Abstract
Background:
Ultrasonic instruments generate aerosols with significantly greater number of bacteria. Preprocedural mouthrinses or chemotherapeutic coolants are used for the reduction of bacterial load in dental aerosols. The use of chlorhexidine as an ultrasonic coolant has been well established. However, this application has not yet been investigated for cinnamon extract which is known to have antibacterial and anti-inflammatory properties in vivo.
Aim:
The aim of this study is to compare and evaluate the efficacy of chlorhexidine and cinnamon extract as an ultrasonic coolant in reduction of aerosol contamination and biofilm formation during ultrasonic scaling in comparison with the distilled water (DW).
Materials and Methods:
Sixty patients diagnosed with moderate-to-severe gingivitis were randomly divided into three groups of twenty patients each undergoing ultrasonic scaling. For Group I, chlorhexidine was used as an ultrasonic coolant; for Group II, cinnamon extract was used; and Group III was served as control where DW was used. The aerosols from ultrasonic units were collected on two blood agar plates at three different positions. Both the plates from each position were incubated aerobically for 48 h. The total number of colony-forming units were counted as mean ± standard deviation and statistically analyzed. In addition, biofilm sampling of dental unit waterlines (DUWLs) was also done to evaluate the effect of these antimicrobials. Apart from microbial examination, clinical parameters such as plaque index and gingival index were also evaluated at baseline and 1-month follow-up.
Results:
Chlorhexidine and cinnamon both were equally effective (P > 0.05) in reducing the bacterial count in aerosols and biofilm in DUWL as compared to DW when used as ultrasonic cooling agent.
Conclusion:
Both cinnamon and chlorhexidine used as an ultrasonic device coolant through DUWLs effectively helped in the reduction of bacterial count in dental aerosols.
Key words: Aerosols, chlorhexidine, cinnamon, coolant, ultrasonics
INTRODUCTION
Transmission of diseases to dental personnel during various procedures has become a source of increased concern to the dental profession. The use of various dental equipment such as high-speed dental handpieces,[1,2] ultrasonic or sonic scalers,[3,4] and three-way syringe[5] may generate aerosols and splatters containing microorganisms from the external environment. Aerosol liquid or solid particles were suspended in air and had a particle size of 50 μ or less. Splatter, on the other hand, is a mixture of air, water, and/or solid substances whose size ranges from 50 μ to several millimeters in diameter visible to the naked eye. These aerosols or splatter contain microorganisms which may potentially cause cross-contamination and infect the dental office and affect the health of both, dental health professionals and the patients.[6]
As mentioned earlier, aerosols are generated during various dental procedures. However, the potential of cross-infection due to aerosols is greater during ultrasonic debridement procedures as there is increased contamination due to blood and viable bacteria present subgingivally.[7,8]
Various approaches are been utilized to minimize the cross-contamination due to microbes in a dental office. This includes use of layered approach, surface decontamination, personal protective barrier use, immunization of dental staff, and preprocedural mouthrinses.[6,9,10,11,12] Extensive research conducted with respect to the use of preprocedural antibacterial mouthrinses has shown positive results.[12,13,14,15]
Ultrasonic debridement is a routinely carried out procedures and requires the use of water as coolant that serves various purposes. It helps in reducing frictional heating, cleansing the treated site, thus aiding in visibility, and speeding up the procedure.[16] However, the use of water as an ultrasonic coolant during debridement procedures increases the chances of aerosol productions, the risk of cross-contamination, and also the chances of transient bacteremia.[17] Thus, to avoid this, various chemotherapeutic agents have been utilized as a coolant.[18] The use of antimicrobials as a coolant serves the dual purpose of not only reducing the bacterial count in aerosols but also helps in continuous irrigation of the treatment site which further enhances the gingival health.[18,19]
Although various antimicrobials have been tried and tested conventionally as mouthrinses, chlorhexidine has emerged as a gold standard. However, its use leads to certain side effects such as staining of teeth and restorations, dryness and soreness of mouth, increased supragingival calculus formation, and alteration in taste.[14] Apart from chlorhexidine, other agents which have been used as ultrasonic device cooling agent include povidone-iodine.[20] Although many studies evaluating the effect of chlorhexidine and povidone-iodine either as preprocedural mouthrinse[14,21] or as an ultrasonic coolant[20,22] have been conducted, the research evaluating the effect of herbal mouthrinse is limited.
Cinnamon extract is one such agent, which could act as cost-effective and clinically efficient mouthrinse as it is known to have various medicinal properties. Cinnamon (Cinnamomum zeylanicum) is a member of Lauraceae family used in dry or ground form. The characteristic aroma of cinnamon is due to the presence of an essential oil cinnamaldehyde. Cinnamon has been historically used as a medicine for cold, flatulence, diarrhea, and nausea.[23] Research conducted regarding its use for clinical purposes has suggested that cinnamon possesses antibacterial, anti-inflammatory, and antifungal property.[23] Based on this evidence, cinnamon extract has been previously evaluated as a mouthrinse for the treatment of gingivitis and promoted the gingival health.[24]
Its molecular formula is C19H22O2 whereas molecular weight is 282.383 g/mol. It has a density of 1.010–1.030 at room temperatures and thickens on exposure to air. Its minimum fatal dose is around 0.5–5 g/kg. However, there are still no data available regarding its shelf life, and further research is indicated.[24]
Extensive research has been conducted evaluating the effect of antimicrobial agents used as ultrasonic coolant on the clinical parameters. However, limited research has been conducted evaluating their effect on aerosol contamination. To the best of our knowledge, there is only one published research where the effect of antimicrobials used as ultrasonic coolant on the aerosols was evaluated. Therefore, the present clinical study was conducted to compare and evaluate the efficacy of chlorhexidine and cinnamon extract used as an ultrasonic coolant on the reduction of bacterial load in aerosols compared with the distilled water (DW). In addition, the additive effect of these antimicrobials on gingival status was also evaluated.
MATERIALS AND METHODS
This was a single-center, placebo-controlled, randomized clinical trial with a three-group parallel design. The study was conducted over a period of 6 months, and participants enrolled were selected from the outpatient department of periodontology. Ethical clearance was obtained from the Institutional Ethical Committee and strictly adhered to the guidelines of the Declaration of Helsinki. A written informed consent was signed by all the patients.
Sample size calculation
The sample size was calculated for α error fixed at <5% (P < 0.005). Based on this calculation, the minimum sample size required in each group was 20 participants. Participants were enrolled in three groups.
Selection criteria
The inclusion criteria of this study were as follows: (i) participants having minimum of 20 permanent teeth, (ii) participants diagnosed with moderate-to-severe gingivitis having a gingival index (GI) score of 2–3, (iii) systemically healthy patients, and (iv) participants indicated for full-mouth scaling in single sitting.
The exclusion criteria of this study were as follows: (i) the presence of any systemic disease, (ii) received antibiotics or nonsteroidal anti-inflammatory drugs in the past 9–11 weeks, (iii) oral prophylaxis within the past 3 months, (iv) pregnant and lactating mothers, and (v) smokers.
Patient selection and randomization
All the clinical assessment and evaluation procedure were performed by one examiner who was blinded. Clinical examination included oral mucosa assessment and gingival health examination. The patients included in the study were initially screened for their GI[25] and plaque index (PI)[26] scores in the first sitting [Table 1]. A total of 60 participants having moderate-to-severe gingivitis from both the sexes with age ranging from 18 to 55 years (mean ± standard deviation [SD] of 29.26 ± 2.8), willing to participate in the study, and having a GI score of 2–3 and a PI score of 2–3 were selected for this study. Patients were recalled after 1-month follow-up for evaluation of the clinical parameters only. The patients were randomly allotted using computer-generated random sequence table to one of the three groups by one examiner while the treatment was performed by another examiner. Three groups included in the study were as follows:
Table 1.
Distances of agar plates from patient’s mouth
| Plate number | Plate position |
|---|---|
| Plate 1a, 1b (C) | 1 foot from patient’s mouth at patient’s chest |
| Plate 2a, 2b (R) | On the right side of patient’s mouth at a distance of 1 foot |
| Plate 3a, 3b (L) | On the left side of patient’s mouth at a distance of 1 foot |
C – Chest; R – Right; L – Left
Group I: Chlorhexidine used as ultrasonic coolant (20 participants)
Group II: Cinnamon extract used as ultrasonic coolant (20 participants)
Group III: DW used as ultrasonic coolant (20 participants).
Cinnamon extract preparation
Fresh cinnamon bark was taken from the botanical garden. It was ground to a fine powder in a mechanical grinder. Ten grams of this finely powdered cinnamon was mixed with 100 ml of sterile deionized water and kept in a water bath in a round-bottomed flask at 55°C–60°C for 5 h and then filtered through sterile filter paper (Whatman®, UK). The aqueous extract was decanted, clarified by filtration through a muslin cloth, and evaporated in a porcelain dish at 40°C, which resulted in the dried extract. This dried extract was suspended in polyethylene glycol 400 (20% w: v) and sterile DW to give a final concentration of 20% w/v. The entire procedure was performed under proper aseptic conditions.[24]
Clinical procedure
All treatment procedures were conducted in a closed operatory where fumigation facility was available. Before the procedure, the surfaces of the operatory were disinfected with ethyl alcohol (70%). Before starting the procedure, the ultrasonic unit was switched on and flushed for 2 min to get rid of contaminated water due to overnight stagnation in waterlines. Thirty minutes before the procedure, a blood agar plate was positioned on the plate 1 spot for a period of 15 min. This was then subjected to microbial assessment in order to check for environmental contamination, if present, in the operatory. The procedure on the patients commenced only after the operator was assured that there is no environmental contamination seen on the agar plate.
Sixty patients who met the inclusion criteria were selected. The type of procedure to be performed was fully explained, and written informed consent was obtained from each patient. Patients were randomly allocated to one of the following three groups: chlorhexidine as ultrasonic coolant (Group I), cinnamon extract as ultrasonic coolant (Group II), and DW as ultrasonic coolant (Group III).
Dental chairs with self-contained water system were selected for the study. The above-mentioned agents were added in the dental unit waterlines (DUWLs). Strict asepsis was observed inside the operatory, and the selected participants were prepared to enter the operatory by wearing headcaps and autoclaved gowns. The participants were instructed to refrain from all the actions that would generate aerosols. Various actions such as conversation, sneezing, and coughing were strictly forbidden. Single-sitting ultrasonic scaling was done for all the patients for a period of 20 min, using ultrasonic scaler. During each scaling procedure, saliva ejector was used. After the completion of the procedure, the patients in each group were asked about any discomfort noticed such as alteration in taste or burning sensation during debridement procedure. Patients were asked to report to dental office if any adverse effects were experienced posttreatment.
Position of agar plates
Blood agar was chosen because it is a general purpose, nonselective, and enriched medium that promotes the growth of microorganisms, such as those sampled from air. Position of the agar plates was chosen based on the findings from a previous study conducted by Yamada et al.[27] for reproducibility of the data. Figure 1 shows the three graphical locations of the blood agar plates placed in operatory room for each treatment group, and fixed distances of the plates were also maintained with respect to the reference point, i.e., the mouth of the patient. Two plates at each position for aerobic culture were placed on the patient's chest, right side, and left side, respectively [Table 1]. Two plates were deliberately placed to check whether amount of colonies formed were almost quantitatively identical.
Figure 1.

Schematic illustration of blood agar plates placed at three different location
Microbial analysis
Analysis of aerosols: The aerosols from the ultrasonic unit were collected on two blood agar plates placed at three different positions, each within a range of 1 foot, in all the three groups. After collecting the samples, plates from each position were incubated aerobically for 48 h.
Analysis of biofilm from dental unit waterlines
The sample of biofilm of a tubing from the DUWLs was taken for each of the three groups by scraping. The sample was placed in saline for 24 h. A volume of 0.1 ml of this saline was then plated on the agar plates for each group using a cotton bud. The plates were then incubated for 48 h aerobically. Colonies of bacteria were counted using classical bacterial counting technique, and they were expressed as number of colony-forming units (CFUs) seen on agar plates.
Statistical analysis
Statistical analysis of the results was done for CFUs using Statistical package for social science (SPSS 20, IBM, Chicago, IL, USA). The ANOVA test was used for continuous variables after confirming normality of the data distribution. The method of Bartlett was used to confirm that the data had a Gaussian distribution. Intergroup analysis of the clinical parameters (GI and PI) was also performed using ANOVA, whereas intragroup analysis was done using Student's t-test. The statistical significance was defined as P < 0.05.
RESULTS
This randomized, placebo-controlled, clinical trial was conducted over a period of February 2017 to July 2017. Flowchart for the study has been shown in Figure 2. Demographic characteristics of the patients according to different experimental group are shown in Table 2. There was no significant difference within the groups with respect to demographic characteristics (P > 0.05). The mean ± SD age of the patients included in the study was 29.26 ± 2.86 years. A total of 58.33% males and 41.67% females participated in the study. No adverse effect was reported by the patients during or after 1-month follow-up [Figure 2].
Figure 2.
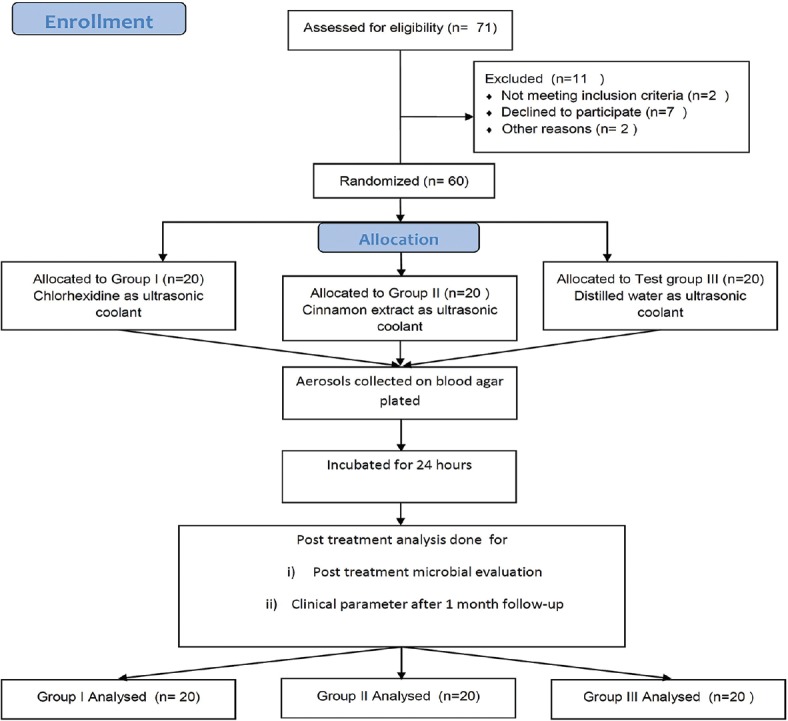
Consort flowchart. n – Sample size
Table 2.
Demographic characteristics of the patients
| Patients | Group I | Group II | Group III | P |
|---|---|---|---|---|
| Age | 29.20±3.05 | 29.30±2.71 | 29.3±2.97 | 0.99* |
| Number of teeth | 29.15±1.63 | 29.45±1.50 | 29.85±1.49 | 0.36* |
| Male/female | 11/9 | 12/8 | 12/8 | 1.28* |
*P>0.05 considered statistically nonsignificant. Group I – Chlorhexidine as ultrasonic coolant; Group II – Cinnamon extract as ultrasonic coolant; Group III – Distilled water as ultrasonic coolant
Table 3 shows the intergroup and intragroup comparison of clinical parameters at baseline and after 1-month follow-up. At baseline, there was no difference statistically with regard to both GI and PI in all the three experimental groups. GI scores of Group I, Group II, and Group III were 2.29 ± 0.20, 2.22 ± 0.17, and 2.31 ± 0.17, respectively, which was statistically nonsignificant (P = 0.21). After 1-month follow-up, these scores were reduced to 0.26 ± 0.14, 0.19 ± 0.03, and 0.57 ± 0.18 in Group I, Group II, and Group III, respectively. This reduction in GI scores was statistically significant (P < 0.05). At baseline, the PI scores of the participants in Group I, Group II, and Group III were 2.41 ± 0.22, 2.37 ± 0.20, and 2.49 ± 0.06, respectively, which was statistically nonsignificant (P = 0.11). These plaque scores were reduced to 1.09 ± 0.09, 1.04 ± 0.11, and 1.30 ± 0.13 in Group I, Group II, and Group III, respectively, which was statistically significant (P < 0.05). Intragroup analysis of both the clinical parameters (GI and PI) after 1-month follow-up revealed a statistically significant difference (P < 0.05).
Table 3.
Values of gingival index and plaque index expressed as mean±standard deviation at baseline and after 1 month
| Experimental group | Baseline | 1 month | P* | |
|---|---|---|---|---|
| Gingival index | Group I | 2.29±0.20 | 0.26±0.14 | <0.05† |
| Group II | 2.22±0.17 | 0.19±0.03 | <0.05† | |
| Group III | 2.31±0.17 | 0.57±0.18 | <0.05† | |
| P‡ | 0.21k | <0.05¶ | ||
| Plaque index | Group I | 2.41±0.22 | 1.09±0.09 | <0.05† |
| Group II | 2.37±0.20 | 1.04±0.11 | <0.05† | |
| Group III | 2.49±0.06 | 1.30±0.13 | <0.05† | |
| P‡ | 0.11k | <0.05¶ |
*P derived from Student’s t-test for intragroup analysis; †Intragroup analysis statistically significant at P<0.05; ‡P derived from ANOVA for intergroup analysis; kIntergroup analysis statistically nonsignificant at P>0.05; ¶Intergroup analysis statistically significant at P<0.05. Group I – Chlorhexidine as ultrasonic coolant; Group II – Cinnamon extract as ultrasonic coolant; Group III – Distilled water as ultrasonic coolant
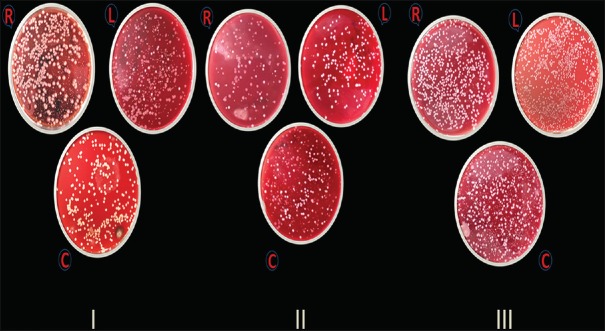
Figure 3 shows the colonies formed on the blood agar plates for all the three experimental groups. Figure 4 shows the graph where illustration of mean ± SD of the CFUs formed is summarized. Table 4 shows the mean ± SD scores of CFUs of all the three groups. In Group I, mean ± SD scores of CFUs formed at the chest, right side, and left side of the patients were 627.35 ± 34.10, 407.6 ± 25.87, and 403.55 ± 16.93, respectively. In Group II, mean ± SD scores of CFUs formed at the chest, right side, and left side of the patients were 575.9 ± 30.41, 419.5 ± 48.21, and 413.5 ± 51.37, respectively. In Group III, mean ± SD scores of CFUs formed at the chest, right side, and left side of the patients were 1396.0 ± 214.93, 1064.05 ± 26.69, and 1009.85 ± 23.29 (mean ± SD), respectively [Table 4].
Figure 3.
Colony-forming units of all three group on blood agar plates
Figure 4.
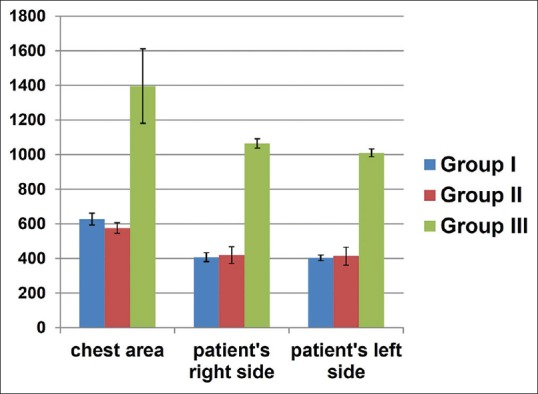
Graph illustrating the mean colony-forming units of all three groups
Table 4.
Colony-forming units according to the different location of all the groups
| Location of the plate | Group I | Group II | Group III | P* |
|---|---|---|---|---|
| Patient’s chest area | 627.35±34.10 | 575.9±30.41 | 1396.15±214.93 | <0.05¶ |
| Patient’s right side | 407.6±25.87 | 419.5±48.21 | 1064.05±26.69 | <0.05¶ |
| Patient’s left side | 403.55±16.93 | 413.5±51.37 | 1009.85±23.29 | <0.05¶ |
| P‡ | <0.05† | <0.05† | <0.05† |
*P derived from ANOVA for intergroup analysis; ¶Intergroup analysis statistically significant at P<0.05; ‡P derived from ANOVA for intragroup analysis; †Intragroup analysis statistically significant at P<0.05. Group I – Chlorhexidine as ultrasonic coolant; Group II – Cinnamon extract as ultrasonic coolant; Group III – Distilled water as ultrasonic coolant
Table 5 shows the pairwise analysis of the CFUs formed at three standardized locations. Microbial analysis of the DUWL biofilm revealed that CFUs in the Group I were 361.80 ± 15.20, in the Group II 279.80 ± 27.80, and in the Group III were 680.0 ± 42.492 [Table 6].
Table 5.
Pairwise comparison of colony-forming units formed for all the three groups
| Location of plates | Groups | Significance* |
|---|---|---|
| Patient’s chest | Group I versus Group II | >0.05‡ |
| Group I versus Group III | <0.05† | |
| Group II versus Group III | <0.05† | |
| Patient’s right side | Group I versus Group II | >0.05‡ |
| Group I versus Group III | <0.05† | |
| Group II versus Group III | <0.05† | |
| Patient’s left side | Group I versus Group II | >0.05‡ |
| Group I versus Group III | <0.05† | |
| Group II versus Group III | <0.05† |
*P derived from independent t-test; ‡P>0.05 considered statistically nonsignificant; †P<0.05 considered statistically significant. Group I – Chlorhexidine as ultrasonic coolant; Group II: Cinnamon extract as ultrasonic coolant; Group III – Distilled water as ultrasonic coolant
Table 6.
Mean±standard deviation of colony-forming units formed at the dental unit waterlines
| Experimental group | Group I | Group II | Group II | P |
|---|---|---|---|---|
| Colony-forming unit count | 361.80±15.20 | 279.80±27.80 | 680.0±42.49 | <0.05† |
| Pairwise comparison | ||||
| Groups |
Significance |
|||
| Group I versus Group II | >0.05‡ | |||
| Group I versus Group III | <0.05† | |||
| Group II versus Group III | <0.05† | |||
‡P>0.05 considered statistically nonsignificant; †P<0.05 considered statistically significant. Group I – Chlorhexidine as ultrasonic coolant; Group II – Cinnamon extract as ultrasonic coolant; Group III – Distilled water as ultrasonic coolant
DISCUSSION
Aerosols generated during different dental procedures potentially spread the infection. Aerosols produced during dental procedures have the potential to spread infection in the dental office. According to the recommendation of the American Dental Association, potentially contaminated aerosols or splatter should be controlled during various dental procedures.[28] Various studies have been conducted evaluating the effect of preprocedural mouthrinses on the dental aerosol contamination and have shown positive results. However, very few studies have been conducted evaluating the effect of antimicrobial mouthrinses used as ultrasonic coolant on the dental aerosols.[22] Thus, the present study was conducted evaluating the effect of chlorhexidine and cinnamon extract as an ultrasonic coolant as compared to DW on the reduction of microbial load in dental aerosols produced. In addition, their effect on the gingival status was also analyzed.
In the present study, antimicrobials as ultrasonic coolant were used instead of preprocedural mouthrinsing due to various reasons. Preprocedural mouthrinsing requires 30–60 s of rinsing period which varies according to various studies. Many studies have shown that 30-s period is enough to reduce bacterial count, but others suggest that 60-s rinsing period is required to produce any effect on bacterial count.[12,29] This suggests that there is still no agreement among the researchers regarding the period of rinsing. On the other hand, using antimicrobial agents as an ultrasonic coolant provides continuous action of the agents over a longer period, thus bypassing the rinsing period as that of preprocedural mouthrinses. In addition, with the use of preprocedural mouthrinses, depth of penetration is less as compared to that of an ultrasonic coolant.[30] Apart from these reasons, the patient's compliance and subjective error in rinsing was also the reason why ultrasonic antimicrobial coolants were chosen in the current study.
In the present study, chlorhexidine 0.2% and cinnamon extract 20% w/v were used. Gupta and Jain 2015[24] evaluated the effect of chlorhexidine and cinnamon extract mouthrinses on gingival status and dental plaque levels. They found that the chlorhexidine group showed the maximum decrease in both plaque and gingival scores, followed by cinnamon extract, but the result was statistically insignificant.[24] Based on this body of evidence, these two mouthrinses were used as ultrasonic coolant in the current study.
In the present study, gingival status of the patients was also analyzed before the scaling procedure and after 1-month follow-up. This was done to evaluate whether the use of chemotherapeutic coolant provides any added advantage or not. The previous studies have been conducted where chlorhexidine has been used as a coolant, but to the best of author's knowledge, this is the first instance where cinnamon extract has been used as an ultrasonic coolant. In the present study, we found that chlorhexidine and cinnamon were equally effective in improving the gingival status and reduction of plaque levels as compared to DW. However, the results of the present study are contradictory to a previous study conducted by Guarnelli et al. 2008[31] where they found no clinical benefits with the use of chlorhexidine as an ultrasonic coolant over the water. However, one thing that needs to be taken in consideration is that the group patients included in our present study were those who were diagnosed with gingivitis as compared to aggressive periodontitis patients. This might have influenced the results of the present study as healing response for both the groups of patients is differs significantly.
In the current study, it was found that CFU counts were highest on the blood agar plates placed at the patient's chest area followed by the patient's right side and left side, respectively. These results are in agreement with a previous study conducted by Gupta et al.[14] and Feres et al.[32] who also found that the highest CFU counts were seen on the blood agar plates placed at the patient's chest area.
In the present study, the highest CFU reduction was seen in chlorhexidine group followed by cinnamon extract and then DW. Although chlorhexidine group had the least CFU counts combined from all three plates, statistically there was no significant difference between the Group I and Group II. These results are in agreement with a previous study conducted by Jawade et al.[22] where they found that chlorhexidine when used as ultrasonic coolant caused the highest reduction in CFU counts as compared to povidone-iodine and DW. Similar results were reported by Bay et al.[29] who found that there was no significant difference in the CFU counts between the patients rinsing with either chlorhexidine or essential oils. Chlorhexidine when used either as mouthrinses or as ultrasonic coolant has shown substantial results and thus been called as a gold standard in plaque control. An important finding from our study was that of cinnamon extract group, which showed comparable benefits as that of chlorhexidine. As reported in literature that chlorhexidine cannot be tolerated by all the patients due to its side effects such as staining of the tooth, dryness of mouth, and slight alteration in taste, cinnamon extract mouthrinses can act as an alternative for the same, and this was proven in a previous study conducted by Gupta and Jain where they found that cinnamon extract mouthrinses had almost similar effects on the gingival status as compared to chlorhexidine.[24] They concluded that cinnamon might prove to be an effective agent owing to its ability to reduce plaque level and gingivitis.
According to Gupta and Jain,[24] the antimicrobial activity of cinnamon on dental plaque and gingivitis, as observed in the present study, may be explained by its active substances: cinnamic aldehyde, an aromatic aldehyde, and eugenol.[24,33] Cinnamon bark is rich in cinnamaldehyde (50.5%), which is highly electronegative and interferes in biological processes involving electron transfer, and reacts with nitrogen-containing components, for example, proteins and nucleic acids, thereby inhibiting the growth of the microorganisms.[24,34]
In the present study, apart from evaluating the CFU counts on blood agar plates placed at a different location, CFUs from DUWLs were analyzed. To the best of our knowledge, this is the first study where DUWLs were analyzed for its disinfection when antimicrobials were used as ultrasonic coolants. It was found that least CFU counts were seen in cinnamon extract group as compared to chlorhexidine and DW group. However, similar to effect on aerosol, there was no statistical difference between the chlorhexidine group and cinnamon extract group.
DUWL analysis was done as they are known to harbor appreciable amounts of bacteria derived from the biofilm on the inner surface of these lines.[4] This continuous reservoir of bacteria carries the potential of causing infection to patients and dental professionals.[4] Thus, it can be said that when antimicrobials are used as a coolant, it can also effectively reduce the bacterial counts in DUWLs although not totally able to eliminate them as suggested by the results of the present study.
While interpreting the results of the current study, its limitation should also be taken into considerations. In the present study, CFU counts of only aerobic bacteria were done. Viruses, anaerobic bacteria, fungi, and those requiring special media for its growth were not analyzed. Moreover, no attempt was made to avoid the fallout of viable bacteria at different positions, which might have underestimated the true extent of bacterial populations in air samples.
Overall, the results of the present study demonstrate that cinnamon extract was as effective as that of chlorhexidine when used as an ultrasonic coolant. To the best of our knowledge, this is the first study where cinnamon extract as an ultrasonic coolant has been compared with that of chlorhexidine. Thus, more randomized controlled clinical trials with larger sample size need to conducted to validate these findings. Longer duration of evaluation will give results that are more predictable and would confirm its stability.
CONCLUSION
Within the limitations of this study, both cinnamon and chlorhexidine when used as an ultrasonic coolant effectively helped in the reduction of bacterial contamination in dental aerosols which was seen by reduction in the CFUs, after adding these agents in the DUWL. Cinnamon extract can also be promoted to be used as a mouthwash as it has no side effects. Moreover, its low cost may motivate the patients at especially low socioeconomic strata for oral hygiene maintenance. This is an encouraging result which clearly favors the promotion of cinnamon among the rural communities, especially belonging to low socioeconomic strata, as cinnamon is easily available, inexpensive, and a safe alternative to chlorhexidine. Furthermore, as the best line of action is prevention of the disease-causing entity and thereby disease itself, these agents can be promoted to be used through DUWLs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bentley CD, Burkhart NW, Crawford JJ. Evaluating spatter and aerosol contamination during dental procedures. J Am Dent Assoc. 1994;125:579–84. doi: 10.14219/jada.archive.1994.0093. [DOI] [PubMed] [Google Scholar]
- 2.Toroǧlu MS, Haytaç MC, Köksal F. Evaluation of aerosol contamination during debonding procedures. Angle Orthod. 2001;71:299–306. doi: 10.1043/0003-3219(2001)071<0299:EOACDD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Rivera-Hidalgo F, Barnes JB, Harrel SK. Aerosol and splatter production by focused spray and standard ultrasonic inserts. J Periodontol. 1999;70:473–7. doi: 10.1902/jop.1999.70.5.473. [DOI] [PubMed] [Google Scholar]
- 4.Timmerman MF, Menso L, Steinfort J, van Winkelhoff AJ, Van der Weijden GA. Atmospheric contamination during ultrasonic scaling. J Clin Periodontol. 2004;31:458–62. doi: 10.1111/j.1600-051X.2004.00511.x. [DOI] [PubMed] [Google Scholar]
- 5.Souza-Gugelmin MC, Lima CD, Lima SN, Mian H, Ito IY. Microbial contamination in dental unit waterlines. Braz Dent J. 2003;14:55–7. doi: 10.1590/s0103-64402003000100010. [DOI] [PubMed] [Google Scholar]
- 6.Leggat PA, Kedjarune U. Bacterial aerosols in the dental clinic: A review. Int Dent J. 2001;51:39–44. doi: 10.1002/j.1875-595x.2001.tb00816.x. [DOI] [PubMed] [Google Scholar]
- 7.Barnes JB, Harrel SK, Rivera-Hidalgo F. Blood contamination of the aerosols produced by in vivo use of ultrasonic scalers. J Periodontol. 1998;69:434–8. doi: 10.1902/jop.1998.69.4.434. [DOI] [PubMed] [Google Scholar]
- 8.Harrel SK, Barnes JB, Rivera-Hidalgo F. Aerosol and splatter contamination from the operative site during ultrasonic scaling. J Am Dent Assoc. 1998;129:1241–9. doi: 10.14219/jada.archive.1998.0421. [DOI] [PubMed] [Google Scholar]
- 9.Cochran MA, Miller CH, Sheldrake MA. The efficacy of the rubber dam as a barrier to the spread of microorganisms during dental treatment. J Am Dent Assoc. 1989;119:141–4. doi: 10.14219/jada.archive.1989.0131. [DOI] [PubMed] [Google Scholar]
- 10.Nejatidanesh F, Khosravi Z, Goroohi H, Badrian H, Savabi O. Risk of contamination of different areas of dentist's face during dental practices. Int J Prev Med. 2013;4:611–5. [PMC free article] [PubMed] [Google Scholar]
- 11.Kanjirath PP, Coplen AE, Chapman JC, Peters MC, Inglehart MR. Effectiveness of gloves and infection control in dentistry: Student and provider perspectives. J Dent Educ. 2009;73:571–80. [PubMed] [Google Scholar]
- 12.Logothetis DD, Martinez-Welles JM. Reducing bacterial aerosol contamination with a chlorhexidine gluconate pre-rinse. J Am Dent Assoc. 1995;126:1634–9. doi: 10.14219/jada.archive.1995.0111. [DOI] [PubMed] [Google Scholar]
- 13.Reddy S, Prasad MG, Kaul S, Satish K, Kakarala S, Bhowmik N, et al. Efficacy of 0.2% tempered chlorhexidine as a pre-procedural mouth rinse: A clinical study. J Indian Soc Periodontol. 2012;16:213–7. doi: 10.4103/0972-124X.99264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta G, Mitra D, Ashok KP, Gupta A, Soni S, Ahmed S, et al. Efficacy of preprocedural mouth rinsing in reducing aerosol contamination produced by ultrasonic scaler: A pilot study. J Periodontol. 2014;85:562–8. doi: 10.1902/jop.2013.120616. [DOI] [PubMed] [Google Scholar]
- 15.Retamal-Valdes B, Soares GM, Stewart B, Figueiredo LC, Faveri M, Miller S, et al. Effectiveness of a pre-procedural mouthwash in reducing bacteria in dental aerosols: Randomized clinical trial Braz Oral Res. 2017;31:e21. doi: 10.1590/1807-3107BOR-2017.vol31.0021. [DOI] [PubMed] [Google Scholar]
- 16.Lea SC, Landini G, Walmsley AD. Thermal imaging of ultrasonic scaler tips during tooth instrumentation. J Clin Periodontol. 2004;31:370–5. doi: 10.1111/j.1600-051X.2004.00491.x. [DOI] [PubMed] [Google Scholar]
- 17.Kinane DF, Riggio MP, Walker KF, MacKenzie D, Shearer B. Bacteraemia following periodontal procedures. J Clin Periodontol. 2005;32:708–13. doi: 10.1111/j.1600-051X.2005.00741.x. [DOI] [PubMed] [Google Scholar]
- 18.Cosyn J, Miremadi SR, Sabzevar MM, De Bruyn H. Clinical effects of an essential oil solution used as a coolant during ultrasonic root debridement. Int J Dent Hyg. 2013;11:62–8. doi: 10.1111/j.1601-5037.2012.00554.x. [DOI] [PubMed] [Google Scholar]
- 19.Greenstein G. Research, Science and Therapy Committee of the American Academy of Periodontology. Position paper: The role of supra- and subgingival irrigation in the treatment of periodontal diseases. J Periodontol. 2005;76:2015–27. doi: 10.1902/jop.2005.76.11.2015. [DOI] [PubMed] [Google Scholar]
- 20.Van der Sluijs M, Van der Sluijs E, Van der Weijden F, Slot DE. The effect on clinical parameters of periodontal inflammation following non-surgical periodontal therapy with ultrasonics and chemotherapeutic cooling solutions: A systematic review. J Clin Periodontol. 2016;43:1074–85. doi: 10.1111/jcpe.12613. [DOI] [PubMed] [Google Scholar]
- 21.Kaur R, Singh I, Vandana KL, Desai R. Effect of chlorhexidine, povidone iodine, and ozone on microorganisms in dental aerosols: Randomized double-blind clinical trial. Indian J Dent Res. 2014;25:160–5. doi: 10.4103/0970-9290.135910. [DOI] [PubMed] [Google Scholar]
- 22.Jawade R, Bhandari V, Ugale G, Taru S, Khaparde S, Kulkarni A, et al. Comparative evaluation of two different ultrasonic liquid coolants on dental aerosols. J Clin Diagn Res. 2016;10:ZC53–7. doi: 10.7860/JCDR/2016/20017.8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burt S. Essential oils: Their antibacterial properties and potential applications in foods – A review. Int J Food Microbiol. 2004;94:223–53. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 24.Gupta D, Jain A. Effect of cinnamon extract and chlorhexidine gluconate (0.2%) on the clinical level of dental plaque and gingival health: A 4-week, triple-blind randomized controlled trial. J Int Acad Periodontol. 2015;17:91–8. [PubMed] [Google Scholar]
- 25.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 26.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 27.Yamada H, Ishihama K, Yasuda K, Hasumi-Nakayama Y, Shimoji S, Furusawa K, et al. Aerial dispersal of blood-contaminated aerosols during dental procedures. Quintessence Int. 2011;42:399–405. [PubMed] [Google Scholar]
- 28.Infection control recommendations for the dental office and the dental laboratory. ADA Council on Scientific Affairs and ADA Council on Dental Practice. J Am Dent Assoc. 1996;127:672–80. doi: 10.14219/jada.archive.1996.0280. [DOI] [PubMed] [Google Scholar]
- 29.Bay NL, Overman PR, Krust-Bray K, Cobb C, Gross KB. Effectiveness of antimicrobial mouthrinses on aerosols produced by an air polisher. J Dent Hyg. 1993;67:312–7. [PubMed] [Google Scholar]
- 30.Pitcher GR, Newman HN, Strahan JD. Access to subgingival plaque by disclosing agentsusing mouth rinsing and direct irrigation. J Clin Periodontol. 1980;7:300–8. doi: 10.1111/j.1600-051x.1980.tb01972.x. [DOI] [PubMed] [Google Scholar]
- 31.Guarnelli ME, Franceschetti G, Manfrini R, Trombelli L. Adjunctive effect of chlorhexidine in ultrasonic instrumentation of aggressive periodontitis patients: A pilot study. J Clin Periodontol. 2008;35:333–41. doi: 10.1111/j.1600-051X.2008.01199.x. [DOI] [PubMed] [Google Scholar]
- 32.Feres M, Figueiredo LC, Faveri M, Stewart B, De Vizio W. The effectiveness of a preprocedural mouthrinse containing cetylpyridinium chloride in reducing bacteria in the dental office. J Am Dent Assoc. 2010;141:415–22. doi: 10.14219/jada.archive.2010.0193. [DOI] [PubMed] [Google Scholar]
- 33.Agaoglu S, Dostbil N, Alemdar S. Antimicrobial activity of some spices used in the meat industry. Bull Vet Inst Pulawy. 2007;51:53–7. [Google Scholar]
- 34.Gupta C, Garg AP, Uniyal RC, Kumari A. Comparative analysis of the antimicrobial activity of cinnamon oil and cinnamon extract on some food-borne microbes. Afr J Microbiol Res. 2008;9:247–51. [Google Scholar]