Abstract
Objective:
The aim of this article is to review the published literature with the purpose of knowing the importance of using various probiotic Streptococcus strains as a preventive and therapeutic method for dental caries management.
Materials and Methods:
Research question was formulated based on the PICO strategy. A comprehensive electronic literature search was conducted across PubMed/Medline, Scopus, and EBSCOhost databases independently by two reviewers. All papers published from 1989 to December 2017 that focused on the use of probiotic Streptococcus strains for caries prevention were included in this review. Inclusion and exclusion criteria were applied to the selected articles, and a customized data extraction sheet was formulated. The selected articles were subjected to quality assessment, and the risk of bias in selected studies was evaluated.
Results:
A total of five articles were included. The overall risk of bias of the selected clinical trials was found to be high risk, and the overall level of evidence of the selected in vitro studies was moderate.
Conclusion:
The two included clinical studies on the use of probiotic Streptococcus strains for caries prevention had high risk of bias. Although in-vitro studies showed promising results, clinical studies have not demonstrated clear clinical outcomes. Thus, there is a vast scope for future research in this field.
Clinical Relevance:
Application of oral probiotics will help reinstate a balanced microbiota and thereby improving oral health. This systematic review focused on evaluating the role played by probiotic Streptococcus strains in the carious lesion incidence.
Keywords: Dental caries, oral health, probiotics
INTRODUCTION
Dental caries still remains one of the most common diseases worldwide, although a decline of the prevalence has been recorded in western countries.[1] It is an endogenous disease that results from a homeostatic imbalance between the host and microbiota.[2] Research indicates a multispecies etiology for dental caries, the mutans streptococci (MS), a cluster of acidogenic, dental plaque-inhabiting streptococcal species, are still recognized as major constituents of most active dental caries lesions.[3]
Caries management strategies have traversed through various paths and are now witnessing a paradigm shift toward preventive approach. The genesis of a dental carious lesion is determined by the interplay of multiple factors.[4] This interplay is best explained by the Keyes’ triad Venn diagram proposed in the 1960s which includes tooth, diet, and dental plaque.[5] Enhancing the inherent defense mechanism of saliva and diminishing the microbial levels seems to be a rational approach. Treatments using antistreptococcal antimicrobials can be effective in the short term to reduce dental plaque levels and to decrease counts of MS, but, as most therapeutic antibiotics have relatively broad-spectrum antimicrobial activity, they indiscriminately destroy both commensal as well as the potentially destructive bacteria, and thereby create population imbalances within the microflora.[3]
Therefore, recent studies aim to identify methods that would selectively inhibit oral pathogens rather than the whole microbial community.[6] One such approach currently researched extensively is the use of probiotics. The application of oral probiotics to help reinstate a balanced microbiota and thereby improving oral health is relatively a new concept. Probiotics are beneficial bacteria that can improve the microecological balance of the host.[7] They confer a health benefit to the host when administered in adequate amounts.[8] Most probiotics are Gram-positive bacteria that belong to the genera Lactobacillus or Bifidobacterium.[9] Studies based on the use of the intestinal probiotics Lactobacillus rhamnosus GG,[10] Lactobacillus reuteri, and Bifidobacterium[11] have each reported achieving reduced levels of Streptococcus mutans. However, these strains have limitations in terms of their colonization of oral tissues.
Hence, a new generation of probiotic strains sourced from the human oral cavity and belonging to commensal species known to have extremely low pathogenic potential has more recently been developed.[12] Streptococcus thermophilus was found to be an effective probiotic for oral health.[13,14] Streptococcus salivarius, a Gram-positive bacterium, is currently being used as a oral probiotic. Two of the most commonly used S. salivarius strains are K12 and M18.[12] S. salivarius strain JH has also been reported to have a potent anti-MS activity.[15]
The aim of this article is to review the published literature with the purpose of knowing the importance of using various probiotic Streptococcus strains as a preventive and therapeutic method for dental caries management. The hypothesis behind this systematic review was that the administration of probiotic Streptococcus strains might play a role in the carious lesion incidence.
MATERIALS AND METHODS
Structured question
Among people with and without dental caries, do probiotic Streptococcus strains have an action against carious incidence?
PICO analysis
Population – people with and without dental caries
Intervention – probiotic Streptococcus strains
Outcome – reduction in microbial count, reduction in new carious lesion, decreased caries risk in cariogram.
Inclusion and exclusion criteria
Inclusion criteria
Studies were included if they focused on the use of probiotic Streptococcus strains for caries prevention. Only original research published in journals was included in this review. Articles published in English alone were included. Only studies in English were collected, due to the virtual absence of research published in other languages.
Exclusion criteria
All in vitro studies and in vivo studies not focusing on administration of probiotic Streptococcus species for caries prevention and studies where probiotic Streptococcus species were administered for other reasons were excluded. Systematic reviews and meta-analysis were also excluded from the review.
Search strategies
Electronic literature search was conducted across PubMed/Medline, Scopus, EBSCOhost, Embase, and ScienceDirect databases. Two preliminary searches were conducted in January 2018 to obtain an overall idea of findings and to polish search terms (MeSH words) and limits. The MeSH Browser was accessed to identify entry terms and compose the final Boolean searches. First, the MeSH terms Dental Caries and Probiotic(s) were associated following which, a combination of keywords derived from the two previous MeSH terms were searched. The keywords used were probiotics dental caries, oral cavity, and probiotic streptococci. Table 1 shows the keywords and search strategy used through the databases. A comparison of the 22 different searches was carried out to delete the repeated studies. All papers published from 1989 to December 2017 were considered for the present review. Abstracts of all selected papers were examined independently by two different evaluators. All studies, which appeared to meet the inclusion and exclusion criteria, were obtained in the full-text format.
Table 1a.
The keywords and search strategy through the databases
| Keywords | PubMed | Scopus | EBSCOhost | Embase | Science direct |
|---|---|---|---|---|---|
| Probiotics, probiotic, dental caries, oral cavity, probiotic streptococci Search string ((((probitoics) OR probiotic)) AND (((dental caries) OR oral cavity) OR oral)) | 2630 | 1457 | 1325 | 1232 | 1863 |
Study selection
Two trained and calibrated reviewers (P. S, N. M. S) independently screened the title and abstract of the selected articles by applying the above-mentioned inclusion and exclusion criteria. Full-text articles were read completely to validate inclusion into the systematic review. Disagreements were settled by discussion with a third reviewer (M. R. S).
Data extraction
Selected articles were read independently by the two reviewers to extract data (P. S, N. M. S). Studies were divided into four types of study design: clinical trials and in vitro studies. Table 2 describes the information extracted from each type of paper. Disagreements between the two reviewers were resolved by discussion with a third reviewer (M. R. S).
Table 2.
The data extracted from each type of study design
| Clinical trials | In vitro studies |
|---|---|
| Author Year Number of participants completed the trial Probiotic strains Control used Probiotic form Probiotic dosage Outcomes assessed Results |
Author Year Model used Carious strains Probiotic strains Outcomes assessed Methods used to assess outcome Results |
Quality assessment of included studies
Each study was assessed using the evaluation method described in the Cochrane Handbook for Systematic Reviews.[16] The risk of bias was assessed individually for selection bias, performance bias, detection bias, attrition bias, and reporting bias. Each domain was classified as having a low, high, or unclear risk of bias.
To analyze the methodological quality of in vitro studies, the clinical appraisal checklist for in vitro studies by Faggion[17] was carefully analyzed and modified to include all relevant contents relating to the methodology based on the research question and PICOS structure. Two authors (P. S, N. M. S) independently scored the articles, and in case of disagreement, consensus was reached with the help of third author (M. R. S). The level of evidence of each article was rated based on the scoring points: low (score 0–4), moderate (score 5–9), and high (score 10–14).
RESULTS
The complete search process used in our systematic review is shown in Figure 1. The number of articles identified from electronic databases is listed in Table 1a. Table 1b shows the full electronic search strategy for PubMed database. A total of 2630 articles meeting the inclusion criteria were identified from the initial search after removing duplicates. Among these, 2504 articles were excluded based on the exclusion criteria. After screening the title and abstract of the remaining 126 articles, 118 articles were excluded as they were found to be irrelevant. The full text of the remaining eight articles was obtained. From this, three papers were excluded as they did not meet the inclusion criteria. A total of five papers were included in the systematic review.
Figure 1.
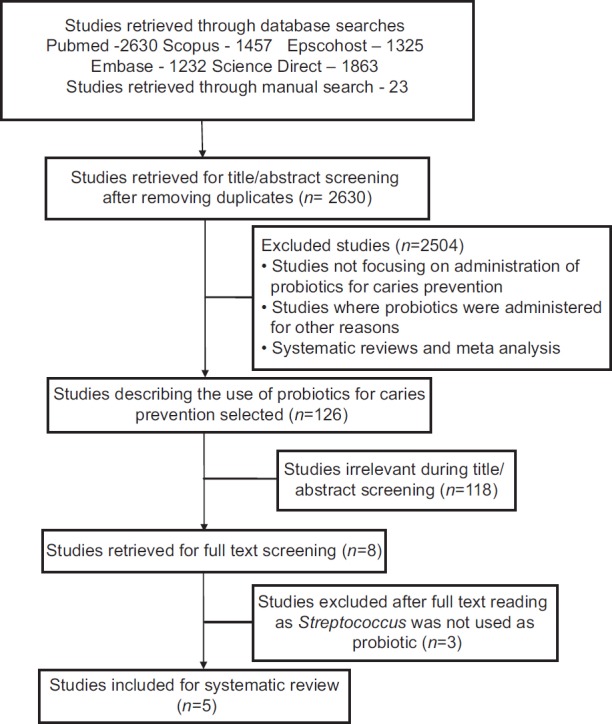
Briefly showing the search process in this systematic review
Table 1b.
The full electronic search strategy for PubMed database
| Search string | No of articles listed |
|---|---|
| Search (dental caries) OR oral cavity | 410,300 |
| Search (probiotics) OR probiotic | 20,475 |
| Search ((((probiotics) OR probiotic)) AND (((dental caries) OR oral cavity) OR oral)) | 2630 |
Among the five papers included in this systematic review, two were clinical trials[3,12] and three were in vitro studies.[15,18,19] The basic characteristics of the included studies are presented in Tables 3 and 4. Both the included clinical trials compared S. salivarius M18 with the control. In the included studies, differences were observed in the following aspects: (a) form of probiotic, (b) probiotic dosage, and (c) outcomes assessed. Among the three in vitro studies included, thought the carious strains used were similar, differences were observed in (a) probiotic strains used, (b) outcomes assessed, and (c) methods used for outcome assessment.
Table 3.
The characteristics of included clinical trials
| Author | Year | Number of participants the completed trial | Control | Probiotic strains | Probiotic form | Probiotic dosage | Outcomes assessed | Results |
|---|---|---|---|---|---|---|---|---|
| Di Pierro et al.[12] | 2015 | 40 (probiotic) 43 (placebo) |
Placebo | S. salivarius M18 | Lozenges | 2 lozenges each day for 3 months | Cariogram Outcome | Use of salivarius M18 increases the chances of avoiding new dental caries development in children |
| Burton et al.[3] | 2013 | 38 (treated group) 38 (control group) |
No treatment | S. salivarius M18 | Dissolving oral tablets | Once a day for 3 months | Salivary levels of S. salivarius, S. mutans, lactobacilli, b-hemolytic streptococci, and Candida species assessed | Cell-culture analyses of sequential saliva samples showed no differences reduced S. mutans counts |
S. mutans: Streptococcus mutans, S. salivarius: Streptococcus salivarius
Table 4.
The characteristics of in vitro studies included
| Author | Year | Model Used | Carious strains | Probiotic Strains | Outcomes assessed | Methods used | Results |
|---|---|---|---|---|---|---|---|
| Schwendicke et al.[18] | 2017 | Biofilm | SM ATCC 25175 SM ATCC 20532 | Streptococcus (TH-4) | Zone of inhibition of SM Biofilm formation inhibition Enamel mineral loss Biofilm bacterial numbers |
Agar diffusion method Transverse microradiography Quantified on BHI agar |
Strong inhibitory effect on S. mutans growth Reduced SM numbers in biofilms than in monospecies Mineral loss by SM×LA-5 biofilms was higher than monospecies Bacterial numbers least in monospecies biofilm and highest in mixed species |
| Walker et al.[15] | 2016 | Not mentioned | SM ATCC 25175 | S. salivarius JH | S. mutans inhibitory activity | Testing dextranase and BLIS activity | Increased anti-S. mutans activity |
| Lee and Kim[19] | 2014 | Biofilm |
S. gordonii ATCC 10558 S. oralis ATCC 9811 S. sanguinis 804 S. mutans ATCC 25175 |
S. thermophilus | Antimicrobial activity Bacterial count Quantify changes in GTFS expression |
Kirby-Bauer disk diffusion Petroff-Hausser counting chamber RT-PCR | Lactobacillus strains formed wider bacteria free zones than S. thermophilus |
BHI: Brain-heart infusion, S. salivarius: Streptococcus salivarius, S. mutans: Streptococcus mutans, S. thermophiles: Streptococcus thermophiles, S. gordonii: Streptococcus gordonii, RT-PCR: Real-time polymerase chain reaction
A total of two clinical trials and three in vitro were assessed for the risk of bias. Figure 2 and 3 shows the risk of bias of included clinical trials. The level of evidence of the included in vitro articles has been presented in Table 5. The overall risk of bias of the selected clinical trials was found to be high risk, and the overall level of evidence of the selected in vitro studies was moderate. In both the clinical trials, patients were not subjected to randomization and blinding. Overall, there was a lack of data on the sample size calculation and information on the incidence of any adverse events.
Figure 2.
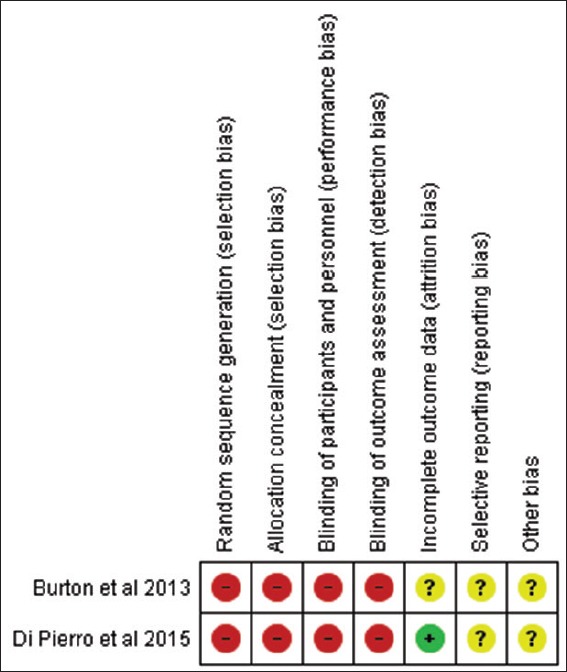
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study
Figure 3.
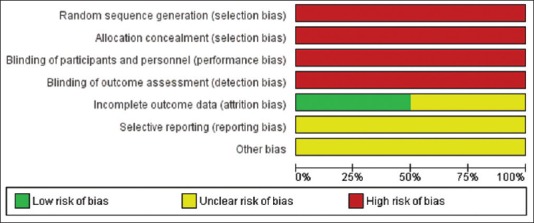
Risk of bias graph depicting each risk of bias item presented as percentages across all included studies
Table 5.
The level of evidence for the in vitro studies included
DISCUSSION
Recent clinical studies have shown that specific probiotic strains can affect oral pathogens by reducing the counts of S. mutans and lactobacilli in the saliva and biofilm.[20,21] Conventional probiotics typically comprised bacteria from intestinal origin (especially lactobacilli and bifidobacteria). However, the realization that oral diseases such as dental caries, periodontal disease, and candidosis can be directly linked to the development of oral microbiota disequilibria has diverted the contemporary probiotic research to the development of products that are capable of establishing a healthy oral microbiota.[22] The antimicrobial action of probiotics can be attributed to their ability to produce lactic and acetic acids that diminish the pH of the medium. These bacteria compete with the pathogenic strains for nutrient sources, releasing hydrogen peroxide and bacteriocins, an action to that of the antibiotics.[23] The mechanism involved is that the undissociated form of the organic acid enters the bacterial cell and dissociates inside the cytoplasm. Lowering of the intracellular pH or the intercellular accumulation of the ionized form of the organic acid leads to death of the pathogen.[24]
The scientific origin of oral probiotics can be traced to the use of mixtures of putative oral commensals producing incompletely characterized inhibitory agents against Streptococcus pyogenes or otitis media pathogens.[25,26] An alternative approach was to target the mutans streptococci using an ultracompetitive bacteriocin-producing S. mutans, genetically modified to attenuate its virulence.[27] Literature search revealed that there was deficiency in research examining the effects of these probiotic Streptococcal strains on salivary microflora. Thus, the role of the probiotic streptococcal strains in caries prevention was the main focus of this systematic review. Results described by various research groups were encouraging, but the scientific evidence is still unclear, and the level of evidence is only moderate. The articles reviewed reported high risk of bias and moderate level of evidence, which limited the conclusions about the real efficacy of various strains of probiotic Streptococcus sps administration in caries lesion prevention.
Among the articles included, S. thermophilus has been probiotic strain of choice in two of the in vitro studies.[18,19] Schwendicke et al.[18] found S. thermophilus to have comparably limited SM growth inhibition and SM biofilm formation inhibition capabilities. It is a relevant probiotic for oral health, as it is aciduric by way of its urease activity, which increases in acidic environments.[13,14] This could allow S. thermophilus to replace other cariogenic species (like lactobacilli) from oral biofilms, thus decreasing their cariogenicity. In contrary, Lee and Kim[19] in his study showed that Lactobacillus strains formed wider bacteria free zones than S. thermophilus.
Another Streptococcal species that is utilized as a commercial oral probiotic of particular importance and the subject of extensive research is S. salivarius. This species is a spherical, Gram-positive, oxidase-negative, and catalase-negative bacterium. S. salivarius is one of the earliest colonizers of the epithelial lining of the human mouth and nasopharynx. The bacterium colonizes the tongue dorsum and pharyngeal mucosa of infants, who acquire the bacterium from their mother within 2 days after birth. The two most well-studied strains of S. salivarius that are currently employed as probiotics are strains K12 and M18. S. salivarius K12 was first isolated from the saliva of a healthy child and has been utilized as a commercial probiotic in New Zealand for over a decade. Clinical trials included in this review[3,12] evaluated the influence of probiotic S. salivarius M18 on the reduction of caries risk.[28,29,30] Burton et al.[3] in their study demonstrated that twice daily dosing with Streptococcus salivarius M18 is an effective way of reducing plaque formation in schoolchildren. The results of the study by Di Pierro et al.[12] showed that the use of S. salivarius M18 increases the chance of avoiding new caries development in children.
Walker et al.[15] in his study showed that oral probiotic S. salivarius JH produces important in vitro inhibitory activity toward strains of S. mutans and Streptococcus sobrinus, the principal species of MS associated with human dental caries. As stated by him, this anti-MS activity appears, at least in part, attributable to the production of SalE, a newly described bacteriocin. Strain JH has also been demonstrated to produce the EPS-hydrolyzing enzyme, dextranase, in larger amounts. Findings of his study indicate that exposure of biofilms to EPS-hydrolyzing enzymes such as dextranase may increase the matrix penetration and killing efficacy of some antibacterial agents. Due to its relatively broad inhibitory spectrum toward oral pathogenic bacteria, and in vitro production of unusually high levels of dextranase activity by strain JH, Walker et al.[15] recommended that the probiotic potential of this strain was further investigated with human subjects.
The current systematic review has shown the lack of clinical research pertaining to the use of probiotic streptococcal strains in caries prevention. As there is paucity of clinical research in this field, the focus of future studies should be on the evaluation of the effect of various streptococcal strains on plaque formation, on modulation of the composition of the oral microbiota and their influence on caries risk. There should be more emphasis on any changes in the mutans streptococcal populations of the host. Further clinical research is vital to recognize specific strains with probiotic activity and also to determine the exact dose, treatment time, and ideal vehicles. The main limitation of this systematic review is that studies in English were only included. Furthermore, the search was confined to specific search engines.
CONCLUSION
Within the limitations of the systematic review, it can be concluded that, the two included clinical studies on the use of probiotic Streptococcus strains for caries prevention had high risk of bias. Although in-vitro studies showed promising results, clinical studies have not demonstrated clear clinical outcomes. As the scientific evidence in this regard is still poor, well-designed high-quality clinical research is required to improve the level of evidence in this area of research.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Cagetti MG, Mastroberardino S, Milia E, Cocco F, Lingström P, Campus G. The use of probiotic strains in caries prevention: A systematic review. Nutrients. 2013;5:2530–50. doi: 10.3390/nu5072530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi N, Nyvad B. Caries ecology revisited: Microbial dynamics and the caries process. Caries Res. 2008;42:409–18. doi: 10.1159/000159604. [DOI] [PubMed] [Google Scholar]
- 3.Burton JP, Drummond BK, Chilcott CN, Tagg JR, Thomson WM, Hale JD, et al. Influence of the probiotic Streptococcus salivarius strain M18 on indices of dental health in children: A randomized double-blind, placebo-controlled trial. J Med Microbiol. 2013;62:875–84. doi: 10.1099/jmm.0.056663-0. [DOI] [PubMed] [Google Scholar]
- 4.Keyes PH. The infectious and transmissible nature of experimental dental caries. Findings and implications. Arch Oral Biol. 1960;1:304–20. doi: 10.1016/0003-9969(60)90091-1. [DOI] [PubMed] [Google Scholar]
- 5.Keyes P, Jordan HV. New York: Academic Press; 1963. Factors Influencing Initiation, Transmission and Inhibition of Dental Caries; pp. 261–83. [Google Scholar]
- 6.Allaker RP, Douglas CW. Novel anti-microbial therapies for dental plaque-related diseases. Int J Antimicrob Agents. 2009;33:8–13. doi: 10.1016/j.ijantimicag.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Lara-Villoslada F, Olivares M, Sierra S, Rodríguez JM, Boza J, Xaus J, et al. Beneficial effects of probiotic bacteria isolated from breast milk. Br J Nutr. 2007;98(Suppl 1):S96–100. doi: 10.1017/S0007114507832910. [DOI] [PubMed] [Google Scholar]
- 8.Ashwell M. Europe, Brussels, Belgium: International Life Sciences Institute; 2002. Concept of Functional Foods. [Google Scholar]
- 9.Vuotto C, Longo F, Donelli G. Probiotics to counteract biofilm-associated infections: Promising and conflicting data. Int J Oral Sci. 2014;6:189–94. doi: 10.1038/ijos.2014.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Näse L, Hatakka K, Savilahti E, Saxelin M, Pönkä A, Poussa T, et al. Effect of long-term consumption of a probiotic bacterium, Lactobacillus rhamnosus GG, in milk on dental caries and caries risk in children. Caries Res. 2001;35:412–20. doi: 10.1159/000047484. [DOI] [PubMed] [Google Scholar]
- 11.Caglar E, Sandalli N, Twetman S, Kavaloglu S, Ergeneli S, Selvi S. Effect of yogurt with Bifidobacterium DN-173 010 on salivary mutans streptococci and lactobacilli in young adults. Acta Odontol Scand. 2005;63:317–20. doi: 10.1080/00016350510020070. [DOI] [PubMed] [Google Scholar]
- 12.Di Pierro F, Zanvit A, Nobili P, Risso P, Fornaini C. Cariogram outcome after 90 days of oral treatment with Streptococcus salivarius M18 in children at high risk for dental caries: Results of a randomized, controlled study. Clin Cosmet Investig Dent. 2015;7:107–13. doi: 10.2147/CCIDE.S93066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotter PD, Hill C. Surviving the acid test: Responses of gram-positive bacteria to low pH. Microbiol Mol Biol Rev. 2003;67:429–53. doi: 10.1128/MMBR.67.3.429-453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arioli S, Ragg E, Scaglioni L, Fessas D, Signorelli M, Karp M, et al. Alkalizing reactions streamline cellular metabolism in acidogenic microorganisms. PLoS One. 2010;5:e15520. doi: 10.1371/journal.pone.0015520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker GV, Heng NC, Carne A, Tagg JR, Wescombe PA. Salivaricin E and abundant dextranase activity may contribute to the anti-cariogenic potential of the probiotic candidate Streptococcus salivarius JH. Microbiology. 2016;162:476–86. doi: 10.1099/mic.0.000237. [DOI] [PubMed] [Google Scholar]
- 16.Page MJ, Cumpston M, Chandler J, Lasserson T. Reporting the review. Draft version for inclusion. Cochrane Handbook for Systematic Reviews of Interventions. Ch. 3. In: Higgins JP, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch V, editors. London: Cochrane; 2018. [Google Scholar]
- 17.Faggion CM., Jr Guidelines for reporting pre-clinical in vitro studies on dental materials. J Evid Based Dent Pract. 2012;12:182–9. doi: 10.1016/j.jebdp.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Schwendicke F, Korte F, Dörfer CE, Kneist S, Fawzy El-Sayed K, Paris S, et al. Inhibition of Streptococcus mutans growth and biofilm formation by probiotics in vitro. Caries Res. 2017;51:87–95. doi: 10.1159/000452960. [DOI] [PubMed] [Google Scholar]
- 19.Lee SH, Kim YJ. A comparative study of the effect of probiotics on cariogenic biofilm model for preventing dental caries. Arch Microbiol. 2014;196:601–9. doi: 10.1007/s00203-014-0998-7. [DOI] [PubMed] [Google Scholar]
- 20.Ahola AJ, Yli-Knuuttila H, Suomalainen T, Poussa T, Ahlström A, Meurman JH, et al. Short-term consumption of probiotic-containing cheese and its effect on dental caries risk factors. Arch Oral Biol. 2002;47:799–804. doi: 10.1016/s0003-9969(02)00112-7. [DOI] [PubMed] [Google Scholar]
- 21.Taipale T, Pienihäkkinen K, Salminen S, Jokela J, Söderling E. Bifidobacterium animalis subsp.lactis BB-12 administration in early childhood: A randomized clinical trial of effects on oral colonization by mutans streptococci and the probiotic. Caries Res. 2012;46:69–77. doi: 10.1159/000335567. [DOI] [PubMed] [Google Scholar]
- 22.Zarco MF, Vess TJ, Ginsburg GS. The oral microbiome in health and disease and the potential impact on personalized dental medicine. Oral Dis. 2012;18:109–20. doi: 10.1111/j.1601-0825.2011.01851.x. [DOI] [PubMed] [Google Scholar]
- 23.Hasslöf P, Hedberg M, Twetman S, Stecksén-Blicks C. Growth inhibition of oral mutans streptococci and candida by commercial probiotic lactobacilli – An in vitro study. BMC Oral Health. 2010;10:18. doi: 10.1186/1472-6831-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makras L, De Vyust L. The in vitro inhibition of gram negative pathogenic bacteria by bifidobacteria is caused by the production of organic acids. Int Dairy J. 2006;16:1049–57. [Google Scholar]
- 25.Sanders CC, Sanders WE., Jr Enocin: An antibiotic produced by Streptococcus salivarius that may contribute to protection against infections due to group A streptococci. J Infect Dis. 1982;146:683–90. doi: 10.1093/infdis/146.5.683. [DOI] [PubMed] [Google Scholar]
- 26.Roos K, Håkansson EG, Holm S. Effect of recolonisation with “interfering” alpha streptococci on recurrences of acute and secretory otitis media in children: Randomised placebo controlled trial. BMJ. 2001;322:210–2. doi: 10.1136/bmj.322.7280.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hillman JD, Mo J, McDonell E, Cvitkovitch D, Hillman CH. Modification of an effector strain for replacement therapy of dental caries to enable clinical safety trials. J Appl Microbiol. 2007;102:1209–19. doi: 10.1111/j.1365-2672.2007.03316.x. [DOI] [PubMed] [Google Scholar]
- 28.Cosseau C, Devine DA, Dullaghan E, Gardy JL, Chikatamarla A, Gellatly S, et al. The commensal Streptococcus salivarius K12 downregulates the innate immune responses of human epithelial cells and promotes host-microbe homeostasis. Infect Immun. 2008;76:4163–75. doi: 10.1128/IAI.00188-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Pierro F, Donato G, Fomia F, Adami T, Careddu D, Cassandro C, et al. Preliminary pediatric clinical evaluation of the oral probiotic Streptococcus salivarius K12 in preventing recurrent pharyngitis and/or tonsillitis caused by Streptococcus pyogenes and recurrent acute otitis media. Int J Gen Med. 2012;5:991–7. doi: 10.2147/IJGM.S38859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horz HP, Meinelt A, Houben B, Conrads G. Distribution and persistence of probiotic Streptococcus salivarius K12 in the human oral cavity as determined by real-time quantitative polymerase chain reaction. Oral Microbiol Immunol. 2007;22:126–30. doi: 10.1111/j.1399-302X.2007.00334.x. [DOI] [PubMed] [Google Scholar]


