Abstract
Background:
A growing body of epidemiologic evidence suggests that neurodegenerative diseases occur less frequently in cancer survivors, and vice versa. While unusual, this inverse comorbidity is biologically plausible and could be explained, in part, by the evolutionary tradeoffs made by neurons and cycling cells to optimize the performance of their very different functions. The two cell types utilize the same proteins and pathways in different, and sometimes opposite, ways. However, cancer and neurodegeneration also share many pathophysiological features.
Objective:
In this review, we compare three overlapping aspects of neurodegeneration and cancer.
Methods:
First, we contrast the priorities and tradeoffs of dividing cells and neurons and how these manifest in disease. Second, we consider the hallmarks of biological aging that underlie both neurodegeneration and cancer. Finally, we utilize information from genetic databases to outline specific genes and pathways common to both diseases.
Conclusions:
We argue that a detailed understanding of the biologic and genetic relationships between cancer and neurodegeneration can guide future efforts in designing disease-modifying therapeutic interventions. Lastly, strategies that target aging may prevent or delay both conditions.
Keywords: Aging, neurodegeneration, carcinogenesis, trade-offs, hallmarks, neurons, dividing cells
1. INTRODUCTION
Neurodegenerative diseases and cancers are often considered to be uniquely distinct but have a complex and intriguing interrelation. Both Alzheimer’s (AD) and Parkinson’s diseases (PD) are less frequent in survivors of many cancers (and vice versa), suggesting that a propensity towards one family of diseases may decrease the risk of the other [1–5]. In contrast, cancer survivors have a higher risk of other agerelated diseases, including non-neurodegenerative dementia, stroke, macular degeneration, and osteoarthritis [2, 6]. However, the inverse association is not consistent across all cancer types; the increased risk of malignant melanoma in patients with PD is the most notable example [7–9]. Cancer treatment may also modify the relationship; with some studies suggesting that chemotherapy treated breast cancer survivors may have lower white matter organization and connectivity when compared to healthy controls [10], and others associating chemotherapy with a lower risk of subsequent AD [6]. Thus, the epidemiologic association is complex and challenged by the difficulty of accounting for the ways in which diagnosis, treatment, and survival from one disease influence the risk of another [11].
While the epidemiologic data may be muddy, there is abundant evidence of a complex biological connection. An expanding body of literature describes genes, proteins, and pathways dysregulated in both cancer and neurodegenerative disease— often in opposite directions. For example, the expression of p53, a known tumor-suppressor gene, is upregulated in AD, PD and Huntington’s disease (HD) [12–15], but downregulated in the large majority of cancers [16]. Pin1, a multifunctional gene hypothesized to function as a molecular timer, is notably upregulated in a number of cancers but is downregulated in AD [17, 18]. On the other hand, there is substantial positive pathophysiological overlap, with oxidative stress, DNA damage, inflammation, metabolic deregulation, and aberrant cell cycle activation playing a central role in both diseases [19]. This rich overlap has spurred substantial interest, especially among researchers hungry for new ways of understanding and treating age-related neurodegeneration [20].
In this article, we further examine the biological and genetic overlap between cancer and neurodegeneration and develop two lines of thought in an effort to elucidate the mixed signals that make up this complex relationship. The first is that inverse associations, such as regulation of the same gene or pathway in opposite directions, or differential use of the same protein, have their origins in teleology – the profoundly different purpose of cycling cells and neurons. The second is that positive biological overlap between sporadic AD and late-onset cancers is generally driven by the common ground of aging on which they occur. These two axes of association are illustrated in Fig. (1). We then provide an overview of the overlapping genes and biological pathways involved in cancer and neurodegeneration, followed by a discussion of ways in which this comparative biology is leading to a broader understanding of both diseases and the development of new therapeutic and preventive strategies.
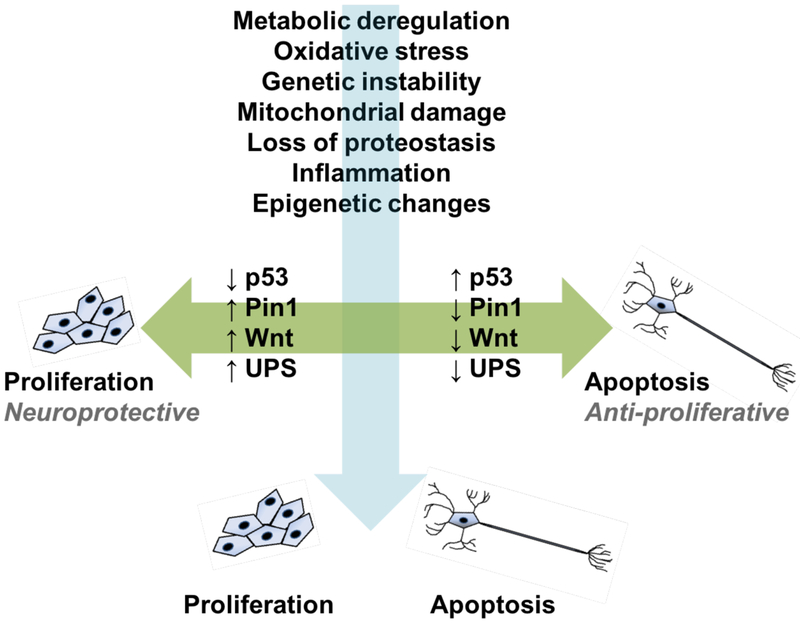
Fig. (1).
Two patterns of association between cancer and neurodegeneration, as depicted in the figure as “proliferation,” and “apoptosis,” respectively. The horizontal axis denotes specific genes, proteins, and pathways that are inversely regulated in cancer and neurodegeneration. The vertical axis shows age-associated pathophysiologic features that are directly associated with both families of diseases.
1.1. A Tale of Two Cells: Insights from Evolutionary Bioiogy
The survival of living things, whether cells or species, depends on preserving the structure and function of genetic material and passing it on with fidelity, meeting energy needs, and maintaining bodily systems in the face of entropy and other forces [21]. Evolutionary tradeoffs, defined as a benefit in one context necessitating a cost in another, have occurred on both the genotypic and phenotypic level over millions of years [22]. Disease risks associated with evolutionary tradeoffs are often diametric; vulnerabilities to one family of diseases can be protective against another [23]. Examples of diametric disease pairs include affective disorders and autism, osteoporosis and osteopenia, and, as we discuss, cancer and neurodegeneration.
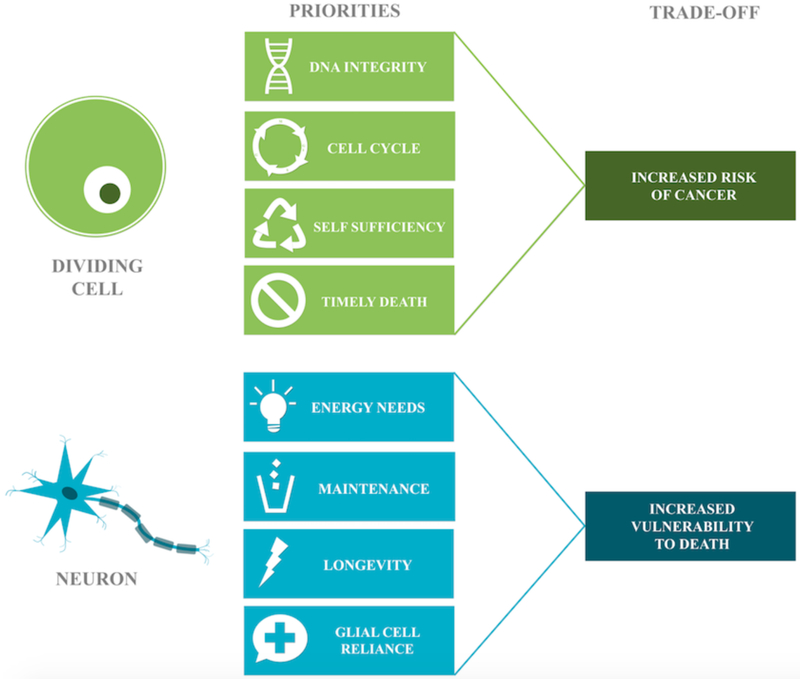
The survival strategy for most tissues in the body is an ongoing replacement. Blood cells have a lifespan of days to months, while the epithelial cells lining organs can live up to two years. Individual cells are interchangeable and can meet their own energy needs; the tissue will survive as long as the cell population is adequate. The tradeoff for this strategy is the risk of cancer. DNA integrity and careful cell-cycle control are thus the overarching priorities of dividing cells, as a single rogue cell can threaten the entire organism. With respect to meeting energy needs, dividing cells are generally self-sufficient. Under typical conditions, dividing cells undergo the more efficient process of oxidative phosphorylation, but can also perform anaerobic glycolysis in the absence of oxygen, or aerobic glycolysis during proliferation. Dysregulation of energy metabolism has been linked to both cancer and aging. A comparison of the tradeoffs made by neurons and dividing cells are listed in Fig. (2).
Fig. (2).
The differential priorities of dividing cells and neurons. Dividing cells prioritize DNA integrity, frequent self-replacement, and bioenergetic self-sufficiency but need to die on time to avoid cancer. The mission of neurons requires longevity and maintenance of networked connections. Their complexity, dependence, and high performance come at the cost of vulnerability to cell death.
A neuron’s purpose is to form networked connections with other neurons and maintain them for the life of the organism, but tradeoffs required for long-term survival make it particularly vulnerable to cell death. While there is evidence that neurogenesis can occur from neural stem cells in the dentate gyrus of the hippocampus, the subventricular zone, and the olfactory bulbs, most neurons in the brain are permanent [24–27]. Because they do not divide, they do not retain all the requisite enzymes to complete mitosis and repair only the DNA they use [28]. As a tradeoff for the specialization required to transmit information, neurons have lost the ability to independently meet many basic survival needs. They rely on nearby astrocytes, pericytes and microglia to perform a variety of critical maintenance tasks. Neurons use an enormous amount of energy and rely on glial cells to create energy precursors for oxidative phosphorylation [29]. These factors make neurons uniquely vulnerable to aging-related changes in energy metabolism.
1.2. Overlap Between Cancer, Neurodegeneration, and Hallmarks of Aging
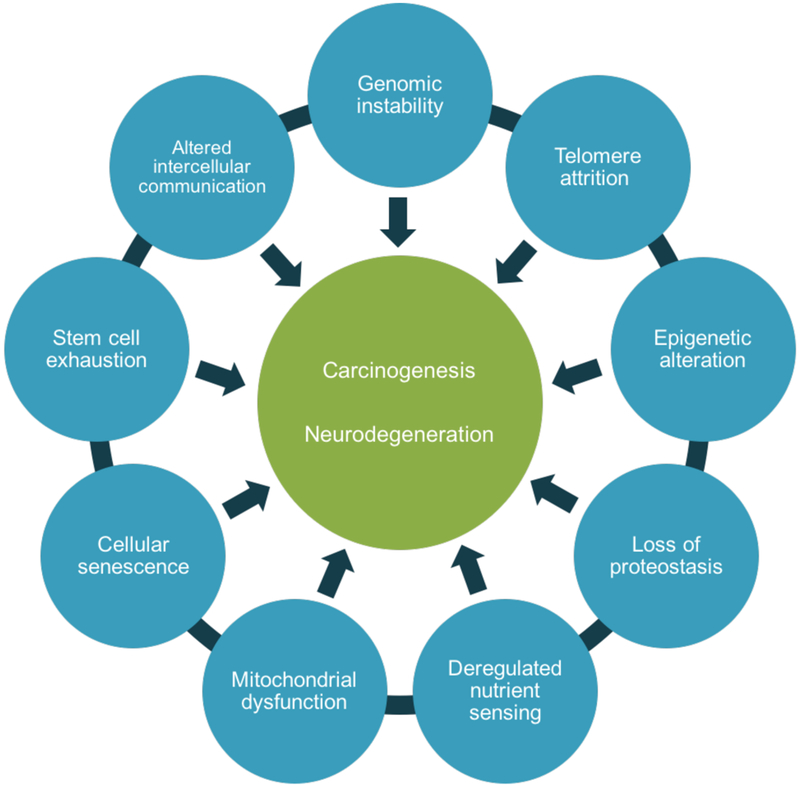
It is well-accepted that aging is a manifestation of the time-dependent accumulation of cellular damage [30]. A review published in 2013 further proposed nine key cellular and molecular hallmarks of physiologic aging: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication [31]. As further discussed below, each of these hallmarks of aging is significant with respect to both carcinogenesis and neurodegeneration, as seen in Fig. (3).
Fig. (3).
Overlap between hallmarks of aging and cancer and neurodegeneration. The nine hallmarks of aging are shared by carcinogenesis and neurodegeneration [31].
1.2.1. Genomic Instability
Cells can experience thousands of potentially toxic molecular lesions every day and have a variety of mechanisms to correct them [32]. A number of human DNA repair gene mutations, most famously BRCA1 and BRCA2, have been shown to strongly increase cancer risk [33, 34]. Most of these mutations precipitate a reduced efficacy of DNA repair processes. Within the past two decades, it has become apparent that epigenetic alterations play a significant role in silencing or reducing DNA repair protein expression [35–37]. Single-strand breaks can block transcriptional elongation by RNA polymerase II [38], and the accumulation of singlestrand breaks has been theorized to contribute to aging and neuronal dysfunction [39].
A seminal paper entitled “The Hallmarks of Cancer” has set the standard for defining the biological basis of cancer [40]. The overlap between the hallmarks of cancer and those of aging is striking; perhaps none is more obvious than genomic instability. Defects in DNA repair are strongly linked to cancer, and many progeroid syndromes are associated with an increased risk of developing both cancer and central nervous system (CNS) disorders [41]. Genomic instability is similarly related to neurodegeneration. A large number of progeroid syndromes are associated with neurodegeneration and/or mental retardation [42]. There is mounting evidence that neurons in patients with AD and other neurodegenerative diseases aberrantly attempt to re-enter the cell cycle as a response to DNA damage. Cell culture and animal models have demonstrated that abnormal activation of the cell cycle precedes the formation of tangles, plaques, and eventual cell death [43, 44]. Defects in various aspects of DNA repair, including nuclear excision repair and base excision repair, have also been linked to neurodegeneration [45, 46].
1.2.2. Telomere Attrition
Telomeres are stretches of repetitive nucleotide sequences at the tail end of each chromosome that over time become truncated due to cellular division. Eventually, when telomeres become too short, the chromosome reaches a “critical length,” after which it can no longer replicate; instead, the cell enters a senescent state. Telomere attrition is classically seen in normal organismal aging [31, 47]; moreover, pathologic alterations in telomeres are also seen in neurodegeneration and cancer. In AD, telomere shortening has been implicated in oxidative stress and inflammation, coupled with cognitive impairment, amyloid pathology, and tau hyper-phosphorylation [48]. Recently, peripheral blood analyses have shown a decrease in telomere length in peripheral blood leukocytes in AD patients, compared to agematched controls, highlighting a potential biomarker for AD [49, 50].
Telomere shortening has been described as “simultaneously a cancer protective mechanism and a pro-aging mechanism” [51]. When telomeres become critically short with aging, chromosomal material is unprotected, potentially leading to a genetic catastrophe from which cancer can arise. In a number of studies, early stages of carcinogenesis have demonstrated evidence of shortened telomeres [52–54]. However, by definition, all cancer cells eventually need to activate telomerase, an enzyme that synthesizes telomeres, in order to become immortal [55]. Thus, telomere attrition is recognized as a risk factor for both age-related carcinogenesis and neurodegeneration.
1.2.3. Epigenetic Alterations
An epigenetic trait was defined as a “stably heritable phenotype resulting from changes in a chromosome without alterations in the DNA sequence” during a recent consensus meeting [56]. There are three pillars of epigenetic mechanisms: DNA methylation, histone modification, and noncoding RNA species, all of which have been implicated in both aging and cancer [57]. Progeroid syndromes can be induced by epigenetic perturbations, and loss- or gain-of-function mutations of epigenetically relevant enzymes, such as the sirtuin SIRT6 have been shown to inversely affect longevity in mice [31, 58, 59]. Many other epigenetic mechanisms can be perturbed in cancer, including oncogene activation [60], histone modification [61], and DNA binding protein dysregulation [62].
The past decade has seen an increase in efforts to understand the epigenetics of neurodegenerative diseases. All three levels of epigenetic gene regulation are also implicated in neurodegeneration [63]. AD, for example, is marked by global hypomethylation and hypohydroxymethylation [64], alterations of histone proteins[65], and elevated expression of certain noncoding RNAs [66]. Several genes associated with amyloid–β processing and methylation homeostasis display significant inter-individual epigenetic variability, which is theorized to contribute to late-onset AD development [67]. There is a growing body of literature linking PD, HD, and many other neurodegenerative diseases to epigenetic alterations, and this will surely increase in coming years. Epigenetic therapy has already been suggested as a potential method for correcting expression levels of dysregulated genes in neurodegenerative disorders as well as cancers.
1.2.4. Loss of Proteostasis
The fourth hallmark of aging is the loss of protein homeostasis or proteostasis, especially in the heat-shock family of proteins [31, 68]. Protein regulators, such as molecular chaperones [69], and control systems, such as the autophagylysosomal system [70] and the ubiquitin-proteosome system (UPS) [71], decline with aging. Neurodegenerative diseases are characterized by a decline in these systems leading to accumulation of distinctive proteopathies. For example, PD is associated with downregulation of the UPS and aggregation of α–synuclein into Lewy bodies [72], while AD is distinguished by impaired chaperone proteins and autophagy, with aggregation of abnormal amyloid–β and tau [73, 74].
Neoplastic cells display a loss of proteostasis, but often in the opposite direction. Because increased protein synthesis is required for unregulated cellular division [75], cancer cells upregulate the UPS and heat shock proteins [76, 77]. These pathways help to drive cellular proliferation, downregulate cell death, and modulate protein folding.
1.2.5. Deregulated Nutrient Sensing
The dysregulation of nutrient sensing is an additional hallmark of aging. Intriguingly, in organisms with constitutively decreased insulin and insulin-like growth factor signaling (IIS), as well as dietary restriction, longevity is extended. This can be attributed to lowered rates of cellular damage due to slower cellular growth and metabolism [31]. Aged cells often attempt to decrease IIS as a protective measure against cellular damage, but this can cross a minimal threshold where the costs of decreased nutrient signaling outweigh the benefits. Just as under typical conditions, where decreased nutrient signaling increases lifespan, the opposite is true for anabolic signaling, which has been shown to accelerate aging [78].
Alterations in nutrient sensing are also characteristically seen in both neurodegeneration and cancer. Nearly 100 years ago, Otto Warburg first observed that cancer cells metabolize glucose differently than normal tissues, performing glycolysis even in the presence of oxygen— a phenomenon now known as the Warburg effect [79, 80]. Aerobic glycolysis, while less efficient than oxidative phosphorylation, is more rapid, allowing hungry cancer cells to outcompete their normal neighbors for energy precursors. It also produces the biomass needed for proliferating cells to meet their increased biosynthesis and redox needs. Altered vasculature provides tumor cells with a continuous supply of oxygen and nutrients for increased demands and liabilities [40, 81]. The PI3K pathway is commonly upregulated in cancers, and one downstream effector in this pathway, AKT1, stimulates ATP generation through a variety of mechanisms [82]. Numerous altered genes across all members of this pathway are associated with an increased risk of cancer [83]. Interestingly, many neoplasms also express mutations that allow them to systematically slow glycolysis, allowing carbohydrates to enter subsidiary pathways that support other necessary processes, such as biomass generation [84].
Neuronal functions are also tightly linked to glucose metabolism, and compromised levels of the nutrient sensor OGlcNAcylation have been shown in AD brains [85]. This corroborates findings from other studies showing that hyperphosphorylated tau is associated with a decrease in OGlcNAcylation [86]. Altered brain glucose metabolism and hyperglycemia have been suggested to contribute to the pathogenesis of AD, and AD has notably been touted as “type 3 diabetes” due to the striking reduction in the utilization of glucose, as well as extensive abnormalities in genes encoding insulin and its related growth factors [87, 88]. AD patients show reduced glucose energy metabolism in affected regions of the brain [89], and the AD brain also shows a marked reduction in GLUT3, the neuronal glucose transporter [90]. There is growing evidence that this hypometabolism may be preceded by a period of hypermetabolism in compensation for bioenergetic insufficiency [91, 92]. This metabolic upregulation may be a similar phenomenon to that seen in cancer and has been coined the “inverse Warburg effect.” Elevated mTOR signaling has also been shown to promote tau pathology in AD and other tauopathies [93].
1.2.6. Mitochondrial Dysfunction
As discussed in the previous section, neurons and dividing cells handle their energy needs in differing ways. Mitochondrial DNA is especially vulnerable to the inevitable loss of molecular integrity that accompanies aging, as well as to oxygen free radicals produced by normal cellular respiration. Mounting evidence suggests that age-related changes in bioenergetics and the metabolic compensation that ensues may be an important driver of both cancer and neurodegeneration, and a potential target for prevention and therapy.
Dysfunction of the mitochondria of dividing cells leads to disruption in DNA repair, and it is well known that these deficiencies increase cancer risk. Specifically, mutations in cell checkpoint genes and human DNA repair have been linked to malignancy [33, 34]. In addition to DNA repair, mitochondrial dysregulation of energy metabolism is also linked to cancer. Cancer cells metabolize glucose via aerobic glycolysis, an inefficient but rapid process. Various other pathways involving the mitochondria are dysregulated in cancers as well, including the PI3K pathway, as discussed prior [82].
As energy metabolism is crucial in neurons, it is not surprising that energetic dysregulation has been linked to neurodegeneration [94, 95]. In a number of AD mouse models, mitochondrial dysfunction in both neurons and glial cells has been demonstrated before the onset of memory impairment or amyloid plaques [96]. Neurons are highly specialized and recruit nearby glial cells for many survival functions, and because neurons are permanent, they accumulate DNA damage over time, and it has been theorized that the accumulation of single-strand breaks contributes to neuronal dysfunction [39]. Recent studies have shown that deleted mitochondrial DNA (mtDNA) is associated with reduced respiratory chain efficiency, and this increases with aging, and the prevalence of mtDNA deletions is particularly high in neurodegenerative disorders such as PD [97, 98]. Astrocytic dysfunction has also been demonstrated in a number of neurodegenerative diseases, including AD [99, 100].
1.2.7. Cellular Senescence
Cellular senescence, the phenomenon by which normal diploid cells cease to continue dividing, was first described in the 1960s when Hayflick and colleagues demonstrated the limited ability of dividing cells to proliferate in culture [101]. Though senescence is canonically associated with loss of replicative capabilities, its phenotype is in reality much more diverse, involving alterations in gene expression, epigenetic regulation, and cellular metabolism [102]. Cellular senescence most notably results from telomere attrition, and can also be induced via non-telomeric DNA damage and derepression of the INK4/ARF locus [51]. The removal of senescent cells in transgenic progeroid mice, as well as nonpregeroid mice, causes an increased resistance to age- associated symptoms, further providing evidence of the association between senescence and aging [103, 104]. Overall, senescence is thought to be a survival response to protect the organism from cancer; ultimately, it leads to the buildup of senescent cells, which in turn can be a driver of age-related disease.
Although neurons are non-dividing cells, senescencelike phenotypes, such as p38MAPK activation, elevated beta–galactosidase, and secretion of interleukin-6, have been noted in Purkinje and cortical neurons following DNA damage [102, 105]. Moreover, senescence of glial cells, namely astrocytes, microglia, and oligodendrocytes, is observed in aging and neurodegeneration and is commonly characterized by dystrophy followed by altered cell functionality [43, 106–109]. These senescent cells in the mammalian brain have been shown to secrete pro-inflammatory senescence associated secretory phenotype (SASP) factors and disrupt cell-cell contacts, thereby leading to neurodegeneration [110–112].
On the other hand, cellular senescence protects against cancer by preventing oncogenic cells from dividing, and abnormalities in senescence have been linked to neoplasia. Of note, inactivation of tumor suppressor proteins p53 (primarily) and p16 (secondarily) causes the reversal of senescence and can trigger cells to re-enter the cell cycle [113]. The relationship between cellular senescence and the tumor microenvironment is complex; while cells may enter a senescent state to prevent pathogenesis, senescent cells can promote the development of age-related cancer by stimulating leukocyte infiltration, tumor growth, and malignant phenotypes. Thus, cellular senescence is an example of antagonistic pleiotropy; a process that protects one from cancer early in life that actually becomes a risk factor for disease at older ages [114].
1.2.8. Stem Cell Exhaustion
Stem cell exhaustion occurs physiologically during the aging process as stem cells decrease in number and slowly lose their ability to differentiate and replenish. The decline in stem cells seems to be an integrative result of multiple types of damage to both cells and their microenvironment [31, 115]. The attenuation of hematopoietic aging in mouse models has been demonstrated by stem cell rejuvenation [116].
Neural stem cells, which are associated with neurogenesis, as well as differentiation into various glial cells, are present in low numbers in isolated regions of the brain. The Wnt signaling pathway has recently been shown to play a critical role in neural stem cell proliferation, and alterations of this pathway are closely related to the pathologic development of AD [117]. Additionally, the neuronal microenvironment is significantly altered in neurodegenerative diseases [118]. This change in the extracellular milieu contributes to exaggerated inflammatory responses through the release of toxic factors, reducing neurogenesis [119]. In a similar vein to stem cell exhaustion, aberrantly regulated stem cells through pathways such as the Wnt signaling pathway have been linked to cancer [120]. Because cancer mutations occur in a series of steps, it is theorized that subsets of tumor cells are in fact malignant “cancer stem cells” [121].
1.2.9. Altered Intercellular Communication
Neurohormonal signaling pathways are often affected in the physiological aging process in the face of globally diffuse increasing inflammatory reactions, decreasing immune responses, and environmental changes [31]. This includes the NF–κB signaling pathway, whose over-activation is a transcriptional signature associated with aging [122]. Inter–organ synchronization of aging has also been described, where senescent cells induce senescence in nearby fibroblasts through a “bystander effect,” using gap junctions and processes involving reactive oxidative species [123].
Altered intercellular communication is also seen in both neurodegeneration and cancer. In cancer, molecular strategies allow for the reprogramming of existing physiological pathways, such as chemokine receptor pair pathways, to promote the survival of tumor cells [124]. Cancer cells have additionally been shown to emit a large number of microvesicles, which function as mediators of intercellular communication [125]. These structures transfer bioactive molecular content to recipient cells, aiding in pro-oncogenic events, such as tumor invasion, angiogenesis, metastasis, drug resistance, and cancer stem cell hierarchy [125].
Proper cell-to-cell communication is integral to the survival of neurons. Indeed, disruptions in this process have been invariably linked to the earliest stages of a number of neurodegenerative disorders, including AD [126], HD [127], and ALS [128]. Moreover, glutamate removal from the synaptic cleft is critical to the process of neurotransmission, and failure to eliminate can lead to glutamate excitotoxicity and, ultimately, neuronal death. This has been demonstrated in ALS, hD, and spinocerebellar ataxia type 7 (SCA7) [129]. Additionally, an elegant review recently described a number of ways in which intercellular communication may be altered in a variety of neurodegenerative diseases by means of disrupted neuron-neuron communication, dysfunctional crosstalk between neurons and glia, and the transmission of pathogenic proteins between cells [130].
2. BIOLOGIC OVERLAP
Genetic diseases commonly have an early age of onset and lead to an early death; as a result, they remain rare in the population. In contrast, chronic age-related diseases such as cancer and neurodegeneration have complex causes that include small contributions from many genes, as well as aging- related changes, environmental, and lifestyle factors [131–136]. The connections between the genetics of PD and cancer are more obvious. In fact, while the familial PD genes are used by the neuron to control protein processing and clearance, in dividing cells, most play some role in development or cell cycle regulation. PARK2 (Parkin) and PARK5 are a critical part of the UPS, the main pathway by which proteins are degraded in cells. These genes have anti-proliferative properties and are often inactivated in cancers [137, 138]. PARK6 may also have anti-proliferative functions [139, 140]. Thus, these PD genes are both neuroprotective and tumor-suppressive. On the other hand, PARK7 (DJ–1) takes part in the UPS, but in the dividing cell antagonizes the tumor suppressor gene PTEN [141–143]. Rather than functioning as a tumor-suppressor, then, PARK7 is an oncogene. Thus, there are “mixed signals” in the overlap, and this may help explain why there are both positive and negative associations between PD and cancer.
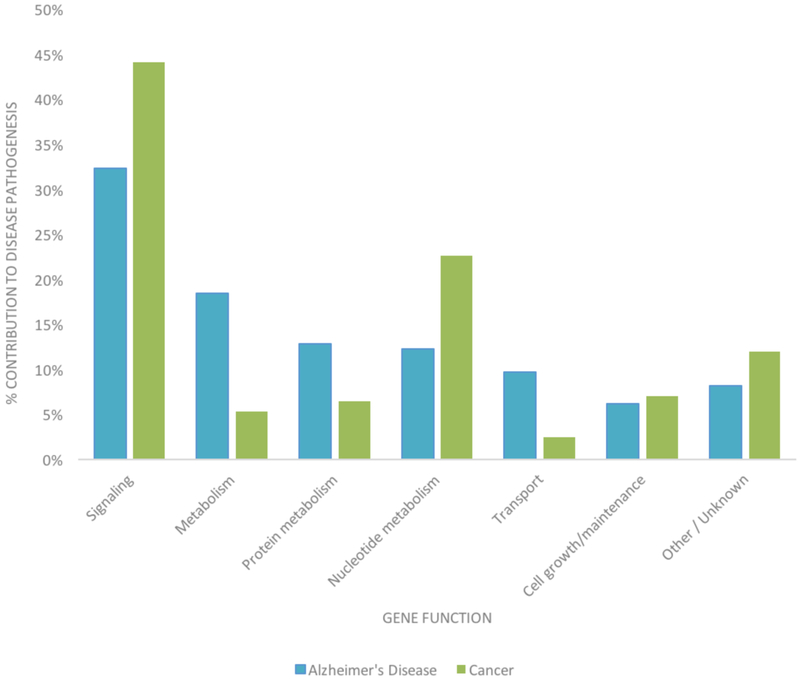
Less is known about the shared genetic component between AD and cancer, as strongly AD–associated genes are not known oncogenes or tumor suppressors. Using summary statistics from large genome wide association studies, we have recently found evidence of a shared genetic component between AD and five cancers (colon, breast, prostate, ovarian, lung) [144]. Our results suggested that gene expression regulators play an important role in the genetic overlap, and that some shared variants modulate the risk of both diseases in the same direction, while others increase the risk of one disease while increasing the risk of the other. Here, we examine the genetic overlap between cancer and AD using publicly available databases. The NetAge database is an online biogerontological database for the study of aging, longevity, and age-related diseases, and it contains lists of genes implicated in cancer and AD [145]. To construct this database, genes were collected, compiled, and manually curated from a number of scientific and publicly available databases and selected according to three criteria: 1) mutations associated with disease frequency, 2) consistent positive or negative association with gene expression, and 3) gene polymorphisms associated with disease susceptibility or predisposition [145]. NetAge classifies each gene within a categorical function and creates a topologic map of other genetic interactions (Fig. 4). It is immediately evident that genes related to nucleotide metabolism are more prominent in cancer than in AD, reflecting the mitotic activity of these cells. General metabolism, protein metabolism, and transport are elevated in AD when compared with cancer, a reflection of the importance of waste clearance and maintenance of cellular structure and function in these long-lived cells.
Fig. (4).
NetAge gene categorization of AD and cancer. In both cancer and AD, the majority of classified genetic contributors are associated with signaling activities. Compared to cancer, in AD, a disproportionate number of genes involved in general metabolism (18.46% vs. 5.28%), protein metabolism (12.82% vs. 6.44%), and transport (9.74% vs. 2.50%) are mutated. Compared to AD, in cancer, a disproportionate number of genes involved in nucleotide metabolism (22.67% vs. 12.31%) are affected.
We then sought to examine the genes most commonly associated with either AD or cancer. For this, we queried information on 20 genes from each family of disease using two databases. AlzGene is a database that lists genes associated with AD [146]. In its listing, it also ranks the top 10 genes associated with sporadic AD according to the HuGENet interim criteria for the cumulative assessment of genetic associations [147, 148]. This ranking is determined by the p– value of association with AD, derived using meta-analysis techniques. We were unable to find a cancer database that ranked cancer genes according to statistical association with the development of cancer and instead used Phenopedia, a component of the integrated knowledge base on human genome epidemiology (HuGE Navigator) [149, 150]. This comprehensive online database ranks genes by a total number of citations in peer-reviewed literature. We used Phenopedia to gather the top 20 genes linked to cancer, as well 10 more genes linked to the sporadic AD, and compared the function of these genes (Table 1). From these data, it is apparent that the genes most associated with either disease directly correspond to the evolutionary tradeoffs associated with their cells of origin. Genes most associated with AD are intimately linked with waste removal and neuroinflammation, while those associated with cancer are mostly implicated in DNA repair, cellular growth, and signaling. While this confirms much of what we already understand about the two disease families, it alone does not address the relation between them.
Table 1.
Top 20 genes linked to AD and cancer. (A) Genes most associated with AD are mainly involved in waste removal and neuroinflammation. (B) Genes most associated with cancer are mainly involved in DNA repair, cellular growth, and signaling.
| Alzheimer’s Disease | Cancer | ||
|---|---|---|---|
| Gene | Function | Gene | Function |
| APOE | Cholesterol packaging | TP53 | Tumor suppressor |
| BIN1 | Modulates tau pathology | KRAS | Signaling |
| CLU | Unknown (chaperone protein) | EGFR | Growth factor |
| ABCA7 | Unknown (transporter protein) | GSTM1 | Detoxification |
| CR1 | Neuroinflammation | BRCA1 | Tumor suppressor |
| PICALM | Waste removal | BRAF | Signaling |
| MS4A6A | Unknown (immune-mediated function) | BRCA2 | Tumor suppressor |
| CD33 | Immunoregulation | GSTT1 | Detoxification |
| MS4A4E | Unknown (immune-mediated function) | XRCC1 | DNA repair |
| CD2AP | Actin cytoskeleton regulation | GSTP1 | Drug metabolism |
| MAPT | Encodes tau | ERCC2 | DNA repair |
| ACE | Blood pressure regulation and waste removal | CYP1A1 | Drug metabolism |
| BDNF | Neuroprotection | PIK3CA | Signaling |
| SORL1 | APOE receptor | TNF | Innate immunity - cytokine |
| MTHFR | Unknown (protein production) | ABCB1 | Detoxification, transportation |
| ILIA | Neuroinflammation | XRCC3 | DNA repair |
| IL6 | Neuroinflammation | IL10 | Neuroinflammation |
| A2M | Neuroinflammation | NAT2 | Drug metabolism |
| IL1B | Neuroinflammation | ERCC1 | DNA repair |
| SLC6A4 | Serotonin transport | VEGFA | Growth factor |
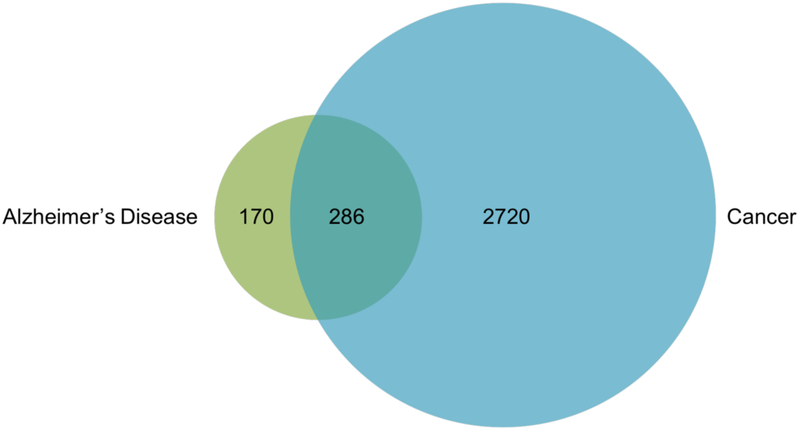
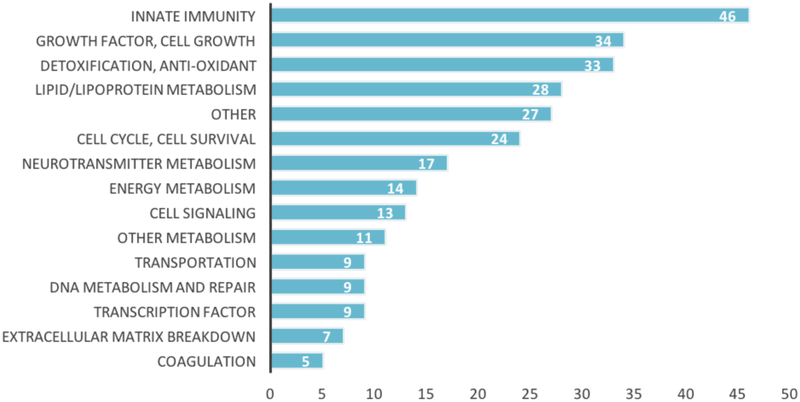
To compare the biological overlap between the two families of disease, we analyzed all cancer and AD genes cited at least two times, according to Phenopedia, and tabulated the total number of mutual genes (Fig. 5). From this database, 3006 genes were associated with cancer and 456 with AD. Of these genes, 286 overlapped between the two families of disease, many of which were in our “Top 20” gene category of one disease, but were located further down the list in the other disease. Notably, this links more than 60% of AD– linked genes to cancer. One drawback to Phenopedia, when compared to NetAge, is that it does not classify genes by function. And while NetAge does make these categorizations, the categories lack detail. Of the 286 overlapping genes from Phenopedia, we manually assigned each gene a more specific functional category, as shown in Figure 6. Interestingly, several of these genes, including AT–1, are implicated in the pathogenesis of both neurodegeneration and cancer, but through different mechanisms due to the multifunctionality of their proteins [151].
Fig. (5).
Overlap between genes associated with AD and cancer. Of the 456 genes associated with AD and the 3006 genes associated with cancer, 286 overlap between the two diseases.
Fig. (6).
Functions of the overlapping genes between AD and cancer. It is evident that genes regulating innate immunity, cell growth, and detoxification are most commonly implicated in both diseases.
3. CLINICAL IMPLICATIONS
A number of important conclusions regarding preventive and therapeutic approaches to age-related cancer and neurodegeneration can be drawn from this review. First, interventions that slow the hallmarks of aging will decrease the risk of both families of diseases. It has already been shown that promoting healthy lifestyle and metabolism can decrease the risk of both cancer and cognitive decline [152, 153]. In addition to fostering healthy metabolism, these interventions are known to decrease markers of inflammation, improve mitochondrial health and decrease oxidative stress [154]. The FINGER trial, a randomized controlled trial (RCT) of regular exercise, modified Mediterranean diet, and cognitive training, either improved or maintained cognitive function at two years [155]. Medications that target various hallmarks of aging are also being explored in both fields. Metformin, a biguanide approved for the treatment of Type 2 diabetes, appears to decrease the risk of cancer, dementia, and other age–related diseases through multiple mechanisms [156–158]. These include a decrease in insulin and IGF–1 levels, inhibition of the mTOR pathway, inhibition of mitochondrial function and decrease in oxidative damage and activation on AMP kinase [159–162]. The “Targeting Aging with Metformin” trial– a large randomized controlled trial for metformin for the prevention of age-related disease– is currently in its early stages [156].
Drugs that decrease inflammation have been tested in cancer field for years and are now being actively investigated for their possible role as neuroprotective agents. Similarly, a number of DNA–methylation and histone acetylation inhibitors are currently in various stages of the drug development process. Senescent cells are also a potential target for therapy because of their presence in many age-related diseases, including both carcinogenesis and neurodegeneration. Due to altered excretion of toxic metabolites caused by aberrant mutations in autophagy-associated genes such as AT– 1/SLC33A1, therapies targeting improved proteostasis involving these genes are being explored in animal models of AD [151, 163]. Senescent cells express unique proteins such as p16INK4a that cause intriguing age-related deterioration and can be used as potential targets in new therapies [164]. Another proposed approach to combating cancer and other agerelated diseases is targeting select epitopes such as SASP on senescent cells [165].
Consideration of the horizontal axis in Fig. (1) that illustrates differentially regulated pathways common to the two diseases raises other therapeutic possibilities. Proteasome inhibitors such as bortezomib inhibit protein degradation, and their role as anticancer agents has been expanding on the past decade. If inhibitors of the proteasome are effective anti-cancer agents, then drugs that enhance proteasome function will likely be neuroprotective. Small molecules that increase proteasome 26S activity are currently being developed for this purpose [166]. However, proteasome activation would need to be carefully targeted to neurons, as it could theoretically promote cancer in other tissues. This is not unreasonable, as proteasome inhibitors should theoretically cause PD in humans, but thus far there is no evidence of this. Inhibitors targeting heat shock proteins have been in development for cancer for years; the effort to modulating the chaperone network of proteins as a strategy for neuroprotection has begun only recently [167, 168]. Modulating a single chaperone protein like Hsp90 has been proposed as a potential therapeutic for both cancer and neurodegeneration [169].
CONCLUSION
We have provided an overview of the complex and intriguing interrelation between age-related neurodegenerative disease and cancer. While some of the connections between the disorders could help explain the pattern of inverse comorbidity seen in epidemiologic studies, others would suggest that the diseases should co-occur. This mixed picture may help explain why an inverse association with neurodegeneration is seen in some cancers but not others. Both positive and inverse associations in the overlapping biology provide new potential directions for developing effective prevention and treatment. The strong overlap between the processes of neurodegeneration, cancer and aging suggests that interventions that target hallmarks of aging, such as metformin, are likely to prevent both diseases.
ACKNOWLEDGEMENTS/FUNDING
Alex Houck is funded by the MSTAR Program (American Federation for Aging Research/NIH Grant # 1T35AG038027–02 9). Sahba Seddighi is funded by the Intramural Research Training Award (IRTA) Fellowship program at the National Institute on Aging. Dr. Driver is funded by a VA Merit Review Award, Clinical Science R&D I01CX000934–01A1.
Footnotes
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
Publisher's Disclaimer: DISCLAIMER: The above article has been published in Epub (ahead of print) on the basis of the materials provided by the author. The Editorial Department reserves the right to make minor modifications for further improvement of the manuscript.
REFERENCES
- [1].Driver JA, Beiser A, Au R, Kreger BE, Splansky GL, Kurth T, et al. Inverse association between cancer and Alzheimer’s disease: results from the Framingham Heart Study. Bmj. 2012; 344: e1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Roe CM, Fitzpatrick AL, Xiong C, Sieh W, Kuller L, Miller JP, et al. Cancer linked to Alzheimer disease but not vascular dementia. Neurology. 2010; 74(2): 106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tabarés-Seisdedos R, Dumont N, Baudot A, Valderas JM, Climent J, Valencia A, et al. No paradox, no progress: inverse cancer comorbidity in people with other complex diseases. The lancet oncology. 2011; 12(6): 604–8. [DOI] [PubMed] [Google Scholar]
- [4].Driver JA, Logroscino G, Buring JE, Gaziano JM, Kurth T. A prospective cohort study of cancer incidence following the diagnosis of Parkinson’s disease. Cancer Epidemiol Biomarkers & Prevention. 2007; 16(6): 1260–5. [DOI] [PubMed] [Google Scholar]
- [5].Shi HB, Tang B, Liu YW, Wang XF, Chen GJ. Alzheimer disease and cancer risk: a meta-analysis. J Cancer Res Clin Oncol 2015; 141(3): 485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Frain L, Swanson D, Cho K, Gagnon D, Lu KP, Betensky RA, et al. Association of cancer and Alzheimer’s disease risk in a national cohort of veterans. Alzheimer’s & Dementia. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pan T, Li X, Jankovic J. The association between Parkinson’s disease and melanoma. Internat J Cancer 2011; 128(10): 2251–60. [DOI] [PubMed] [Google Scholar]
- [8].Walter U, Heilmann E, Voss J, Riedel K, Zhivov A, Schad SG, et al. Frequency and profile of Parkinson’s disease prodromi in patients with malignant melanoma. J Neurol Neurosurg Psychiatry. 2016; 87(3): 302–10. [DOI] [PubMed] [Google Scholar]
- [9].Hu HH, Kannengiesser C, Lesage S, Andre J, Mourah S, Michel L, et al. PARKIN Inactivation Links Parkinson’s Disease to Melanoma. J Natl Cancer Inst. 2016; 108(3). [DOI] [PubMed] [Google Scholar]
- [10].Kesler SR, Watson CL, Blayney DW. Brain network alterations and vulnerability to simulated neurodegeneration in breast cancer. Neurobiology of aging. 2015; 36(8): 2429–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ganguli M. Cancer and dementia: disease and associated disorders. 2015; 29(2): 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hooper C, Meimaridou E, Tavassoli M, Melino G, Lovestone S, Killick R. p53 is upregulated in Alzheimer’s disease and induces tau phosphorylation in HEK293a cells. Neurosci Lett 2007; 418(1): 34–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bretaud S, Allen C, Ingham PW, Bandmann O. p53–dependent neuronal cell death in a DJ–1 –deficient zebrafish model of Parkinson’s disease. J Neurochem 2007; 100(6): 1626–35. [DOI] [PubMed] [Google Scholar]
- [14].Bae B-I, Xu H, Igarashi S, Fujimuro M, Agrawal N, Taya Y, et al. p53 mediates cellular dysfunction and behavioral abnormalities in Huntington’s disease. Neuron. 2005; 47(1): 29–41. [DOI] [PubMed] [Google Scholar]
- [15].Chang JR, Ghafouri M, Mukerjee R, Bagashev A, Chabrashvili T,Sawaya BE. Role of p53 in Neurodegenerative Diseases. Neurodegener Dis. 2012; 9(2): 68–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Meek DW. Regulation of the p53 response and its relationship to cancer. Biochem J. 2015; 469(3): 325–46. [DOI] [PubMed] [Google Scholar]
- [17].Lu KP, Finn G, Lee TH, Nicholson LK. Prolyl cis-trans isomerization as a molecular timer. Nature chemical biology. 2007; 3(10): 619–29. [DOI] [PubMed] [Google Scholar]
- [18].Driver JA, Ping Lu K. Pin1: a new genetic link between Alzheimer’s disease, cancer and aging. Curr Aging Sci 2010; 3(3): 158–65. [DOI] [PubMed] [Google Scholar]
- [19].Driver JA. Inverse association between cancer and neurodegenerative disease: review of the epidemiologic and biological evidence. Biogerontol 2014; 15(6): 547–57. [DOI] [PubMed] [Google Scholar]
- [20].Snyder HM, Ahles T, Calderwood S, Carrillo MC, Chen H, Chang C-C, et al. Exploring the nexus of Alzheimer’s disease and related dementias with cancer and cancer therapies. Alzheimer’s & Dementia. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kennedy SR, Loeb LA, Herr AJ. Somatic mutations in aging, cancer and neurodegeneration. Mech Ageing Dev 2012; 133(4): 118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Crespi BJ, Go MC. Diametrical diseases reflect evolutionary-genetic tradeoffs: Evidence from psychiatry, neurology, rheumatology, oncology and immunology. Evol Med Public Health. 2015; 2015(1): 216–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Crespi B. Autism and cancer risk. Autism Res. 2011; 4(4): 302–10. [DOI] [PubMed] [Google Scholar]
- [24].Ming G–l, Song H. Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions. Neuron. 2011; 70(4): 687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn A-M, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nature medicine. 1998; 4(11): 1313–7. [DOI] [PubMed] [Google Scholar]
- [26].Alvarez–Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci 2002; 22(3): 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bédard A, Parent A. Evidence of newly generated neurons in the human olfactory bulb. Develop Brain Res 2004; 151(1): 159–68. [DOI] [PubMed] [Google Scholar]
- [28].Fishel ML, Vasko MR, Kelley MR. DNA repair in neurons: so if they don’t divide what’s to repair? Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2007; 614(1): 24–36. [DOI] [PubMed] [Google Scholar]
- [29].Bélanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metabolism 2011; 14(6): 724–38. [DOI] [PubMed] [Google Scholar]
- [30].Moskalev AA, Shaposhnikov MV, Plyusnina EN, Zhavoronkov A, Budovsky A, Yanai H, et al. The role of DNA damage and repair in aging through the prism of Koch-like criteria. Ageing Res Rev. 2013; 12(2): 661–84. [DOI] [PubMed] [Google Scholar]
- [31].López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The Hallmarks of Aging. Cell. 2013; 153(6): 1194–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hoeijmakers JH. DNA damage, aging, and cancer. N Engl J Med. 2009; 361(15): 1475–85. [DOI] [PubMed] [Google Scholar]
- [33].Antoniou A, Pharoah P, Narod S, Risch HA, Eyfjord JE, Hopper J, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. The American Journal of Human Genetics. 2003; 72(5): 1117–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ford D, Easton D, Stratton M, Narod S, Goldgar D, Devilee P, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The American Journal of Human Genetics. 1998; 62(3): 676–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Baylin SB, Herman JG. DNA hypermethylation in tumorigenesis: epigenetics joins genetics. Trends in Genetics. 2000; 16(4): 168–74. [DOI] [PubMed] [Google Scholar]
- [36].Esteller M Epigenetic lesions causing genetic lesions in human cancer: promoter hypermethylation of DNA repair genes. European journal of cancer. 2000; 36(18): 2294–300. [DOI] [PubMed] [Google Scholar]
- [37].Lahtz C, Pfeifer GP. Epigenetic changes of DNA repair genes in cancer. Journal of molecular cell biology. 2011; 3(1): 51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kathe SD, Shen GP, Wallace SS. Single-stranded breaks in DNA but not oxidative DNA base damages block transcriptional elongation by RNA polymerase II in HeLa cell nuclear extracts. J Biol Chem. 2004; 279(18): 18511–20. [DOI] [PubMed] [Google Scholar]
- [39].Brasnjevic I, Hof PR, Steinbusch HW, Schmitz C. Accumulation of nuclear DNA damage or neuron loss: molecular basis for a new approach to understanding selective neuronal vulnerability in neurodegenerative diseases. DNA Repair (Amst). 2008; 7(7): 1087–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hanahan D, Weinberg Robert A. Hallmarks of Cancer: The Next Generation. Cell. 2011; 144(5): 646–74. [DOI] [PubMed] [Google Scholar]
- [41].Navarro CL, Cau P, Lévy N. Molecular bases of progeroid syndromes. Human Mol Genetics 2006; 15(Suppl 2): R151–R61. [DOI] [PubMed] [Google Scholar]
- [42].Rolig RL, McKinnon PJ. Linking DNA damage and neurodegeneration. Trends in Neurosciences. 2000; 23(9): 417–24. [DOI] [PubMed] [Google Scholar]
- [43].Yang Y, Mufson EJ, Herrup K. Neuronal cell death is preceded by cell cycle events at all stages of Alzheimer’s disease. The Journal of neuroscience. 2003; 23(7): 2557–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lee H-g, Casadesus G, Zhu X, Castellani RJ, McShea A, Perry G, et al. Cell cycle re-entry mediated neurodegeneration and its treatment role in the pathogenesis of Alzheimer’s disease. Neurochemistry international. 2009; 54(2): 84–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pan L, Penney J, Tsai L-H. Chromatin regulation of DNA damage repair and genome integrity in the central nervous system. Journal of molecular biology. 2014; 426(20): 3376–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Federico A, Cardaioli E, Da Pozzo P, Formichi P, Gallus GN, Radi E. Mitochondria, oxidative stress and neurodegeneration. Journal of the neurological sciences. 2012; 322(1): 254–62. [DOI] [PubMed] [Google Scholar]
- [47].Blasco MA. Telomere length, stem cells and aging. Nature chemical biology. 2007; 3(10): 640–9. [DOI] [PubMed] [Google Scholar]
- [48].Cai Z, Yan L-J, Ratka A. Telomere shortening and Alzheimer’s disease. Neuromolecular medicine. 2013; 15(1): 25–48. [DOI] [PubMed] [Google Scholar]
- [49].Panossian L, Porter V, Valenzuela H, Zhu X, Reback E, Masterman D, et al. Telomere shortening in T cells correlates with Alzheimer’s disease status. Neurobiol Aging. 2003; 24(1): 77–84. [DOI] [PubMed] [Google Scholar]
- [50].Lukens JN, Van Deerlin V, Clark CM, Xie SX, Johnson FB. Comparisons of telomere lengths in peripheral blood and cerebellum in Alzheimer’s disease. Alzheimer’s & Dementia. 2009; 5(6): 463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007; 130(2): 223–33. [DOI] [PubMed] [Google Scholar]
- [52].DePinho RA. The age of cancer. Nature. 2000; 408(6809): 248. [DOI] [PubMed] [Google Scholar]
- [53].Kuhn E, Meeker A, Wang T-L, Sehdev AS, Kurman RJ, Shih I-M. Shortened telomeres in serous tubal intraepithelial carcinoma: an early event in ovarian high-grade serous carcinogenesis. The American journal of surgical pathology. 2010; 34(6): 829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hu H, Zhang Y, Zou M, Yang S, Liang X-Q. Expression of TRF1, TRF2, TIN2, TERT, KU70, and BRCA1 proteins is associated with telomere shortening and may contribute to multistage carcinogenesis of gastric cancer. J Cancer Res Clin Oncol 2010; 136(9): 1407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kim NW, Piatyszek MA, Prowse KR, Harley CB. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994; 266(5193): 2011. [DOI] [PubMed] [Google Scholar]
- [56].Berger SL, Kouzarides T, Shiekhattar R, Shilatifard A. An operational definition of epigenetics. Genes & development. 2009;23(7): 781–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Gonzalo S Epigenetic alterations in aging. Journal of Applied Physiology. 2010; 109(2): 586–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006; 124(2): 315–29. [DOI] [PubMed] [Google Scholar]
- [59].Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, et al. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012; 483(7388): 218–21. [DOI] [PubMed] [Google Scholar]
- [60].Jaenisch R, Bird A.Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals.Nature genetics. 2003; 33: 245–54. [DOI] [PubMed] [Google Scholar]
- [61].Hake S, Xiao A, Allis C. Linking the epigenetic ‘language’of covalent histone modifications to cancer. British J Cancer 2004; 90(4): 761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001; 293(5532): 1068–70. [DOI] [PubMed] [Google Scholar]
- [63].Qureshi IA, Mehler MF. Advances in epigenetics and epigenomics for neurodegenerative diseases. Current neurology and neuroscience reports. 2011; 11(5): 464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Chouliaras L, Mastroeni D, Delvaux E, Grover A, Kenis G, Hof PR, et al. Consistent decrease in global DNA methylation and hydroxymethylation in the hippocampus of Alzheimer’s disease patients. Neurobiology of aging. 2013; 34(9): 2091–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ogawa O, Zhu X, Lee H-G, Raina A, Obrenovich ME, Bowser R, et al. Ectopic localization of phosphorylated histone H3 in Alzheimer’s disease: a mitotic catastrophe? Acta neuropathologica. 2003; 105(5): 524–8. [DOI] [PubMed] [Google Scholar]
- [66].Faghihi MA, Modarresi F, Khalil AM, Wood DE, Sahagan BG, Morgan TE, et al. Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of β-secretase. Nature Med 2008; 14(7): 723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wang S-C, Oelze B, Schumacher A. Age-specific epigenetic drift in late-onset Alzheimer’s disease. PloS one. 2008; 3(7): e2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Koga H, Kaushik S, Cuervo AM. Protein homeostasis and aging: The importance of exquisite quality control. Ageing Res Rev 2011; 10(2): 205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Calderwood SK, Murshid A, Prince T. The shock of aging: molecular chaperones and the heat shock response in longevity and aging-a mini-review. Gerontology. 2009; 55(5): 550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Rubinsztein DC, Mariño G, Kroemer G. Autophagy and aging. Cell. 2011; 146(5): 682–95. [DOI] [PubMed] [Google Scholar]
- [71].Tomaru U, Takahashi S, Ishizu A, Miyatake Y, Gohda A, Suzuki S, et al. Decreased proteasomal activity causes age-related phenotypes and promotes the development of metabolic abnormalities. Am J Pathol 2012; 180(3): 963–72. [DOI] [PubMed] [Google Scholar]
- [72].Spillantini MG, Schmidt ML, Lee VM-Y, Trojanowski JQ, Jakes R, Goedert M. α-Synuclein in Lewy bodies. Nature. 1997; 388(6645): 839–40. [DOI] [PubMed] [Google Scholar]
- [73].Price JL, Davis P, Morris J, White D. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiol Aging. 1991; 12(4): 295–312. [DOI] [PubMed] [Google Scholar]
- [74].Houck AL, Hernández F, Ávila J. A Simple Model to Study Tau Pathology. J Exper Neurosci 2016; 10: 31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annual review of biochemistry. 2009; 78: 959–91. [DOI] [PubMed] [Google Scholar]
- [76].Neckers L Heat shock protein 90: the cancer chaperone Heat Shock Proteins in Cancer: Springer; 2007. p. 231–52. [DOI] [PubMed] [Google Scholar]
- [77].Frezza M, Schmitt S, Ping Dou Q. Targeting the ubiquitin- proteasome pathway: an emerging concept in cancer therapy. Current topics in medicinal chemistry. 2011; 11(23): 2888–905. [DOI] [PubMed] [Google Scholar]
- [78].Fontana L, Partridge L, Longo VD. Extending healthy life span— from yeast to humans. science. 2010; 328(5976): 321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. The Journal of general physiology. 1927; 8(6): 519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Warburg O On the origin of cancer cells. Science. 1956; 123(3191): 309–14. [DOI] [PubMed] [Google Scholar]
- [81].Efeyan A, Comb WC, Sabatini DM. Nutrient-sensing mechanisms and pathways. Nature 2015; 517(7534): 302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Fan Y, Dickman KG, Zong W-X. Akt and c-Myc differentially activate cellular metabolic programs and prime cells to bioenergetic inhibition. J Biological Chem 2010; 285(10): 7324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Polivka J, Janku F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacology & therapeutics. 2014; 142(2): 164–75. [DOI] [PubMed] [Google Scholar]
- [84].Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nature Reviews Cancer. 2011; 11(2): 85–95. [DOI] [PubMed] [Google Scholar]
- [85].Liu F, Iqbal K, Grundke-Iqbal I, Hart GW, Gong C-X. O- GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer’s disease. Proceedings of the National Acad Sci United States of America 2004; 101(29): 10804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lefebvre T, Ferreira S, Dupont-Wallois L, Bussiere T, Dupire M-J, Delacourte A, et al. Evidence of a balance between phosphorylation and O-GlcNAc glycosylation of Tau proteins—a role in nuclear localization. Biochimica et Biophysica Acta (BBA)- General Subjects. 2003; 1619(2): 167–76. [DOI] [PubMed] [Google Scholar]
- [87].Steen E, Terry BM, J Rivera E, Cannon JL, Neely TR, Tavares R, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease-is this type 3 diabetes? Journal of Alzheimer’s disease. 2005; 7(1): 63–80. [DOI] [PubMed] [Google Scholar]
- [88].Demetrius LA, Driver JA. Preventing Alzheimer’s disease by means of natural selection. J Royal Society Interface 2015; 12(102): 20140919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Sakamoto S, Ishii K, Sasaki M, Hosaka K, Mori T, Matsui M, et al. Differences in cerebral metabolic impairment between early and late onset types of Alzheimer’s disease. Journal of the neurological sciences. 2002; 200(1): 27–32. [DOI] [PubMed] [Google Scholar]
- [90].Simpson IA, Chundu KR, Davies-Hill T, Honer WG, Davies P. Decreased concentrations of GLUT1 and GLUT3 glucose transporters in the brains of patients with Alzheimer’s disease. Annals of Neurology. 1994; 35(5): 546–51. [DOI] [PubMed] [Google Scholar]
- [91].Cohen AD, Price JC, Weissfeld LA, James J, Rosario BL, Bi W, et al. Basal cerebral metabolism may modulate the cognitive effects of Abeta in mild cognitive impairment: an example of brain reserve. J Neurosci. 2009; 29(47): 14770–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Ossenkoppele R, Madison C, Oh H, Wirth M, van Berckel BN, Jagust WJ. Is verbal episodic memory in elderly with amyloid deposits preserved through altered neuronal function? Cereb Cortex. 2014; 24(8): 2210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Caccamo A, Magrì A, Medina DX, Wisely EV, López-Aranda MF,Silva AJ, et al. mTOR regulates tau phosphorylation and degradation: implications for Alzheimer’s disease and other tauopathies. Aging Cell. 2013; 12(3): 370–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Beal MF. Does impairment of energy metabolism result in excitotoxic neuronal death in neurodegenerative illnesses? Ann Neurol. 1992; 31(2): 119–30. [DOI] [PubMed] [Google Scholar]
- [95].Yin F, Boveris A, Cadenas E. Mitochondrial energy metabolism and redox signaling in brain aging and neurodegeneration. Antioxid Redox Signal. 2014; 20(2): 353–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Trushina E, Nemutlu E, Zhang S, Christensen T, Camp J, Mesa J, et al. Defects in mitochondrial dynamics and metabolomic signatures of evolving energetic stress in mouse models of familial Alzheimer’s disease. PloS one. 2012; 7(2): e32737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nature Genetics. 2006; 38(5): 518–20. [DOI] [PubMed] [Google Scholar]
- [98].Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nature genetics. 2006; 38(5): 515–7. [DOI] [PubMed] [Google Scholar]
- [99].Mei X, Ezan P, Giaume C, Koulakoff A. Astroglial connexin immunoreactivity is specifically altered at β-amyloid plaques in β- amyloid precursor protein/presenilin1 mice. Neuroscience. 2010; 171(1): 92–105. [DOI] [PubMed] [Google Scholar]
- [100].Stobart JL, Anderson CM. Multifunctional role of astrocytes as gatekeepers of neuronal energy supply. Imaging and monitoring astrocytes in health and disease. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Hayflick L The limited in vitro lifetime of human diploid cell strains. Experimental cell research. 1965; 37(3): 614–36. [DOI] [PubMed] [Google Scholar]
- [102].Tan FCC, Hutchison ER, Eitan E, Mattson MP. Are There Roles for Brain Cell Senescence in Aging and Neurodegenerative Disorders? Biogerontology. 2014; 15(6): 643–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, Van De Sluis B, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011; 479(7372): 232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA, et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife. 2015; 4: e12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Jurk D, Wang C, Miwa S, Maddick M, Korolchuk V, Tsolou A, et al. Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell. 2012; 11(6): 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Chinta SJ, Lieu CA, DeMaria M, Laberge RM, Campisi J,Andersen JK. Environmental stress, ageing and glial cell senescence: a novel mechanistic link to Parkinson’s disease? Journal of Internal Medicine. 2013; 273(5): 429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Flanary BE, Sammons NW, Nguyen C, Walker D, Streit WJ. Evidence that aging and amyloid promote microglial cell senescence. Rejuvenation research. 2007; 10(1): 61–74. [DOI] [PubMed] [Google Scholar]
- [108].Streit WJ, Sammons NW, Kuhns AJ, Sparks DL. Dystrophic microglia in the aging human brain. Glia. 2004; 45(2): 208–12. [DOI] [PubMed] [Google Scholar]
- [109].Bitto A, Sell C, Crowe E, Lorenzini A, Malaguti M, Hrelia S, et al. Stress-induced senescence in human and rodent astrocytes. Experimental cell research. 2010; 316(17): 2961–8. [DOI] [PubMed] [Google Scholar]
- [110].Benarroch EE, editor Neuron-astrocyte interactions: partnership for normal function and disease in the central nervous system Mayo Clinic Proceedings; 2005: Elsevier. [DOI] [PubMed] [Google Scholar]
- [111].Magistretti PJ. Neuron-glia metabolic coupling and plasticity. Journal of Experimental Biology. 2006; 209(12): 2304–11. [DOI] [PubMed] [Google Scholar]
- [112].Chinta SJ, Woods G, Rane A, Demaria M, Campisi J, Andersen JK. Cellular senescence and the aging brain. Experimental gerontology. 2015; 68: 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Beauséjour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J 2003; 22(16): 4212–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Campisi J, Aging, cellular senescence, and cancer. Annual review of physiology. 2013; 75: 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Behrens A, van Deursen JM, Rudolph KL, Schumacher B. Impact of genomic damage and ageing on stem cell function. Nature cell biology. 2014; 16(3): 201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Akunuru S, Geiger H. Aging, Clonality, and Rejuvenation of Hematopoietic Stem Cells. Trends Mol Med 2016; 22(8): 701–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Yao H, Sun L, Lian J, Zhang M, Liu D. Correlation of Alzheimer’s disease with Wnt signaling pathway and neural stem cells. 2015.
- [118].Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nature Reviews Neurology. 2014; 10(4): 217–24. [DOI] [PubMed] [Google Scholar]
- [119].Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011; 477(7362): 90–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005; 434(7035): 843–50. [DOI] [PubMed] [Google Scholar]
- [121].Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nature Reviews Cancer. 2012; 12(2): 133–43. [DOI] [PubMed] [Google Scholar]
- [122].Adler AS, Sinha S, Kawahara TL, Zhang JY, Segal E, Chang HY.Motif module map reveals enforcement of aging by continual NF- kB activity. Genes & development. 2007; 21(24): 3244–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Nelson G, Wordsworth J, Wang C, Jurk D, Lawless C, Martin-Ruiz C, et al. A senescent cell bystander effect: senescence-induced senescence. Aging cell. 2012; 11(2): 345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Hendrix MJ, Seftor EA, Seftor RE, Kasemeier-Kulesa J, Kulesa PM, Postovit L-M. Reprogramming metastatic tumour cells with embryonic microenvironments. Nature Reviews Cancer 2007; 7(4): 246–55. [DOI] [PubMed] [Google Scholar]
- [125].Lee TH, D’Asti E, Magnus N, Al-Nedawi K, Meehan B, Rak J, editors. Microvesicles as mediators of intercellular communication in cancer—the emerging science of cellular ‘debris’ Seminars in immunopathology; 2011: Springer. [DOI] [PubMed] [Google Scholar]
- [126].Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S,Ramakrishnan S, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016; 352(6286): 712–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Estrada-Sánchez AM, Rebec GV. Role of cerebral cortex in the neuropathology of Huntington’s disease. Motor Cortex Microcircuits (Frontiers in Brain Microcircuits Series). 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Casas C, Manzano R, Vaz R, Osta R, Brites D. Synaptic Failure: Focus in an Integrative View of ALS. Brain Plasticity. 2014(Preprint): 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009; 187(6): 761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Garden GA, La Spada AR. Intercellular (mis) communication in neurodegenerative disease. Neuron. 2012; 73(5): 886–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Balmain A, Gray J, Ponder B. The genetics and genomics of cancer. Nature genetics. 2003; 33: 238–44. [DOI] [PubMed] [Google Scholar]
- [132].Epigenetics Herceg Z. and cancer: towards an evaluation of the impact of environmental and dietary factors. Mutagenesis. 2007; 22(2): 91–103. [DOI] [PubMed] [Google Scholar]
- [133].Gatz M, Reynolds CA, Fratiglioni L, Johansson B, Mortimer JA, Berg S, et al. Role of genes and environments for explaining Alzheimer disease. Archives General Psychiatry 2006; 63(2): 168–74. [DOI] [PubMed] [Google Scholar]
- [134].Escott-Price V, Sims R, Bannister C, Harold D, Vronskaya M, Majounie E, et al. Common polygenic variation enhances risk prediction for Alzheimer’s disease. Brain. 2015; 138(12): 3673–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Escott-Price V, Nalls MA, Morris HR, Lubbe S, Brice A, Gasser T, et al. Polygenic risk of Parkinson disease is correlated with disease age at onset. Annals of neurology. 2015; 77(4): 582–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Kieburtz K, Wunderle KB. Parkinson’s disease: evidence for environmental risk factors. Movement Disorders. 2013; 28(1): 8–13. [DOI] [PubMed] [Google Scholar]
- [137].Inzelberg R, Samuels Y, Azizi E, Qutob N, Inzelberg L, Domany E, et al. Parkinson disease (PARK) genes are somatically mutated in cutaneous melanoma. Neurology Genetics. 2016; 2(3): e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Kato N, Yamamoto H, Adachi Y, Ohashi H, Taniguchi H, Suzuki H, et al. Cancer detection by ubiquitin carboxyl-terminal esterase L1 methylation in pancreatobiliary fluids. World Journal of Gastroenterology: WJG. 2013; 19(11): 1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Matsushima-Nishiu M, Unoki M, Ono K, Tsunoda T, Minaguchi T, Kuramoto H, et al. Growth and gene expression profile analyses of endometrial cancer cells expressing exogenous PTEN. Cancer Res. 2001; 61(9): 3741–9. [PubMed] [Google Scholar]
- [140].Unoki M, Nakamura Y. Growth-suppressive effects of BPOZ and EGR2, two genes involved in the PTEN signaling pathway. Oncogene. 2001; 20(33): 4457–65. [DOI] [PubMed] [Google Scholar]
- [141].Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003; 299(5604): 256–9. [DOI] [PubMed] [Google Scholar]
- [142].Kim RH, Peters M, Jang Y, Shi W, Pintilie M, Fletcher GC, et al. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell. 2005; 7(3): 263–73. [DOI] [PubMed] [Google Scholar]
- [143].Xiong H, Wang D, Chen L, Choo YS, Ma H, Tang C, et al. Parkin, PINK1, and DJ-1 form a ubiquitin E3 ligase complex promoting unfolded protein degradation. J Clin Invest. 2009; 119(3): 650–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Feng Y-CA, Cho K, Lindstrom S, Kraft P, Cormack J, Liang L, et al. Investigating the genetic relationship between Alzheimer’s disease and cancer using GWAS summary statistics. Human genetics. 2017; 136(10): 1341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Tacutu R, Budovsky A, Fraifeld VE. The NetAge database: a compendium of networks for longevity, age-related diseases and associated processes. Biogerontology 2010; 11(4): 513–22. [DOI] [PubMed] [Google Scholar]
- [146].Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nature Genetics. 2007; 39(1): 17–23. [DOI] [PubMed] [Google Scholar]
- [147].Ioannidis JP, Boffetta P, Little J, O’Brien TR, Uitterlinden AG, Vineis P, et al. Assessment of cumulative evidence on genetic associations: interim guidelines. Inter J Epidemiol 2008; 37(1): 120–32. [DOI] [PubMed] [Google Scholar]
- [148].Khoury MJ, Bertram L, Boffetta P, Butterworth AS, Chanock SJ, Dolan SM, et al. Genome-wide association studies, field synopses, and the development of the knowledge base on genetic variation and human diseases. Am J Epidemiol 2009; 170(3): 269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Yu W, Gwinn M, Clyne M, Yesupriya A, Khoury MJ. A navigator for human genome epidemiology. Nature Genetics. 2008; 40(2): 124–5. [DOI] [PubMed] [Google Scholar]
- [150].Yu W, Clyne M, Khoury MJ, Gwinn M. Phenopedia and Genopedia: Disease-centered and gene-centered views of the evolving knowledge of human genetic associations. Bioinformatics. 2010; 26(1): 145–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Peng Y, Li M, Clarkson BD, Pehar M, Lao PJ, Hillmer AT, et al. Deficient import of acetyl-CoA into the ER lumen causes neurodegeneration and propensity to infections, inflammation, and cancer. Journal of Neuroscience. 2014; 34(20): 6772–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Ford ES, Bergmann MM, Kröger J, Schienkiewitz A, Weikert C, Boeing H. Healthy living is the best revenge: findings from the European Prospective Investigation Into Cancer and Nutrition- Potsdam study. Archives Internal Med 2009; 169(15): 1355–62. [DOI] [PubMed] [Google Scholar]
- [153].Yaffe K, Barnes D, Nevitt M, Lui L-Y, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Archiv internal Med 2001; 161(14): 1703–8. [DOI] [PubMed] [Google Scholar]
- [154].Leeuwenburgh C, Heinecke JW. Oxidative stress and antioxidants in exercise. Current medicinal chemistry. 2001; 8(7): 829–38. [DOI] [PubMed] [Google Scholar]
- [155].Ngandu T, Lehtisalo J, Solomon A, Levalahti E, Ahtiluoto S,Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015; 385(9984): 2255–63. [DOI] [PubMed] [Google Scholar]
- [156].Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a Tool to Target Aging. Cell Metab. 2016; 23(6): 1060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Duncan BB, Schmidt MI. Metformin, cancer, alphabet soup, and the role of epidemiology in etiologic research. Am Diabetes Assoc; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Hsu C-C, Wahlqvist ML, Lee M-S, Tsai H-N. Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. Journal of Alzheimer’s Disease. 2011; 24(3): 485–93. [DOI] [PubMed] [Google Scholar]
- [159].Ahmed S, Mahmood Z, Javed A, Hashmi SN, Zerr I, Zafar S, et al. Effect of Metformin on Adult Hippocampal Neurogenesis: Comparison with Donepezil and Links to Cognition. J Mol Neurosci. 2017. [DOI] [PubMed] [Google Scholar]
- [160].El-Mir MY, Detaille D, G RV, Delgado-Esteban M, Guigas B, Attia S, et al. Neuroprotective role of antidiabetic drug metformin against apoptotic cell death in primary cortical neurons. J Mol Neurosci. 2008; 34(1): 77–87. [DOI] [PubMed] [Google Scholar]
- [161].Kickstein E, Krauss S, Thornhill P, Rutschow D, Zeller R, Sharkey J, et al. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc Natl Acad Sci USA 2010; 107(50): 21830–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Mielke JG, Taghibiglou C, Wang YT. Endogenous insulin signaling protects cultured neurons from oxygen-glucose deprivation-induced cell death. Neuroscience. 2006; 143(1): 165–73. [DOI] [PubMed] [Google Scholar]
- [163].Peng Y, Kim MJ, Hullinger R, O’Riordan KJ, Burger C, Pehar M, et al. Improved proteostasis in the secretory pathway rescues Alzheimer’s disease in the mouse. Brain. 2016; 139(3): 937–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Naylor R, Baker DJ, Deursen J. Senescent cells: a novel therapeutic target for aging and age-related diseases. Clinical Pharmacology & Therapeutics. 2013; 93(1): 105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Tchkonia T, Zhu Y, Van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Investigat 2013; 123(3): 966–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Leestemaker Y, de Jong A, Witting KF, Penning R, Schuurman K, Rodenko B, et al. Proteasome Activation by Small Molecules. Cell Chem Biol. 2017. [DOI] [PubMed] [Google Scholar]
- [167].Calderwood SK, Murshid A. Molecular Chaperone Accumulationin Cancer and Decrease in Alzheimer’s Disease: The Potential Roles of HSF1. Front Neurosci. 2017; 11: 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Jinwal UK, Koren J, O’Leary JC, Jones JR, Abisambra JF, Dickey CA. Hsp70 ATPase Modulators as Therapeutics for Alzheimer’s and other Neurodegenerative Diseases. Mol Cell Pharmacol. 2010; 2(2): 43–6. [PMC free article] [PubMed] [Google Scholar]
- [169].Kitson RR, Moody CJ. An improved route to 19-substituted geldanamycins as novel Hsp90 inhibitors-potential therapeutics in cancer and neurodegeneration. Chemical Communications. 2013; 49(76): 8441–3. [DOI] [PMC free article] [PubMed] [Google Scholar]