Abstract
Background:
To date, HIV incidence studies among men who have sex with men (MSM) across sub-Saharan Africa have focused on studying sexual risk practices with less focus on sexual networks.
Setting:
TRUST/RV368 conducted in Abuja and Lagos, Nigeria recruited MSM using respondent driven sampling (RDS) and followed HIV negative men for incident infection over 4 years.
Methods:
Four-hundred forty-one HIV-uninfected MSM underwent a parallel rapid HIV testing algorithm every 3 months for up to 18 months. HIV incidence per 100 person-years (PY) and 95% confidence intervals (CIs) were estimated using Poisson regression. Individual and network characteristics were examined using multivariable Cox proportional hazards regression adjusted and unadjusted for RDS-weights.
Results:
Among cohort members with a median age of 23 years (interquartile range [IQR]: 20–27), 81 HIV infections occurred over 527 PY (incidence 15.4/100 PY; 95% CI: 12.3–19.0). The incidence rate was highest among 16–19 year olds as compared to those 25 years or older (30.9/100 PY; 95% CI: 22.1–45.3 vs. 6.9/100 PY; 95% CI: 4.2–10.9, respectively). Individual determinants included receptive partnerships, condomless sex, no history of testing for HIV, and rectal gonorrhea. Sexual networks were larger and consisted of an older sexual partner, though there was no clustering by recruitment networks.
Conclusions:
These HIV incidence data reinforce the unmet HIV prevention needs among young MSM in Nigeria. Even in the context of emerging HIV diagnostic and prevention strategies, structural challenges including stigma and criminalization of same-sex practices highlight the need for novel implementation approaches in the context of MSM-friendly services.
Keywords: HIV, incidence, MSM, sub-Saharan Africa, egocentric network, respondent driven sampling
INTRODUCTION
The global HIV pandemic among key populations, particularly among men who have sex with men (MSM), remains unabated regardless of effective antiretroviral prevention and treatment strategies1. In societies where the general public is intolerant of MSM and same-sex behavior is criminalized, pervasive homophobia in the healthcare setting hinders the engagement of MSM and uptake of HIV prevention services2, 3. In Nigeria, up to 24% of medical students and 39% of non-medical students believed that MSM should be denied healthcare services and HIV prevention services4. Additionally, Nigerian MSM experienced an increase in fear and avoidance of health care settings after the passage of the Same-Sex Marriage Prohibition Act in January 20145. Avoidance of healthcare services among Nigerian MSM is increased when stigma experiences synergize with suicidal ideation6. Such an environment negatively impacts linkage to HIV test and treat programs, undermining the effective control of HIV.
In Nigeria, prevalence estimates of HIV are much higher among MSM (17–66%)7–9 as compared to the reproductive aged adults (4%)7. Phylodynamic modeling of our cohort at one year of follow up estimated an incidence of 7.9 per 100 susceptible person-years [PY] (95% confidence interval [CI]: 7.0–10.4) and 9.2% of female infections in the region were attributed to the MSM population10. Other studies of MSM in Kenya, South Africa, Mali, Côte d’Ivoire, and Senegal have reported HIV incidence rates that range from 6.8 to 16.0 per 100 PY11–15. The combination of the high burden of disease and the sociopolitical context is likely to exacerbate the HIV incidence rate in Nigeria, which has yet to be fully described.
In many western settings, epidemiologic studies have focused on individual level factors such as unsafe sexual practices, high rates of partner exchange and concurrency in sexual partnerships16. These individual behaviors provide avenues for discussion with healthcare personnel who readily interact with patients on behavior change. However, in settings where those types of relationships are less common, MSM may engage more closely with their sexual and social networks. Networks providing stronger social support increased uptake of HIV testing, ART initiation, and levels of viral suppression among Nigerian MSM17. Therefore, factors that capture characteristics of the network may provide greater insight and novel opportunities for behavior change interventions18. Studies of these determinants in a well-characterized MSM cohort in sub-Saharan Africa deepen our understanding of the intersection of individual and sexual network factors that are vital for HIV control among MSM in highly stigmatizing environments.
The TRUST/RV368 study was premised on undertaking comprehensive investigation of these factors to inform strategies for innovative intervention. The current analysis measures incidence over 4 years and investigates epidemiological and sexual/social network characteristics in addition to traditional individual-level predictors driving incident infection. Individual-level predictors of incident HIV include rectal gonorrhea and receptive anal intercourse11, 12. Other correlates included genital ulcers, exclusive sex with men, low education, condomless sex and group sex11, 12.
METHODS
Study design and population
Between March 2013 and March 2018, a prospective combination HIV prevention and treatment study (TRUST/RV368) recruited MSM using respondent driven sampling (RDS) in Abuja and Lagos, Nigeria as previously described5, 19, 20. Initial “seed” participants, who were well connected within the target population, initiated recruitment by providing coupons to up to three of their eligible peers. Those peers recruited 3 of their peers and this process continued with each new “wave” of peers until target enrollment numbers were met. Recruitment was completed in Lagos in September 2016 and remains ongoing in Abuja. Eligible participants were born male, age ≥16 years (Abuja) or ≥18 years (Lagos), reported anal intercourse in the past year, and provided informed consent in English or Hausa. Participants were followed-up every 3 months for 18 months. At each visit, participants were administered a structured survey instrument through in-person interviews, received a physical exam, and provided biological specimens for HIV and STI diagnostics. HIV counseling and testing were repeated every 3 months for those who were HIV-uninfected. Participants eligible for inclusion in these analyses were HIV-uninfected at baseline and had at least 1 follow-up HIV diagnostic test result.
Ethical considerations
The institutional review boards at the Nigerian Federal Capital Territory Health Research Ethics Committee, the Nigerian Ministry of Defense in Nigeria, the University of Maryland Baltimore, and the Walter Reed Army Institute of Research reviewed and approved the research protocol. All participants provided informed consent. Unique study identifiers for each participant de-identified the study data collected and analyzed.
Laboratory Procedures
At each 3-month visit, whole blood was tested for HIV using rapid test kits (Alere Determine, Waltham MA, USA; Trinity biotech Uni-Gold HIV, Wicklow, Ireland; and Chembio Diagnostics HIV-½ Stat Pak test, Medford NY, USA for discordant results) as outlined by the parallel testing algorithm for high-risk individuals in Nigeria21. At each visit, anal swabs and urine were collected and tested for Neisseria gonorrhoeae and Chlamydia trachomatis using the Aptima Combo 2 CT/NG Assay (Hologic, San Diego, CA). Participants testing positive for HIV and/or bacterial sexually transmitted infections (STIs) were offered antiretroviral or antibiotic therapy as clinically indicated.
Exposures and outcome
The primary outcome was incident HIV infection. The primary predicting variables included individual (demographic, behavioral, biological) and network related factors. Time-independent variables were assessed at baseline and included demographic factors (site, age, education, employment status, sexual orientation, and gender) and behavioral factors over the past year (number of male and female sexual partners, receptive and insertive partnerships, sex in exchange for money or gifts, testing for HIV, and drug use). Time-varying variables were assessed at the most recent visit i.e. preceding HIV acquisition for those who seroconverted or at the preceding visit for those who remained uninfected. These included concurrent sexual partnerships, meeting partners online, condomless sex during last sexual encounter, rectal or urethral chlamydia or gonorrhea, and alcohol use. If the one-visit lag was missing, the next most recent was filled forward and the time difference was not accounted for in the analysis.
The participant (ego) was asked to report up to 5 of his most recent sexual partners (alters) along with their demographic and behavioral information. Ego-centric network data were used to describe respondents’ network characteristics. The social network was a tertile categorization of the number of MSM the participant knew personally in the past two years. The sexual network was a tertile categorization of the most recent reported alters in the past year. Known linkages within a sexual network were defined by the number of alters who knew each other. Density of a sexual network was the number of known linkages divided by the total possible linkages available for the number of reported alters and further categorized into three groups (0%, 1–50%, 51–100%). Strength of friendship ranged from 0 (acquaintance) to 10 (best friend) for each alter. Reported friendship ratings were averaged for the total number of alters and divided into three groups (low (0–3), medium (4–6), high (7–10)). Demographic and behavioral characteristics of the alters were calculated as the proportion of alters with that characteristic and further divided a priori into three categories (0%, 1–50%, 51–100%).
Statistical Analysis
Of the 858 MSM who were HIV-uninfected at enrollment, 441 had follow-up HIV testing results and were included in these analyses. A sensitivity analysis compared those who defaulted to those included in the analysis to evaluate potential biases in the incidence estimates. The quality and equilibrium of the RDS sample was also evaluated to verify that the HIV incidence estimates were not biased by the sampling strategy. The proportion of incident infections was estimated for 5 categories of recruitment waves (1–5, 6–10, 11–15, 16–20, 21–28) and tested using a trend t-test. Incidence by recruitment seed was evaluated using a Chi-square test. Convergence and bottleneck plots were generated using RDS Analyst (v. 0.56)22 to further confirm equilibrium in the sample population. RDS weights were generated using RDS-II in RDS Analyst separately for Abuja and Lagos sites. The distribution of seroconverters within the recruitment chains was visualized using Netdraw23. Cumulative hazards of infection by individual age and those with a sexual network comprised of an older partner were estimated by Kaplan-Meier survival curves and compared with the log-rank test. Since the crude incidence rates among stratified proportions of older alters (1–50%, 51–100%) were similar (18.73 vs. 18.71), the two groups were pooled for the Kaplan-Meier analysis. Crude incidence rates with 95% CIs for HIV and loss to follow up were calculated per 100 PY using Poisson regression models. Bivariate and multivariable Cox proportional hazards regression models were used to analyze the relationship between demographic, behavioral, biological and alter characteristics and time to HIV infection. Observation time began at first HIV-negative test for all participants. For participants who remained HIV-uninfected, time of exposure ended with the last available HIV-negative test result. For those who seroconverted, the time of exposure ended at the midpoint between last HIV-negative test date and first HIV-positive test date. All variables significantly associated with HIV incidence in bivariate analysis (p<0.05) were included in the multivariable model. Variables that were insignificant (p≥0.05) were removed in a backwards stepwise approach to obtain the most parsimonious model. Both bivariate and multivariable models, adjusted and un-adjusted for RDS-weights, were presented. Missing observations accounted for less than 5% of the total sample and were retained in the models with indicators. Data were analyzed using Statistical Analysis Software (SAS) version 9.4 (SAS Institute, Cary, NC) and Stata Statistical Software: Release 13 (Stata Corp LP, College Station, TX).
RESULTS
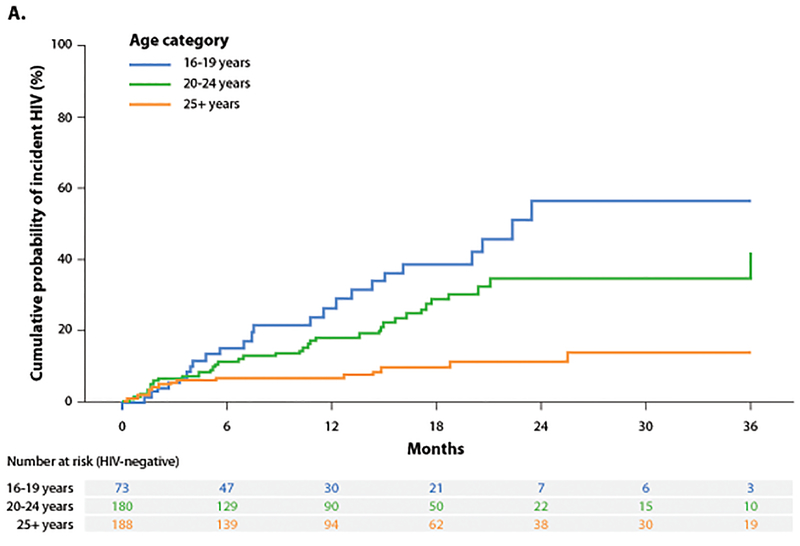
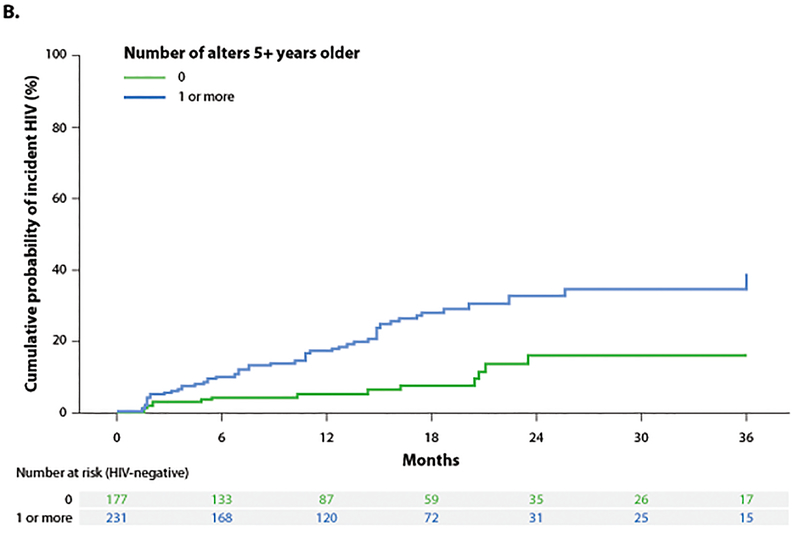
Participants had a median age of 23 years (interquartile range [IQR]: 20–27) and reported a median number of 1 (IQR: 0–2) of same aged alters, a median of 0 (IQR: 0–0) of younger alters and a median number of 1 (IQR: 0–1) older alters. Eighty-one HIV infections occurred over 527 PY resulting in an incidence rate of 15.4/100 PY (95% CI: 12.3–19.0). Incidence was 4 fold higher among 16–19 year olds and nearly 3 fold higher among 20–24 year olds as compared to those 25 years or older (Figure 1A). Incidence was 3 fold higher for those with a sexual network comprised of an older alter as compared to one with similar aged alters (Figure 1B).
Figure 1.
A-B. Cumulative probability of incident HIV stratified by age of individual and reported partners.
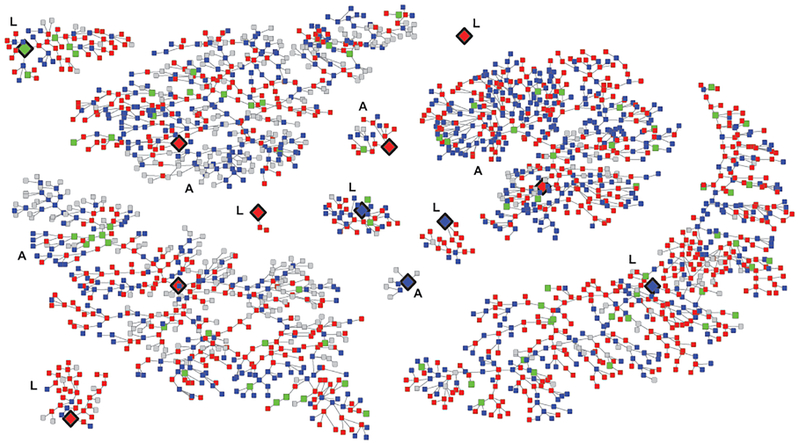
Of the 12 recruitment chains, 1 seed was unproductive (Figure 2). The depth of the recruitment chains ranged from 3 to 28 waves in Abuja and 0 to 26 waves in Lagos. HIV seroconversion did not differ by recruitment network indicated by seed of origin. The proportion of incident infections between early waves of recruitment versus later waves of recruitment were similar (wave 1–5: 58%, wave 6–10: 53%, wave 11–15: 44%, wave 16–20: 46%, wave 21–28: 52%) (p>0.05). HIV incidence reached convergence (or equilibrium) after the recruitment of 100–300 participants and remained stable as the number of recruited participants increased. Bottleneck plots indicated stable estimates in HIV incidence per seed. Therefore, the RDS diagnostic analysis suggested the sampling strategy yielded a representative cross-section of the MSM population as it relates to HIV incidence.
Figure 2. Seroconverters within the recruitment chains of the cohort, TRUST/RV368.
Note: Large diamonds with black rims are seeds, red squares are HIV+, blue squares are HIV-, green squares are seroconverters, grey squares are unknown HIV status. Letters A and L indicate networks originating in Abuja or Lagos.
In bivariate analyses, a number of demographic, behavioral and biological risk factors were significantly associated with HIV seroconversion (Table 1). Younger age was associated with increased risk of a new HIV infection. Behavioral risk factors included receptive anal intercourse, condomless sex at last anal sex, sex in exchange for money or gifts, and no history of HIV testing. Having insertive sex was protective of incident infection. Men with a laboratory diagnosed rectal gonorrhea at the preceding visit were at increased risk of HIV. Concurrent sexual relationships were not significantly associated in this analysis. Significant sexual network determinants included more reported alters in sexual network, a stronger friendship on average with sexual partners, older sexual partners by 5 years, a larger proportion of partners with a higher SES, partners with more education, some regular partners, and a higher proportion perceived to be at high risk for HIV (Table 2).
Table 1.
Incidence and bivariate analysis of individual level risk factors of incident HIV infection
| Characteristic | Cases/person-years | Incidence/100 person-years* | Unadjusted HRs (95% CI)** | RDS-adjusted HRs (95% CI)*** |
|---|---|---|---|---|
| Overall | 81/527 | 15.36 | ||
| Demographic | ||||
| Site | ||||
| Abuja | 47/358 | 13.15 | Ref. | Ref. |
| Lagos | 34/170 | 20.00 | 1.40 (0.9–2.2) | 1.09 (0.6–2.0) |
| Age (years) | ||||
| 25+ | 17/246 | 6.92 | Ref. | Ref. |
| 20–24 | 40/204 | 19.59 | 2.66 (1.5–4.7) | 2.55 (1.6–4.0) |
| 16–19 | 24/78 | 30.92 | 4.11 (2.2–7.7) | 3.30 (1.5–7.2) |
| Education | ||||
| > Senior Secondary School | 25/236 | 10.59 | Ref. | Ref. |
| ≤ Senior Secondary School | 56/291 | 19.22 | 1.73 (1.1–2.8) | 2.26 (0.9–5.6) |
| Employment | ||||
| Employed/student | 55/383 | 14.36 | Ref. | Ref. |
| Unemployed | 24/121 | 19.83 | 1.33 (0.8–2.1) | 1.12 (0.6–2.3) |
| Sexual Orientation | ||||
| Bisexual | 47/377 | 12.45 | Ref. | Ref. |
| Homosexual | 34/147 | 23.11 | 1.78 (1.1–2.8) | 1.20 (0.8–1.7) |
| Gender Identification | ||||
| Male only | 58/452 | 12.84 | Ref. | Ref. |
| Female or both | 22/73 | 30.20 | 2.23 (1.4–3.6) | 1.46 (0.7–3.2) |
| Behavioral | ||||
| Number of male sexual partners† | ||||
| 0–1 | 8/67 | 11.99 | Ref. | Ref. |
| 2–4 | 28/184 | 15.25 | 1.33 (0.6–2.9) | 0.72 (0.3–1.9) |
| 5+ | 43/259 | 16.61 | 1.49 (0.7–3.2) | 1.28 (0.5–3.1) |
| Number of female sexual partners† | ||||
| 0 | 50/218 | 22.95 | Ref. | Ref. |
| 1+ | 31/308 | 10.06 | 0.46 (0.3–0.7) | 0.82 (0.4–1.5) |
| Concurrent sexual partnerships | ||||
| No | 42/310 | 13.56 | Ref. | Ref. |
| Yes | 39/218 | 17.91 | 1.28 (0.8–2.0) | 0.98 (0.7–1.3) |
| Met male partners online | ||||
| No | 8/144 | 5.55 | Ref. | Ref. |
| Yes | 73/383 | 19.05 | 3.39 (1.6–7.0) | 1.55 (0.5–4.8) |
| Receptive sex† | ||||
| No | 9/219 | 4.12 | Ref. | Ref. |
| Yes | 67/289 | 23.22 | 5.27 (2.6–10.6) | 2.77 (1.6–4.9) |
| Insertive sex† | ||||
| No | 30/86 | 34.81 | Ref. | Ref. |
| Yes | 47/427 | 11.02 | 0.33 (0.2–0.5) | 0.42 (0.2–0.9) |
| Condomless sex at last anal sex | ||||
| No | 49/388 | 12.64 | Ref. | Ref. |
| Yes | 32/140 | 22.91 | 1.91 (1.2–3.0) | 1.85 (1.3–2.6) |
| Sex in exchange for money or gifts† | ||||
| No | 43/334 | 12.89 | Ref. | Ref. |
| Yes | 35/180 | 19.48 | 1.48 (0.9–2.3) | 1.78 (1.0–3.1) |
| Ever tested for HIV | ||||
| Yes | 57/423 | 13.47 | Ref. | Ref. |
| No | 24/100 | 23.97 | 1.74 (1.1–2.8) | 2.13 (1.6–2.9) |
| Drank alcohol in past month | ||||
| No | 47/234 | 20.06 | Ref. | Ref. |
| Yes | 33/287 | 11.49 | 0.60 (0.4–0.9) | 0.63 (0.4–1.0) |
| Ever injected drugs | ||||
| No | 78/522 | 14.95 | Ref. | Ref. |
| Yes | 3/6 | 54.91 | 3.79 (1.2–12.1) | 1.39 (0.3–7.5) |
| Biological | ||||
| Urethral Chlamydia | ||||
| No | 58/483 | 12.00 | Ref. | Ref. |
| Yes | 7/33 | 21.35 | 1.72 (0.8–3.8) | 0.78 (0.3–2.3) |
| Rectal Chlamydia | ||||
| No | 5¾63 | 11.45 | Ref. | Ref. |
| Yes | 12/53 | 22.86 | 1.99 (1.1–3.7) | 2.70 (0.8–9.5) |
| Urethral Gonorrhea | ||||
| No | 6¼82 | 12.66 | Ref. | Ref. |
| Yes | 4/34 | 11.67 | 1.00 (0.4–2.8) | 1.10 (0.7–1.8) |
| Rectal Gonorrhea | ||||
| No | 42/465 | 9.03 | Ref. | Ref. |
| Yes | 21/49 | 42.67 | 4.55 (2.7–7.7) | 4.63 (2.1–10.4) |
Abbreviations: HR, hazard ratios; CI, confidence intervals; P, p-value.
in past year
Incidence per 100 person-years calculated using Poisson Regression.
Crude Hazard ratios calculated using Cox Proportional Hazards Model.
Adjusted for RDS-weights. Bolded indicates p<0.05.
Table 2.
Bivariate analysis of alter-related risk factors of incident HIV infection
| Characteristic | Cases/person-years | Incidence/100 person-years* | Unadjusted HRs (95% CI)** | RDS-adjusted HRs (95% CI)*** |
|---|---|---|---|---|
| Social Network (No. of known MSM) | ||||
| ≤10 | 34/216 | 15.73 | Ref. | Ref. |
| 11–20 | 20/117 | 17.16 | 1.11 (0.6–1.9) | 1.88 (0.8–4.4) |
| ≥21 | 27/193 | 13.97 | 0.94 (0.6–1.6) | 1.13 (0.7–2.0) |
| Sexual Network (up to 5 reported alters) | ||||
| 0–1 | 4/145 | 2.76 | Ref. | Ref. |
| 2–3 | 22/186 | 11.81 | 4.10 (1.4–11.9) | 5.16 (1.3–19.9) |
| 4–5 | 41/190 | 21.60 | 7.47 (2.7–20.9) | 6.91 (2.2–21.6) |
| Density within reported sexual network | ||||
| No linkages | 24/166 | 14.47 | Ref. | Ref. |
| Less than 50% linked | 21/81 | 25.98 | 1.76 (1.0–3.2) | 2.96 (0.9–9.9) |
| More than 50% linked | 2½01 | 10.47 | 0.73 (0.4–1.3) | 0.93 (0.3–3.4) |
| Average strength of friendship with alters | ||||
| low | 4/72 | 5.57 | Ref. | Ref. |
| medium | 25/205 | 12.19 | 2.06 (0.7–5.9) | 2.19 (1.0–4.9) |
| high | 37/240 | 15.45 | 2.48 (0.9–7.0) | 3.75 (1.5–9.2) |
| Percentage of Alters older by 5+ years | ||||
| 0% | 14/230 | 6.09 | Ref. | Ref. |
| 1–50% | 25/134 | 18.73 | 2.93 (1.5–5.6) | 4.00 (1.7–9.2) |
| 51–100% | 27/144 | 18.71 | 3.01 (1.6–5.7) | 4.32 (1.5–12.3) |
| Percentage of Alters with higher SES | ||||
| 0% | 7/150 | 4.66 | Ref. | Ref. |
| 1–50% | 15/140 | 10.73 | 2.25 (0.9–5.5) | 1.48 (0.3–6.7) |
| 51–100% | 45/224 | 20.08 | 4.05 (1.8–9.0) | 5.82 (1.4–25.0) |
| Percentage of Alters with more education | ||||
| 0% | 24/287 | 8.36 | Ref. | Ref. |
| 1–50% | 9/51 | 17.78 | 1.95 (0.9–4.2) | 2.91 (1.8–4.6) |
| 51–100% | 32/168 | 19.02 | 2.24 (1.3–3.8) | 4.31 (1.5–12.1) |
| Percentage of Alters are regular partners | ||||
| 0% | 10/147 | 6.81 | Ref. | Ref. |
| 1–50% | 38/264 | 14.39 | 2.00 (1.0–4.0) | 3.21 (1.5–6.9) |
| 51–100% | 19/107 | 17.78 | 2.38 (1.1–5.1) | 4.56 (1.0–21.4) |
| Percentage of Alters are casual partners | ||||
| 0% | 5/82 | 6.12 | Ref. | Ref. |
| 1–50% | 22/104 | 21.07 | 3.44 (1.3–9.1) | 2.28 (0.9–6.1) |
| 51–100% | 40/332 | 12.06 | 2.07 (0.8–5.2) | 1.07 (0.2–5.2) |
| Percentage of Alters who identify as bisexual | ||||
| 0% | 10/108 | 9.30 | Ref. | Ref. |
| 1–50% | 16/123 | 13.00 | 1.42 (0.6–3.1) | 0.75 (0.3–2.2) |
| 51–100% | 39/284 | 13.75 | 1.47 (0.7–2.9) | 1.21 (0.7–2.1) |
| Percentage of Alters perceived as high risk for HIV | ||||
| 0% | 46/392 | 11.74 | Ref. | Ref. |
| 1–50% | 13/88 | 14.77 | 1.22 (0.7–2.3) | 1.51 (0.5–4.7) |
| 51–100% | 5/27 | 18.78 | 1.55 (0.6–3.9) | 2.44 (1.2–4.9) |
Abbreviations: No, number; MSM, men who have sex with men; SES, socio-economic status; HR, hazard ratios; CI, confidence intervals; P, p-value.
Incidence per 100 person-years calculated using Poisson Regression.
Crude Hazard ratios calculated using Cox Proportional Hazards Model.
Adjusted for RDS-weights. Bolded indicates p<0.05.
In the multivariable analysis, self-reported receptive partnerships, condomless sex, no history of testing for HIV, having rectal gonorrhea, sexual networks with more alters, and those with older partners remained significantly associated with HIV incidence (Table 3). Age of the participant did not remain significant because it was highly correlated with a number of risk behaviors. Decreasing age was significantly associated with engaging in receptive anal sex, no experience testing for HIV, having rectal gonorrhea, having older alters, having alters of higher SES, and having more educated alters (all ptrend < 0.01). Variables removed in backwards selection were age, transactional sex, proportion of regular alters, insertive sex, proportion likely at higher risk for HIV, strength of friendship, proportion with higher SES, proportion with more education.
Table 3.
Multivariable analysis of individual and network level predictors of incident HIV Infection
| Characteristic | Adjusted HRs (95% CI)* | Adjusted HRs (95% CI) with RDS-Weights** |
|---|---|---|
| Receptive sex (self report)† | ||
| No | Ref. | Ref. |
| Yes | 2.99 (1.4–6.2) | 1.81 (1.6–2.1) |
| Condomless sex at last anal sex | ||
| No | Ref. | Ref. |
| Yes | 1.30 (0.8–2.1) | 1.63 (1.2–2.3) |
| Ever tested for HIV | ||
| Yes | Ref. | Ref. |
| No | 1.46 (0.9–2.4) | 1.58 (1.2–2.0) |
| Rectal Gonorrhea | ||
| No | Ref. | Ref. |
| Yes | 4.05 (2.4–6.9) | 4.49 (2.2–9.3) |
| Sexual Network (up to 5 reported alters) | ||
| 0–1 | Ref. | Ref. |
| 2–3 | 4.95 (1.6–14.9) | 9.17 (2.0–42.7) |
| 4–5 | 8.05 (2.8–23.5) | 11.12 (3.9–32.0) |
| Alters 5+ years older | ||
| 0% | Ref. | Ref. |
| 1–50% | 1.87 (1.0–3.7) | 3.27 (1.3–8.0) |
| 51–100% | 2.72 (1.4–3.3) | 6.00 (2.3–15.4) |
Abbreviations: HR, hazard ratios; CI, confidence intervals.
in the past year
Cox proportional hazards model was adjusted for self-reported receptive sex in past year, condomless sex, ever tested for HIV, rectal Gonorrhea, number of reported alters, older age of alters. Other factors significant in bivariate analyese were no longer significant in saturated model and removed by backwards selection.
Same adjusted Cox proportional hazards model but with RDS-weights.
Sensitivity analysis
Enrollees in the cohort who only had a single HIV test result (n=417) were significantly different from those retained in the analysis on individual risk factors. Those who defaulted from the study were more likely to be younger, have low education, reported more concurrent partnerships, less likely to meet partners online, more likely to engage in transactional sex, less likely to have an HIV test prior to enrollment, and more likely to report a smaller social network (Supplementary Table 1). Those with a single HIV test did not complete the second baseline visit 2 weeks later, and as a result, differences in biological and network characteristics could not be assessed because of insufficient data. The overall attrition rate at 18 months of follow-up was 62.3 cases/100PY. Attrition was similar for those who were younger as compared to those who were 25 years old or older (age 16–19 years, HR: 1.1, 95% CI: 0.7–1.8; age 20–24 years, HR: 1.1, 95% CI: 0.5–2.4). Attrition was 50% higher for those who did not have any older partners as compared to those with an older partner (HR: 1.5, 95% CI: 1.1–2.2).
DISCUSSION
The findings document high incidence among MSM in this sub-Saharan African cohort where avoidance to HIV prevention services may be the result of intolerance and criminalization of MSM behavior2, 3, 5, 6. By integrating individual behavior and network-based measures of risk, the current analysis provides novel insights on new HIV infections among Nigerian MSM. Individual factors include receptive partnerships, condomless sex, no history of testing for HIV, and rectal gonorrhea, many of which were highly correlated with young age. Network defined factors include more sexual partners and one comprised of at least one older member by 5 or more years. Recruitment networks and depth within the recruitment chains did not influence seroconversion. The high observed rate reinforce our previous estimate10 and highlight the public health impact of Nigeria’s socio-political environment on transmission rates. As such, treatment as prevention and comprehensive and innovative preventative interventions in non-stigmatizing venues have significant public health implications.
Previously reported incidence rates among MSM in sub-Saharan Africa (6.8 – 16.0 per 100 PY)11–15 highlight that the reported incidence in the current study are among the highest observed thus far, underscoring that the HIV epidemic among MSM in this setting continues unabated. The lower estimates of earlier studies may be attributed to differences in sampling methodology. Two of the studies originated from a vaccine preparedness cohort where fewer of the most vulnerable MSM, men who exclusively had sex with men (17%, 77/449 MSM), were enrolled11, 12. The other studies were small in size and lacked sufficient follow-up to conduct robust multivariable analyses13–15. With greater precision, our findings are consistent with the vaccine preparedness cohort in identifying receptive anal intercourse and laboratory diagnosed gonorrhea (a marker of unprotected sex), as drivers of incident infection.
Our analysis uniquely observed an especially vulnerable risk for adolescent MSM where incidence was 30% higher in the 16–19 year olds as compared to the 20 and 24 year olds and 400% higher than those over 25 years of age. Furthermore, the finding that linkages to older partners in sexual networks heighten risk three fold demonstrates the importance of interventions such as PrEP and treatment as prevention to reduce transmission from older to younger MSM. However, empowering these young MSM to embrace these prevention interventions with their older partners in the context of a stigmatizing and discriminatory environment has its challenges. As seen with adolescent girls who are at greatest risk of HIV from their older male partners in sub-Saharan Africa, there are a number of economic, social and psychological barriers that hinder self-advocacy for sexual health24–26. Our finding of a 70% increased risk of incident HIV among MSM who accept gifts in payment for sex supports this hypothesis. Non-stigmatizing clinics may be one way to provide access to prevention interventions as well as a place to seek social and psychological support outside of sexual networks.
This study suggests that the composition of sexual networks is an important determinant of HIV risk. The ego-centric sexual networks provided greater insight on characteristics that may influence a person’s risk profile. Many demographic and socio-behavioral traits of members in a sexual network create a clustering of social norms and risk behaviors. In Nigeria, networks providing stronger social support increased uptake of HIV testing, ART initiation, and levels of viral suppression among MSM17. Furthermore, the size of the sexual network influences the likelihood of exposure to HIV and STIs. Nigerian MSM with larger sexual networks had higher incidence of bacterial STIs27. With HIV and STIs unlikely to be randomly distributed among sexual networks, the characteristics of the network offer greater insight on those at higher risk. Combining both the individual and sexual network characteristics provides a higher dimensional view of those at greatest risk for new HIV infections and provides a context for developing targeted interventions that refocus sexual risk taking norms in the networks.
In this study, those who did not undergo an HIV test prior to study enrollment were at increased risk of a new HIV infection. In Nigeria, recent findings suggest a synergistic negative relationship between sexual stigma and suicidal ideation on HIV testing6. Prior stigma experiences generated psychological distress and increased feelings of vulnerability, both of which decreased willingness to seek services at general health facilities. MSM who are reluctant to undergo HIV testing may be more inclined to seek out older partners, who presumably offer a greater sense of stability and support. Sex in exchange for money or gifts may also be an indication of younger MSM in age-disparate relationships that provide a mixture of emotional and financial support, although transactional sex did not retain significance in the final model. Community-based MSM friendly venues with a “one-stop shop” for treatment and prevention services when implemented effectively provide an emotionally supportive environment to foster self-advocacy among young MSM and ultimately increase awareness and testing for HIV.
This study has some limitations. First, the estimated HIV incidence rates may be biased because of incomplete data on those with one HIV diagnostic test. Loss to follow-up is a challenge with any study of a hard-to-reach population and especially when those lost were disproportionately younger and were not aware of their HIV status28–29. The non-random recruitment methodology may have resulted in recruitment of certain sub-groups within the MSM community, but RDS diagnostics suggest the sample converged and is a representative cross-section of MSM as it relates to HIV incidence. Since adding RDS-weights to the models may bias the findings, both weighted and un-weighted are presented. Third, there were some “micro clusters” of incident infection that suggest yet to be identified linkages between the newly infected in the social/sexual recruitment networks but this would be best evaluated by phylogenetic analysis of genetic clusters. The lack of an association with age may be driven by more proximal factors that cluster among younger MSM, such as not testing for HIV and having rectal gonorrhea. The sampling design allowed each person to recruit 3 of their peers, but did not capture whether those 3 peers comprised their sexual network or knew other sexual partners in the recruitment chain. Understanding the bi-directional sexual connections may provide a better measure for understanding whether recruitment networks influenced seroconversion and harbored areas of high transmission. Lastly, this study originated in two metropolitan cities in Nigeria and may not be generalizable to areas outside these cities.
HIV incidence was very high among young MSM, an important key population within the mixed HIV epidemic of Nigeria and in the larger sub-Saharan context. Our data suggest that these young men had not sought their HIV status before entering the cohort, had a number of biological and behavioral risk factors, and had larger sexual networks with increasing numbers of older partners that together contributed to the transmission of HIV. HIV prevention and treatment interventions in stigmatizing and discriminatory free settings are a public health priority for MSM in sub-Saharan Africa. While individual-level risks offer an avenue of intervention, these data suggest the importance of working within the sexual networks as an alternative for men who are afraid to access HIV healthcare prevention services.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the study participants and staff at the TRUST/RV368 clinics for their contributions and commitment to this research. This manuscript is dedicated to the first seed of our cohort who died. We also thank Dr. Søren Bentzen for his review of the statistical analysis.
Conflicts of Interest and Source of Funding: TC has received a speaker fee from Gilead Sciences. The research reported in this publication was supported by funding the U.S National Institutes of Health [R01 MH099001, R01 AI120913, R01 MH110358]; the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense [W81XWH-11-2-0174]; Fogarty Epidemiology Research Training for Public Health Impact in Nigeria program [D43TW010051]; and the President’s Emergency Plan for AIDS Relief through a cooperative agreement between the Department of Health and Human Services/Centers for Disease Control and Prevention, Global AIDS Program, and the Institute for Human Virology-Nigeria [NU2GGH002099].
Footnotes
Publisher's Disclaimer: Disclaimer: The content is solely the responsibility of the authors and should not be construed to represent the positions of the National Institutes of Health, the U.S. Army or the Department of Defense or the Department of Health and Human Services. The investigators have adhered to the policies for protection of human subjects as prescribed in AR-70.
Prior presentation: Presented as a poster presentation at the 9th IAS Conference on HIV Science; July 23–26, 2017; Paris, France.
REFERENCES
- 1.Beyrer C, Baral SD, Collins C, et al. The global response to HIV in men who have sex with men. Lancet 2016;388:198–206. [DOI] [PubMed] [Google Scholar]
- 2.Devi S Uganda takes “another step backward” with HIV bill. Lancet 2014;383:1960. [DOI] [PubMed] [Google Scholar]
- 3.Arreola S, Santos GM, Beck J, et al. Sexual stigma, criminalization, investment, and access to HIV services among men who have sex with men worldwide. AIDS Behav 2015;19:227–34. [DOI] [PubMed] [Google Scholar]
- 4.Sekoni AO, Jolly K, Gale NK, et al. Provision of Healthcare Services to Men Who Have Sex with Men in Nigeria: Students’ Attitudes Following the Passage of the Same-Sex Marriage Prohibition Law. LGBT Health 2016;3:300–7. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz SR, Nowak RG, Orazulike I, et al. The immediate eff ect of the Same-Sex Marriage Prohibition Act on stigma, discrimination, and engagement on HIV prevention and treatment services in men who have sex with men in Nigeria: analysis of prospective data from the TRUST cohort. Lancet HIV 2015;2:e299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez-Hart C, Bradley C, German D, et al. The Synergistic Impact of Sexual Stigma and Psychosocial Well-Being on HIV Testing: A Mixed-Methods Study Among Nigerian Men who have Sex with Men. AIDS Behav 2018;22:3905–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Agency for the Control of AIDS (NACA). 2015 Global AIDS Response Country Report. Abuja, Nigeria, 2015. [Google Scholar]
- 8.Vu L, Adebajo S, Tun W, et al. High HIV prevalence among men who have sex with men in Nigeria: implications for combination prevention. J Acquir Immune Defic Syndr 2013;63:221–7. [DOI] [PubMed] [Google Scholar]
- 9.Keshinro B, Crowell TA, Nowak RG, et al. High prevalence of HIV, chlamydia and gonorrhoea among men who have sex with men and transgender women attending trusted community centres in Abuja and Lagos, Nigeria. J Int AIDS Soc 2016;19:21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volz EM, Ndembi N, Nowak R, et al. Phylodynamic analysis to inform prevention efforts in mixed HIV epidemics. Virus Evol 2017;3:vex014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders EJ, Okuku HS, Smith AD, et al. High HIV-1 incidence, correlates of HIV-1 acquisition, and high viral loads following seroconversion among MSM. AIDS 2013;27:437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price MA, Rida W, Mwangome M, et al. Identifying at-risk populations in Kenya and South Africa: HIV incidence in cohorts of men who report sex with men, sex workers, and youth. J Acquir Immune Defic Syndr 2012;59:185–93. [DOI] [PubMed] [Google Scholar]
- 13.Lane T, Osmand T, Marr A, et al. Brief Report: High HIV Incidence in a South African Community of Men Who Have Sex With Men: Results From the Mpumalanga Men’s Study, 2012–2015. J Acquir Immune Defic Syndr 2016;73:609–11. [DOI] [PubMed] [Google Scholar]
- 14.Drame FM, Crawford EE, Diouf D, et al. A pilot cohort study to assess the feasibility of HIV prevention science research among men who have sex with men in Dakar, Senegal. J Int AIDS Soc 2013;16 Suppl 3:18753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Couderc C, Dembele Keita B, Anoma C, et al. Is PrEP Needed for MSM in West Africa? HIV Incidence in a Prospective Multicountry Cohort. J Acquir Immune Defic Syndr 2017;75:e80–2. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan PS, Carballo-Dieguez A, Coates T, et al. Successes and challenges of HIV prevention in men who have sex with men. Lancet 2012;380:388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramadhani HO, Ndembi N, Nowak RG, et al. Individual and Network Factors Associated With HIV Care Continuum Outcomes Among Nigerian MSM Accessing Health Care Services. J Acquir Immune Defic Syndr 2018;79:e7–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amirkhanian YA. Social networks, sexual networks and HIV risk in men who have sex with men. Curr HIV/AIDS Rep 2014;11:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baral SD, Ketende S, Schwartz S, et al. Evaluating respondent-driven sampling as an implementation tool for universal coverage of antiretroviral studies among men who have sex with men living with HIV. J Acquir Immune Defic Syndr 2015;68 Suppl 2:S107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charurat ME, Emmanuel B, Akolo C, et al. Uptake of treatment as prevention for HIV and continuum of care among HIV-positive men who have sex with men in Nigeria. J Acquir Immune Defic Syndr 2015;68 Suppl 2:S114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.FMOH. Laboratory-Based HIV Rapid Test Validation in Nigeria. 2007.
- 22.RDS Analyst: Software for the Analysis of Respondent-Driven Sampling Data, Version 0.56, URL http://hpmrg.org. [computer program] , 2016.
- 23.Borgatti S NetDraw Software for Network Visualization. Analytic Technologies: Lexington, KY: 20002. [Google Scholar]
- 24.Dellar RC, Dlamini S, Karim QA. Adolescent girls and young women: key populations for HIV epidemic control. J Int AIDS Soc 2015;18:19408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gregson S, Nyamukapa CA, Garnett GP, et al. Sexual mixing patterns and sex-differentials in teenage exposure to HIV infection in rural Zimbabwe. Lancet 2002;359:1896–903. [DOI] [PubMed] [Google Scholar]
- 26.Kelly RJ, Gray RH, Sewankambo NK, et al. Age differences in sexual partners and risk of HIV-1 infection in rural Uganda. J Acquir Immune Defic Syndr 2003;32:446–51. [DOI] [PubMed] [Google Scholar]
- 27.Ramadhani HO, Liu H, Nowak RG, et al. Sexual partner characteristics and incident rectal Neisseria gonorrhoeae and Chlamydia trachomatis infections among gay men and other men who have sex with men (MSM): a prospective cohort in Abuja and Lagos, Nigeria. Sex Transm Infect 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pattanasin S, Wimonsate W, Chonwattana W, et al. Loss to follow-up and bias assessment among a cohort of Thai men who have sex with men in Bangkok, Thailand. Int J STD AIDS 2016; 27(3): 196–206. [DOI] [PubMed] [Google Scholar]
- 29.Tang W, Huan X, Zhang Y, et al. Factors associated with loss-to-follow-up during behavioral interventions and HIV testing cohort among men who have sex with men in Nanjing, China. PloS One 2015; 10(1):e115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.