103/2231-0762.149022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Hemophilia Foundation. Hemophilia A (Factor VIII Deficiency) 2006. [Last accessed on 2018 May 25]. Available from: http://www.hemophilia.org/NHFWeb/MainPgs/MainNHF.aspx?menuid=180&contentid=45&rptname=bleeding .
- 9.Rogaev EI, Grigorenko AP, Faskhutdinova G, Kittler EL, Moliaka YK. Genotype analysis identifies the cause of the “royal disease”. Science. 2009;326:817. doi: 10.1126/science.1180660. [DOI] [PubMed] [Google Scholar]
- 10.Lawn RM, Vehar GA. The molecular genetics of hemophilia. Sci Am. 1986;254:48–54. doi: 10.1038/scientificamerican0386-48. [DOI] [PubMed] [Google Scholar]
- 11.World Federation of Hemophilia. Report on the Annual Global Survey 2016. Published by World Federation of Hemophilia. 2017 [Google Scholar]
- 12.Escobar MA, Brewer A, Caviglia H, Forsyth A, Jimenez-Yuste V, Laudenbach L, et al. Recommendations on multidisciplinary management of elective surgery in people with haemophilia. Haemophilia. 2018;24:1–10. doi: 10.1111/hae.13549. [DOI] [PubMed] [Google Scholar]
- 13.Rasaratnam L, Chowdary P, Pollard D, Subel B, Harrington C, Darbar UR, et al. Risk-based management of dental procedures in patients with inherited bleeding disorders: Development of a dental bleeding risk assessment and treatment tool (DeBRATT) Haemophilia. 2017;23:247–54. doi: 10.1111/hae.13122. [DOI] [PubMed] [Google Scholar]