Abstract
Background:
Biosynthesized silver nanoparticles (AgNPs) have been proposed as effective antimicrobial agents against endo–perio pathogens. Determination of cytotoxicity is important for effective clinical use.
Aim:
The aim is to determine the cytotoxicity of fungal-derived AgNPs on human gingival fibroblast (HGF) cell line using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.
Materials and Methods:
HGF cell cultures were trypsinized and adjusted to 5 × 103 cells/ml and 100-μl cell suspension (50,000 cells/well) and were added to 96-well plate. After 24 h, 100 μl of AgNPs (8–512-μg/ml concentrations) was added and incubated at 37°C for 24 h in 5% CO2 atmosphere. Controls were used without AgNPs. MTT (1 mg/ml) was added and incubated for 4 h at 37°C in 5% CO2 atmosphere. Microscopic examination was done, and absorbance was measured using a microplate reader at a wavelength of 540 nm. Percentage growth inhibition was calculated, and the concentration of AgNPs needed to inhibit cell growth by 50% (CTC50) was generated.
Results:
CTC50 was found at a concentration of 260 μg/ml. AgNPs exerted less cytotoxicity against HGF cell line and increased with increase in the concentration of AgNPs.
Conclusion:
Fungal-derived AgNPs are safe to healthy cells at a concentration <260 μg/ml. Therefore, they can be effectively used for the treatment of endo–perio lesions.
Keywords: Antimicrobial agents, biosynthesized silver nanoparticles, cytotoxicity, endo–perio lesions
INTRODUCTION
The pulp–periodontal interrelationship can be considered as a single biologic unit with several paths of communication which spread the infection from pulp to periodontium and vice versa.[1] They can get affected individually or combined; when both tissues are involved, they are called endo–perio lesions.[2]
The main objective of the treatment is to control the bacterial infection. Periodontal and endodontic therapies can be grouped into three broad categories as follows: mechanical debridement and cleaning to eliminate bacteria; treatment aimed at killing or affecting the metabolism of the infectious microorganism, such as use of antiseptics and antimicrobial agents; and treatment of the tissues/environment that are affected by the infectious microorganisms.[3] Along with mechanical debridement, the antimicrobial agents are commonly employed.[3] In endodontic therapy, apart from root canal debridement, root canal disinfectants such as root canal irrigants, intracanal medicaments (ICMs), sealers, and three-dimensional obturation are employed to control the disease process.[4] During periodontal therapy, along with mechanical cleaning, antimicrobial agents such as systemic drugs, local drug delivery, and mouth rinses are used for controlling infection.[3] However, even after using the most contemporary treatment approaches, complete elimination of the bacteria from the tissues is not possible because of the complex anatomy of the tooth system and also, increase in the number of resistant strains to the available antimicrobial agents.[5] Microorganisms causing the endo–perio lesions are usually biofilm mediated and are resistant to most of the conventional antimicrobial agents, leading to persistent and refractory infections.[6] Therefore, it is important to design and develop newer antimicrobial agents that overcome these limitations.
Nanotechnology has evolved as a favorable tool in medical field and dentistry. Nanoparticles offer an attractive alternative to conventional antimicrobial agents.[5,7] Among the nobel metals, silver (Ag) is considered in the field of biological system, living organisms, and medicine.[8] Silver nanoparticles (AgNPs) can be effectively used as antimicrobial agents because of their broad spectrum of activity and biocompatibility.[9]
Biosynthesis of AgNPs using biological entities such as bacteria, fungi, herbal extracts, and yeasts is an ongoing research in nanotechnology.[10] Biosynthesized AgNPs exhibit effective antimicrobial activity, and the process of synthesis using biological entities does not use chemicals that cause hazards to human health and environment.[11]
Endophytic fungi live inside the internal tissues of plants and do not cause any side effects. Several researchers have reported endophytic fungal flora as a source of various bioactive compounds with potential advantages such as they are rich sources of bioactive secondary metabolites with unique structures for the production of AgNPs.[8,12]
Although the biosynthesis of AgNPs using endophytic fungi and their application as antimicrobial agents against endo–perio pathogens have been reported in earlier studies,[13,14,15] little is known about their cytotoxicity. The aim of the present study was to evaluate the cytotoxicity of biosynthesized AgNPs produced using endophytic fungi on human gingival fibroblast (HGF) cell lines using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay.
MATERIALS AND METHODS
AgNPs were produced using endophytic fungi Fusarium semitectum as described earlier.[6,7] Briefly, the fungal isolates were grown on potato dextrose agar plates and inoculated in 100-ml malt glucose yeast peptone broth and incubated at 29°C for 72 h for the fungal biomass to grow. The biomass was filtered using Whatman filter paper number 1 and washed with sterile distilled water several times. About 25 g of the biomass was then added to flasks containing 100 ml of sterile distilled water and incubated for 48 h. The filtrate was mixed with 1-mM concentration of aqueous solution of silver nitrate and kept in dark at 29°C for 24 h and used further.
For cytotoxicity evaluation, the HGF cell lines were obtained from the National Centre for Cell Sciences Pune, India. Stock cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% inactivated fetal bovine serum (FBS), penicillin (100 IU/ml), and streptomycin (100 μg/ml) in a humidified atmosphere of 5% CO2 at 37°C until confluent. The cells were dissociated with TPVG solution (0.2% trypsin, 0.02% EDTA, and 0.05% glucose in phosphate-buffered solution). The viability of the cells was checked under inverted microscope, centrifuged, and subcultured into a T-75 cm2 tissue culture flask.
The HGF cell count was adjusted to 5 × 103 cells/ml using DMEM containing 10% FBS. To each well of the 96-well microtiter plate, 100 μl of the diluted cell suspension (50,000 cells/well) was added. After 24 h, when a monolayer was formed, the supernatant was discarded, washed with the medium, and 100 μl of various test concentrations of AgNPs ranging from 8 to 512 μg/ml was added onto the monolayer in microtiter plates and incubated at 37°C for 24 h in 5% CO2 atmosphere. Controls were prepared with an equivalent volume of cells in culture media without AgNPs.
After 24 h, culture medium containing MTT (1 mg/ml) in PBS was added to each well. The plates were gently shaken and incubated for another 4 h at 37°C in 5% CO2 atmosphere. The supernatant was removed, 100 μl of dimethyl sulfoxide was added, and the plates were gently shaken to solubilize the formed formazan. Microscopic examination was done, and observations were noted [Figure 1a-c]. The absorbance was measured using a microplate reader at a wavelength of 540 nm. The percentage growth inhibition was calculated using the following formula, and the concentration of AgNPs needed to inhibit cell growth by 50% (CTC50) values was generated from the dose–response curves.
Figure 1.
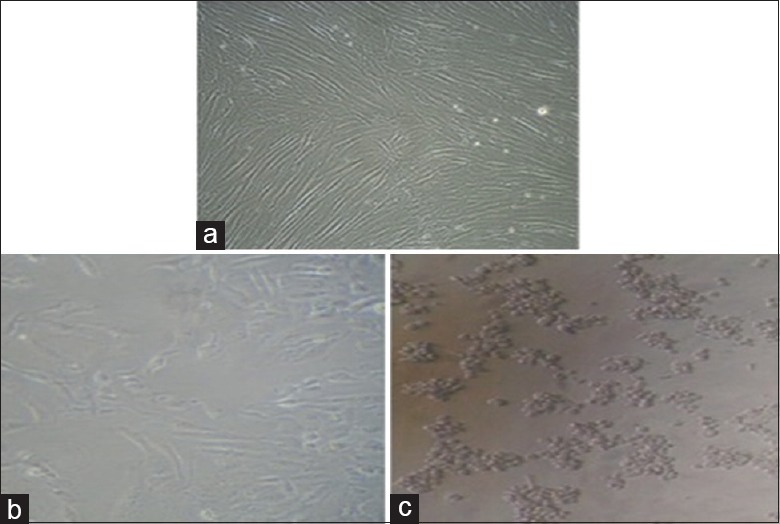
Human gingival fibroblast cell lines, (a) control and treated with (b) 258 μg/ml silver nanoparticles and (c) 512 μg/ml silver nanoparticles
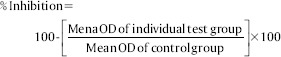
RESULTS
At a minimum concentration of 8 μg/ml of AgNPs, the percentage inhibition was found to be 2.11%. The maximum cytotoxicity of 65.24% was found at the concentration of 512 μg/ml. At a concentration of 256 μg/ml, AgNPs exhibited 46.36% inhibition. AgNPs exerted less cytotoxicity against HGF cell line and increased with increase in the concentration of AgNPs. Graphical representation showed CTC50 at a concentration 260 μg/ml. Therefore, a concentration <260 μg/ml will be effective against diseased cells and is safe to the healthy cells [Table 1 and Graph 1].
Table 1.
Percentage inhibition on human gingival fibroblast cell lines determined by MTT cytotoxicity assay
| Test compound | Test concentration (µg/ml) | Percentage inhibition HGF cell line |
|---|---|---|
| Control | 0 | 0.00 |
| AgNPs | 512 | 65.24 |
| 256 | 46.36 | |
| 128 | 27.23 | |
| 64 | 10.32 | |
| 32 | 5.10 | |
| 16 | 3.34 | |
| 8 | 2.11 |
AgNPs: Silver nanoparticles, HGF: Human gingival fibroblast
Graph 1.
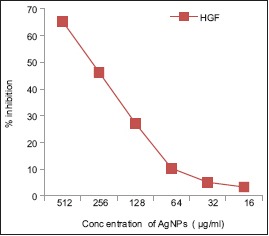
Graphical representation of inhibition of cell growth by 50% of silver nanoparticles against human gingival fibroblast -1 cell line
DISCUSSION
The mortality rate of endo–perio lesions is more than 50% today.[16] Most of the endo–perio pathogens are resistant to the available antimicrobial agents; therefore, newer methods to control the disease process, prevent persistent or refractory lesions, and achieve high success rate is the need of the hour.[14]
Biosynthesized AgNPs have emerged as novel antimicrobial agents with effective antimicrobial activity against several pathogens including the pathogens associated with endo–perio lesions.[10,14] AgNPs act synergistically in distinct targets, and there is no interference with antimicrobial resistance mechanisms.[17] AgNPs can penetrate the tissues owing to their extremely small size and high surface area, hence the potential to use for resistant microbes.[18]
Biosynthesized AgNPs offer an attractive alternative as antimicrobial agents in the treatment of endodontic, periodontal, and the combined lesions and are effective against the endo–perio pathogens.[5,14] Exposure to AgNPs as antimicrobial agents for endodontic or periodontal therapy involves intact contact with tissues or administration; therefore, understanding the properties of AgNPs and cytotoxicity evaluation is important for effective use of AgNPs for clinical applications. Cytotoxicity studies of nanoparticles are widely conducted on in vitro models due to ease in execution, control, and interpretation, and they are initial studies which mimic in vivo conditions. Cytotoxicity can be studied in vitro by means of several qualitative and quantitative tests; however, quantitative tests are most suitable as they quantify the number of living cells.[19]
MTT assay was chosen for the present study because it is a quantitative test recommended for biocompatibility testing, simple to perform, and allows distinguishing nonviable from viable cells by microscopic analysis. It is a colorimetric method that measures the reduction of yellow MTT by mitochondrial succinate dehydrogenase. Since reduction of MTT can only occur in metabolically active cells and cellular reduction is only catalyzed by living cells, it is possible to quantify the percentage of living cells in a solution.[19]
Despite the widespread use of AgNPs in medical field, relatively few in vitro studies have been conducted on healthy cells, most of which are conducted on tumor cells.[20] Different cell lines have been used to assess cytotoxicity because primary/diploid human cells, mainly oral fibroblasts, are believed to be more biologically relevant tools for these experiments compared to the permanent cells.[21] However, literature is scarce regarding the cytotoxic studies of endophytic fungal-derived AgNPs on oral cells like HGF cells which are considered to be common in vitro model for biocompatibility studies[22] and are therefore used in the present study.
Han et al.[23] reported the cytotoxicity of biosynthesized AgNPs on human lung epithelial adinocarcinoma cell lines with IC50 values of 20 μg/ml compared to the synthetic AgNPs with IC50 values of 70 μg/ml, indicating that biosynthesized AgNPs are much effective at a minimum dose compared to synthetic AgNPs.
In the present study, at a minimum concentration of 8 μg/ml, the percentage inhibition was found to be 2.11%. The maximum cytotoxicity of 65.24% was found at the concentration of 512 μg/ml. At a concentration of 256 μg/ml, AgNPs exhibited 46.36% inhibition. Fifty percent inhibition (CTC50) was found at a concentration of 260 μg/ml. Therefore, a concentration <260 μg/ml will be effective against diseased cells and is safe to the healthy cells.
The results of the present study indicate that AgNPs exerted less cytotoxicity against HGF cell line and increased with increase in the concentration of AgNPs, which is in accordance to the study by Magdi et al.[24]
The present study shows an insight for research in endodontic and periodontal therapy; biosynthesized AgNPs can be used as root canal irrigants, ICMs, root canal sealers, etc.; can be used as an alternative to topical antiseptics and antimicrobial agents; and can be used for local drug delivery during periodontal therapy, increasing the success rate.
CONCLUSION
The biosynthesized AgNPs derived from endophytic fungi Fusarium semitectum exhibit effective less cytotoxicity against HGF cell line, and are therefore safe to use. Hence, the present study shows an insight for the use of biosynthesized AgNPs as antimicrobial agents for successful treatment of endodontic, periodontal, and the combined lesions. However, further in vivo and in vitro studies evaluating the antimicrobial efficacy using nonculture methods, cytotoxicity by different methods other than MTT assay, effectiveness when employed as root canal irrigants and root canal sealers, and clinical trials should be conducted for the effective and confident use of these biosynthesized AgNPs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Mjör IA, Nordahl I. The density and branching of dentinal tubules in human teeth. Arch Oral Biol. 1996;41:401–12. doi: 10.1016/0003-9969(96)00008-8. [DOI] [PubMed] [Google Scholar]
- 2.Storrer CM, Bordin GM, Pereira TT. How to diagnose and treat periodontal endodontic lesions? RSBO. 2012;9:427–33. [Google Scholar]
- 3.Padmanabhan P. Antimicrobials in treatment of periodontal disease – A review. IOSR J Dent Med Sci. 2013;4:19–23. [Google Scholar]
- 4.Kishen A. Advanced therapeutic options for endodontic biofilms. Endod Top. 2010;22:99–123. [Google Scholar]
- 5.Halkai KR, Mudda JA, Shivanna V, Rathod V, Halkai R. Biosynthesised silver nanoparticles from fungi as antimicrobial agents for endo-perio lesions – A review. Ann Res Rev Biol. 2016;10:1–7. [Google Scholar]
- 6.Halkai KR, Mudda JA, Shivanna V, Rathod V, Halkai R. Antibacterial efficacy of biosynthesized silver nanoparticles against Enterococcus faecalis biofilm: An in vitro study. Contemp Clin Dent. 2018;9:237–41. doi: 10.4103/ccd.ccd_828_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pagonis TC, Chen J, Fontana CR, Devalapally H, Ruggiero K, Song X, et al. Nanoparticle-based endodontic antimicrobial photodynamic therapy. J Endod. 2010;36:322–8. doi: 10.1016/j.joen.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh D, Rathod V, Ninganagouda S, Herimath J, Kulkarni P. Biosynthesis of silver nano particle by endophytic fungi Pencillium sp. Isolated from Curcuma longa (Turmeric) and its antibacterial activity against pathogenic gram negative bacteria. J Pharm Res. 2013;7:448–53. [Google Scholar]
- 9.Neal AL. What can be inferred from bacterium-nanoparticle interactions about the potential consequences of environmental exposure to nanoparticles? Ecotoxicology. 2008;17:362–71. doi: 10.1007/s10646-008-0217-x. [DOI] [PubMed] [Google Scholar]
- 10.Halkai KR, Halkai R, Mudda JA, Shivanna V, Rathod V. Antibiofilm efficacy of biosynthesized silver nanoparticles against endodontic-periodontal pathogens: An in vitro study. J Conserv Dent. 2018;21:662–6. doi: 10.4103/JCD.JCD_203_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandhu SS, Shukla H, Shukla S. Biosynthesis of silver nanoparticles by endophytic fungi: Its mechanism, characterization techniques and antimicrobial potential. Afr J Biotechnol. 2017;16:683–98. [Google Scholar]
- 12.Halkai KR, Mudda JA, Shivanna V, Rathod V, Halkai R. Evaluation of antibacterial efficacy of fungal-derived silver nanoparticles against Enterococcus faecalis. Contemp Clin Dent. 2018;9:45–8. doi: 10.4103/ccd.ccd_703_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halkai KR, Mudda JA, Shivanna V, Rathod V, Halkai RS. Evaluation of antibacterial efficacy of biosynthesized silver nanoparticles derived from fungi against endo-perio pathogens Porphyromonas gingivalis, bacillus pumilus, and Enterococcus faecalis. J Conserv Dent. 2017;20:398–404. doi: 10.4103/JCD.JCD_173_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halkai KR, Mudda JA, Shivanna V, Rathod V, Halkai RS. Biosynthesis, characterization and antibacterial efficacy of silver nanoparticles derived from endophytic fungi against P.gingivalis. J Clin Diagn Res. 2017;11:ZC92–6. doi: 10.7860/JCDR/2017/29434.10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parolia A, Gait TC, Porto IC, Mala K. Endo-perio lesion: A dilemma from 19th until 21st century. J Interdisip Dent. 2013;3:2–11. [Google Scholar]
- 16.Ansari MA, Khan HM, Khan AA, Malik A, Sultan A, Shahid M, et al. Evaluation of antibacterial activity of silver nanoparticles against MSSA and MRSA on isolates from skin infections. Biol Med. 2011;3:141–6. [Google Scholar]
- 17.Sapra P, Patel BD, Patel DV, Borkhataria CH. Review: Recent advances in periodontal formulations. Int J Pharm Chem Anal. 2014;1:65–74. [Google Scholar]
- 18.Stone V, Johnston H, Schins RP. Development of in vitro systems for nanotoxicology: Methodological considerations. Crit Rev Toxicol. 2009;39:613–26. doi: 10.1080/10408440903120975. [DOI] [PubMed] [Google Scholar]
- 19.Hanks CT, Wataha JC, Sun Z. In vitro models of biocompatibility: A review. Dent Mater. 1996;12:186–93. doi: 10.1016/s0109-5641(96)80020-0. [DOI] [PubMed] [Google Scholar]
- 20.Vivek R, Kannan S. Green biosynthesis of silver nanoparticles from Annona squamosa leaf extracts and its in vitro cytotoxic effect on MCF-7 cells. Process Biochem. 2012;47:2405–10. [Google Scholar]
- 21.Hauman CH, Love RM. Biocompatibility of dental materials used in contemporary endodontic therapy: A review. Part 1. Intracanal drugs and substances. Int Endod J. 2003;36:75–85. doi: 10.1046/j.1365-2591.2003.00631.x. [DOI] [PubMed] [Google Scholar]
- 22.Chiang SL, Jiang SS, Wang YJ, Chiang HC, Chen PH, Tu HP, et al. Characterization of arecoline-induced effects on cytotoxicity in normal human gingival fibroblasts by global gene expression profiling. Toxicol Sci. 2007;100:66–74. doi: 10.1093/toxsci/kfm201. [DOI] [PubMed] [Google Scholar]
- 23.Han JW, Gurunathan S, Jeong JK, Choi YJ, Kwon DN, Park JK, et al. Oxidative stress mediated cytotoxicity of biologically synthesized silver nanoparticles in human lung epithelial adenocarcinoma cell line. Nanoscale Res Lett. 2014;9:459. doi: 10.1186/1556-276X-9-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magdi HM, Mourad MH, Elaziz MM. Biosynthesis of silver nanoparticles using fungi and biological evaluation of mycosynthesized silver nanoparticles. Egypt J Exp Biol. 2014;10:1–12. [Google Scholar]


