Abstract
Context:
One of the common oral bacterial infectious diseases is dental caries. Control of dental plaque formed by Streptococcus mutans and Streptococcus sobrinus leads to prevention and treatment of caries. Chitosan (1-4, 2-amino-2-deoxy-b-D-glucan), a deacetylated derivative from chitin, is an antimicrobial polysaccharide that exerts broad-spectrum activity against pathogenic bacteria and has been suggested as a preventive and therapeutic material for dental caries.
Aim:
The aim of this investigation is whether chitosan has effective antimicrobial and antibiofilm properties against common cariogenic microorganisms.
Materials and Methods:
The effect of 0.019–5 mg/ml of high-molecular-weight (HMW) and low-molecular-weight (LMW) chitosan on S. mutans and S. sobrinus was evaluated, and minimal inhibitory concentration (MIC) and minimal bactericide concentration (MBC) were determined. In addition, the effects of HMW and LMW of chitosan on bacterial adhesion to surfaces and biofilm formation were assayed by tube method.
Results:
The results showed that chitosan is capable of inhibiting S. mutans and S. sobrinus growth (P = 0.001). MIC of HMW chitosan for S. mutans and S. sobrinus was 0.62 mg/mL and MIC of LMW chitosan for S. mutans and S. sobrinus was 0.62 mg/mL, 1.25 mg/mL, respectively. MBC of HMW chitosan for S. mutans and S. sobrinus was 1.25 mg/mL, respectively, and MBC of LMW chitosan for S. mutans and S. sobrinus was 1.25 and 2.5 mg/ml, respectively. On the other hand, HMW chitosan was more effective than LMW chitosan. In addition, S. mutans showed equal MIC and MBC values for both MWs chitosan, but S. sobrinus was more resistant to LMW chitosan. Regarding biofilm growth, chitosan inhibited S. mutans and S. sobrinus adhesion and biofilm formation. The results of tube test showed weak adherence and biofilm formation in concentration of 0.312 and 0.625 mg/ml, but 1.25 and 2.5 mg/ml concentrations of both MWs could completely inhibit biofilm formation.
Conclusion:
These results display the potential of chitosan to be used as an effective antibacterial and antibiofilm agent for oral hygiene and health care.
Keywords: Chitosan, minimal inhibitory concentration, Streptococcus mutans, Streptococcus sobrinus
INTRODUCTION
Periodontitis is caused by specific microorganisms in a susceptible host. Most oral bacteria are harmless under normal circumstances but under specific conditions disease can occur.
The initial settlers of dental surface constitute a highly selective part of the oral microbiota, which are the Streptococcus species. Many researchers believe that Streptoccocus mutans is the principal microbiological agent in the etiology of dental caries.[1]
S. mutans and S. sobrinus contribute the metabolism a great variety of carbohydrates, which are main factors to contribute the pathogenesis because they generate low pH and cause the consequent demineralization of the tooth enamel, due to its high capacity in the carbohydrates metabolism; in addition, extracellular polysaccharides increase the adhesion of the oral microorganisms facilitating the demineralization instead of remineralization.[2,3]
Production of Polysaccharide intercellular adhesin (PIA) in bacteria is dependent on the ica operon (icaADBC) because gene expression of icaADBC is polysaccharide intracellular adhesin. This protein causes cell-to-cell adhesion and finally biofilm production. Biofilm is removed difficult so the resistance of bacteria to antibiotic increases.
Some methods have been used to eradicate colonization of bacteria such as DNA plasmids, vaccine, antibodies against these bacteria or using substances on the dental structure such as chitosan, silver, and zinc oxide.[4]
Chitosan is obtained by deacetylation of chitin, has antimicrobial activity against great variety of microorganisms, including algae, fungi, and bacteria.[5]
Since chitosan is a natural product and has little side-effects, furthermore, there is limited information about high-molecular-weight (HMW) and low-molecular-weight (LMW) chitosan activities; the purpose of the present research was to compare antibacterial and antiadhesion activities of two chitosan with different molecular weights (MW) against common cariogenic microorganisms such as Streptococcus mutans and Streptococcus sobrinus. Therefore, if it has antimicrobial and antibiofilm properties, there is possibility of using chitosan as an alternative antimicrobial in toothpaste, mouthwash for oral hygiene, and health care.
It has been proven that the antimicrobial agents such as bandaging materials and dressings generally lead to cytotoxicity, leading to pathogen resistance or delaying the healing process, but in the case of chitosan, there is almost no need to use antibacterial substances, the implants themselves, change bandages, or dressings when they are applied because the antimicrobial effects come directly from the membrane.[6]
MATERIALS AND METHODS
Sources of chitosan, microorganisms
Chitosan, characterized by two different MW 600–800 KDa with degree of deacetylation >75% and 100 KDa with degree of deacetylation 75%–85%, was purchased from Thermo Fisher (ACROS). The strains of bacteria which were obtained from Iranian Research Organization for Science and Technology include S. mutans (PTCC) and S. sobrinus (PTCC). Microorganisms were grown in Trypticase soy broth (TSB), blood agar at 37°C in a atmosphere containing 5% CO2. Then, growths were confirmed using Gram staining.
Preparation of chitosan
Chitosan was dispersed in a 1% (v/v) glacial acetic acid (Merck, Germany) for preparation of chitosan solutions 2% (w/v) and kept stirring at 50°C overnight for complete dissolution of their particles. Then, the solutions were autoclaved at 121°C for 15 min and were stored at 4°C until use.[7]
Determination of antimicrobial activity of chitosan
Minimal inhibitory concentration and minimal bactericide concentration determination
The minimum inhibitory concentrations (MICs) and the minimum bactericidal concentration (MBC) of HMW and LMW of chitosan on S. mutans and S. sobrinus were determined according to the National Committee for Clinical Laboratory Standards (NCCLS) guideline, and broth microdilution method was used.
Two-fold serial dilutions of HMW and LMW of chitosan solutions were prepared in TSB to give the final concentration of 0.019–5 mg/mL. 100 μL of each dilution of HMW and LMW chitosan solutions was placed into every well of sterile 96-well microplates.
We prepared inoculum of 0.5 McFarland (108 colony-forming units (CFU/ml) of S. mutans and S. sobrinus from overnight cultures in TSB (company name) with 1% sucrose separately and inoculated 100 μL of microorganism suspensions into wells of microplate containing different concentrations of chitosan.[8] Wells containing culture medium and bacteria were used as negative control. All experiments were performed in triplicate. Both microplates were incubated in GasPak jars at 37°C and a microaerophilic environment during 48 h.[9] The determination of MIC (NCCLS) was based on turbidity, and the growth of Streptococci was measured at 630 nm using a microplate reader (AWARENESS, Technology INC, Stat fax 2100).
The MBC is complementary to the MIC and is the lowest concentration of an antibacterial agent that kills 99% of bacteria. MBC was determined by subculturing broth dilution MIC on MHA with defibrinated sheep blood. Plates were incubated in a microaerophilic environment at 37°C for 24 h. If no growth was observed, the original well contained no living bacteria, and the LMW and HMW chitosan were considered as being bactericidal at that concentration.[7,10]
Agar diffusion
Bacterial suspensions were prepared based on Clinical and Laboratory Standards Institute guideline. We prepared suspensions of 0.5 McFarland (108 CFU/ml) of S. mutans and S. sobrinus from overnight cultures, then they were cultured on MHA with defibrinated sheep blood by sterile swab. Antibacterial activities of both chitosan with different MWs were investigated by making 6-mm-diameter wells on agar using sterile Pasteur pipettes. The bottoms of wells were sealed by pouring 20 μl of molten Mueller-Hinton agar (MHA), then different diluted chitosan were added to different wells of plates. Plates were incubated in microaerophilic environment at 37°C for 24 h, then the inhibition zone was measured. We used Gas pack A for making microaerophilic environment.[11]
Determination of antibiofilm activity of chitosan
Tube method
One of the methods for the detection of biofilm production is tube method that is qualitative method.[12] Bacterial suspensions were prepared 106 CFU of S. mutans and S. sobrinus from overnight cultures in TSB with 1% sucrose. Transparent 15 ml tubes containing 2 ml of bacterial suspensions and 2 ml of different dilutions of HMW and LMW chitosan, respectively, according to the MIC results (0.312–2.5 mg/ml) were prepared, and they were incubated in horizontal position at 37°C for 72 h under microaerophilic atmosphere.
Tube with chitosan and noninoculated medium as negative and tube without chitosan and inoculated medium as positive control were used under similar condition. After incubation, the contents of tubes were removed and washed with phosphate-buffered saline and dried. Then, tubes were stained with crystal violet solution (Merck, Germany) for 5 min. Staining solution was removed, and tubes were washed with water and dried in inverted position at room temperature overnight. Biofilm formation was scored as 0: absent, 1: weak, 2: moderate, and 3: strong.[3,13,14]
Statistical analysis
The data were statistically analyzed by Mann–Whitney and Kruskal–Wallis tests on SPSS version 22. A value of P < 0.05 was considered statistically significant.
RESULTS
Figure 1 shows the colonies of S. mutans and S. sobrinus on Trypticase soy agar with sheep blood overnight.
Figure 1.
The colonies of Streptococcus mutans (a) and Streptococcus sobrinus (b) on Trypticase soy agar with sheep blood
The MIC and MBC of HMW and LMW chitosan on S. mutans PTCC 1683 and S. sobrinus PTCC 1601 are showed in Table 1. MIC of HMW chitosan for S. mutans and S. sobrinus was 0.62 mg/mL, and MIC of LMW chitosan for S. mutans and S. sobrinus was o. 62 mg/mL, 1.25 mg/mL.
Table 1.
Minimum inhibitory concentrations and minimum bactericidal concentrations of high-molecular-weight and low-molecular-weight chitosan on Streptococcus mutans and Streptococcus sobrinus
| Microorganism | HMW (mg/mL) | LMW (mg/mL) | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| Streptococcus mutans | 0.62 | 1.25 | 0.62 | 1.25 |
| Streptococcus sobrinus | 0.62 | 1.25 | 1.25 | 2.5 |
MIC: Minimum inhibitory concentration, MBC: Minimum bactericidal concentration, HMW: High molecular weight, LMW: Low molecular weight
MBC of LMW chitosan for S. mutans and S. sobrinus was 1.25 and 2.5 mg/ml and MBC of HMW chitosan for S. mutans and S. sobrinus was 1.25 mg/mL. On the other hand, S. mutans showed equal MIC and MBC values for both MWs chitosan, but S. sobrinus was more resistant to LMW chitosan.
S. mutans and S. sobrinus were susceptible to HMW and LMW chitosan, but the HMW and LMW chitosan exerted higher antibacterial activities against S. mutans (MIC 0.62, MBC 1.25 mg/ml) than S. sobrinus (MIC 1.25, MBC 2.5 mg/ml). As expected, the MBC values obtained were higher than those obtained for the MIC since in general higher concentrations of chitosan are needed to destroy the bacteria [Table 1].
The growth of S. mutans and S. sobrinus was inhibited by HMW and LMW chitosan. Graph 1 shows that both chitosan led to complete elimination of the initial population, so the growth of S. mutans and S. sobrinus was decreased >90% in 0.625–1.25 concentrations of HMW and LMW chitosan. Based on Kruskal–Wallis test, there were significant differences between different concentrations of HMW chitosan to each other (0.019–5 mg/ml) as well as LMW chitosan on S. mutans (P = 0.001). Furthermore, there were significant differences between different concentrations of HMW chitosan to each other (0.019–5 mg/ml) as well as LMW chitosan on S. subrinus (P = 0.001). Mann–Whitney test showed that there were significant differences between S. mutans and S. sobrinus by LMW at concentration 0.019–1.25 mg/ml and HMW at concentration 0.019–0.625 mg/ml (P < 0.05), but there were no significant differences between S. mutans and S. sobrinus by LMW chitosan at concentration 2.5–5 mg/ml and HMW chitosan at concentration 1.25–5 mg/ml (P > 0.05) [Graph 1].
Graph 1.
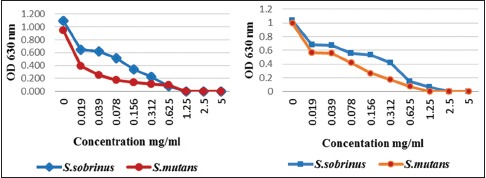
Effect of high-molecular-weight and low-molecular-weight chitosan on the growth of oral streptococci
Both chitosan showed more antibacterial activity on S. mutans than S. sobrinus and HMW chitosan exerted more activity than LMW, so the growth of S. mutans decreased >50% at the concentration of 0.019 mg/ml but LMW chitosan caused a decrease of 50% growth at the concentration between 0.039 and 0.078 mg/ml. As result, OD increased in low concentration of chitosan indicating an increase of bacterial growth. Based on Mann–Whitney test, significant differences were observed in the effect of HMW and LMW chitosan on S. mutans as well S. sobrinus in comparison to their control (P < 0.05).
Comparison between HMW and LMW chitosan on S. mutans showed that there were significant differences between them at concentrations of 0.019–0.625 mg/ml (P < 0.05) and 0.019–1.25 mg/ml on S. sobrinus (P < 0.05) [Graph 2].
Graph 2.
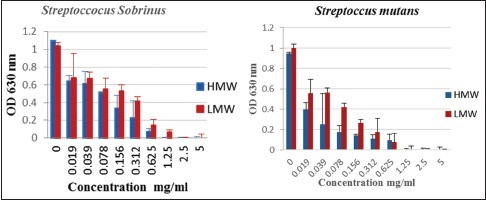
Comparison between high-molecular-weight and low-molecular-weight chitosan on oral Streptococci
Table 2 presents the inhibition zone diameters determined by agar diffusion for S. mutans and S. sobrinus. Approximately 7–12 mm Inhibitory zones were observed for both of bacteria when HMW and LMW chitosan was affected at concentration of 1.25–5 mg/ml, respectively.
Table 2.
Inhibition zones of high-molecular-weight and low-molecular-weight chitosan against oral streptococci
| Concentration (mg/ml) | Zone of inhibition (mm) | |||
|---|---|---|---|---|
| Streptococcus sobrinus | Streptococcus mutans | |||
| HMW | LMW | HMW | LMW | |
| 5 | 11.5 | 11.2 | 11.7 | 11 |
| 2.5 | 9.2 | 9 | 8.8 | 8.5 |
| 1.25 | 7.1 | 7 | 7 | 6.8 |
HMW: High molecular weight, LMW: Low molecular weight
Tube test
Qualitative evaluation of biofilm formation was considered positive when a visible film lined the wall and bottom of the tube, and biofilm formation was estimated as 0 – absent, 1 – weak, 2 – moderate, or 3 – strong. In our study, chitosan showed inhibitory effect upon S. mutans and S. sobrinus. The results of tube test showed adherence and biofilm formation in concentration of 0.312 and 0.625 mg/ml, but 1.25 and 2.5 mg/ml concentrations of both MWs could completely inhibit biofilm formation with no difference between MW and bacteria [Table 3 and Figure 2].
Table 3.
Inhibitory effect of high-molecular-weight and low-molecular-weight chitosan (mg/ml) upon biofilm formation in tube method
| C | 0.312 mg/ml | 0.625 mg/ml | 1.25 mg/ml | 2.5 mg/ml | |
|---|---|---|---|---|---|
| Streptococcus mutans | +++ | + | + | 0 | 0 |
| Streptococcus sobrinus | +++ | + | + | 0 | 0 |
0: No biofilm, +: Weak, +++: Strong
Figure 2.
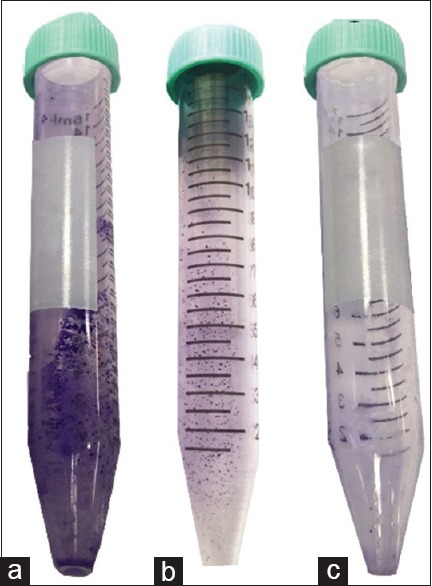
Tube method for antibiofilm test (a): strong (b): weak (c): absent
DISCUSSION
Chitosan has some properties including biocompatibility, biodegradability, no toxicity, high bioactivity, selective permeability and antimicrobial activity which has effect on a great variety of microorganisms such as pathogenic bacteria.
In our study, the MIC and the MBC of HMW and LMW chitosan on S. mutans and S. sobrinus were determined to assess their antimicrobial activity. Since higher concentrations of chitosan are required to kill the bacteria, the MBC values obtained were higher than those obtained for the MIC.[15] The results are shown in Table 1.
Our results indicated that HMW and LMW chitosan caused a decrease in the growth of oral bacteria, so the antimicrobial effect strengthened as the concentration of chitosan increased and optimal density of growth decreased; both f chitosan led to complete elimination of the initial population so the growth of S. mutans and S. sobrinus was decreased by >90% in 0.625–1.25 mg/ml concentrations of chitosan (P = 0.001).
HMW and LMW chitosan showed antibacterial activities against S. mutans (MIC – 0.62, MBC – 1.25 mg/mL), but HMW chitosan had more antibacterial activities than LMW chitosan against S. sobrinus (HMW chitosan: MIC – 0.62, MBC – 1.25 mg/mL) (LMW chitosan: MIC – 1.25, MBC – 2.5 mg/ml), so MIC and MBC of chitosan depended on the bacteria being studied and MW of chitosan. HMW chitosan exerted more activity than LMW so the growth of S. mutans decreased >50% at the concentration of 0.019 mg/ml, but LMW chitosan caused a decrease of 50% growth at the concentration between 0.039 and 0.078 mg/ml. Some authors believed that the antimicrobial activity of chitosan is related to its MW. Zheng and Zhu indicated that increasing chitosan MW resulted in enhanced antimicrobial effect on Gram-positive bacteria because the chitosan of higher MW forms a membrane which inhibits nutrient adsorption.[16]
Benhabiles et al. reported inhibitory effect of chitosan related to MW and type of bacteria.[15] Goy investigated about the effect of HMW chitosan (400,000 g/mol) on Gram-positive and Gram-negative bacteria. The chitosan is consistently more active against the Gram-positive S. aureus than Gram-negative E. coli.[17]
In this study, HMW and LMW chitosan reduced adhesion and biofilm formation of S. mutans and S. sobrinus. The results of tube test showed adherence and biofilm formation in concentration of 0.312 and 0.625 mg/ml, but 1.25 and 2.5 mg/ml concentrations of both MWs could completely inhibit biofilm formation with no difference between MW and bacteria. Hence, the conclusion is that chitosan caused a reduction in bacterial adhesion.
Costa et al. investigated the comparison of chitosan mouthwash with two commercial mouthwashes on biofilm formation of S. mutans, Lactobacillus acidophilus, Enterococcus faecium, Candida albicans, and Prevotella intermedia. They reported that chitosan containing mouthwash had more activity than both commercial mouthwashes.[18]
Hayashi et al. reported that chewing gum containing chitosan can effectively suppress the growth of cariogenic bacteria such as Streptococci in saliva, and the count of S. mutans decreased 1 h after chewing the gum.[19]
Kong et al. reported that chitosan microspheres with a high DD (97.5%) lead to higher positive charge density, which confers stronger antibacterial activity than moderate DD (83.7%) against Staphylococcus aureus at pH 5.5. Takahashia et al. found that a higher DD with more positive charge was especially successful in inhibiting the growth of S. aureus, suggesting that antibacterial activity of chitosan toward S. aureus enhanced with increasing DD.[20,21]
The mechanism of antimicrobial activity of chitosan is not yet fully determined; several mechanisms are suggested. Some researchers believe that amino groups of chitosan in contact with physiological fluids are protonated and when bound to anionic groups of the microorganisms cause agglutination of the microbial cells and inhibition of growth.[22]
Chitosan in the form of bioadhesive polymer has quite enough duration of action to show antibacterial activity. On the other hand, its great potential for improving the delivery of drugs into the oral cavity as well as across the oral mucosa justifies its evaluation for antibacterial activity in addition to its good properties of biodegradability, biocompatibility, and low toxicity. Chitosan appears to have great potential in applications for oral mucosal delivery and treatment.
Zheng Zhu believed that the antimicrobial activity of the chitosan is directly related with the absorption of the polysaccharide to the bacterium, which will cause alterations in the cell wall structure, consequently, in the permeability of the cell membrane.[16]
CONCLUSION
It is concluded that HMW and LMW chitosan have a significant antibacterial effect on common oral bacteria such as S. mutans and S. sobrinus and inhibit biofilm formation which indicates a bright future in biomaterial application, and there is possibility of using chitosan as an alternative antimicrobial in toothpaste, mouthwash for oral hygiene, or health care.
Financial support and sponsorship
This research project has been funded by Babol University of Medical Sciences (No: 42225).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Featherstone JD. The science and practice of caries prevention. J Am Dent Assoc. 2000;131:887–99. doi: 10.14219/jada.archive.2000.0307. [DOI] [PubMed] [Google Scholar]
- 2.Carvalho MM, Thayza CMS, Emerson PS, Pedro T, Fabio S. Chitosan as an oral antimicrobial agent, Science against microbial pathogens: communicating current research and technological advances 2011. 1:542–50. [Google Scholar]
- 3.Mathur T, Singhal S, Khan S, Upadhyay DJ, Fatma T, Rattan A, et al. Detection of biofilm formation among the clinical isolates of staphylococci: An evaluation of three different screening methods. Indian J Med Microbiol. 2006;24:25–9. doi: 10.4103/0255-0857.19890. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez-Sierra JF, Ruiz F, Pena DC, Martinez-Gutierrez F, Martinez AE, Guillen AD, et al. The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomed Nanotechnol Biol Med. 2008;4:237–240. doi: 10.1016/j.nano.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Croisier F, Jerome C. Chitosan-based biomaterials for tissue engineering. Eur Polym J. 2013;49:780–92. [Google Scholar]
- 6.Ueno H, Mori T, Fujinaga T. Topical formulations and wound healing applications of chitosan. Adv Drug Deliv Rev. 2001;52:105–15. doi: 10.1016/s0169-409x(01)00189-2. [DOI] [PubMed] [Google Scholar]
- 7.Costa EM, Silva S, Pina C, Tavaria FK, Pintado MM. Evaluation and insights into chitosan antimicrobial activity against anaerobic oral pathogens. Anaerobe. 2012;18:305–9. doi: 10.1016/j.anaerobe.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Aliasghari A, Rabbani Khorasgani M, Vaezifar S, Rahimi F, Younesi H, Khoroushi M, et al. Evaluation of antibacterial efficiency of chitosan and chitosan nanoparticles on cariogenic streptococci: An in vitro study. Iran J Microbiol. 2016;8:93–100. [PMC free article] [PubMed] [Google Scholar]
- 9.Pasquantonio G, Greco C, Prenna M, Ripa C, Vitali LA, Petrelli D, et al. Antibacterial activity and anti-biofilm effect of chitosan against strains of Streptococcus mutans isolated in dental plaque. Int J Immunopathol Pharmacol. 2008;21:993–7. doi: 10.1177/039463200802100424. [DOI] [PubMed] [Google Scholar]
- 10.Qi L, Xu Z, Jiang X, Hu C, Zou X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr Res. 2004;339:2693–700. doi: 10.1016/j.carres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Islam M, Masum SH, Mahbub KR, Zahurul Haque MD. Antibacterial activity of crab-chitosan against Staphylococcus aureus and Escherichia coli. J Adv Scic Res. 2011;2 [Google Scholar]
- 12.Christensen GD, Simpson WA, Bisno AL, Beachey EH. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982;37:318–26. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hassan A, Usman J, Kaleem F, Omair M, Khalid A, Iqbal M, et al. Evaluation of different detection methods of biofilm formation in the clinical isolates. Braz J Infect Dis. 2011;15:305–11. [PubMed] [Google Scholar]
- 14.Costa EM, Silva S, Tavaria FK, Pintado MM. Study of the effects of chitosan upon Streptococcus mutans adherence and biofilm formation. Anaerobe. 2013;20:27–31. doi: 10.1016/j.anaerobe.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Benhabiles S, Salah R, Lounici H, Drouiche N, Goosen MF, Mameri N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 2012;29:48–56. [Google Scholar]
- 16.Zheng LY, Zhu JA. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr Polym. 2003;54:27–530. [Google Scholar]
- 17.Goy RC, Morais ST, Assis OB. Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E-coli and S.Aureus growth. Rev Bras Farmacognosia Brazilian J Pharmacogn. 2016;26:122–7. [Google Scholar]
- 18.Costa EM, Silva S, Madureira AR, Cardelle-Cobas A, Tavaria FK, Pintado MM, et al. A comprehensive study into the impact of a chitosan mouthwash upon oral microorganism's biofilm formation in vitro. Carbohydr Polym. 2014;101:1081–6. doi: 10.1016/j.carbpol.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi Y, Ohara N, Ganno T, Yamaguchi K, Ishizaki T, Nakamura T, et al. Chewing chitosan-containing gum effectively inhibits the growth of cariogenic bacteria. Arch Oral Biol. 2007;52:290–4. doi: 10.1016/j.archoralbio.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Kong M, Chen XG, Liu CS, Yu LJ, Ji QX, Xue YP, et al. Preparation and antibacterial activity of chitosan microspheres in a solid dispersing system. Front Mater Sci China. 2008;2:214–20. [Google Scholar]
- 21.Takahashia T, Imai M, Suzuki I, Sawai J. Growth inhibitory effect on bacteria of chitosan membranes regulated with deacetylation degree. Biochem Eng J. 2008;40:485–91. [Google Scholar]
- 22.Avadi MR, Sadeghi AM, Tahzibi A, Bayati K, Pouladzadeh M, Zohuriaan-Mehr MJ, et al. Diethylmethyl chitosan as an antimicrobial agent: Synthesis, characterization and antibacterial effects. Eur Polym J. 2004;40:1355–61. [Google Scholar]


