Abstract
Context:
Periodontally accelerated osteogenic orthodontics (PAOO) combines alveolar corticotomy, bone graft materials, and the application of orthodontic forces for rapid correction of malocclusions.
Aims:
The present study aims to primarily assess differences in orthodontic treatment duration, bone quality around corticotomy sites, postoperative healing, and subjective pain when corticotomy was done conventionally and with the placement of recombinant human bone morphogenetic protein-2 (rhBMP-2).
Settings and Design:
Thirty individuals participated in this study. Individuals were randomly assigned into each of the following experimental groups; C + BMP: Corticotomy with 0.5 μg/mL rhBMP-2 and C: Corticotomy only.
Materials and Methods:
Clinical parameters included recording the duration of the treatment period, visual analog scale scores and early wound healing index scores. The evaluation of bone density was performed at baseline, 3 months, and 6 months by using RVG.
Statistical Analysis Used:
Two-way analysis of variance and post hoc multiple comparison tests were used to compare data between test and control groups at different time points.
Results:
rhBMP-2 application was effective in reducing the overall treatment time and resulted in an increase in bone density around corticotomy sites at the end of the treatment period when compared to conventional corticotomy procedure. Placement of rhBMP-2 neither delayed wound healing nor affected participant pain scores.
Conclusions:
From this trial conducted over a period of 6 months, rhBMP-2 has the potential to function as a regenerative material in PAOO.
Key words: Bone morphogenetic protein 2, orthodontics, osteogenesis, periodontal ligament, periodontics
INTRODUCTION
To accelerate tooth movement, corticotomy procedure can be performed by perforating the cortical layer to the depth of the medullary bone.[1] During bone healing, a regional acceleratory phenomenon (RAP), which is a natural localized reaction of the soft- and hard-tissues, occurs in the periodontium in response to the injury and is associated with increased bone turnover and decreased bone density.[2,3] Advantages of the corticotomy technique include a decrease in treatment times, increased tooth movement without periodontally compromising the patient, and greater posttreatment stability due to the subsequent increase in bone volume through the dynamic demineralization–remineralization process.[4]
Kole in 1959 first performed corticotomy technique to accelerate tooth movement using the theory of bone block movements. He performed interdental, and osteotomy cuts in the cortical bone as cortical bone offers greater resistance to tooth movement.[5,6] A more invasive surgical orthodontic therapy, referred to as accelerated osteogenic orthodontics (AOO), was introduced by Wilcko et al. This technique requires palatal and vestibular approach with decortication using rotary systems and orthodontic appliances.[7]
Wilcko et al. modified the conventional AOO technique by combining corticotomy with alveolar grafting and referred to it as periodontally accelerated osteogenic orthodontics (PAOO).[8] In this technique, vertical corticotomies are joined with horizontal corticotomies in the upper or lower portion of the apex and 0.5 mm perforations are made on the vestibular and lingual cortical bone at the thickest area to induce maximum bleeding. Later, a bone graft is placed to stimulate osteoblastic activity. The placement of graft material helps in alveolar increase and remodeling.[8] When corticotomy was combined with bone grafting, it initiates the RAP and accelerates the tooth movement and also reduces the chances of gingival recession. This also results in decreased alveolar mineralization; the less mineralized the alveolar bone, the more easily the teeth move.[9] The placement of a bone graft results in an increase in bone density and volume around corticotomy sites at the end of the treatment period.[10,11,12,13,14]
Various alternatives to particulate bone grafts are being explored in corticotomy procedure to decrease the duration of the lag phase of orthodontic treatment and to increase the rate of tooth movement by enhancing the proliferation of osteoblasts.[10,11] At present, the feasibility of biological molecules such as prostaglandins, vascular endothelial growth factor, and basic fibroblast growth factor, are being evaluated to expedite and facilitate orthodontic tooth movement.[12,13] In this context, the role of various osteogenic proteins, including the bone morphogenetic proteins (BMPs), are being explored as an alternative for alveolar grafting.[14]
BMPs are a category of proteins that are involved in bone formation and repair. Among these, BMP-2 is one of the most potent members of the BMP family and has been used for correcting intrabony, supra-alveolar, furcation, and fenestration defects due to its osteoinductive property.[15,16] BMPs are also essential for the differentiation of osteoclasts, but their role in this process remains unclear.[17] Treatment of osteoclasts with exogenous BMP-2 directly enhances receptor activator of nuclear factor kappa-B ligand (RANKL)-stimulated differentiation of osteoclast precursors in vitro and stimulates survival and resorptive activity of mature osteoclasts that accelerates tooth movement.[18]
To the best of the author's knowledge, the synergistic use of corticotomy and BMP-2, in human subjects has not been described to date.[19] The present short-term study aims to primarily assess differences in orthodontic treatment duration, bone quality around the corticotomy sites, postoperative healing, and subjective pain when corticotomy was done conventionally and with the placement of recombinant human BMP-2 (rhBMP-2).
MATERIALS AND METHODS
Study design
This study is a double-blind, randomized controlled clinical trial in systemically healthy individuals undergoing orthodontic therapy and willing to participate in the study over 6 months period. Approval from the Institutional Ethical Committee was obtained, and informed consent was taken from all the individuals.
Source of data
Sample size calculation
The sample size for the present study was calculated using proportional power calculation. A minimum sample size of 24 was needed to detect a bone density difference of 50 hounsfield unit (HU) when the power of the test is 0.80 at a significance level of 0.05.
From a patient pool of 64 individuals, 30 individuals (mean age: 23.62 ± 6.23 years; 17 females) with moderate crowding of lower incisors based on Little's index and willing to undergo orthodontic treatment with extraction therapy of lower 1st premolars and PAOO were included in the study. Smokers and individuals with severe crowding of anterior teeth, periodontitis, and systemic diseases were excluded from the study.
Presurgical protocol, randomization, and blinding
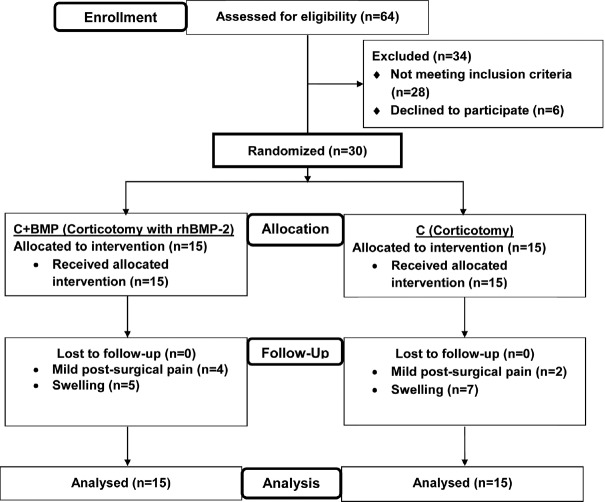
Using block randomization, individuals were randomly divided into two groups. Group A: 15 individuals treated with conventional corticotomy (C) and Group B: 15 individuals treated with corticotomy with rhBMP-2 (C + BMP). The sequence of procedures was as follows; premolar extractions were done after the treatment had been planned and a waiting period of 4 weeks was allowed for the healing of extraction socket. After a thorough phase-I periodontal therapy, banding and bonding were subsequently performed and the individuals were reassessed after 1 week for their oral hygiene compliance. The individuals were recalled for the surgical procedure after 2 weeks, and a 0.012 NiTi archwire was engaged into the brackets on the day the corticotomy. Individuals were blinded to intervention assignment. The orthodontist who performed the relevant treatment after PAOO was different from the orthodontist randomizing the individuals into C or C + BMP groups and hence was blinded to the treatment received by the patient. One periodontist performed the PAOO surgeries and another periodontist blinded to the therapy received by the individuals recorded the clinical and radiographic data pertinent to the study. The blind was not broken until the end of the study period [Figure 1].
Figure 1.
Trial profile and CONSORT diagram showing the flow of participants through each stage in the current study. n – number of subjects, C – Conventional corticotomy, BMP – Bone morphogenetic protein
Preparation of recombinant human bone morphogenetic protein-2 gel
A total of 20 μg of lyophilized human BMP-2 (ACRO Biosystems, Beijing, China) was reconstituted in sterile 100 μg/ml 20 mM Acetic Acid. A 1 μg/ml mixture was made by dissolving the reconstituted mixture into 1 ml of 10 mM Na-butyrate solution (Pro Lab Marketing Pvt. Ltd, New Delhi, India). Five batches of 1 μg/ml rhBMP-2 were prepared at a time and were stored at −20°C for up to 48 h. Subsequently, 5 ml of the solution was dissolved in a solvent mixture (ethanol: Propylene glycol: Water in the ratio of 50:30:20). Triethanolamine was added to adjust the pH to above 7.4. The solution was then gelled by adding 8% hydroxypropyl cellulose and set aside for 24 h. The in situ gel was prepared for the concentration of approximately 0.5 μg/mL and was stored at 4°C.
Surgical phase
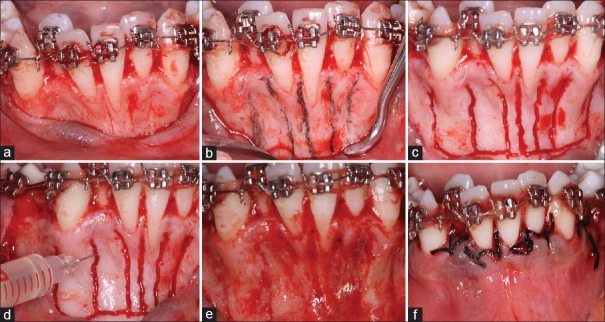
Corticotomy was performed as follows. After achieving profound anesthesia, a sulcular incision was given from mesiolabial line angle of mandibular right 2nd premolar extending along the crest of the edentulous area of 1st premolar up to the mesiolabial line angle of the mandibular left 2st premolar. A full-thickness flap was raised on the labial side of lower anterior exposing the cortical bone. Vertical grooves were marked on the bone surface using a sterile pencil. A 701 surgical bur was used to make the vertical grooves. These grooves were made all along the surface of the tooth starting 1–2 mm from the alveolar crest and extended 1–2 mm below the apices of the teeth. Horizontal subapical cuts were given joining the vertical cuts. After hemostasis, careful irrigation of the surgical site was done. In C + BMP-2 group, rhBMP-2 gel, which is a sticky stable material at room temperature, was placed across the corticotomy cuts and the flaps were sutured [Figure 2].
Figure 2.
Surgical procedure; full thickness mucoperiosteal flaps were raised (a) and the planned osteotomy cuts were marked with a sterile pencil (b). Vertical and sub-apical corticotomy cuts were given (c) and the recombinant human bone morphogenetic protein-2 gel was applied after securing hemostasis (d). The gel clings onto the osteotomy sites as a film if properly applied (e). Primary closure was obtained after gel application (f)
Orthodontic treatment duration analysis
Initiation of orthodontic force was done within 5–7 days by delivering a force of 130–150 g as measured with a Dontrix gauge. Appliance activation was done every 2 weeks until the alignment was considered to be satisfactory. At every visit, the amount of crowding relieved was measured using digital Vernier calipers and photographs. The time taken to unravel the crowding in the pretreatment and posttreatment study models and photographs was recorded.
Radiographic analysis
Radiographs were taken with the digitalized RVG machine (Carestream Dental RVG 5200®, Kodak, New Delhi, India) at 60 kVp/2 mA with the inactive interface. Immediately after the procedure, intraoral periapical through RVG were obtained. At baseline, 3, and 6 months, RVG was taken, and these RVG images were utilized to evaluate bone mineral density through HUs. Bone mineral density was measured, interdentally halfway along the length of the root from canine to canine, as HUs within a specific region of interest.
Measurement of bone density
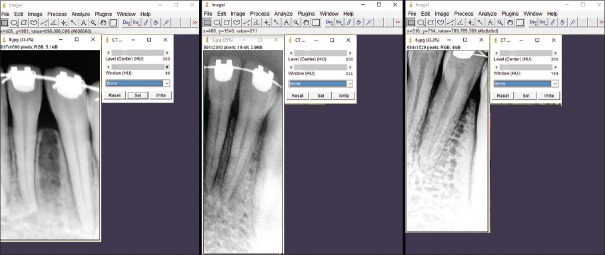
ImageJ was used to measure bone density values. ImageJ was used to automatically displays the HUs in the status bar when placing the curser on the image; for example: x = 404, y = 218, value = −896.00 (31872) where − 896 is your HU at the pixel locationp(x, y). The values were validated by using the computed tomography (CT) Window Level plugin which allows 16-bit digital imaging and communications in medicine (DICOM) grayscale images to be displayed with Window and Level specified in HUs, as is standard for CT scans. In a valid image, the plugin will display the image using any Window and Level settings specified in the DICOM header. If no such settings are specified the display defaults to the full range from minimum to maximum [Figure 3].
Figure 3.
Image J was used to automatically display the Hounsfield units in the status bar when placing the curser on the image. The values were validated by using the computed tomography window level plugin which allows 16-bit digital imaging and communications in medicine grayscale images to be displayed with Window and Level specified in Hounsfield units, as is standard for computed tomography scans
Measures of wound healing and pain
Early wound healing index (EHI)[20] was graded as: (1) Complete flap closure-no fibrin line in inter-proximal area; (2) Complete flap closure– fine fibrin line in inter-proximal area; (3) Complete flap closure-fibrin clot in the proximal area; (4) Incomplete flap closure– partial necrosis of inter-proximal tissue; (5) Incomplete flap closure– partial necrosis of inter-proximal tissue.
EHI was recorded at 1 and 2 weeks after surgery. Visual analog scale (VAS)[21] was recorded immediately after surgery and at 1st and 2nd weeks after the surgical procedure.
Statistical analysis
Data was analyzed by using Prism8® (GraphPad Software, San Diego, USA). Data were summarized by the mean ± standard deviation for continuous data and percentages for categorical data. The comparison between two-time points was done by Wilcoxon's signed test for scored data. The comparison between test and control was done by Mann–Whitney U-test for scored data. The comparison between different time points within the group was done by repeated one-way analysis of variance (ANOVA) and followed by post hoc multiple comparison test for continuous data. The comparison between test and control groups for different time points was done by repeated two-way ANOVA test and followed by post hoc multiple comparison test for continuous data. All values of P ≤ 0.05 were considered as statistically significant and P ≤ 0.001 was considered as highly statistically significant.
RESULTS
All enrolled individuals (n = 30) completed the treatment, and there were no dropouts. Mild postsurgical pain (n = 6) and swelling (n = 12) were the most common postsurgical complaints. However, no untoward effects or complications were seen.
Intragroup comparison
Orthodontic treatment duration
The mean Little's index values for the C and C + BMP groups were 10.77 and 10.10, respectively. The treatment period ranged from 7.5–18.1 weeks to 67–127 days (mean = 12.22 weeks; 102.90 days) in the C group and 7.2–14.1 weeks/28–127 days (mean = 10.22 weeks; 71.73 days) in the C + BMP group.
Bone density
The mean bone density values (in HU) in C + BMP group at baseline, 3 months, and 6 months were 51.22 ± 44.21, 254.89 ± 65.17, and 626.56 ± 122.72, respectively, and in C group were 67.6 ± 24.13, 192.8 ± 24.83, and 429.4 ± 118.46, respectively. A highly significant increase in bone density in the C + BMP group (P ≤ 0.001) and significant increase in bone density in the C group (P ≤ 0.05) was seen from baseline to 3 months and 6 months.
Early wound healing index
The difference in median EHI scores in the C + BMP and C groups was 2 and 1 at 1 week and 2 weeks, respectively. This reduction in intragroup EHI scores from 1 week to 2 weeks was significant in both the groups (P ≤ 0.05).
Postoperative pain (visual analog scale score)
The median reduction in VAS scores was from 5 to 4, 1–0 and 0.5–0 in the C + BMP group and 5–4.5, 1.5–0.5, and 0.5–0 in the C group at the immediate postoperative period, 1 week, and 2 weeks, respectively. This intragroup reduction in VAS scores was highly statistically significant in C + BMP group (P ≤ 0.001) and significant in C group (P ≤ 0.05) [Table 1].
Table 1.
Intergroup comparisons of early wound healing index and visual analog scale at different time based intervals using Mann-Whitney test
| Parameter | Duration | Group | Range | Median | IQR | P |
|---|---|---|---|---|---|---|
| EHI | 1 week | C + BMP (n=15) | 2-3 | 2 | 3-2 | 0.400† |
| C (n=15) | 2-3 | 2 | 1.5-1 | |||
| 2 weeks | C + BMP (n=15) | 1-2 | 1 | 1.5-1 | 0.478† | |
| C (n=15) | 1-2 | 1 | 1.5-1 | |||
| VAS | Postoperative | C + BMP (n=15) | 4-5 | 4 | 5-4 | 0.228† |
| C (n=15) | 4-5 | 5 | 5-4.5 | |||
| 1 week | C + BMP (n=15) | 0-1 | 1 | 1-0 | 0.0204* | |
| C (n=15) | 0-2 | 1 | 1.5-0.5 | |||
| 2 weeks | C + BMP (n=15) | 0-1 | 0 | 0.5-0 | 0.930† | |
| C (n=15) | 0-1 | 0 | 0.5-0 |
†Not significant (P>0.05); *Significant (P≤0.05). n - Number of subjects; EHI - Early wound healing index; VAS - Visual analog scale; C - Conventional corticotomy; C+BMP - Corticotomy with rhBMP-2; BMP - Bone morphogenetic proteins; IQR - Interquartile range; P - Significance level
Intergroup comparisons
Orthodontic treatment duration
There was no significant difference in the mean Little's index values between both the groups. Intergroup comparison of the treatment period showed a significant (P = 0.049) to highly statistically significant (P = 0.0003) difference in weeks and days between C and C + BMP groups [Table 2].
Table 2.
Intergroup treatment period comparison between the two groups in weeks and days
| Treatment period | Groups | Minimum-maximum | Mean±SD | P |
|---|---|---|---|---|
| Weeks | C (n=15) | 7.5-18.1 | 12.22±3.287 | 0.049* |
| C + BMP (n=15) | 7.2-14.1 | 10.22±2.015 | ||
| Days | C (n=15) | 67-127 | 102.90±16.99 | 0.0003** |
| C + BMP (n=15) | 28-127 | 71.73±23.95 |
*Significant (P≤0.05); **Highly significant (P≤0.001). C - Conventional corticotomy; C+BMP - Corticotomy with rhBMP-2; BMP - Bone morphogenetic proteins; SD - Standard deviation; n - Number of subjects; P - Significance level
Bone density
At baseline and 3 months, there were no significant differences in bone density between both the groups (P ≥ 0.05). However at 6 months, there was statistically significant difference in bone density (P ≤ 0.05) between both the treatment groups [Table 3].
Table 3.
Intergroup comparison of bone density at different time-based intervals in Hounsfield units
| Duration | Group | HU (mean±SD) | P |
|---|---|---|---|
| Baseline | C + BMP (n=15) | 51.22±44.21 | 0.463† |
| C (n=15) | 67.6±24.13 | ||
| 3 months | C + BMP (n=15) | 254.89±65.17 | 0.066† |
| C (n=15) | 192.8±24.83 | ||
| 6 months | C + BMP (n=15) | 626.56±122.72 | 0.013* |
| C (n=15) | 429.4±118.46 |
*Significant (P≤0.05); †Not significant (P>0.05). C - Conventional corticotomy; C + BMP - Corticotomy with rhBMP-2; BMP - Bone morphogenetic proteins; HUs - Hounsfield units; SD - Standard deviation; n - Number of subjects; P - Significance level
Early wound healing index
At the end of 1st week and 2nd week, no statistically significant differences in wound healing were observed in both the groups (P ≥ 0.05) [Table 1].
Postoperative pain (visual analog scale score)
1st day: The median values in VAS score between C + BMP and C groups were 4 and 5, respectively. The pain felt by individuals in C + BMP group was similar to C group, and the differences were not statistically significant (P ≥ 0.05). At the end of 1st week, C + BMP group showed more reduction in pain and results were statistically significant (P ≤ 0.05). At the end of 2 weeks, no statistically significant differences were observed among the two groups (P ≥ 0.05) [Table 1].
DISCUSSION
Long et al.[22] evaluated the effectiveness of accelerating orthodontic tooth movement and found that corticotomy is an effective and safe procedure and hastens orthodontic tooth treatment.[3,23,24,25,26] In this study, the treatment duration for individuals treated with corticotomy alone was 7.5–18.1 weeks. This is in agreement with the studies of Kole[23] and Shoreibah et al.,[24] who reported a treatment time of 6–12 weeks and 17.5 weeks respectively. Wilcko et al.,[25] reported that corticotomy technique reduces the treatment time by one-thirds when compared to conventional orthodontics and Gantes et al.,[3] stated that there are no adverse effects of corticotomy procedure on the periodontal apparatus during the treatment period. The treatment period for subjects treated with corticotomy and rhBMP-2 was significantly lesser than individuals treated with corticotomy alone (7.2–14.1 weeks vs. 7.5–18.1 weeks). A direct comparison with other studies is not possible as human trials have not been reported as yet on rhBMP-2 in PAOO. BMP-2 directly enhances RANKL-stimulated differentiation of osteoclast precursors in vitro and stimulates survival and resorptive activity of mature osteoclasts reducing bone mineralization.[18] When compared to conventional corticotomy, this decrease in mineralization occurs to a greater degree when BMP-2 is applied, reducing bone resistance to orthodontic movement explaining the decreased treatment time.[15,16,17,18,19]
In this study, corticotomy was performed, and rhBMP-2 was placed in the surgical site owing to its high osteogenic potential, and subsequently, bone density was measured. In a study conducted by Wilcko et al.,[26] where alveolar augmentation with demineralized freeze dried bone allograft/xenograft or alloplastic graft was done for teeth with corticotomy-assisted orthodontic tooth movement, there was a significant difference in the bone density values from baseline to 3 months and 6 months. Between both the treatment groups, the bone density values at the baseline and 3 months were not significant. However after 6 months, there was a significant difference (P ≤ 0.05) in the bone density attributable to the high osteoinductive property of BMP-2.[10,11,12,13,14,15,16,17,18,19] The observations are in agreement with the study by Bahammam[27] who stated that there was a significant increase in bone density from immediate posttreatment to 9 months in 3 groups where corticotomy was performed. The mean increase was 0.87% in modified corticotomy group, 31.99% in PAOO with bovine-derived xenograft group, and 13.71% in PAOO with bioactive glass group indicating that the net percentage increase in bone density was greater in bovine-derived xenograft group than in bioactive glass group and in modified corticotomy group. In this study, the mean bone density after 6 months in the C + BMP group was 626.56 HU (vs. 429.4 HU in the control group). Bone density may have increased significantly due to increase in bone turnover and decrease of bone density because osteoclasts and osteoblasts are increased due to a RAP.[26]
Contrary to the observations of the present study, Iglesias-Linares et al.,[19] in a study on Wistar rats concluded that there was little observable enhancement when two antagonistic approaches such as BMP-2 and corticotomy were combined on the tension and pressure sides as compared to the controls. Nowzari et al.,[28] had performed corticotomy procedure with the use of autogenous particulate grafting and have not observed any increase in thickness of maxillary and mandibular buccal plates. Autogenous bone graft is known to undergo extensive resorption when used in a lateral augmentation procedure.[29,30] Shoreibah et al.[24] compared corticotomy-facilitated orthodontics in adults using a modified technique (in which subapical horizontal cut was not given) versus traditional therapy in orthodontic tooth movement. They concluded that there was only reduction in duration of orthodontic therapy with no observable changes in bone density. However, Shoreibah et al.[31] conducted another study, in which they compared conventional corticotomy procedure and corticotomy with bone graft material and measured the bone density values. Six months posttreatment, bone density values in corticotomy group were 17.59% less than pretreatment values, while bone density values of corticotomy with bone graft group were 25.85% more than pretreatment values. In a study conducted by Stenport et al.,[32] BMP-2 failed to induce supracrestal bone growth around partially inserted implants in a dog model.[32,33]
In most of the studies carried out using rhBMP-2, it was delivered using absorbable collagen sponge for bone augmentation.[32,33] However in this study, rhBMP-2 was applied in the form of gel onto the corticotomy cuts. The advantage of rhBMP-2 in the gel form is that it can be easily applied to the surgical area. The concentration of rhBMP-2 used in dental applications vary between 0.5 and 2.5 μg/ml.[32,33,34,35] In this study, the authors chose to use the gel at a minimum required concentration of 0.5 μg/ml. It was found that higher concentration of rhBMP-2 stimulates recruitment and differentiation of osteoclasts, thus possibly promoting the resorption of newly formed bone.[32,33,34,35]
In this study, mild postsurgical pain was observed in both the study groups. Vercellotti and Podesta,[34] performed corticotomy procedure using piezosurgery and autogenous bone/bone graft substitute and noticed mild postoperative swelling of the face on the 3rd day after surgery with no inflammation of oral tissues. Nevins et al.[35] reported that absorbable collagen sponge implants impregnated with rhBMP-2 did not provoke immune, toxic, or adverse responses. In a study by Rajasekaran and Krishna Nayak,[36] mild pain was reported that persisted for 3 days. Thind et al.,[37] observed negligible root resorption when PAOO was performed with a piezosurgical unit and surgical bur.
CONCLUSIONS
In view of the present findings, both corticotomy with rhBMP-2 and conventional corticotomy are effective in accelerating tooth movement. From this short-term trial conducted over a period of 6 months, rhBMP-2 has the potential to function as a regenerative material in PAOO. As an osteoinductive material, rhBMP-2 can reduce the anticipated loss of dentoalveolar bone and can increase regional bone density.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Baloul SS, Gerstenfeld LC, Morgan EF, Carvalho RS, Van Dyke TE, Kantarci A, et al. Mechanism of action and morphologic changes in the alveolar bone in response to selective alveolar decortication-facilitated tooth movement. Am J Orthod Dentofacial Orthop. 2011;139:S83–101. doi: 10.1016/j.ajodo.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 2.Frost HM. The biology of fracture healing. An overview for clinicians. Part I. Clin Orthop Relat Res. 1989;248:283–93. [PubMed] [Google Scholar]
- 3.Gantes B, Rathbun E, Anholm M. Effects on the periodontium following corticotomy-facilitated orthodontics. Case reports. J Periodontol. 1990;61:234–8. doi: 10.1902/jop.1990.61.4.234. [DOI] [PubMed] [Google Scholar]
- 4.Wilcko MT, Wilcko WM, Pulver JJ, Bissada NF, Bouquot JE. Accelerated osteogenic orthodontics technique: A 1-stage surgically facilitated rapid orthodontic technique with alveolar augmentation. J Oral Maxillofac Surg. 2009;67:2149–59. doi: 10.1016/j.joms.2009.04.095. [DOI] [PubMed] [Google Scholar]
- 5.Ibarra BD, Climent MH, Calvo PL. La expansion quirurgica de la cresta alveolar mediante corticotomia. Gaceta Dental. 2011;22:130–49. [Google Scholar]
- 6.Andrade MS, Sierra CG, Hernandez CH. Ortodonica acelerada periodontalmante: fundamentos biologicos tecnicas quirurgicas. Rev Max Periodontal. 2011;2:12–6. [Google Scholar]
- 7.Wilcko MT, Wilcko WM, Bissada NF. An evidence-based analysis of periodontally accelerated orthodontic and osteogenic techniques: A synthesis of scientific perspectives. Semin Orthod. 2008;14:305–16. [Google Scholar]
- 8.Blazquez EK, Villalonga PG, Coral AM, Perez AP. La corticotomia alveolar selective como coadyuvante al tratamiento de ortodoncia. Rev Esp Ortod. 2010;40:215–30. [Google Scholar]
- 9.Mao LX, Shen GF, Fang B, Xia YH, Ma XH, Wang B, et al. Bone grafting, corticotomy, and orthodontics: Treatment of cleft alveolus in a Chinese cohort. Cleft Palate Craniofac J. 2013;50:662–70. doi: 10.1597/12-034R. [DOI] [PubMed] [Google Scholar]
- 10.Davidovitch Z. Tooth movement. Crit Rev Oral Biol Med. 1991;2:411–50. doi: 10.1177/10454411910020040101. [DOI] [PubMed] [Google Scholar]
- 11.Krishnan V, Davidovitch Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop. 2006;129:469.e1–32. doi: 10.1016/j.ajodo.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Seifi M, Badiee MR, Abdolazimi Z, Amdjadi P. Effect of basic fibroblast growth factor on orthodontic tooth movement in rats. Cell J. 2013;15:230–7. [PMC free article] [PubMed] [Google Scholar]
- 13.Kohno S, Kaku M, Kawata T, Fujita T, Tsutsui K, Ohtani J, et al. Neutralizing effects of an anti-vascular endothelial growth factor antibody on tooth movement. Angle Orthod. 2005;75:797–804. doi: 10.1043/0003-3219(2005)75[797:NEOAAE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Wu X, Shi W, Cao X. Multiplicity of BMP signaling in skeletal development. Ann N Y Acad Sci. 2007;1116:29–49. doi: 10.1196/annals.1402.053. [DOI] [PubMed] [Google Scholar]
- 15.Wang EA. Bone morphogenetic proteins (BMPs): Therapeutic potential in healing bony defects. Trends Biotechnol. 1993;11:379–83. doi: 10.1016/0167-7799(93)90096-R. [DOI] [PubMed] [Google Scholar]
- 16.Hollinger JO, Uludag H, Winn SR. Sustained release emphasizing recombinant human bone morphogenetic protein-2. Adv Drug Deliv Rev. 1998;31:303–18. doi: 10.1016/s0169-409x(97)00126-9. [DOI] [PubMed] [Google Scholar]
- 17.Kanatani M, Sugimoto T, Kaji H, Kobayashi T, Nishiyama K, Fukase M, et al. Stimulatory effect of bone morphogenetic protein-2 on osteoclast-like cell formation and bone-resorbing activity. J Bone Miner Res. 1995;10:1681–90. doi: 10.1002/jbmr.5650101110. [DOI] [PubMed] [Google Scholar]
- 18.Itoh K, Udagawa N, Katagiri T, Iemura S, Ueno N, Yasuda H, et al. Bone morphogenetic protein 2 stimulates osteoclast differentiation and survival supported by receptor activator of nuclear factor-kappaB ligand. Endocrinology. 2001;142:3656–62. doi: 10.1210/endo.142.8.8300. [DOI] [PubMed] [Google Scholar]
- 19.Iglesias-Linares A, Yañez-Vico RM, Moreno-Fernandez AM, Mendoza-Mendoza A, Solano-Reina E. Corticotomy-assisted orthodontic enhancement by bone morphogenetic protein-2 administration. J Oral Maxillofac Surg. 2012;70:e124–32. doi: 10.1016/j.joms.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 20.Fickl S, Thalmair T, Kebschull M, Böhm S, Wachtel H. Microsurgical access flap in conjunction with enamel matrix derivative for the treatment of intra-bony defects: A controlled clinical trial. J Clin Periodontol. 2009;36:784–90. doi: 10.1111/j.1600-051X.2009.01451.x. [DOI] [PubMed] [Google Scholar]
- 21.Seymour RA, Charlton JE, Phillips ME. An evaluation of dental pain using visual analogue scales and the Mcgill pain questionnaire. J Oral Maxillofac Surg. 1983;41:643–8. doi: 10.1016/0278-2391(83)90017-4. [DOI] [PubMed] [Google Scholar]
- 22.Long H, Pyakurel U, Wang Y, Liao L, Zhou Y, Lai W, et al. Interventions for accelerating orthodontic tooth movement: A systematic review. Angle Orthod. 2013;83:164–71. doi: 10.2319/031512-224.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kole H. Surgical operations on the alveolar ridge to correct occlusal abnormalities. Oral Surg Oral Med Oral Pathol. 1959;12:515–29. doi: 10.1016/0030-4220(59)90153-7. [DOI] [PubMed] [Google Scholar]
- 24.Shoreibah EA, Salama AE, Attia MS, Abu-Seida SM. Corticotomy-facilitated orthodontics in adults using a further modified technique. J Int Acad Periodontol. 2012;14:97–104. [PubMed] [Google Scholar]
- 25.Wilcko WM, Wilcko MT, Bouquot JE, Ferguson DJ. Accelerated orthodontics with alveolar reshaping. J Orthop Pract. 2000;10:63–70. [Google Scholar]
- 26.Wilcko WM, Wilcko T, Bouquot JE, Ferguson DJ. Rapid orthodontics with alveolar reshaping: Two case reports of decrowding. Int J Periodontics Restorative Dent. 2001;21:9–19. [PubMed] [Google Scholar]
- 27.Bahammam MA. Effectiveness of bovine-derived xenograft versus bioactive glass with periodontally accelerated osteogenic orthodontics in adults: A randomized, controlled clinical trial. BMC Oral Health. 2016;16:126. doi: 10.1186/s12903-016-0321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nowzari H, Yorita FK, Chang HC. Periodontally accelerated osteogenic orthodontics combined with autogenous bone grafting. Compend Contin Educ Dent. 2008;29:200–6. [PubMed] [Google Scholar]
- 29.von Arx T, Cochran DL, Hermann JS, Schenk RK, Buser D. Lateral ridge augmentation using different bone fillers and barrier membrane application. A histologic and histomorphometric pilot study in the canine mandible. Clin Oral Implants Res. 2001;12:260–9. doi: 10.1034/j.1600-0501.2001.012003260.x. [DOI] [PubMed] [Google Scholar]
- 30.Kim SJ, Park YG, Kang SG. Effects of corticision on paradental remodeling in orthodontic tooth movement. Angle Orthod. 2009;79:284–91. doi: 10.2319/020308-60.1. [DOI] [PubMed] [Google Scholar]
- 31.Shoreibah EA, Ibrahim SA, Attia MS, Diab MM. Clinical and radiographic evaluation of bone grafting in corticotomy-facilitated orthodontics in adults. J Int Acad Periodontol. 2012;14:105–13. [PubMed] [Google Scholar]
- 32.Stenport VF, Roos-Jansåker AM, Renvert S, Kuboki Y, Irwin C, Albrektsson T, et al. Failure to induce supracrestal bone growth between and around partially inserted titanium implants using bone morphogenetic protein (BMP): An experimental study in dogs. Clin Oral Implants Res. 2003;14:219–25. doi: 10.1034/j.1600-0501.2003.00861.x. [DOI] [PubMed] [Google Scholar]
- 33.Razzouk S, Sarkis R. BMP-2: Biological challenges to its clinical use. N Y State Dent J. 2012;78:37–9. [PubMed] [Google Scholar]
- 34.Vercellotti T, Podesta A. Orthodontic microsurgery: A new surgically guided technique for dental movement. Int J Periodontics Restorative Dent. 2007;27:325–31. [PubMed] [Google Scholar]
- 35.Nevins M, Kirker-Head C, Nevins M, Wozney JA, Palmer R, Graham D, et al. Bone formation in the goat maxillary sinus induced by absorbable collagen sponge implants impregnated with recombinant human bone morphogenetic protein-2. Int J Periodontics Restorative Dent. 1996;16:8–19. [PubMed] [Google Scholar]
- 36.Rajasekaran UB, Krishna Nayak US. Effect of prostaglandin E1 versus corticotomy on orthodontic tooth movement: An in vivo study. Indian J Dent Res. 2014;25:717–21. doi: 10.4103/0970-9290.152170. [DOI] [PubMed] [Google Scholar]
- 37.Thind SK, Chatterjee A, Arshad F, Sandhu PS, Thind MS, Nahin J, et al. Aclinical comparative evaluation of periodontally accelerated osteogenic orthodontics with piezo and surgical bur: An interdisciplinary approach. J Indian Soc Periodontol. 2018;22:328–33. doi: 10.4103/jisp.jisp_359_16. [DOI] [PMC free article] [PubMed] [Google Scholar]