Abstract
Background.
Influenza A/H7N9 viruses are undergoing antigenic drift since their emergence in 2013, and vaccination strategies are needed for pandemic preparedness. Two doses of adjuvanted monovalent inactivated influenza A/H7N9 vaccine (IIV1 A/H7N9) are needed for optimal serological responses. However, administering 2 doses in a pandemic setting might be challenging. We evaluated the immunogenicity of “boosting” with IIV1 A/H7N9 in subjects “primed” 8 years previously with IIV1 A/H7N7.
Methods.
We administered 1 booster dose containing 45mcg of IIV1 A/H7N9 hemagglutinin to 17 recipients of 2 prior doses of IIV1 A/H7N7, and to 10 influenza A/H7-naïve subjects. We tested their post-boosting sera for antibodies (Ab) against homologous influenza A/H7N9 using a hemagglutination inhibition assay; and compared their Ab titers to those in stored sera from recipients of AS03-adjuvanted IIV1 A/H7N9 against 9 strains of influenza A/H7N9 viruses.
Results.
The percentage of subjects with Ab titers ≥40 on Days 9 and 29 post boosting, respectively, was 65% and 41% in primed subjects and 10% and 0% in unprimed subjects. The Ab titers in recipients of AS03-adjuvanted IIV1 A/H7N9 were higher than those in the prime-boost group against a panel of influenza A/H7N9 viruses, except for 2 highly pathogenic strains.
Conclusions.
Priming with IIV1 A/H7 results in serological responses following a delayed boost with 1 dose of unadjuvanted IIV1 A/H7N9, despite lack of antibody response after the prime. Optimizing prime-boost approaches would benefit pandemic preparedness.
Keywords: Influenza A/H7N9, prime-boost, vaccines, pandemic, avian, influenza
INTRODUCTION
In 2013, the novel influenza A/H7N9 virus emerged as a cause of human disease in Eastern China, and since then 1,567 laboratory-confirmed cases have been reported to the World Health Organization (WHO) resulting in 615 deaths (as of March 2nd, 2018). Influenza A/H7N9 infections have occurred in 6 epidemic waves, with the 5th wave causing a higher number of cases (766 cases) and spanning a wider geographic distribution than its predecessors [1, 2]. The circulating influenza A/H7N9 viruses have evolved in these successive waves. Genotypically, fewer circulating genotypes caused most of the disease in wave 5 [3]. Antigenically, some strains isolated in the 5th wave have accumulated enough mutations in the hemagglutinin (HA) gene for ferret antisera raised against 2013 influenza A/H7N9 isolates to have no or low reactivity against these strains [4]. Moreover, highly pathogenic influenza A/H7N9 virus strains (HPAI) were identified in the 5th wave, which may have implications for vaccine development considerations [4]. During these waves, the virus maintained its ability to bind both human and avian sialic acid receptors [4]. While sustained human-to-human transmission of influenza A/H7N9 viruses has not occurred, the potential for reassortment and host adaptation exists, which could result in an influenza pandemic with enormous morbidity and mortality [5].
The closure of live poultry markets in major urban areas has resulted in a reduction in the number of cases of influenza A/H7N9 cases in those regions and a shift of the epidemic to suburban and rural communities [6]. Vaccination remains the most important measure to control influenza in humans. Studies have shown that unadjuvanted monovalent inactivated influenza A/H7N9 and A/H7N7 vaccines (IIV1 A/H7N9 and IIV1 A/H7N7, respectively) are poorly immunogenic. The putative seroprotective hemagglutination inhibition antibody (HAI Ab) titer of 40 was reached in 0% and 2% of recipients of 2 doses of IIV1 H7N7 and IIV1 H7N9, respectively, containing 15mcg of HA per dose. Increasing the HA dose in IIV1 A/H7N7 to 90mcg and in IIV1 A/H7N9 to 45mcg did not result in significantly improved responses [7, 8]. Improvement in the serologic responses to IIV1 A/H7N9 was achieved with the addition of oil-in-water based adjuvants and at dose-sparing HA levels [7, 9].
Administering 2 doses of adjuvanted vaccines to the general population after the declaration of a pandemic is logistically challenging, given the manufacturing, distribution, and administration time constraints. Administering one dose of vaccine would be more desirable in a pandemic setting. A prime-boost scenario, whereby the population is primed with an avian influenza vaccine based on a circulating strain that is hypothesized to have a pandemic potential, then boosted by a single dose of a vaccine based on the related (but antigenically drifted) strain that has been shown to cause sustained human-to-human transmission, is potentially more achievable. There are published data to support such a heterologous prime-boost strategy. For example, in unprimed individuals, 2 doses of homologous IIV1 A/H5N1 at the 90 mcg HA per dose level are needed to achieve an HAI Ab titer of ≥40 in 58% of individuals [10]. However, in a heterologous prime-boost strategy, 37 subjects who received two priming doses of recombinant HA influenza A/H5N1 clade 0 vaccine in 1997–1998 were given a single dose of IIV1 A/H5N1 (clade 1) ~ 8 years later. On Day 28 post boosting, 70% of the subjects had seroprotective HA titers against the clade 1 strain [11].
In 2014, Babu et al. demonstrated that administering 2 doses of live attenuated influenza A/H7N7 vaccine followed by a homologous IIV H7N7 18–24 months later resulted in a 69% seroconversion rate against homologous and heterologous strains [12]. This approach resulted in a rapid increase of quantity, epitope diversity, and affinity of H7 head-specific antibodies [13]. There are no published data demonstrating serological responses following a heterologous prime-boost with IIV H7 subtypes. In 2008 our group demonstrated that 2 doses of unadjuvanted IIV1 A/H7N7 at the 7.5, 15, 45 or 90mcg HA per dose were poorly immunogenic in young adults, with only 5% of subjects achieving an HAI Ab titer of ≥40 after 2 doses of 90mcg HA [8]. To test the priming achieved by this poorly immunogenic IIV1 A/H7N7, we boosted subjects from the aforementioned trial, and influenza A/H7 vaccine-naïve individuals with 1 dose of IIV1 A/H7N9 at the 45mcg HA dosage level, and assessed the immunologic response by HAI and virus microneutralization (MN) Ab assays. To assess the breadth of the serological responses generated by the heterologous prime-boost approach, we tested post-boosting sera from the study subjects against a panel of 9 influenza A/H7N9 strains isolated from the 1st and 5th epidemic waves and compared it to the responses generated by 2 doses of homologous IIV1 A/H7N9 at the 3.75, 7.5 or 15mcg HA dosage with AS03 adjuvant, using stored sera from a previously conducted study (DMID 13–0033, NCT01942265) [7].
METHODS
Study design.
This was a Phase 2 open-label study of non-pregnant healthy subjects, 19–50 years of age, who received 2 doses of IIV1 A/H7N7 at any dosage level in clinical trial DMID 07–0023 (NCT00546585), or IIV A/H7 naïve-subjects, evaluating the safety and immunogenicity of 1 dose of IIV1 A/H7N9 at the 45mcg HA dosage level. Full eligibility criteria are provided on clinicaltrials.gov, NCT02586792. We defined Group 1 subjects as prior recipients of IIV1 A/H7N7, and Group 2 as influenza A/H7-naïve subjects.
DMID 07–0023 was a randomized, double-blind study in which 126 subjects were enrolled and randomized to 5 groups of 25 subjects who received 2 doses (28 days apart) of placebo or IIV1 A/H7N7 at the 7.5mcg, 15mcg, 45mcg or 90mcg HA dosage level. The study was conducted in 2008. The DMID 07–0023 study vaccine was an A/H7N7 egg-based, formalin inactivated reassortant IIV1 produced by Sanofi Pasteur. The HA donor virus was A/mallard/Netherlands/12/2000 (H7N3), the NA donor virus was A/mallard/Netherlands/2/2000 (H10N7), and the internal genes were influenza A/H1N1-derived (A/Puerto Rico 8/34 (PR8) and A/Johannesburg/82/96).
Stored sera from DMID 13–0033 subjects who agreed to future use research were assayed to compare the breadth of the serologic response generated by the heterologous prime-boost approach to that achieved following receipt of 2 doses of homologous AS03-adjuvanted IIV1 H7N9. DMID 13–0033 was a double blind, randomized study evaluating the safety and immunogenicity of 2 doses of IIV1 A/H7N9 given intramuscularly (IM) at different HA dosage levels (3.75mcg, 7.5mcg, 15mcg, and 45mcg), with or without AS03 (GSK) or MF59 (Novartis/Seqirus) adjuvants. For the current study, we only tested sera from subjects who received study vaccine containing AS03 at the 3 HA dosage levels.
Study Procedures.
All subjects received 1 dose of IIV1 A/H7N9 at the 45mcg HA dosage level as a single 0.75mL IM injection in the deltoid region. Subjects recorded pre-specified injection site and systemic reactions on a memory aid for 8 days after vaccination (vaccination day is Day 1). Adverse events (AE) were collected for 28 days after study vaccination and graded as mild (Grade 1) if they did not interfere with subjects’ daily activities and required no treatment, moderate (Grade 2) if they caused some interference with subjects’ daily activities or required therapeutic measures, and severe (Grade 3) if they interrupted the subjects’ daily activities or incapacitated him/her. Serious adverse events (SAEs) data were collected for 6 months after study injection and were defined as Guillain-Barré syndrome or any AE that results in death, life threatening event, hospitalization or prolongation of hospitalization, persistent or significant incapacity, birth defect/congenital anomaly, or AE that requires a medical intervention to prevent death, threat to life or hospitalization. Venous blood was collected on Days 1 (pre-vaccine), 9 and 29 for HAI and MN Ab assays.
The study was approved by the Institutional Review Board at Baylor College of Medicine, under protocol number H-37922. All subjects provided written consent to study participation prior to any study procedure.
Study vaccine.
The study product is IIV1 A/H7N9 vaccine manufactured by Sanofi Pasteur using the same manufacturing process as the seasonal IIV, except for a minor modification in the phosphate buffered saline (PBS) diluent in the formulation step. This vaccine (lot number UD16397) was derived from the influenza A/Shanghai/2/2013 virus (1st wave strain). The HA content of the bulk inactivated influenza A/H7N9 vaccine was determined by reversed-phase high-performance liquid chromatography (RP-HPLC) instead of single radial immunodiffusion (SRID) assay, as the SRID reagents were not available from regulatory authorities at the time of formulation. Once the reagents became available, the HA content of the vaccine was determined to be approximately 50% higher (74.25 mcg of HA per dose) than the targeted HA content on the label (45 mcg). In this protocol, we maintained the labeling of the original HA content of the RP-HPLC-based IIV A/H7N9 formulations.
Laboratory assays.
HAI and MN Ab assays against the homologous influenza A/Shanghai/2/2013 (H7N9), and influenza A/H7N7 viruses were performed at a single central laboratory (Southern Research, Birmingham, Alabama), as previously described [14]. Serum samples were tested in duplicate and the geometric mean titer (GMT) of replicate results was used for analysis. The initial dilution was defined as 1:10 per US Food and Drug Administration recommendations; serum samples without activity were scored as 5. Seroconversion was defined as either a pre-vaccination HAI titer <10 and a post-vaccination HAI titer ≥40 or a pre- vaccination HAI titer ≥10 and a minimum four-fold rise in post-vaccination HAI antibody titer. In addition, 2 serum aliquots were shipped to Joint Institute of Virology (Shantou University-The University of Hong Kong) for testing against A/Shanghai/2/2013 (wave 1; Shg/2/13), A/Anhui/1/2013 (wave 1; Anh/1/13), A/chicken/Dongguan/A3217/2016 (wave 5, clade B; chk/Don/A3217/16), A/chicken/Guangzhou/372/2017 (wave 5, clade B; chk/Gua/372/17), A/Zhejiang/5/2017 (wave 5, Clade C; Zhe/5/17), A/chicken/Huzhou/849/2017 (wave 5, Clade C; chk/Huz/849/17), A/chicken/Shantou/39/2017 (wave 5, Clade C; chk/Sha/39/17), A/chicken/Shantou/9204/2016 (wave 5, Clade C, HPAI; chk/Sha/9204/16), and A/Guangzhou/17SF003RG/2017 (wave 5, Clade C, HPAI; Gua/17SF003RG/17). Briefly, sera were thawed and treated with receptor-destroying enzyme (Denka Seiken Co., Ltd.) to remove non-specific inhibitors, then heat-inactivated at 56°C for 30 min. The sera were then absorbed with turkey red blood cells to minimize non-specific agglutination. Antibody titers were determined by testing serial two-fold dilutions from 1:10 to 1:1280 in 96-well microtiter plates with 0.5% turkey erythrocytes using four hemagglutination units. Any uncertain results were resolved by repeat testing in duplicate. Positive and negative control sera (ferret sera) were also tested at the same time with virus back-titration performed. The HAI titer value is the reciprocal of the last dilution of serum that completely inhibited hemagglutination. Two results were obtained for each subject-visit and the GMT was computed and used as the subject’s visit-specific response for all subsequent calculations.
Statistical analysis.
The sample size for this study was based on the number of subjects who participated in DMID 07–0023, received 2 doses of IIV1 A/H7N7 or 1 or 2 doses of placebo, and consented to future contact, and it was not designed to test any specific null hypothesis. After difficulty recruiting placebo recipients from the previous trial, the protocol was amended to enroll A/H7 vaccine-naïve subjects who did not enroll in DMID 07–0023 to increase the precision for estimates of serologic responses in this group, and improve power for comparisons between groups.
The two co-primary immunologic endpoints included the proportion of subjects who had an HAI Ab titer of ≥ 40 and the proportion of subjects who met the definition of seroconversion 28 days after vaccination (Day 29). Since all subjects were seronegative at baseline, these 2 endpoints were identical and for simplicity only the proportion of subjects with a titer ≥ 40 is presented. As secondary endpoints, the proportion of subjects with MN Ab titer ≥ 40, and HAI and MN Ab GMTs were also calculated. Comparisons were conducted to evaluate differences in response between study groups using a Fisher’s exact test to compare the percentage of subjects with titer ≥ 40 and a non-parametric Wilcoxon test for comparisons of Ab titer magnitude. Statistical significance was considered at a level of α = 0.05 and all tests were two-sided. Analysis was performed using SAS 9.4 (SAS Institute, Cary, NC). As the study objective was to obtain preliminary estimates of immune responses using the prime-boost vaccination strategy, analyses were not adjusted for multiple comparisons and because missing data were minimal, imputation was not performed.
RESULTS
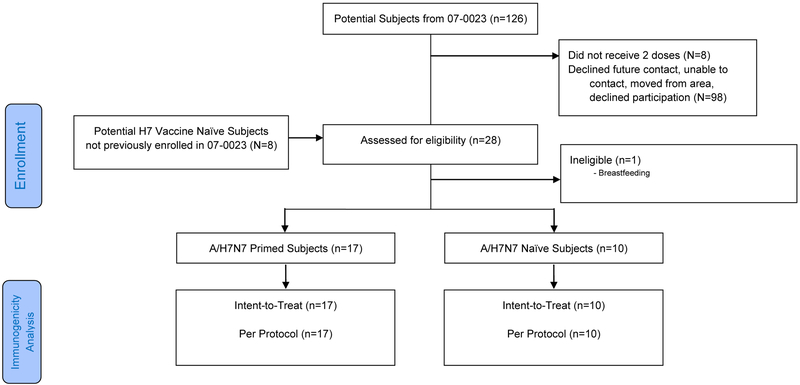
We attempted to contact all 126 subjects previously enrolled in DMID 07–0023 (Figure 1, Consort Diagram). A total of 20 subjects (18 previous vaccine recipients and 2 previous placebo recipients) were screened for the current study. One subject was not eligible (breastfeeding). We enrolled 19 subjects from the DMID 07–0023 study (7.5mcg HA, N = 7; 15mcg HA, N = 2; 45mcg HA; N = 6, 90mcg HA; N = 2, placebo, n=2), and an additional 8 IIV1 A/H7-naïve subjects. All subjects were enrolled at Baylor College of Medicine in Houston, Texas, between January 12th and July 1st, 2016. Subjects were 52% male, and 67% were non-Hispanic White (Table 1).
Figure 1.
Study Flow Chart (CONSORT diagram)
Table 1.
Baseline Demographic Characteristics of Study Subjects.
| Influenza A/H7N7 vaccine recipients (N=17) |
Influenza A/H7N7 vaccine naïve (N=10) |
All subjects (N=27) |
|
|---|---|---|---|
| Female | 8 (47%) | 6 (60%) | 14 (52%) |
| Hispanic | 2 (12%) | 3 (30%) | 5 (19%) |
| Unknown | 0 | 1 (10%) | 1 (4%) |
| Median (range) | 40 (31–48) | 38.5 (29–49) | 39 (29–49) |
Safety and Reactogenicity.
The IIV1 A/H7N9 vaccine was well tolerated. A total of 18 (67%, 95% CI 46–85%) subjects reported at least one solicited systemic or injection site reaction, and similar event rates were reported in both groups. The most prevalent solicited injection site reaction was tenderness (35%, 95% CI 14–62% in Group 1, and 50%, 95% CI 19–81% in Group 2). All injection site reactions were graded as mild or moderate in intensity. There were 11 subjects in Group 1 (65%, 95% CI 38–86%) and 2 subjects (20%, 95% CI 3–56%) in Group 2 who reported solicited systemic reactions, all were mild or moderate in intensity. There were 6 unsolicited AEs (5 in Group 1 and 1 in Group 2) in 6 subjects, all were mild or moderate in intensity, and none were assessed as related to study product. There were no SAEs reported during the 6-month follow up duration.
Immunogenicity.
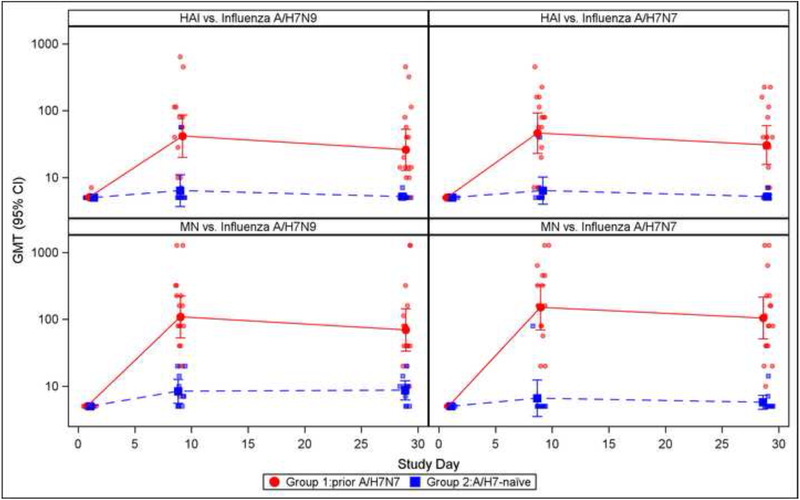
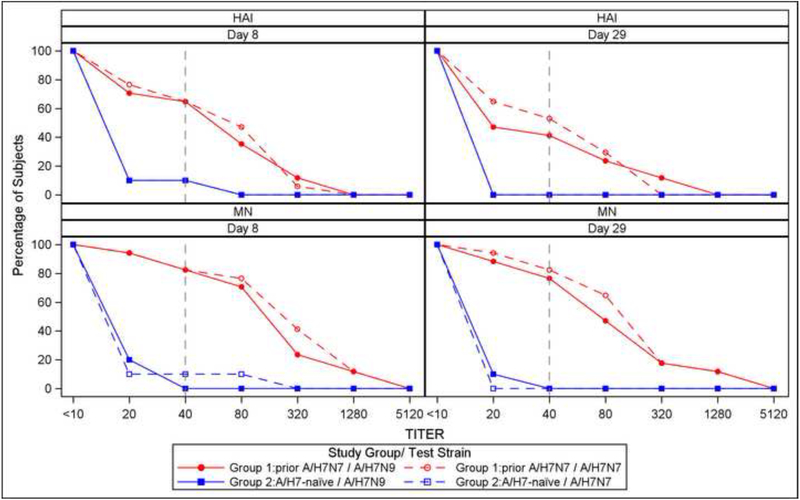
At baseline, all subjects had HAI and MN Ab titers that were below the limit of detection against influenza A/H7N9 and A/H7N7. Using the A/H7N9 virus as a test strain, HAI Ab GMTs in Group 1 rose to 41.7 on Day 9 and then declined to 26.1 on Day 29; in contrast, HAI Ab GMTs in group 2 were 6.4 and 5.2 on Days 9 and 29, respectively. The difference between the 2 groups was statistically significant (P<0.001 for the 2 time points). On Days 9 and 29 post vaccination, 65% and 41% (respectively) of subjects in Group 1 had HAI Ab titers ≥40, compared to 10 and 0% (respectively) in Group 2. The difference between the 2 groups was statistically significant (P<0.001 and P=0.026 for Days 9 and 29 comparisons, respectively). For MN Abs, subjects in Group 1 had GMTs of 108.6 and 69.4 on Days 9 and 29 (respectively), compared to GMTs of 8.4 and 8.7 in Group 1 subjects on Days 9 and 29 (respectively). The difference between the 2 groups was statistically significant (P<0.001 for the 2 time points). On Days 9 and 29, 82% and 76% of subjects (respectively) in Group 1, compared to 0% and 0% in Group 2 achieved a MN Ab titer of ≥40 (P<0.001 for the 2 time points). A similar pattern was observed in the Ab responses using influenza A/H7N7 virus as a test strain (Figure 2).
Figure 2.
Hemagglutination inhibition antibody titers following influenza A/H7N9 in A/H7N7 primed and A/N7-naïve individuals for the prime and boost strains. Panel A displays Geometric mean titers (GMT) with corresponding 95% confidence intervals; post-vaccination titers were significantly higher (p<0.05 by Wilcoxon test) for previously primed subjects at all time points for both prime and boost strains. Panel B displays reverse cumulative distributions of HAI and MN titers by post vaccination visit; a reference line is shown at the seroprotective titer (1:40).
Breadth of immune response.
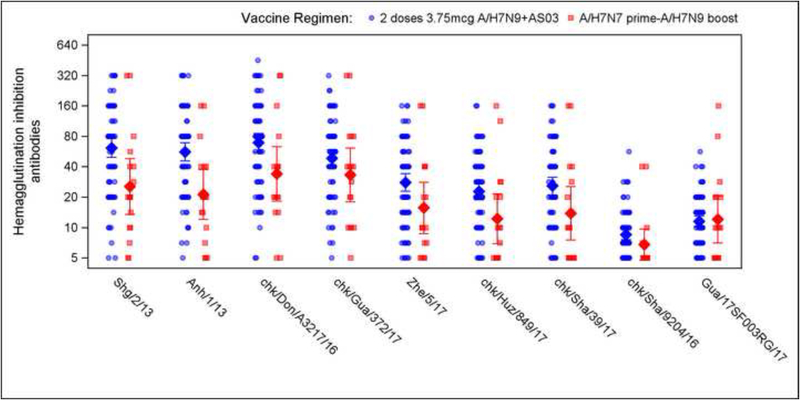
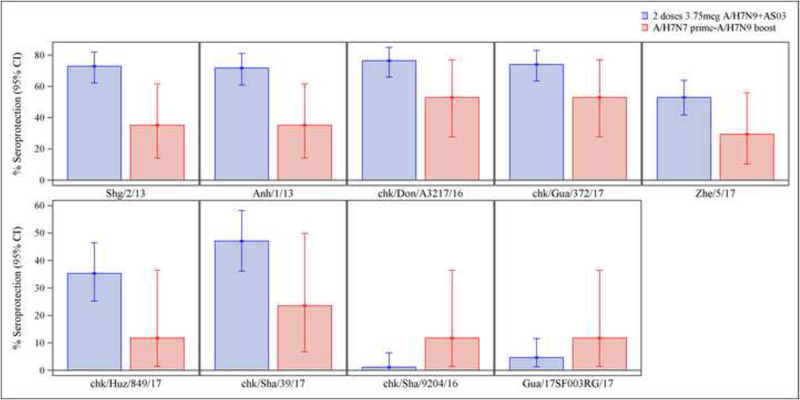
We compared the breadth of the Ab responses on Day 29 after the boost in our study to that observed 21 days after 2 doses of 3.75mcg HA, 7.5mcg HA or 15mcg HA of IIV A/H7N9, all doses given with AS03 adjuvant in a previous study (DMID 13–0033). The test strains represented influenza A/H7N9 viruses from the 1st and 5th epidemic waves. Following the heterologous boost, sera cross-reacted with the panel of variant viruses. However, for all tested strains, the HAI antibodies GMTs were higher in the recipients of the 2 AS03-adjuvanted IIV1 A/H7N9 doses than in those boosted with a single dose of IIV1 H7N9, except for the 2 HPAI strains (Figure 3, Table 2). Of note, subjects who were influenza A/H7-naïve had no to minimal detection of HAI GMTs, seroprotection and seroconversion rates against all tested strains following a single dose of IIV1 H7N9. For the 2 vaccine strategies, the highest HAI Ab GMTs, seroprotection and seroconversion rates were observed against the Wave 5-Clade B - A/chicken/Dongguan/A3217/2016 virus (Strain 3), and the lowest antibody responses were against the 2 Clade C HPAI viruses (Figure 3 and Table 2).
Figure 3.
Hemagglutination inhibition antibody titers (2A) and seroprotection rates (2B) following influenza A/H7N9 vaccine boost (current study) and in stored sera following AS03-adjuvanted influenza A/H7N9 vaccine against 9 influenza A/H7N9 virus strains from epidemic waves 1 and 5. (See Methods section for virus designation).
Table 2.
Hemagglutination inhibition antibodies against influenza A/H7N9 strains after 2 doses of AS03 adjuvanted vaccine or an unadjuvanted influenza A/H7N9 vaccine boost (primed only).
| 2 vaccine doses with AS03 adjuvant | Group 1§ | P value* | |||
|---|---|---|---|---|---|
| HA dosage | 3.75 mcg (N=22) | 7.5 mcg (N=29) | 15 mcg (N=34) | 45 mcg (N=17) | |
| Baseline * | |||||
| GMT (95% CI) | 5.2 (4.8 – 5.5) | 5.8 (5.0 – 6.8) | 5.3 (4.9 – 5.7) | 5.0 ( - ) | |
| Titer ≥40 - % (95% CI) | 0 (0–15) | 0 (0–12) | 0 (0–4) | 0 (0–20) | |
| Post Vaccination, Strain 1, Wave 1 - A/Shanghai/2/2013 | |||||
| GMT (95% CI) | 68.3 (45.5 – 102.7) | 55.9 (36.8 – 84.9) | 62.0 (44.0 – 87.4) | 25.5 (13.5 – 48.2) | 0.0029 |
| Titer ≥40 - % (95% CI) | 73 (50–89) | 69 (49–85) | 76 (59–89) | 35 (14–62) | 0.0098 |
| Post Vaccination, Strain 2, Wave 1 - A/Anhui/1/2013 | |||||
| GMT (95% CI) | 65.2 (44.1 – 96.3) | 53.3 (35.8 – 79.4) | 53.2 (37.9 – 74.7) | 21.3 (12.1 – 37.5) | 0.0013 |
| Titer ≥40 - % (95% CI) | 73 (50–89) | 69 (49–85) | 74 (56–87) | 35 (14–62) | 0.0119 |
| Post Vaccination, Strain 3, Wave 5-Clade B - A/Chicken/Dongguan/A3217/2016 | |||||
| GMT (95% CI) | 80.0 (52.6 – 121.6) | 63.0 (42.0 – 94.4) | 68.0 (48.8 – 94.7) | 34.0 (18.3 – 63.2) | 0.0134 |
| Titer ≥40 - % (95% CI) | 77 (55–92) | 72 (53–87) | 79 (62–91) | 53 (28–77) | 0.0716 |
| Post Vaccination, Strain 4, Wave 5-Clade B - A/Chicken/Guangzhou/372/2017 | |||||
| GMT (95% CI) | 53.1 (36.3 – 77.8) | 46.7 (33.0 – 66.2) | 47.6 (34.7 – 65.1) | 33.3 (18.1 – 61.3) | 0.1189 |
| Titer ≥40 - % (95% CI) | 77 (55–92) | 69 (49–85) | 76 (59–89) | 53 (28–77) | 0.1673 |
| Post Vaccination, Strain 5, Wave 5-Clade C - A/Zhejiang/5/2017 | |||||
| GMT (95% CI) | 33.6 (23.2 – 48.8) | 25.7 (17.4 – 37.9) | 26.6 (19.3 – 36.6) | 15.7 (8.7 – 28.2) | 0.0205 |
| Titer ≥ 40 - % (95% CI) | 64 (41–83) | 48 (29–67) | 50 (32–68) | 29 (10–56) | 0.1104 |
| Post Vaccination, Strain 6, Wave 5-Clade C - A/Chicken/Huzhou/849/2017 | |||||
| GMT (95% CI) | 25.3 (17.1 – 37.5) | 21.5 (15.1 – 30.5) | 22.1 (16.6 – 29.5) | 12.3 (7.0 – 21.6) | 0.0077 |
| Titer ≥ 40 - % (95% CI) | 45 (24–68) | 28 (13–47) | 35 (20–54) | 12 (1–36) | 0.0841 |
| Post Vaccination, Strain 7, Wave 5-Clade C - A/Chicken/Shantou/39/2017 | |||||
| GMT (95% CI) | 29.2 (19.3 – 44.2) | 26.0 (17.9 – 37.9) | 23.8 (17.4 – 32.4) | 13.9 (7.5 – 25.5) | 0.0138 |
| Titer ≥ 40 - % (95% CI) | 50 (28–72) | 48 (29–67) | 44 (27–62) | 24 (7–50) | 0.1071 |
| Post Vaccination, Strain 8, Wave 5-Clade C (HPAI) - A/Chicken/Shantou/9204/2016 | |||||
| GMT (95% CI) | 9.0 (6.9 – 11.7) | 8.7 (6.9 – 10.9) | 8.2 (7.0 – 9.4) | 6.8 (4.8 – 9.7) | 0.0072 |
| Titer ≥ 40 - % (95% CI) | 0 (0–15) | 3 (0–18) | 0 (0–10) | 12 (1–36) | 0.0713 |
| Post Vaccination, Strain 9, Wave 5-Clade C (HPAI) - A/Guangzhou/17SF003RG/2017 | |||||
| GMT (95% CI) | 10.7 (8.1 – 14.0) | 12.1 (9.7 – 15.1) | 11.7 (9.6 – 14.1) | 12.0 (7.0 – 20.5) | 0.5267 |
| Titer ≥ 40 - % (95% CI) | 9 (1–29) | 3 (0–18) | 3 (0–15) | 12 (1–36) | 0.2611 |
The baseline antibody titers were measured against strain 1; the baseline against the other strains were similar.
Seroprotection and seroconversion titers were nearly identical. So we present only seroprotection data.
P value in comparing the responses in the recipients of 1 dose of unadjuvanted boost vaccination at the 45mcg HA dosage (N=17) to a combined group of recipients of 2 doses of adjuvanted vaccine at all HA dosages (N=85) using a Fisher’s exact test for comparisons of proportional endpoints, and Wilcoxon comparisons of titers.
Group 1 subjects are prior recipients of IIV1 A/H7N7.
Discussion
In this study we demonstrate that the prime-boost approach was safe, and well-tolerated. More importantly, we found that in subjects with no detectable HAI and MN Ab responses following 2 doses of unadjuvanted IIV1 A/H7N7, priming (immunological memory) did occur, as evidenced by a prompt rise in HAI and MN Ab against the boost and priming strains following a single dose of unadjuvanted IIV1 A/H7N9 administered 8 years later.
The rises in HAI Ab were modest, with GMTs on Day 29 post vaccination of 26.1 and seroprotection rates of 41% against the boost strain in primed individuals. In comparison, a single dose of 45mcg IIV H7N9 resulted in HAI GMT’s below the limit of detection and no seroprotection in unprimed individuals. Two doses of AS03-adjuvanted IIV H7N9 resulted in higher levels of HAI GMTs and seroprotection rates than the heterologous prime-boost approach with unadjuvanted vaccines. However, and in a pandemic scenario, the former approach requires the administration of 2 doses of antigenically-matched adjuvanted vaccines, which given the time and economic constraints in the face of a rapidly sweeping pandemic may result in fewer individuals being vaccinated globally. The prime-boost approach allows for antigenic mismatch in the priming series, is logistically and economically more feasible, and would probably result in more individuals being vaccinated. It is unknown if a larger number of individuals who are vaccinated using the prime-boost approach with resulting lower HAI Ab levels would lead to better overall vaccine effectiveness, than a smaller number of individuals vaccinated with resulting higher antibody titers. HAI Ab levels are but one determinant of protection in the setting of a pandemic, and it is possible that previous vaccination with a related strain, such as during a priming scenario, can result in protection via other mechanisms, including anti-neuraminidase Abs [15, 16].
A lower HAI Ab GMT against the homologous strain may signify diminished breadth of the response against drifted influenza strains, as shown in previous studies [17]. In our study, we also found that the HAI Ab GMTs generated by the prime-boost approach were lower against an array of divergent influenza A/H7N9 strains than the ones generated by 2 doses of adjuvanted IIV1 H7N9. This may have bearing on the use of adjuvants in the priming, as it may improve responses following delayed boosting with a heterologous strain, as demonstrated with influenza A/H5 strains [18]. MN Ab assays were not performed to test the breadth of the response against the 9 strains. While HAI and MN Abs usually show a strong correlation, MN Ab assays are more sensitive against influenza A/H7, and we predict the MN Ab levels to be higher. Similar to findings from ferret sera assays, the HPAI A/H7N9 strains were the most antigenically distinct viruses using human sera in our study [4]. The HPAI A/H7N9 strains were present during the 5th wave, but were not the most prevalent strains isolated from humans, and their epidemiological significance remains unknown.
While our study and previous ones demonstrate the feasibility and potential merits of a prime-boost approach, many uncertainties remain. The optimal priming regimen is unclear. Priming options against pandemic influenza with favorable immunogenicity data include DNA vaccines, live attenuated vaccines, adjuvanted and unadjuvanted inactivated vaccines, mRNA-based vaccines, and recombinant vaccines. These regimens were evaluated in different studies using different influenza strains and in different populations. Hence it is difficult to speculate about the best approach. In addition to different priming regimens, it is possible that a single priming dose, especially if adjuvanted, will be sufficient to prime the immune system. The minimum time needed between the heterologous prime and the boost is another variable that should be evaluated, although in general longer periods result in improved responses and expansion of Ab epitope repertoire [19]. An NIH-sponsored study evaluating some of these different permutations of prime-boost approaches using adjuvanted and unadjuvanted IIV A/H7N9 is currently underway.
One limitation of the present study is the small sample size. The fact that we detected patterns of responses with IIV1 H7N7 prime-A/H7N9 boost that are consistent with previous descriptions is reassuring, and confirms that memory responses develop despite the almost complete lack of detectable serologic responses following the priming series. The small sample size did not allow for an examination of the role of the HA dosage in the priming series in the response to the boost, although in an exploratory analysis we found no apparent trend. Another limitation is that the 2 trials from which sera were used to test against divergent strains were non-contemporaneous. But both were performed within the Vaccine Treatment and Evaluation Units, with comparable quality control and processing methods, and the testing against the panel of influenza A/H7N9 viruses took place in 1 laboratory at the same time.
In summary, vaccination with 2 doses of unadjuvanted IIV1 A/H7N7 resulted in immune priming to a delayed heterologous boost, despite lack of detectable serologic responses following the priming series. An exploration of the role of alternate prime-boost approaches and the role of alternate correlates of protection against pandemic influenza would help inform the design of pandemic preparedness strategies.
Highlights:
Inactivated influenza A/H7N7 vaccine results in development of memory responses.
Cross-reactive antibodies develop post heterologous influenza A/H7 prime-boost.
The inclusion of an AS03 adjuvant results in broadly cross-reactive antibodies.
Acknowledgements
We would like to thank our study subjects (DMID 07–0023, DMID 13–0033 and DMID 13–0044), Dr. Hoonmo Lee Koo, Dr. Jose Serpa-Alvarez, Dr. Jeanne S. Sheffield, Dr. Richard Webby, Dr. Maria Deloria Knoll, The Division of Microbiology and Infectious Diseases at the National Institutes of Health team: Wendy Buchanan, Suzanne Murray, Soju Chang, Valerie Riddle, Teresa Hauguel and Chris Roberts.
The DMID 13–0033 Vaccine Study Group consists of: Lisa A. Jackson, James D. Campbell, Sharon E. Frey, Kathryn M. Edwards, Wendy A. Keitel, Karen L. Kotloff, Andrea A. Berry, Irene Graham, Robert L. Atmar, Buddy Creech, Isaac Thomsen, Shital M. Patel, Andres F. Gutierrez, Edwin L. Anderson, Hana M. El Sahly, Heather Hill, Diana L. Noah, Abbie Bellamy.
GSK were given the opportunity to review the manuscript for medical accuracy and protection of intellectual property, however editorial control resided fully with the authors only.
Funding for this project was provided by the National Institutes of Health, contracts HHSN272201300015I, HHSN272200800002C, HHSN272201500002C, and HHSN272201400006C; Hong Kong Research Grant Council (17127714) and The National Key Plan for Scientific Research and Development of China (2016YFD0500302).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflicts of interest pertaining to the conduct and reporting of this clinical trial
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ClinicalTrials.gov identifier: NCT02586792
References
- [1].Wu H, Wang X, Xue M, Xue M, Wu C, Lu Q, et al. Spatial characteristics and the epidemiology of human infections with avian influenza A(H7N9) virus in five waves from 2013 to 2017 in Zhejiang Province, China. PLoS One. 2017;12:e0180763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Su S, Gu M, Liu D, Cui J, Gao GF, Zhou J, et al. Epidemiology, Evolution, and Pathogenesis of H7N9 Influenza Viruses in Five Epidemic Waves since 2013 in China. Trends Microbiol. 2017;25:713–28. [DOI] [PubMed] [Google Scholar]
- [3].Ding X, Luo J, Quan L, Wu A, Jiang T. Evolutionary genotypes of influenza A (H7N9) viruses over five epidemic waves in China. Infect Genet Evol. 2017;55:269–76. [DOI] [PubMed] [Google Scholar]
- [4].Zhu W, Zhou J, Li Z, Yang L, Li X, Huang W, et al. Biological characterisation of the emerged highly pathogenic avian influenza (HPAI) A(H7N9) viruses in humans, in mainland China, 2016 to 2017. Euro Surveill. 2017;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Belser JA, Gustin KM, Pearce MB, Maines TR, Zeng H, Pappas C, et al. Pathogenesis and transmission of avian influenza A (H7N9) virus in ferrets and mice. Nature. 2013;501:556–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cheng W, Wang X, Shen Y, Yu Z, Liu S, Cai J, et al. Comparison of the three waves of avian influenza A(H7N9) virus circulation since live poultry markets were permanently closed in the main urban areas in Zhejiang Province, July 2014-June 2017. Influenza Other Respir Viruses. 2018;12:259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jackson LA, Campbell JD, Frey SE, Edwards KM, Keitel WA, Kotloff KL, et al. Effect of Varying Doses of a Monovalent H7N9 Influenza Vaccine With and Without AS03 and MF59 Adjuvants on Immune Response: A Randomized Clinical Trial. JAMA. 2015;314:237–46. [DOI] [PubMed] [Google Scholar]
- [8].Couch RB, Patel SM, Wade-Bowers CL, Nino D. A randomized clinical trial of an inactivated avian influenza A (H7N7) vaccine. PLoS One. 2012;7:e49704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mulligan MJ, Bernstein DI, Winokur P, Rupp R, Anderson E, Rouphael N, et al. Serological responses to an avian influenza A/H7N9 vaccine mixed at the point-of-use with MF59 adjuvant: a randomized clinical trial. JAMA. 2014;312:1409–19. [DOI] [PubMed] [Google Scholar]
- [10].Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006;354:1343–51. [DOI] [PubMed] [Google Scholar]
- [11].Goji NA, Nolan C, Hill H, Wolff M, Noah DL, Williams TB, et al. Immune responses of healthy subjects to a single dose of intramuscular inactivated influenza A/Vietnam/1203/2004 (H5N1) vaccine after priming with an antigenic variant. J Infect Dis. 2008;198:635–41. [DOI] [PubMed] [Google Scholar]
- [12].Babu TM, Levine M, Fitzgerald T, Luke C, Sangster MY, Jin H, et al. Live attenuated H7N7 influenza vaccine primes for a vigorous antibody response to inactivated H7N7 influenza vaccine. Vaccine. 2014;32:6798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Halliley JL, Khurana S, Krammer F, Fitzgerald T, Coyle EM, Chung KY, et al. High-Affinity H7 Head and Stalk Domain-Specific Antibody Responses to an Inactivated Influenza H7N7 Vaccine After Priming With Live Attenuated Influenza Vaccine. J Infect Dis. 2015;212:1270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Noah DL, Hill H, Hines D, White EL, Wolff MC. Qualification of the hemagglutination inhibition assay in support of pandemic influenza vaccine licensure. Clin Vaccine Immunol. 2009;16:558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Couch RB, Atmar RL, Franco LM, Quarles JM, Wells J, Arden N, et al. Antibody correlates and predictors of immunity to naturally occurring influenza in humans and the importance of antibody to the neuraminidase. J Infect Dis. 2013;207:974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wan H, Qi L, Gao J, Couzens LK, Jiang L, Gao Y, et al. Comparison of the Efficacy of N9 Neuraminidase-Specific Monoclonal Antibodies against Influenza A(H7N9) Virus Infection. J Virol. 2018;92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Keitel WA, Atmar RL, Nino D, Cate TR, Couch RB. Increasing doses of an inactivated influenza A/H1N1 vaccine induce increasing levels of cross-reacting antibody to subsequent, antigenically different, variants. J Infect Dis. 2008;198:1016–8. [DOI] [PubMed] [Google Scholar]
- [18].Galli G, Hancock K, Hoschler K, DeVos J, Praus M, Bardelli M, et al. Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proc Natl Acad Sci U S A. 2009;106:7962–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Khurana S, Wu J, Dimitrova M, King LR, Manischewitz J, Graham BS, et al. DNA priming prior to inactivated influenza A(H5N1) vaccination expands the antibody epitope repertoire and increases affinity maturation in a boost-interval-dependent manner in adults. J Infect Dis. 2013;208:413–7. [DOI] [PMC free article] [PubMed] [Google Scholar]