Abstract
Context:
Periodontitis and diabetes are universally prevalent diseases which are interlinked with each other. Inflammatory products released both by the microorganisms, and the host plays a pivotal role in causing both the diseases. Pentraxins are acute-phase proteins which are often found to be elevated in inflammatory states. Anti-inflammatory agents have a very important role to play in curbing infection of which, aspirin and omega-3 fatty acids (O3FAs) are being administered often nowadays. Nonsurgical periodontal therapy (NSPT) remains the gold standard of treatment, and other agents have been used as adjuvants only, to increase the efficacy of treatment.
Aims:
This study compares the effects of low-dose aspirin versus O3FAs when used as adjuvants to NSPT in patients with diabetes and chronic periodontitis.
Settings and Design:
A total of 42 patients (mean age of 30–65 years) from a diabetic center who were diagnosed with Type II diabetes and chronic periodontitis were included in the study.
Materials and Methods:
This study was done in the department of periodontics of a tertiary referral care hospital in Hyderabad, in collaboration with a reputed diabetic center.
Statistical Analysis Used:
Intragroup comparison was done using the paired t-test for continuous data and Wilcoxon signed-rank test for score data. Intergroup comparison was compiled using independent t-test. All P < 0.05 were considered statistically significant.
Results:
Intragroup comparison at baseline and 3 months after NSPT showed statistically significant results (P < 0.001) in all the three groups pertaining to the clinical (gingival index, probing pocket depth, and clinical attachment level) and biochemical (glycosylated hemoglobin and pentraxin) parameters. However, the intergroup comparison showed a significant improvement in Group II related to pentraxin levels only (P < 0.001).
Conclusions:
O3FAs proved to be better than low-dose aspirin and placebo after NSPT.
Key words: Chronic periodontitis, diabetes mellitus, lipoxins, nonsurgical periodontal therapy, pentraxin, resolvins
INTRODUCTION
Periodontal disease is initiated by plaque formation. Plaque is a biofilm which is laden with microorganisms and food debris and if left to mature, causes periodontitis. It involves a complex interplay of inflammatory products such as cytokines, chemokines, and matrix metalloproteinase released both by the putative pathogens and the host. When the host response is not able to ward off the toxic insult by the microorganisms, tissue injury occurs culminating in pocket formation, attachment loss, and bone loss. Diabetes mellitus is an endocrine disorder related to the insufficient release of insulin-causing hyperglycemia. Insulin resistance could occur due to the improper functioning of the pancreas or could also be due to the noxious products released by the periodontal pathogens.[1]
It has often been observed that both diseases are interlinked. The chronic inflammatory condition leading to detrimental inflammatory events has been observed in both the diseases. In many studies, it has been stated that the systemic levels of the inflammatory mediators, such as C-reactive protein, tumor necrosis factor-alpha, and interleukin-6, are elevated in periodontitis which may aggravate diabetes in immunocompromised individuals.[2,3,4] Hence, plasma pentraxin (PTX3) which belongs to the C-reactive protein family could serve as a good marker of the inflammatory status. Oxidative stress is one of the major determining factors of chronic inflammation, and it has been studied that the neutrophils are hyperactive in these conditions and express reactive oxygen species which in turn stimulate pro-inflammatory pathways which cause insulin resistance in patients with diabetes and periodontitis.[5] Nonsurgical periodontal therapy has shown positive results in patients afflicted with both the conditions. Host-modulation therapy in the form of nonsteroidal anti-inflammatory drugs, bisphosphonates, has shown promising results but they have their side effects; hence, the newer lipid mediators, such as low-dose aspirin and omega-3 fatty acids (O3FAs), are being evaluated. A study has been conducted using low-dose aspirin with O3FAs in patients with diabetes and periodontitis;[6] however, their efficacy individually has not been compared, thus this study aimed to do so.
MATERIALS AND METHODS
This randomized clinical study was carried out in the Department of Periodontics of a tertiary referral care center in collaboration with a reputed diabetic center in Hyderabad. The Institutional Ethical Committee approved this study, and it was also registered (NCT03599401). Participants were enrolled from December 2016 to May 2017 and were asked to sign the consent form before the commencement of the study.
To achieve a difference of 0.43 in the levels of PTX3 pre- and post-drug administration in test groups with power at 80% and level of significance 5%, it was analyzed that 42 samples would be sufficient for enrollment into the study.
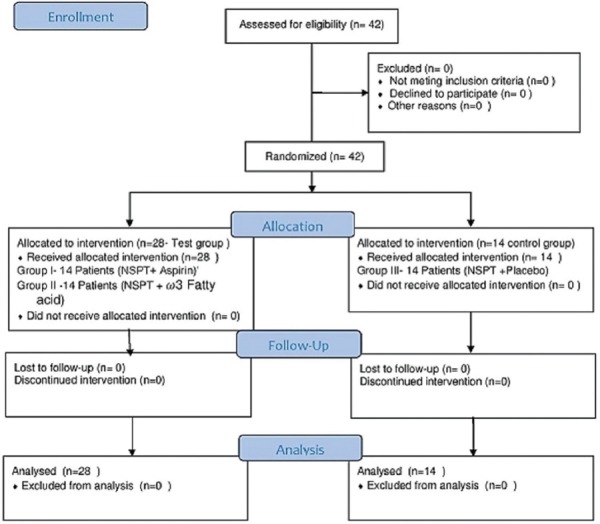
42 patients of both sexes were selected from the outpatient ward of the diabetic center and were equally divided into three groups. After scaling and root planning (Nonsurgical periodontal therapy [NSPT]), 14 participants in Group I (Test group) received 75 mg of aspirin orally (ecosprin 75 mg, USV Private Ltd.) once a day for 3 months, 14 participants in Group II (Test Group) received 500 mg of O3FAs orally (fish oil O3FA, Inlife group) twice a day for 3 months, and the control group (Group III) comprising of 14 patients received placebo (empty gelatin capsules) orally twice a day for 3 months [Figure 1].
Figure 1.
CONSORT flow diagram. n – Number of patients
Inclusion criteria
Patients with Type 2 diabetes having glycosylated hemoglobin (HbAIc) values ≥6.5%, as per the American Diabetes Association criteria and taking metformin 500 mg/day by oral route and having chronic periodontitis as per the American Academy of Periodontology guidelines which included the presence of minimum of 15 natural teeth and at least four teeth with one or more sites with probing depth (PD) ≥5 mm and clinical attachment level (CAL) ≥4 mm were included in the study.[7,8]
Exclusion criteria
Patients with systemic diseases other than Type II diabetes mellitus, participants having undergone periodontal therapy within 12 months, or taking antibiotics within 3 months, smokers, pregnant and lactating mothers, and participants who were intolerant to aspirin and O3FAs were excluded from the study.
Clinical variables
All the patients underwent clinical examination, wherein the extent of periodontitis was measured using a Williams probe. The indices measuring both the gingival status (gingival index [GI]) and periodontal condition (probing pocket depth [PPD] and CAL) were recorded preoperatively and 3 months after scaling and root planing.[9,10,11]
Biochemical parameters
Collection of blood sample and serum separation
Random blood samples (2 ml) were collected by venipuncture of antecubital vein. About 1 mL was placed in two separate test tubes. About 1 mL of whole blood was used for HbA1c estimation. 10 min after collection, the other test tube containing 1 mL blood was subjected to centrifugation at 3000 rpm for 10 min. The supernatant straw-colored fluid (plasma) was separated into two storage vials for PTX3.
Assessment of glycosylated hemoglobin
HbA1c estimation was done at baseline and 3 months using spectrophotometric analysis (Erbachem 5 plus v2 Transasia Bio-medicals Limited, Mumbai, India) [Figure 2].
Figure 2.

Analysis of HbA1c by spectrophotometer
Assessment of plasma pentraxin
It was done at baseline and 3 months after NSPT by commercial kit (Boster Biological Technologies, Co. Ltd., USA). The assay employs the quantitative sandwich enzyme immunoassay technique [Figure 3].
Figure 3.

Plasma pentraxin3 evaluation by ELISA
Reagents used
Antibody-coated microtiter strips, Biotin-labeled antibody concentrate (×100), Streptavidin-horseradish peroxidase conjugate, Master standard, Dilution buffer, Biotin-Ab diluent, Wash solution concentrate (×10), Substrate solution, and Stop solution [Figure 4].
Figure 4.

Reagents used for ELISA
Primary and Secondary outcomes
Change in plasma pentraxin and glycated hemoglobin levels from baseline to 3 months was the primary outcomes assessed. Change in GI, PD, and CAL levels was the secondary outcomes assessed.
Statistical analysis
The statistical analysis made the use of Statistical Package for Social Sciences (SPSS) version 22.0 (Armonk NY: IBM United States. IBM Corp. 2013) and was summarized using mean ± standard deviation for continuous data and median ± interquartile range for score data. Categorical data were calculated in percentages. The intragroup comparison between baseline and 3 months was done using the paired t-test for continuous data and Wilcoxon signed-rank test for score data. The intergroup comparison was done using independent t-test. All P < 0.05 was considered as statistically significant.
RESULTS
Intragroup comparison: All the three groups showed a statistically significant improvement pertaining to all the clinical parameters such as GI, PD, and CAL from baseline to 3 months (P < 0.001). Even, the HbA1c showed marked improvement from baseline to 3 months in all the three groups. Pentraxin levels also dropped from baseline to 3 months in all the groups [Tables 1-3].
Table 1.
Intragroup comparison in Group I (aspirin+nonsurgical periodontal therapy)
| Parameters | Group I | n | Minimum | Maximum | Mean±SD | P |
|---|---|---|---|---|---|---|
| GI | Baseline | 14 | 1.6 | 2.6 | 2.04±0.35 | <0.001** |
| 3 months | 14 | 0.6 | 1.8 | 1.24±0.35 | ||
| PD | Baseline | 14 | 6.0 | 7.0 | 6.43±0.51 | <0.001** |
| 3 months | 14 | 4.0 | 5.0 | 4.43±0.51 | ||
| CAL | Baseline | 14 | 5.0 | 6.0 | 5.43±0.51 | <0.001** |
| 3 months | 14 | 3.0 | 4.0 | 3.43±0.51 | ||
| Hb1Ac% | Baseline | 14 | 6.9 | 11.7 | 8.97±1.46 | <0.001** |
| 3 months | 14 | 5.7 | 8.5 | 6.98±0.88 | ||
| Ptxpg/ml | Baseline | 14 | 63.45 | 2643.0 | 359.13±698.67 | 0.001** |
| 3 months | 14 | 35.94 | 268.60 | 101.42±72.31 |
**P<0.05 statistically significant. n – Sample size, SD – Standard deviation; GI – Gingival index; PD – Probing pocket depth; CAL – Clinical attachment level; Hb1Ac – Glycosylated hemoglobin; Ptx – Pentraxin; P– Probability value
Table 3.
Intragroup comparison in the control group (placebo+nonsurgical periodontal therapy)
| Parameter | Group III | n | Minimum | Maximum | Mean±SD | P |
|---|---|---|---|---|---|---|
| GI | Baseline | 14 | 1.2 | 3 | 1.96±0.44 | <0.001** |
| 3 months | 14 | 0.2 | 2 | 1.14±0.57 | ||
| PD | Baseline | 14 | 6.0 | 7 | 6.43±0.51 | <0.001** |
| 3 months | 14 | 4.0 | 5 | 4.43±0.51 | ||
| CAL | Baseline | 14 | 5.0 | 6 | 5.43±0.51 | <0.001** |
| 3 months | 14 | 3.0 | 4 | 3.43±0.51 | ||
| Hb1Ac% | Baseline | 14 | 6.6 | 10 | 7.54±0.82 | <0.001** |
| 3 months | 14 | 6.2 | 9 | 7.25±0.81 | ||
| Ptxpg/ml | Baseline | 14 | 62.780 | 7374 | 721.29±1934.03 | 0.001** |
| 3 months | 14 | 57.01 | 1378 | 212.44±347.01 |
**P<0.05 statistically significant. n – Sample size; SD – Standard deviation; GI – Gingival index; PD – Probing pocket depth; CAL – Clinical attachment level; Hb1Ac – Glycosylated hemoglobin; Ptx – Pentraxin; P – Probability value
Table 2.
Intragroup comparison in Group II ( omega-3 fatty acid+nonsurgical periodontal therapy)
| Parameter | Group II | n | Minimum | Maximum | Mean±SD | P |
|---|---|---|---|---|---|---|
| GI | Baseline | 14 | 1.5 | 2.5 | 2.03±0.30 | <0.001** |
| 3 months | 14 | 0.3 | 1.9 | 1.26±0.44 | ||
| PD | Baseline | 14 | 6.0 | 7.0 | 6.71±0.47 | <0.001** |
| 3 months | 14 | 4.0 | 5.0 | 4.71±0.47 | ||
| CAL | Baseline | 14 | 5.0 | 6.0 | 5.71±0.47 | <0.001** |
| 3 months | 14 | 3.0 | 4.0 | 3.71±0.47 | ||
| Hb1Ac% | Baseline | 14 | 6.8 | 11.4 | 8.079±1.15 | <0.001** |
| 3 months | 14 | 6.0 | 10.6 | 7.136±1.21 | ||
| Ptxpg/ml | Baseline | 14 | 20.60 | 198.00 | 264.47±497.47 | 0.009* |
| 3 months | 14 | 35.62 | 125.70 | 76.63±25.65 |
**P<0.05 statistically significant, n – Sample size; SD – Standard deviation; *GI – Gingival index; PD – Probing pocket depth; CAL – Clinical attachment level; Hb1Ac – Glycosylated hemoglobin; Ptx – Pentraxin; P– Probability value
Intergroup comparison: When a comparison was made between Group I and Group II after 3 months of NSPT, it was observed that there was no significant difference related to the clinical parameters (GI, PD, and CAL) and the HbA1c levels. However, related to the PTX3 levels, it was observed that patients in Group II (O3FA + NSPT) had marked improvement when compared to Group I (low-dose aspirin + NSPT) (P = 0.035) [Table 4].
Table 4.
Intergroup comparison between Group I and Group II
| ParametersAfter 3 months | Groups | n | Minimum | Maximum | Mean±SD | P |
|---|---|---|---|---|---|---|
| GI | Group I | 14 | 0.6 | 1.8 | 1.24±0.35 | 0.888 NS |
| Group II | 14 | 0.3 | 1.9 | 1.26±0.44 | ||
| PD | Group I | 14 | 4.0 | 5.0 | 4.43±0.51 | 0.136 NS |
| Group II | 14 | 4.0 | 5.0 | 4.71±0.47 | ||
| CAL | Group I | 14 | 3.0 | 4.0 | 3.43±0.51 | 0.136 NS |
| Group II | 14 | 3.0 | 4.0 | 3.71±0.47 | ||
| Hb1Ac% | Group I | 14 | 5.7 | 8.5 | 6.98±0.88 | 0.698 NS |
| Group II | 14 | 6.0 | 10.6 | 7.136±1.21 | ||
| Ptxpg/ml | Group I | 14 | 35.94 | 268.60 | 101.42±72.31 | 0.035* |
| Group II | 14 | 35.62 | 125.70 | 76.63±25.65 |
*P<0.05 statistically significant; *GI – Gingival index; PD – Probing pocket depth; CAL – Clinical attachment level; NS – Not significant; Hb1Ac – Glycosylated hemoglobin; Ptx – Pentraxin; n – Sample size; SD – Standard deviation; P– Probability value
Comparing results between Group I and Group III also, it was found that there was no difference between the groups pertaining to the clinical parameters as well as the HbA1c levels 3 months after NSPT, however, PTX3 levels were markedly lower in Group I (low-dose aspirin + NSPT) when compared to Group III (placebo + NSPT) (P < 0.001) [Table 5].
Table 5.
Intergroup comparison between Group I and Group III
| Parameters After 3 months |
Groups | n | Minimum | Maximum | Mean ± SD | P |
|---|---|---|---|---|---|---|
| GI | Group I | 14 | 0.6 | 1.8 | 1.24±0.35 | 0.584 NS |
| Group III | 14 | 0.2 | 2 | 1.14±0.57 | ||
| PD | Group I | 14 | 4.0 | 5.0 | 4.43±0.51 | 1 NS |
| Group III | 14 | 4.0 | 5 | 4.43±0.51 | ||
| CAL | Group I | 14 | 3.0 | 4.0 | 3.43±0.51 | 1 NS |
| Group III | 14 | 3.0 | 4 | 3.43±0.51 | ||
| Hb1Ac% | Group I | 14 | 5.7 | 8.5 | 6.98±0.88 | 0.403 NS |
| Group III | 14 | 6.2 | 9 | 7.25±0.81 | ||
| Ptxpg/ml | Group I | 14 | 35.94 | 268.60 | 101.42±72.31 | <0.001** |
| Group III | 14 | 57.01 | 1378 | 212.44±347.01 |
**P < 0.05 statistically significant. GI – Gingival index; PD – Probing pocket depth; CAL – Clinical attachment level; Hb1Ac – Glycosylated hemoglobin; Ptx – Pentraxin; n – Sample size; SD – Standard deviation; NS – Not significant; P – Probability value
Hence also, when matching the results between Group II and Group III, it was deciphered that all the clinical parameters as well as HbA1c levels did not show significant difference; however, PTX3 levels were markedly less in Group II (O3FA + NSPT) when compared to Group III (placebo + NSPT) (P < 0.001) [Table 6].
Table 6.
Intergroup comparison between Group II and Group III
| Parameters After 3 months |
Groups | n | Minimum | Maximum | Mean±SD | P |
|---|---|---|---|---|---|---|
| GI | Group II | 14 | 0.3 | 1.9 | 1.26±0.44 | 0.536 NS |
| Group III | 14 | 0.2 | 2 | 1.14±0.57 | ||
| PD | Group II | 14 | 4.0 | 5.0 | 4.71±0.47 | 0.136 NS |
| Group III | 14 | 4.0 | 5 | 4.43±0.51 | ||
| CAL | Group II | 14 | 3.0 | 4.0 | 3.71±0.47 | 0.136 NS |
| Group III | 14 | 3.0 | 4 | 3.43±0.51 | ||
| Hb1Ac% | Group II | 14 | 6.0 | 10.6 | 7.136±1.21 | 0.771 NS |
| Group III | 14 | 6.2 | 9 | 7.25±0.81 | ||
| Ptxpg/ml | Group II | 14 | 35.62 | 125.70 | 76.63±25.65 | <0.001** |
| Group III | 14 | 57.01 | 1378 | 212.44±347.01 |
**P<0.05 statistically significant. GI – Gingival index; PD – Probing pocket depth; CAL – Clinical attachment level; Hb1Ac – Glycosylated hemoglobin; Ptx – Pentraxin; n – Sample size; SD – Standard deviation; NS – Not significant; P – Probability value
Thus, pertaining to PTX3 levels, the greatest improvement was observed in Group II (O3FA + NSPT), followed by Group I ((low-dose aspirin + NSPT). The least improvement was observed in Group III (placebo + NSPT).
DISCUSSION
Periodontitis is a polymicrobial disease which is initiated by the formation and maturation of plaque. Plaque is a biofilm which facilitates the growth and survival of the putative periodontal pathogens which cannot be easily eliminated by the host. In addition to the microbial insult, other risk factors, such as smoking and genetic factors, have a very important role to play in the progression of periodontitis. Equally crucial as a risk factor is diabetes. It has been observed that diabetics have an increased propensity for periodontitis and respond poorly to therapy.[12]
Diabetics are more prone for micro- and macrovascular complications such as retinopathy, nephropathy, and importantly atherosclerosis of the large blood vessels causing coronary artery disease, stroke, and pain in the lower extremities due to improper blood circulation. Neuropathy is also a very common feature in them.[13]
In the US National Health and Nutrition Examination Survey III, it was observed that adults with a HbA1c level of >9% had increased prevalence of periodontitis when matched with nondiabetics of the same age, ethnicity socioeconomic, and smoking status.[14]
In another cross-sectional study conducted, it was seen that among the 1400 participants examined, diabetics were 2.3 times more prone to periodontal attachment loss.[15]
Inflammation is seen commonly in both diabetes and periodontitis, and PTX3 which belongs to the long-acting pentraxin family has proved to be a highly sensitive and specific marker in inflammatory states.[16]
A study conducted by researchers on 163 participants, evaluated the association between the elevated levels of PTX3 and the development and/or progression of diabetic retinopathy (DR). They concluded that plasma PTX3 is positively associated with DR development and progression and may be a more accurate predictor of DR development than high-sensitive C-reactive protein.[17]
In a study done on 50 participants, (25 with peripheral arterial disease (PAD) and periodontitis and 25 with periodontitis and without PAD) to examine the correlation between PAD and periodontal disease by examining the levels of inflammatory cytokines, PTX3, and high-sensitive C-reactive protein and periodontal clinical parameters, it was observed that the periodontal clinical parameters as well as the biochemical parameters were higher in the group with PAD and periodontitis.[18]
In this study, also both the clinical (GI, PD, and CAL) and biochemical parameters (PTX3 and HbA1c) were elevated in both the test groups as well as the control group [Tables 1-3].
In patients with diabetes and periodontitis, it was observed that nonsurgical periodontal therapy can facilitate metabolic control and improve insulin sensitivity, by reducing peripheral inflammatory cytokine levels.[19]
In a meta-analysis, 639 identified studies were screened. A total of 371 patients with periodontitis and diabetes were included in the study. The follow-up after NSPT was assessed for 3–9 months. It was concluded that periodontal treatment leads to an improvement of glycemic control in Type 2 diabetic patients for at least 3 months.[20]
In another meta-analysis, ten trials of 1135 patients were included in the study. A randomized controlled trial was selected if it investigated periodontal therapy for diabetic participants compared with a control group who received no periodontal treatment for at least 3 months of the follow-up period. The primary outcome was hemoglobin A1c (HbA1c) and secondary outcomes were periodontal parameters including PPD and CAL. After 3 months, treatment substantially lowered HbA1c compared with no treatment after periodontal therapy. However, there was no significant change in HbA1c levels between the groups after 6 months.[21]
This study tallied with the meta-analysis, as there was an improvement in the HbA1c levels 3 months after NSPT in all the three groups [Tables 1-3].
Inflammation in a susceptible host causes progression of any disease and periodontitis and diabetes also follow the same trend. Therefore, the resolution of inflammation is of prime importance to control and stop the spread of disease. Aspirin plays an important role in the generation of lipoxin. It inhibits the synthesis of prostaglandins (PGs) and also cyclooxygenase-2 (COX-2) by acetylation of COX-2, which generates a new still more active enzyme, 15R-LO. The end product is 15E LX or aspirin-triggered lipoxin which is more potent than LXA4 in combating infection.[22]
A study was conducted on 308 cardiac patients, 162 taking low-dose aspirin (75 mgms to 150 mgms-test group) for <6 months and 146 participants who were not taking aspirin (control group). The indices measured where the oral hygiene index simplified, clinical attachment loss, and bleeding index. It was observed that there was no marked difference pertaining to the oral hygiene index in both groups.
The bleeding index was more in the test group; however, the clinical attachment loss was 2.01 ± 0.69 mm in the study group and 2.38 ± 0.49 mm in the control group indicating that patients in the test group had significantly less CAL than the control group.[23]
Other researchers studied 392 males ≥50 years, with ≥6 natural teeth who were either nonsmokers or former smokers. Test participants were identified who took aspirin (≤300 mg) for ≥2 years. The control group included those who did not take aspirin. In both smokers and former-smokers, low-dose aspirin therapy for ≥2-year duration was correlated with a lower level of periodontal attachment loss than individuals who were not taking daily aspirin therapy. This may indicate a protective association of aspirin in the treatment of periodontitis in older adults.[24]
This study agreed with the previous study. Low-dose aspirin (75 mg) was administered once daily to the patients in Group I (after NSPT for 3 months). A significant mean reduction in the PTX3 levels has been observed that is from 359.13 pg/ml to 101.42 pg/ml, 3 months after NSPT in these patients. The intergroup comparison between Group I (aspirin) and Group III (placebo) also showed significantly more mean reduction of PTX3 levels in Group I, proving the beneficial role of aspirin as an anti-inflammatory therapeutic agent.
Dietary fatty acids, particularly the omega-3 polyunsaturated fatty acids (PUFA]), are known to clinically reduce inflammation in humans. These essential fatty acids and their precursor, the vegetable oil α-linoleic acid, are widely believed to act by the prevention of conversion of arachidonate to pro-inflammatory eicosanoids or serving as an alternative substrate producing less-potent products. Novel-oxygenated products of omega-3 PUFA“s generated by enzymatic pathways similar to the production of LX are called “resolvins,” and exhibit potent actions in the resolution of inflammation. Eicosapentaenoic acid and its derivative docosahexaenoic acid are held to be beneficial in a wide range of human inflammatory disorders, including cardiovascular diseases, rheumatoid arthritis, Alzheimer's disease, lung fibrosis, and inflammatory bowel disease, as well as periodontal disease.[25]
Studies done by some researchers highlighted the anti-inflammatory properties of O3FAs. After conducting many studies, they concluded that O3FAs had strong antibacterial activity against various oral pathogens.[26]
Some researchers evaluated whether prophylactic usage of O3FA provides the possible effects of therapy on the gingival tissue levels of PGE2, PGF2α, platelet-activating factor (PAF), and leukotriene B4 (LTB4) in endotoxin-induced periodontitis in rats. It was concluded that therapeutic O3FA significantly reduced the gingival tissue levels of PGE2, PGF2α, LTB4, and PAF in experimental periodontitis. Furthermore, the prophylactic usage of O3FA provided additional beneficial effects to the therapeutic administration by decreasing the gingival tissue levels of these mediators to levels seen in healthy tissue.[27]
Other researchers studied about fish oil and its constituents and were of the opinion that fish oil showed decreased osteoclast activation in vitro.[28]
The bioactive products released from O3FAs produced dramatic improvement in the tissue, and bone levels of periodontitis induced in some animal models according to the research by some clinicians.[29]
In another study, a total of 344 Type 2 diabetic patients with a history of O3FA supplementation for managing hypertriglyceridemia were included. Reduction in urine albumin-to-creatinine ratio (ACR) and glomerular filtrate rate were examined. Serum total cholesterol, triglyceride, and urine ACR significantly reduced after O3FA supplementation. Patients treated with higher doses of O3FA (4G/day) showed better results when compared to the patients who were administered lower doses.[30]
This study also proved the efficacy of O3FAs when used as an adjunct to NSPT. O3FAs were administered twice daily (1000 mgms/day) for 3 months after NSPT in Group II. It was observed that there was a marked reduction in all the clinical and biochemical parameters from baseline to 3 months within the group. When an intergroup comparison was made, it was analyzed that PTX3 levels were significantly reduced only in Group II (76.63 pg/ml) when compared to Group I (101.42 pg/ml) and Group III (212.44 pg/ml).
Drug safety/tolerance: It was observed that there was no drop out of patients in both the test groups due to intolerance. Furthermore, there were no side effects pertaining to aspirin and O3FAs during the study.
Limitation
Both low-dose aspirin and O3FAs were administered only for 3 months. Their true benefits could have surfaced if administered at least for 6 months.
CONCLUSIONS
This study reiterates the beneficial role of NSPT in periodontal therapy, as a significant improvement was obtained in relation to all the parameters within each group from baseline to 3 months. It was observed that there was a better response after NSPT in both the test groups, when compared to the placebo group, proving their efficacy as anti-inflammatory agents. However, when an intergroup comparison was made Group II (O3FAs) proved to be more efficacious in reducing PTX3 levels when compared to Group I (aspirin) and Group III (placebo). Further studies using O3FAs as well as aspirin for a longer period of time should be performed to confirm their role in treating periodontitis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors would like to acknowledge the help, support, and guidance from, Dr. N. G. Sastry, director and consultant, Dr. Mohan's Diabetic Centre, Domalaguda, Hyderabad. The authors are also thankful to Mr. Yanadi Reddy, Statistician, for his assistance in compiling the statistical analysis.
REFERENCES
- 1.Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K, et al. Periodontitis and diabetes: A two-way relationship. Diabetologia. 2012;55:21–31. doi: 10.1007/s00125-011-2342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bretz WA, Weyant RJ, Corby PM, Ren D, Weissfeld L, Kritchevsky SB, et al. Systemic inflammatory markers, periodontal diseases, and periodontal infections in an elderly population. J Am Geriatr Soc. 2005;53:1532–7. doi: 10.1111/j.1532-5415.2005.53468.x. [DOI] [PubMed] [Google Scholar]
- 3.Engebretson S, Chertog R, Nichols A, Hey-Hadavi J, Celenti R, Grbic J. Plasma levels of tumour necrosis factor-alpha in patients with chronic periodontitis and type 2 diabetes. J Clin Periodontol. 2007;34:18–24. doi: 10.1111/j.1600-051X.2006.01017.x. [DOI] [PubMed] [Google Scholar]
- 4.Paraskevas S, Huizinga JD, Loos BG. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J Clin Periodontol. 2008;35:277–90. doi: 10.1111/j.1600-051X.2007.01173.x. [DOI] [PubMed] [Google Scholar]
- 5.Allen EM, Matthews JB, O'Halloran DJ, Griffiths HR, Chapple IL. Oxidative and inflammatory status in type 2 diabetes patients with periodontitis. J Clin Periodontol. 2011;38:894–901. doi: 10.1111/j.1600-051X.2011.01764.x. [DOI] [PubMed] [Google Scholar]
- 6.Elwakeel NM, Hazaa HH. Effect of omega 3 fatty acids plus low-dose aspirin on both clinical and biochemical profiles of patients with chronic periodontitis and type 2 diabetes: A randomized double blind placebo-controlled study. J Periodontal Res. 2015;50:721–9. doi: 10.1111/jre.12257. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl 1):S62–9. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Machtei EE, Christersson LA, Grossi SG, Dunford R, Zambon JJ, Genco RJ, et al. Clinical criteria for the definition of “established periodontitis”. J Periodontol. 1992;63:206–14. doi: 10.1902/jop.1992.63.3.206. [DOI] [PubMed] [Google Scholar]
- 9.Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38(Suppl):610–6. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 10.Listgarten MA, Mao R, Robinson PJ. Periodontal probing and the relationship of the probe tip to periodontal tissues. J Periodontol. 1976;47:511–3. doi: 10.1902/jop.1976.47.9.511. [DOI] [PubMed] [Google Scholar]
- 11.Pihlstrom BL. Measurement of attachment level in clinical trials: Probing methods. J Periodontol. 1992;63:1072–7. doi: 10.1902/jop.1992.63.12s.1072. [DOI] [PubMed] [Google Scholar]
- 12.Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS. Advances in the pathogenesis of periodontitis: Summary of developments, clinical implications and future directions. Periodontol 2000. 1997;14:216–48. doi: 10.1111/j.1600-0757.1997.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 13.Matthews DC. The relationship between diabetes and periodontal disease. J Can Dent Assoc. 2002;68:161–4. [PubMed] [Google Scholar]
- 14.Tsai C, Hayes C, Taylor GW. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol. 2002;30:182–92. doi: 10.1034/j.1600-0528.2002.300304.x. [DOI] [PubMed] [Google Scholar]
- 15.Grossi SG, Zambon JJ, Ho AW, Koch G, Dunford RG, Machtei EE, et al. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J Periodontol. 1994;65:260–7. doi: 10.1902/jop.1994.65.3.260. [DOI] [PubMed] [Google Scholar]
- 16.Kathariya R, Jain H, Gujar D, Singh A, Ajwani H, Mandhyan D, et al. Pentraxins as key disease markers for periodontal diagnosis. Dis Markers. 2013;34:143–51. doi: 10.3233/DMA-130963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang HS, Woo JE, Lee SJ, Park SH, Woo JM. Elevated plasma pentraxin 3 levels are associated with development and progression of diabetic retinopathy in Korean patients with type 2 diabetes mellitus. Invest Ophthalmol Vis Sci. 2014;55:5989–97. doi: 10.1167/iovs.14-14864. [DOI] [PubMed] [Google Scholar]
- 18.Boyapati R, Chinthalapani S, Ramisetti A, Salavadhi SS, Ramachandran R. Association of pentraxin and high-sensitive C-reactive protein as inflammatory biomarkers in patients with chronic periodontitis and peripheral arterial disease. J Indian Soc Periodontol. 2018;22:112–5. doi: 10.4103/jisp.jisp_290_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christgau M, Palitzsch KD, Schmalz G, Kreiner U, Frenzel S. Healing response to non-surgical periodontal therapy in patients with diabetes mellitus: Clinical, microbiological, and immunologic results. J Clin Periodontol. 1998;25:112–24. doi: 10.1111/j.1600-051x.1998.tb02417.x. [DOI] [PubMed] [Google Scholar]
- 20.Teeuw WJ, Gerdes VE, Loos BG. Effect of periodontal treatment on glycemic control of diabetic patients: A systematic review and meta-analysis. Diabetes Care. 2010;33:421–7. doi: 10.2337/dc09-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Han X, Guo X, Luo X, Wang D. The effect of periodontal treatment on hemoglobin A1c levels of diabetic patients: A systematic review and meta-analysis. PLoS One. 2014;9:e108412. doi: 10.1371/journal.pone.0108412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serhan CN. Systems approach to inflammation resolution: Identification of novel anti-inflammatory and pro-resolving mediators. J Thromb Haemost. 2009;7(Suppl 1):44–8. doi: 10.1111/j.1538-7836.2009.03396.x. [DOI] [PubMed] [Google Scholar]
- 23.Faizuddin M, Tarannum F, Korla N, Swamy S. Association between long-term aspirin use and periodontal attachment level in humans: A cross-sectional investigation. Aust Dent J. 2012;57:45–50. doi: 10.1111/j.1834-7819.2011.01650.x. [DOI] [PubMed] [Google Scholar]
- 24.Drouganis A, Hirsch R. Low-dose aspirin therapy and periodontal attachment loss in ex- and non-smokers. J Clin Periodontol. 2001;28:38–45. doi: 10.1034/j.1600-051x.2001.280106.x. [DOI] [PubMed] [Google Scholar]
- 25.Schuster GU, Bratt JM, Jiang X, Pedersen TL, Grapov D, Adkins Y, et al. Dietary long-chain omega-3 fatty acids do not diminish eosinophilic pulmonary inflammation in mice. Am J Respir Cell Mol Biol. 2014;50:626–36. doi: 10.1165/rcmb.2013-0136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang CB, Ebersole JL. A novel bioactivity of omega-3 polyunsaturated fatty acids and their ester derivatives. Mol Oral Microbiol. 2010;25:75–80. doi: 10.1111/j.2041-1014.2009.00553.x. [DOI] [PubMed] [Google Scholar]
- 27.Sun D, Krishnan A, Zaman K, Lawrence R, Bhattacharya A, Fernandes G, et al. Dietary n-3 fatty acids decrease osteoclastogenesis and loss of bone mass in ovariectomized mice. J Bone Miner Res. 2003;18:1206–16. doi: 10.1359/jbmr.2003.18.7.1206. [DOI] [PubMed] [Google Scholar]
- 28.Hasturk H, Kantarci A, Ohira T, Arita M, Ebrahimi N, Chiang N, et al. RvE1 protects from local inflammation and osteoclast- mediated bone destruction in periodontitis. FASEB J. 2006;20:401–3. doi: 10.1096/fj.05-4724fje. [DOI] [PubMed] [Google Scholar]
- 29.Vardar S, Buduneli E, Türkoǧlu O, Berdeli AH, Baylas H, Başkesen A, et al. Therapeutic versus prophylactic plus therapeutic administration of omega-3 fatty acid on endotoxin-induced periodontitis in rats. J Periodontol. 2004;75:1640–6. doi: 10.1902/jop.2004.75.12.1640. [DOI] [PubMed] [Google Scholar]
- 30.Han E, Yun Y, Kim G, Lee YH, Wang HJ, Lee BW, et al. Effects of omega-3 fatty acid supplementation on diabetic nephropathy progression in patients with diabetes and hypertriglyceridemia. PLoS One. 2016;11:e0154683. doi: 10.1371/journal.pone.0154683. [DOI] [PMC free article] [PubMed] [Google Scholar]