Abstract
Background:
Disinfection of the prepared cavity can be a crucial step in the longevity of restorations. The objective of this study was to compare the antimicrobial action (AMA) of silver diamine fluoride-potassium iodide combination (SDF-KI) with 2% chlorhexidine gluconate (CHX) and to compare the alteration in bond strength and microleakage while using SDF-KI and CHX as cavity cleansers in resin-modified glass ionomer cement (RMGIC) restorations.
Materials and Methods:
Samples were grouped as follows: Group 1: Polyacrylic acid (PAA), Group 2: CHX, Group 3: SDF-KI, and Group 4: Distilled water (CTRL). AMA was assessed by measuring the zone of inhibition of the above-mentioned materials by dispensing them into the punch hole prepared on agar plates with an inoculum of Streptococcus mutans. For assessing the effect of the cavity cleansers on the bond strength of RMGIC, they were applied to the dentinal samples prepared from freshly extracted noncarious molars. After the surface was treated, cylindrical restoration of RMGIC was placed and allowed to set. The shear bond strength was then evaluated using a universal testing machine. Rhodamine-B dye penetration was viewed under a fluorescent microscope to evaluate the microleakage of RMGIC following surface treatment of the standardized cavities prepared on the cervical third of freshly extracted noncarious premolars.
Results:
SDF-KI (34 ± 0.8 mm) showed potent AMA followed by CHX (23.9 ± 0.7 mm) and PAA (12.7 ± 0.8 mm). SDF-KI showed a drastic increase in the bond strength when compared to the PAA, CHX, and CTRL groups. Although the application of SDF-KI showed the least microleakage among all the groups, it was not statistically significant.
Conclusion:
The application of SDF-KI and CHX is useful against S. mutans in an in vitro study. Although SDF-KI group showed the least microleakage among the groups, it was not statistically significant. SDF-KI application has shown a drastic increase in the bond strength of RMGIC although further research is required for the suitable reasoning of the phenomenon.
Keywords: Antimicrobial action, bond strength, chlorhexidine gluconate, microleakage, potassium iodide, silver diamine fluoride
INTRODUCTION
Even after proper cavity preparation, tooth sensitivity can be seen in a lot of patients.[1] Sensitivity is a result of a marginal gap at the tooth–restoration interface called microleakage and can be exaggerated by the presence of potent microorganisms remaining even after cavity preparation, resulting in secondary or residual caries.[2] Various studies have reflected that bacteria left in the cavity preparation can stay viable for a long duration.[3] Therefore, disinfection of the tooth before restoration is vital.
Application of silver fluoride after applying stannous fluoride has given positive results in inhibiting the caries progression in the primary molars in children.[4] Silver desensitizes the exposed dentinal tubules.[5] Wang et al.[6] reported that Ag/ZnO nanocomposite exhibited low cytotoxicity and improved antibacterial activity against Streptococcus mutans when compared to pure ZnO nanorods. Silver diamine fluoride (Ag(NH3)2F), referred to as SDF, is a recently introduced cavity cleanser which is reported to have multiple beneficial effects such as inhibition of demineralization, conservation of collagen from degradation, increasing microhardness of dentine post application and inhibiting the active growth of cariogenic bacteria.[7] In spite of the above-mentioned benefits of SDF, a not so significant side effect is the staining of tooth and the restorative material caused by the residual silver ions. This can be reduced by the application of potassium iodide (KI) which reacts with silver ions to form silver iodide which forms a white-colored product.[8]
2% Chlorhexidine gluconate (CHX), a bis-biguanide, is a broad-spectrum antimicrobial agent used as a disinfectant and is available in many forms such as solution, gels, and spray. It is shown to disinfect the dentinal tubules and gets adsorbed onto the dentin. CHX could be considered clinically advantageous over sodium hypochlorite as even though both show similar antimicrobial activity, CHX is relatively nontoxic, has broad-spectrum antimicrobial action (AMA), and has residual action with less potential for adverse effects.[9]
The restorative material makes a huge difference in the clinical success of a restoration. Resin-modified glass ionomer cement (RMGIC) due to its superior biocompatibility, chemical adhesion, anticariogenic property, and lower moisture sensitivity has been advocated to be used as a liner below composite resin restorations in deep caries management. It is also indicated for the restoration of cervical lesions. RMGIC is also the material of choice in individuals with active caries and high caries risk.[10]
Therefore, the purpose of this study was to compare the AMA of SDF-KI with CHX and to compare the alteration in bond strength and microleakage with the use of SDF-KI as well as CHX cavity cleansers in RMGIC restorations.
MATERIALS AND METHODS
Study groups
The samples were grouped in the following manner: Group 1 (positive control) - polyacrylic acid (PAA), Group 2 (test) - CHX, Group 3 (test) - SDF-KI, and Group 4 (negative control) - distilled water. Each group was compared for their AMA (n = 10), bond strength (n = 8), and microleakage (n = 8).
Antimicrobial action
The standard strain of S. mutans NCTC10449 was used to test the antimicrobial activity of the above four different cavity cleansing materials. The bacterium was grown in 3 mL of brain–heart infusion broth for 24 h at 37°C to form an inoculum. The inoculum was adjusted to the density of 0.5 McFarland standard. Muller–Hinton sheep blood agar was used to check the antibacterial property. About 15 mL of the agar medium was dispensed in 90-mm diameter Petri dish with a thickness of 4–5 mm. The culture plates were stored at 4°C until further use. After drying the plate at 37°C for 30 min, 100 μL of standardized bacterial inoculum was dispensed with a micropipette and lawn culture was done by spreading evenly using a sterile glass spreader. Initially, four wells of 5 mm diameter and 2 mm depth were made in agar plate with agar punchers. These wells were incorporated with cavity cleansers according to the standardized method as mentioned in Table 1. Later, four separate Muller–Hinton sheep blood agars were used for different groups. All the procedures were carried out under sterile precautions in a Type II biosafety cabinet. The plates were incubated aerobically for 24 h at 37°C under 5% CO2. The zone of inhibition in diameter around the material in the punched wells was measured in millimeters (mm) after 24 h. The experiment was repeated for ten times, and the results were expressed as mean diameters with standard deviations. The antimicrobial testing was done by disc diffusion method also, but it was found that agar punch well method was better than the disc diffusion. Comparison of the mean values of the zones of inhibition of S. mutans in the four groups was carried out using one-way ANOVA and Tukey's honestly significant difference (HSD) post hoc test. The comparison was done using SPSS Version 20.0 (SPSS Inc., Chicago IL), and P < 0.05 was considered statistically significant (95% confidence interval).
Table 1.
Procedure of cavity cleanser application to test each aspect of the study
| Material | Application | ||
|---|---|---|---|
| Antimicrobial action | Bond strength | Microleakage | |
| All tooth preparations were rinsed for 20 s and air dried for 5 s | |||
| PAA (Dentine conditioner, GC, Cooperation, Tokyo, Japan) | Dispense 0.01 mL of 10% PAA in the punched well-using micropipette | 10% PAA applied by micro brush on the cavity surface for 20 s Rinsed thoroughly with water. Gently dried without desiccation by blotting with a cotton pellet | |
| CHX gluconate 2% solution (Asep - RC, Anabond Stedman Pharma Research (P) Ltd, India) | Dispense 0.01 mL of 2% CHX solution in punched well using micropipette | Application of 2% CHX on tooth surface by micro-brush for 20 s, air dried for 5 s. PAA solution was then applied by micro-brush for 20 s and rinsed with water followed by blot drying | |
| SDF and KI (Riva Star, SDI, Bayswater, Australia) | 0.01 mL of SDF+KI dispensed in the agar well using micropipette | SDF solution applied to dentine surface, and then KI applied to dentine surface both using a standardized micro brush until the creamy white solution turned clear. The reaction products were washed off with copious distilled water and then blot dried[11] | |
| Distilled water | 0.01 mL of distilled water dispensed in agar well using a micropipette | Cavity washed with distilled water and blot dried | |
CHX: 2% Chlorhexidine Gluconate, PAA: Polyacrylic acid, SDF: Silver diamine fluoride, KI: Potassium iodide
Bond strength
Sample selection and storage
Thirty-two noncarious extracted human molars with no wear defects, fracture line, or cracks were included in the study. Surface debridement was done with a hand-scaler, cleaning with a rubber cup applied with slurry of pumice, and was subsequently stored in distilled water at 4°C until use.
Sample preparation
The teeth were then partially submerged in to separate self-cure acrylic resin blocks of 1 cm × 1 cm × 2 cm in size. The teeth were sectioned through a plane parallel to the longitudinal axis at the level of the middle third to expose a flat dentine surface. Slow-speed diamond abrasive disc was used. The tooth surface was then ground flat using silicon carbide rough paper of 180-grit. The dentin surface was polished with 600-grit silicon carbide paper to standardize the smear layer.
Tooth surface treatment
Eight teeth each were assigned to four groups in no particular order, and respective conditioning was performed according to the procedure as outlined in Table 1.
Placement of resin-modified glass ionomer cement
RMGIC (GC Gold Label 2 LC, GC Cooperation, Tokyo, Japan) mixed as per the manufacturer instruction was packed into a hollow cylindrical-shaped plastic scaffold (2 mm length, 4 mm diameter) of a specific dimension, condensed on to the dentin surface, and polymerized using visible light curing (VLC) unit (Elipar 2500, 3M ESPE, St. Paul, MN, USA) for 30 s. The prepared samples were then stored in temperature of 37°C and 100% humidity for 24 h.
Shear bond strength analysis
The specimens were evaluated for the bond strength of RMGIC to dentin. Samples positioned into a positioning jig and tested for shear strength with an Instron Testing Machine (Instron Corporation, Canton, MA, USA) using at a crosshead speed of 0.5 mm/min. The strengths of the samples were calculated and expressed in MPa. Statistical analysis of the mean of the shear bond strength values obtained in the various groups were done using one-way ANOVA and Tukey's HSD post hoc test with P value set at <0.05.
Microleakage
Sample selection
Thirty-two noncarious extracted human premolars with no wear defects, fracture line, or cracks were included in the microleakage study. Surface debridement was done with the hand-scaling instrument, cleaning with a rubber cup and slurry of pumice, and was subsequently stored in distilled water at temperature of 4°C until further use.
Sample preparation
A standardized Class V cavity was prepared on the buccal surface of each tooth using tungsten carbide straight fissure bur under air-water cooling. The bur was replaced after every five preparations. The dimensions of the preparations measured 5 mm in length, 3 mm in width, and 2 mm in depth with the occlusal margin placed in enamel and the gingival margin placed in dentin. A William's graduated periodontal probe (GDC, India) was used to confirm the dimensions of the cavity.
Cavity cleansing
Eight teeth each were assigned to four groups in no particular order, and respective cavity conditioning was performed as in the procedure as outlined in Table 1.
Placement of resin-modified glass ionomer cement
RMGIC was mixed according to the manufacturer's directions and filled into the cavity. Immediately after the restorations were placed, a transparent mylar matrix was adapted over RMGIC restorations during the initial setting for 2 min and was polymerized using VLC unit for 30 s. The mylar matrix was removed, and the unfinished restorations were instantaneously coated with tooth varnish according to the manufacturer's instructions. Excess material was removed with a BP knife (Bard-parker). Once the varnish dried, the teeth were stored in distilled water for 24 h at 37°C. The restorations were finished to contour with finishing bur under air-water spray using a high-speed hand piece. Medium, fine, and superfine discs were used in sequence under air-water spray using a slow-speed hand piece to refine the restoration. Teeth were then stored in distilled water at 37°C.
Preparation of samples for microleakage
The specimens were layered with two coats of nail varnish, leaving a 1 mm window peripheral to the cavity margins. During the process of placement of nail varnish, a moistened cotton pellet was placed on top of the restoration to prevent desiccation. Teeth apex was embedded in wax and was inverted so that the crown remain submerged in a solution of 2% Rhodamine-B dye (Lobachemie, India) for 24 h at 37°C under vacuum. Only the coronal portion of teeth was covered with the dye to prevent leakage through the root apices. Specimens were removed from the dye solution and were sectioned longitudinally in a buccolingual direction through the center of the restorations using a low-speed diamond disc. The section was evaluated with a fluorescent microscope (Olympus CX41, Olympus Microscopy Europa) at ×10 to determine the level of dye penetration at the occlusal and gingival margins, by two evaluators who were blinded to the experimental groups.
Dye scoring criteria
The level of dye penetration was analyzed according to a 0–3 ordinal scoring system as mentioned in Figure 1. Score 0 is the absence evidence of dye penetration; Score 1 is dye penetration present along the occlusal/gingival wall but less than half of the cavity depth; Score 2 is dye penetration present along the occlusal/gingival wall involving more than half of the cavity depth but not extending on to the axial wall; and Score 3 is dye penetration present along the occlusal/gingival wall as well as full cavity depth and extending to axial wall. Statistical analysis of the results was done using Fisher's exact value.
Figure 1.
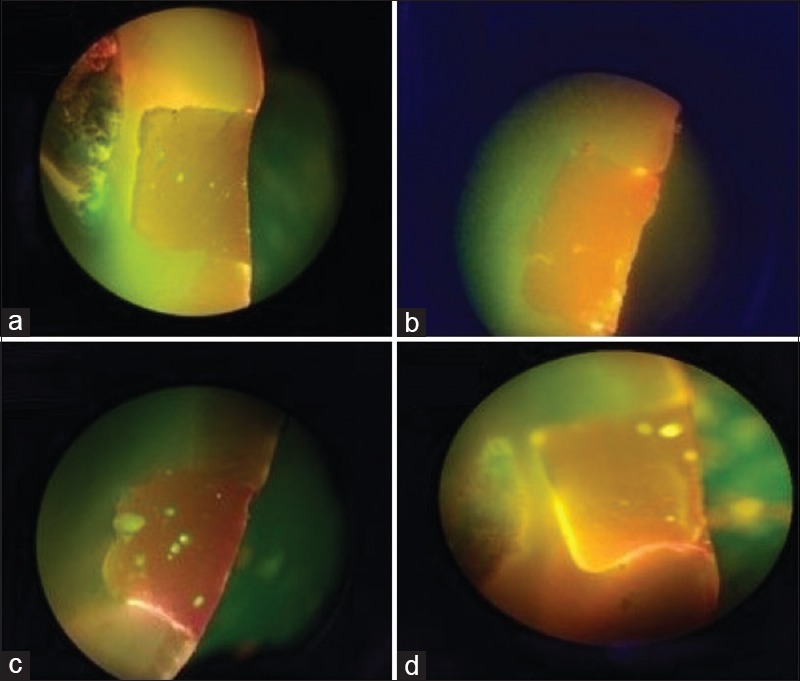
(a) No evidence of dye penetration (Score 0); (b) dye penetration along the occlusal wall which is less than half of the cavity depth (Score 1); (c) dye penetration along the occlusal wall which is more than half of the cavity depth but not extending on to the axial wall (Score 2); (d) dye penetration along the occlusal wall to the full cavity depth and extending on to the axial wall (Score 3)
RESULTS
Antimicrobial action
The comparison of AMA (mean ± standard deviation in mm) of cavity cleansers is shown in Table 2. The highest zone of inhibition was elicited by SDF-KI (34 ± 0.8) followed by CHX (23.9 ± 0.7), PAA (12.7 ± 0.8), and least by CTRL 0. In comparison to control, significant improvement (P < 0.001) was seen in all PAA, CHX, and SDF-KI, with SDF-KI having the highest antimicrobial activity followed by CHX and the least shown by PAA.
Table 2.
Antimicrobial action of various cavity cleansers against Streptococcus mutans
| Groups | n | Zone of inhibition mean (mm) | SD (mm) | P | Post hoc Tukey test |
|---|---|---|---|---|---|
| Group 1 (PAA) | 10 | 12.7 | 0.8 | <0.001 | All subgroups are significant with each other |
| Group 2 (CHX) | 10 | 23.9 | 0.7 | ||
| Group 3 (SDF-KI) | 10 | 34 | 0.8 | ||
| Group 4 (CTRL) | 10 | 0 | 0 |
CHX: 2% Chlorhexidine Gluconate, PAA: Polyacrylic acid, SDF: Silver diamine fluoride, KI: Potassium iodide, CTRL: Distilled water, SD: Standard deviation
Bond strength
A significant increase in bond strength was seen when the cavity cleanser SDF-KI (22.3 ± 4.4) unit is MPa was used in comparison to CTRL (10.5 ± 2.7). However, the use of CHX (9.7 ± 5.3) and PAA (8.9 ± 4.6) had statistically insignificant effect on the bond strength in comparison to control [Table 3].
Table 3.
Variation in bond strength of resin modified glass ionomer cement after application of various cavity cleansers
| Groups | n | Bond strength mean (MPa) | SD | P | Post hoc Tukey test |
|---|---|---|---|---|---|
| Group 1 (PAA) | 8 | 8.9 | 4.6 | <0.001 | The significance was seen between PAA and SDF-KI CHX and SDF-KI SDF-KI and CTRL |
| Group 2 (CHX) | 8 | 9.7 | 5.3 | ||
| Group 3 (SDF-KI) | 8 | 22.3 | 4.4 | ||
| Group 4 (CTRL) | 8 | 10.5 | 10.5 |
CHX: 2% Chlorhexidine Gluconate, PAA: Polyacrylic acid, SDF: Silver diamine fluoride, KI: Potassium iodide, CTRL: Distilled water, SD: Standard deviation
Microleakage
Microleakage scores have been assigned by the depth of dye penetration [Table 4]. Rating 1 being the least penetration was attached to 50% of SDF-KI, 37.5% of CHX, 25% of CTRL, and 12.5% of PAA samples. Score 2 being moderate penetration was assigned to 75% of PAA, 50% of SDF-KI, 50% of CHX, and 50% of CTRL samples. Rating 3 being severe dye penetration was assigned to 25% of CTRL, 12.5% of CHX, 12.5% of PAA, and 0% of SDF-KI samples. The results depict that SDF-KI has the highest resistance to microleakage followed by CHX, PAA, and least resistance seen in CTRL, but the values were nonsignificant.
Table 4.
Comparison of microleakage using various cavity cleansers
| Scores | Groups | Total | |||
|---|---|---|---|---|---|
| PAA | CHX | SDF-KI | CTRL | ||
| Score 1 | |||||
| Count | 1 | 3 | 4 | 2 | 10 |
| Percentage within group | 12.5 | 37.5 | 50 | 25 | 31.2 |
| Score 2 | |||||
| Count | 6 | 4 | 4 | 4 | 18 |
| Percentage within group | 75 | 50 | 50 | 50 | 56.2 |
| Score 3 | |||||
| Count | 1 | 1 | 0 | 2 | 4 |
| Percentage within group | 12.5 | 12.5 | 0 | 25 | 12.5 |
| Total | |||||
| Count | 8 | 8 | 8 | 8 | 32 |
| Percentage within group | 100 | 100 | 100 | 100 | 100 |
Fisher’s exact value of 4.585, the P value of 0.645. CHX: 2% Chlorhexidine Gluconate, PAA: Polyacrylic acid, SDF: Silver diamine fluoride, KI: Potassium iodide, CTRL: Distilled water
DISCUSSION
The cavity preparation should be free of all pathogenic bacteria before restoration to prevent any possibility of secondary caries.[12] The currently available medicament does not have substantial efficacy. Thus, a newly available drug SDF has been evaluated in this research. SDF was cleared by the Food and Drug Administration, United States, for market use, in August 2014.[13] Another cavity cleanser available is CHX, a cationic agent which acts by disinfecting the dentinal tubules and gets adsorbed onto the dentin which is a useful broad-spectrum antimicrobial agent, is nontoxic to the pulp, and has residual action.[9] Thus, the present study compares the various properties using SDF and CHX as cavity cleansers.
The results of this research showed a higher zone of inhibition in SDF in contrast to CHX against S. mutans on blood agar. S. mutans was found to be the primary causative bacteria for dental caries.[14] Hall et al.[15] proved in a study that addition of 20 ppm of Ag(NO)3 in a nutrient broth retarded growth of S. mutans and Staphylococcus aureus, which proves that silver salts act individually as growth inhibitor at this concentration rather than bactericidal, although unspecified higher levels were bactericidal SDF has a few disadvantages such as pulpal irritation, dental staining, and oral soft tissue irritation, which need to be addressed for it to be acceptable as a cavity cleanser.[11] A solution to this problem is to apply KI after application of SDF as it reacts to form silver iodide, a creamy white precipitate which reduced the staining action of SDF.[8] A study by Knight et al.[16] showed that AgF or AgF/KI treated demineralized dentine disks resisted the formation of an S. mutans biofilm during a 14-day exposure to the organism, rather than control disks and those treated with KI alone which showed marked biofilm formation. Silver ions (Ag+) can hamper metabolic activity of bacteria by reacting with intra-cytoplasmic sulfur-containing enzymes, disrupt bacterial replication by binding with the phosphorus-containing DNA molecules, and bond electrostatically to the negatively charged bacterial cell wall (due to a high concentration of carboxylate groups), thus preventing bacterial aggregation.[17] SDF application under GIC restorations in primary teeth has shown an advantageous pulpal response and improved reparative dentine formation.[18]
CHX, a cationic agent (biguanide group; 4-chlorophenyl radical), has a broad-spectrum antibacterial action. The cationic nature of the compound forms a bond on the bacterial surface with anionic compounds (lipopolysaccharide at Gram-negative bacteria and phosphate groups of teichoic acid at Gram-positive bacteria) which are capable of altering its integrity. The increase in the cytoplasmic membrane permeability causes precipitation of cytoplasmic proteins, interference in cell metabolism, alteration of cellular osmotic balance, inhibits the action of membrane ATPase, and hinders the anaerobic process.[19]
The use of CHX as a cavity cleanser before RMGIC restoration did not affect its bond strength to dentin. This is in accordance to the previous studies by Cunningham and Meiers[10] and Sekhar et al.[20] However, the use of SDF-KI hastened a drastic increase in the bond strength of RMGIC in contrast to samples of PAA, CHX, or control. Yamaga et al.[21] also showed an increase in bond strength GIC when used concomitantly with SDF as cavity cleanser. This contrast in results could be attributed to the variation in the application procedure of SDF-KI, wherein the etched the cavity surface was rinsed off of SDF-KI after its application prior to the placement of GIC. The possible hypothesis for the drastic increase in bond strength after the use of SDF-KI preceding the placement of RMGIC could be as mentioned below.
The carboxylic acid of RMGIC may bond to silver phosphate (formed after reaction between the tooth surface and SDF)[22] and silver iodide precipitate (formed after reaction between SDF and KI)[5] plugged in the dentinal tubules.[23] Improved chemical bonding of GIC could also be attributed to the hardened dentin surface as a result of SDF.[24] Hydroxylapatite and fluoroapatite formed on exposed organic matrix can also contribute to increased bond strength.[7] The insoluble precipitate of calcium fluoride, silver phosphate, and silver protein formed after the application of SDF has shown to decrease the loss of calcium and phosphorous from the carious lesions. Better bonding can be a result of this as well.[25] Reduced collagen degradation and promotion of remineralization by the anti-matrix metalloprotease action of SDF could also have improved the chemical bond of GIC to the collagen fibrils.[24,25] Fixation of the organic material by SDF, leading to enhanced interlocking to the dentinal tubules, could be a reason for increased bond strength.[21]
As per the results of the study, the least leakage, even though not statistically significant, was seen in the SDF-KI group when compared to other study groups. This could be attributed to precipitate formed during the reaction between SDF and KI.[23] Although the results of the present study suggest favorable outcome after the use of SDF-KI combination as a cavity disinfectant, further studies are required to substantiate this. In addition, studies are also required to assess the effect of SDF on the adhesion of RMGIC as well as composite resin on caries affected dentin to better simulate clinical condition.
CONCLUSION
It has been observed in this research that SDF-KI combination has superior AMA than CHX. A significant increase in the bond strength can be seen when using SDF-KI as cavity cleansing agent. Although clear reasoning of the increase in bond strength was not achievable, thus further studies are required. The values of microleakage were not significantly affected by using SDF-KI or CHX as cavity cleansers.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Grover PS, Hollinger J, Lorton L. A review of the incidence of pain after an operation treatment visit: Part I. J Prosthet Dent. 1984;51:224–5. doi: 10.1016/0022-3913(84)90266-x. [DOI] [PubMed] [Google Scholar]
- 2.Brännström M. The cause of postrestorative sensitivity and its prevention. J Endod. 1986;12:475–81. doi: 10.1016/S0099-2399(86)80202-3. [DOI] [PubMed] [Google Scholar]
- 3.Crone FL. Deep dentinal caries from a microbiological point of view. Int Dent J. 1968;18:481–8. [PubMed] [Google Scholar]
- 4.Craig GG, Powell KR, Cooper MH. Caries progression in primary molars: 24-month results from a minimal treatment programme. Community Dent Oral Epidemiol. 1981;9:260–5. doi: 10.1111/j.1600-0528.1981.tb00342.x. [DOI] [PubMed] [Google Scholar]
- 5.Craig GG, Knight GM, McIntyre JM. Clinical evaluation of diamine silver fluoride/potassium iodide as a dentine desensitizing agent. A pilot study. Aust Dent J. 2012;57:308–11. doi: 10.1111/j.1834-7819.2012.01700.x. [DOI] [PubMed] [Google Scholar]
- 6.Wang S, Wu J, Yang H, Liu X, Huang Q, Lu Z. Antibacterial activity and mechanism of Ag/ZnO nanocomposite against anaerobic oral pathogen Streptococcus mutans. J Mater Sci Mater Med. 2017;28:23. doi: 10.1007/s10856-016-5837-8. [DOI] [PubMed] [Google Scholar]
- 7.Mei ML, Ito L, Cao Y, Li QL, Lo EC, Chu CH. Inhibitory effect of silver diamine fluoride on dentine demineralisation and collagen degradation. J Dent. 2013;41:809–17. doi: 10.1016/j.jdent.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Obwegeser H, Von Wachter R. The treatment of hyperesthetic dentin with nascent silver iodide. Zahnarztl Welt. 1954;9:429–30. [PubMed] [Google Scholar]
- 9.Ercan E, Ozekinci T, Atakul F, Gül K. Antibacterial activity of 2% chlorhexidine gluconate and 5.25% sodium hypochlorite in infected root canal: In vivo study. J Endod. 2004;30:84–7. doi: 10.1097/00004770-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham MP, Meiers JC. The effect of dentin disinfectants on shear bond strength of resin-modified glass-ionomer materials. Quintessence Int. 1997;28:545–51. [PubMed] [Google Scholar]
- 11.Rosenblatt A, Stamford TC, Niederman R. Silver diamine fluoride: A caries “silver-fluoride bullet”. J Dent Res. 2009;88:116–25. doi: 10.1177/0022034508329406. [DOI] [PubMed] [Google Scholar]
- 12.Kidd EA. How ’clean’ must a cavity be before restoration? Caries Res. 2004;38:305–13. doi: 10.1159/000077770. [DOI] [PubMed] [Google Scholar]
- 13.Horst JA, Ellenikiotis H, Milgrom PL. UCSF protocol for caries arrest using silver diamine fluoride: Rationale, indications and consent. J Calif Dent Assoc. 2016;44:16–28. [PMC free article] [PubMed] [Google Scholar]
- 14.Krzyściak W, Jurczak A, Kościelniak D, Bystrowska B, Skalniak A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur J Clin Microbiol Infect Dis. 2014;33:499–515. doi: 10.1007/s10096-013-1993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall RE, Bender G, Marquis RE. Inhibitory and cidal antimicrobial actions of electrically generated silver ions. J Oral Maxillofac Surg. 1987;45:779–84. doi: 10.1016/0278-2391(87)90202-3. [DOI] [PubMed] [Google Scholar]
- 16.Knight GM, McIntyre JM, Craig GG, Mulyani, Zilm PS, Gully NJ, et al. Inability to form a biofilm of Streptococcus mutans on silver fluoride- and potassium iodide-treated demineralized dentin. Quintessence Int. 2009;40:155–61. [PubMed] [Google Scholar]
- 17.Hamama HH, Yiu CK, Burrow MF. Effect of silver diamine fluoride and potassium iodide on residual bacteria in dentinal tubules. Aust Dent J. 2015;60:80–7. doi: 10.1111/adj.12276. [DOI] [PubMed] [Google Scholar]
- 18.Gotjamanos T. Pulp response in primary teeth with deep residual caries treated with silver fluoride and glass ionomer cement (’atraumatic’ technique) Aust Dent J 1996. 41:328–34. doi: 10.1111/j.1834-7819.1996.tb03142.x. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins S, Addy M, Wade W. The mechanism of action of chlorhexidine. A study of plaque growth on enamel inserts in vivo . J Clin Periodontol. 1988;15:415–24. doi: 10.1111/j.1600-051x.1988.tb01595.x. [DOI] [PubMed] [Google Scholar]
- 20.Sekhar A, Anil A, Thomas MS, Ginjupalli K. Effect of various dentin disinfection protocols on the bond strength of resin modified glass ionomer restorative material. J Clin Exp Dent. 2017;9:e837–41. doi: 10.4317/jced.53725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamaga M, Koide T, Hieda T. Adhesiveness of glass ionomer cement containing tannin-fluoride preparation (HY agent) to dentin – An evaluation of adding various ratios of HY agent and combination with application diammine silver fluoride. Dent Mater J. 1993;12:36–44. doi: 10.4012/dmj.12.36. [DOI] [PubMed] [Google Scholar]
- 22.Knight GM, McIntyre JM, Craig GG, Mulyani, Zilm PS, Gully NJ, et al. Differences between normal and demineralized dentine pretreated with silver fluoride and potassium iodide after an in vitro challenge by Streptococcus mutans. Aust Dent J. 2007;52:16–21. doi: 10.1111/j.1834-7819.2007.tb00460.x. [DOI] [PubMed] [Google Scholar]
- 23.Selvaraj K, Sampath V, Sujatha V, Mahalaxmi S. Evaluation of microshear bond strength and nanoleakage of etch-and-rinse and self-etch adhesives to dentin pretreated with silver diamine fluoride/potassium iodide: An in vitro study. Indian J Dent Res. 2016;27:421–5. doi: 10.4103/0970-9290.191893. [DOI] [PubMed] [Google Scholar]
- 24.Zhao IS, Mei ML, Burrow MF, Lo EC, Chu CH. QEffect of silver diamine fluoride and potassium iodide treatment on secondary caries prevention and tooth discolouration in cervical glass ionomer cement restoration. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18020340. pii: E340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uzel I, Ulukent O, Coguluthe D. Effect of silver diamine fluoride on microleakage of resin composite. J Int Dent Med Res. 2014;6:105–8. [Google Scholar]


