Abstract
This review describes the history, development, and evolution of cell‐based replacement therapy for Parkinson's disease (PD), from the first pioneering trials with fetal ventral midbrain progenitors to future trials using stem cells as well as reprogrammed cells. In the spirit of Tom Isaacs, the review takes parallels to the storyline of Star Wars, including the temptations from the dark side and the continuous fight for the light side of the Force. It is subdivided into headings based on the original movies, spanning from A New Hope to the Last Jedi.
Keywords: cell reprogramming, human embryonic stem cells, human‐induced pluripotent stem cells, induced neurons, Parkinson's disease, Star Wars, transplantation
Abbreviations
- DA
Dopaminergic
- GMP
Good manufacturing practice
- hESCs
Human embryonic stem cells
- HLA
Human leukocyte antigen
- iNs
Induced neurons
- iPSCs
Induced pluripotent stem cells
- MHC
Major histocompatibility complex
- NIH
National Institutes of Health
- PD
Parkinson's disease
- PET
Positron emission tomography
- UPDRS
Unified Parkinson's Disease Rating Scale
- VM
Ventral midbrain
1. INTRODUCTION
It was with great sadness that we all received the news about Tom's passing. He was a force for good in the Parkinson's disease (PD) community and he will be greatly missed. For Tom, nothing was too serious that you could not joke about it. It is in the spirit of Tom that we write this review on cell‐based therapies for PD. We take inspiration from his spectacular Star Wars themed talk at the World Parkinson Congress in Portland, OR, USA, September 2016 (Figure 1), to discuss the past, present, and future of cell transplantation in PD (Box 1).
Figure 1.

Pictures from Tom Isaacs’ Star Wars themed talk at the World Parkinson Congress, Portland, OR, USA, 2016 entitled “Stem cells, what they mean to people with Parkinson's disease”. Tom, depicted as Darth Vader, used these images to illustrate “a kind of Stem Cell Wars in which stem cells can come from either a dodgy source usually combined with far‐fetched and false claims about their efficacy. Or they come from a reliable source like those being studied by the previous speakers here today. So, we need to stand by these scientists, who, hopefully, in the long run will be able to offer us a source of stem cells and therapies which are evidence based”
Box 1. Thirty years ago, in a galaxy near, near by….
1.
In an attempt to replace the lost dopaminergic (DA) cells in Parkinson's disease (PD), two Jedi Knights and their Padawans led a desperate mission to develop cell‐replacement therapy using fetal ventral mesencephalic tissue. With the first small clinical trials yielding positive results, two bigger clinical trials were then conducted. Little did they know that these new trials would result in a negative outcome. It was a dark time for the rebellion. Evading the negative aura surrounding cell‐based therapy for PD at the time, and building on lessons learned from these failed trials—a group of freedom fighters established a new and improved clinical trial in Europe, Transeuro. Meanwhile in the same galaxy, a small band of rebels took upon themselves to generate authentic DA neurons from pluripotent stem cells, with the hope to develop a cell therapy even more powerful than the first. When completed, this stem cell rebellion will pave the way to a new era of scientific prosperity and reprogramming, restoring balance to the Force, and bestowing freedom of movement for the PD patients of the galaxy.
2. THE ORIGINAL TRILOGY
2.1. A new hope—fetal cell trials
The release of Star Wars in 1977 forever changed the movie industry, and around the same time, our view of the brain's reparative capacity was also fundamentally altered. At that time, a series of experimental and preclinical studies conducted in Lund, Sweden showed that catecholaminergic and cholinergic neuroblasts derived from rat and human fetal ventral midbrain (VM) and the basal forebrain nuclei, respectively, could survive and innervate when transplanted into the rodent brain (Bjorklund & Stenevi, 1977; Björklund, Stenevi, & Svendgaard, 1976; Stenevi, Björklund, & Svendgaard, 1976). These studies suggested that the brain is more plastic than previously thought, and that brain repair may be possible. Of particular relevance for the PD transplantation field is that transplanted dopaminergic (DA) neuroblasts were shown to mediate functional recovery in the 6‐OHDA lesion model (Bjorklund, Dunnett, Stenevi, Lewis, & Iversen, 1980; Bjorklund & Stenevi, 1979; Bjorklund, Stenevi, Dunnett, & Iversen, 1981; Brundin, Nilsson, Gage, & Björklund, 1985). Following these positive preclinical studies using fetal VM, two individuals with PD were transplanted in Lund in 1987 (Lindvall et al., 1989), followed by two additional patients that was transplanted using an improved procedure in 1989. The first clinical benefits could be monitored in Patient 3 as early as 3 months posttransplantation. The clinical improvement was substantiated by PET studies using 6‐L‐[18F]‐fluorodopa, suggesting graft survival at 5 months (Lindvall et al., 1990). The fourth patient of this series also showed clinical benefits, with a complete withdrawal of Levodopa treatment 3 years posttransplantation, and exhibiting only mild Parkinsonian symptoms a decade after surgery (Piccini et al., 1999). The success of the procedure with Patients 3 and 4 brought optimism and motivated the transplantation of 14 additional patients in Lund over the next decade, in an open‐label manner (Brundin et al., 2000; Lindvall et al., 1990, 1994; Wenning et al., 1997). It also encouraged other centers to lead similar clinical studies (Freed et al., 1992; Freeman et al., 1995; Mendez et al., 2000, 2002; Redmond et al., 1993; Widner et al., 1992). While the results of these programs were variable from patient to patient, it was considered successful with an overall decrease in the Unified PD Rating Scale (UPDRS) motor score (part III) (Barker, Drouin‐Ouellet, & Parmar, 2015).
2.2. The empire strikes back—NIH‐funded trials
The lift on the ban of federal funding for research using fetal tissue in 1993 enabled two National Institutes of Health (NIH) funded double‐blind placebo‐controlled trials to take place in the mid‐1990s. This was seen as an expected and valuable follow‐up to the open‐label studies. However, the design of these trials were significantly different than the earlier open‐label studies (Barker, Drouin‐Ouellet, et al., 2015). The Colorado/Columbia trial transplanted 20 patients with severe PD, with another 20 receiving sham‐transplantation surgeries (13 of which whom received a graft 1 year after sham surgery) (Freed et al., 2001). In this study, tissue was stored for up to 4 weeks before being transplanted and none of the patients received immunosuppressive drugs. The primary outcome of the trial was negative, despite a modest improvement observed in younger patients (Freed et al., 2001). This, combined with the development of graft‐induced dyskinesias in five grafted patients, lead to the conclusion that this approach needed further refinement. In the Tampa/Mount Sinai/Rush trial, 23 patients were transplanted with either one or four donors and 11 patients randomized into sham surgery. Similar to the first NIH‐founded trial (Freed et al., 2001), there was no significant effect of the transplantation on the primary and secondary endpoints. In addition, more than half the grafted patients developed graft‐induced dyskinesias (GID) (Olanow et al., 2003). As these two double‐blind, placebo‐controlled trials failed to show significant clinical benefit—likely reflective of the study design—what was initially seen as a great opportunity, took a turn for the worse. As a result, the overall view on cell‐based replacement therapy for PD was then considered inefficacious, leading to the field being left dormant for the coming years.
2.3. Return of the Jedi—Transeuro
In the scientific transplantation community, however, the conclusion that transplantation of fetal VM tissue was not efficacious for PD was considered premature. For one, it was clear that some patients in the early open‐label trials showed remarkable clinical improvements (Lindvall et al., 1990; Piccini et al., 1999). Moreover, longer term follow‐ups of the patients from the double‐blind, placebo‐controlled studies suggested that more time (3–5 years) was needed for the graft to provide significant clinical improvement (Ma et al., 2010). Around the same time, continuous follow‐up clinical data as well as postmortem evaluation of the grafts from the earlier open‐labeled studies demonstrated that long‐lasting improvement (for a minimum of 10 years, and so far up to 20 years), without the need for any DA medication, could be achieved in a subset of patients (Kefalopoulou et al., 2014; Piccini et al., 1999; Politis et al., 2011). Taken together, a more comprehensive evaluation from the open‐label, as well as from the double‐blind, placebo‐controlled trials, indicated that this approach can work very well over long time, but that the outcome is highly variable.
In retrospect, many shortcomings in the trial design of the two double‐blind, placebo‐controlled studies, including the immunosuppressive regime, patient selection, cell preparation, handling and storage of the fetal VM, surgical methods, and site of grafting, were identified (reviewed in (Barker, Barrett, Mason, & Bjorklund, 2013)). Stratification of the data showed that younger patients with a good response to DA medications and less severe motor scores at baseline responded well to the DA cell‐replacement therapy. Moreover, in the only NIH‐funded study using immunosuppression, the clinical improvement seen in the transplanted patients declined shortly after the discontinuation of immunosuppression. This suggests that premature cessation of immunosuppression has an adverse effect on the clinical efficacy of the transplant—likely by causing an inflammatory response that impairs the cells’ abilities to mature, innervate and integrate into the circuitry. This is substantiated by the prominent microglial response observed around the grafts in the two postmortem cases (Olanow et al., 2003). Thus, despite the failure of the two NIH‐funded studies due to suboptimal trial designs, it was thought that a significant amount of valuable information could be assembled from those results and be useful to improve future clinical trials. With this in mind, a task‐force was created with the aim of reanalyzing all studies published using fetal VM tissue in PD patients and several criteria necessary for a positive outcome were identified (Barker et al., 2013). This in turn led in 2009 to the initiation of a new multicenter trial sponsored by the European Union, TRANSEURO, which includes:
Selection of young patients (≤65 years old) with (a) clinically less advanced PD, (b) a predominant dorsal striatal DA loss and (c) without any prior disabling levodopa‐induced dyskinesias
Fetal VM tissue stored in good manufacturing practice (GMP)‐compliant reagents and methods for a maximum of 4 days
Increase in the number of fetal VM grafted (3–4 per side) with the aim to reach a minimum yield of 100,000 surviving nigral DA cells in the patient's brain
Optimal dissection techniques to limit the number of serotoninergic neurons to reduce the appearance of GIDs
Tissue placed using refined neurosurgery methods in the posterior putamen using 5–7 tracts
Triple immunosuppression therapy for 12 months
Longer time for primary endpoint (36 months) and a longer follow‐up period
Eleven patients have been grafted in the TRANSEURO trial. The first transplantation was performed in Cambridge in May 2015 and the last patient was grafted in Lund in March 2018. Initiation of Transeuro trial led to a revitalized interest in cell‐replacement therapy for PD, returning hope to the field that cell‐replacement therapy could work.
3. THE PREQUELS
After the original Star Wars trilogy, the prequels gave an insight into what happened prior to that day on Tatooine when the droid R2‐D2 delivered the holographic message from Princess Leia pleading for help. In summary, while some important information was revealed in these prequels, the story was inconsistent, it was evident that computer‐generated characters could not replace real actors, and Jar Jar Binks was terrible. Thus, there are striking similarities with the prequels of the fetal cell trilogy and the development of cell transplantation for PD. In an era before consensus was reached that authentic DA neurons are needed for effective repair, all sorts of catecholaminergic cells, for example, from retinal pigment epithelium and adrenal medulla were used with the expectation that they would functionally replace the DA (Allen, Burns, Tulipan, & Parker, 1989; Bakay et al., 2004; Goetz et al., 1989; Olanow et al., 1990; Stover et al., 2005; Watts et al., 2003). However, the use of nonneuronal cells—which did not produce appropriately regulated DA at high enough levels to achieve functional recovery and which survived poorly after grafting‐ instead of authentic VM progenitors, all ended up bearing disappointing results. Lesson learned: authenticity is crucial for a good outcome.
Even though the prequels were not successful in their own right, they were certainly necessary to better shape new developments. The message from the prequels is today perhaps more relevant than ever as the sequel trilogy is unraveling—with new therapies being developed based on stem cells and reprogrammed cells (see below). Entering this new and exciting era, we need to remember the valuable lessons learnt from the prequels: you need the real thing to succeed. In the transplantation field, this means that the cells that we make from stem cells or via reprogramming need to be authentic and functional midbrain (A9) DA neurons, acting in a similar manner and potency as fetal DA neurons.
4. THE SEQUELS
4.1. The force awakens—stem cells
Despite the encouraging results, work with human fetal tissue presents a number of ethical and logistical problems as well as issues of standardization, and thus does not represent a realistic therapeutic option in the future. For large‐scale applications, readily available, renewable, and bankable cells are therefore absolutely necessary, and this is why the field has turned to stem cells. Among the different stem cell sources available (Figure 2), human embryonic stem cells (hESCs) seem the most likely to go to clinical trials next in PD (Barker, Parmar, Studer, & Takahashi, 2017). Since the generation of hESCs were first reported (Thomson et al., 1998), a number of protocols on how to differentiate them into neurons, and further specify them into DA neurons have been reported (reviewed in (Barker, Drouin‐Ouellet, et al., 2015)). Newer protocols where DA neurons are formed via a floor plate intermediate were the first to show satisfactory functionality after transplantation (Kirkeby et al., 2012; Kriks et al., 2011), and since then, a number of studies have documented their authenticity, function, and potency (Chen et al., 2016; Grealish et al., 2014, 2015; Steinbeck et al., 2015). These cells can now be obtained under GMP compliant conditions (Kirkeby, Nolbrant, et al., 2017; Nolbrant, Heuer, Parmar, & Kirkeby, 2017), and are on the verge of entering clinical trials (Barker et al., 2017; Kirkeby, Parmar, & Barker, 2017; Studer, 2017). These rapid developments in the field have greatly renewed enthusiasm and created new hope for cell‐based repair in PD. However, while working together on a global level to bring stem cell‐based therapies to the clinic in an accelerated manner (Barker, Studer, Cattaneo, & Takahashi, 2015), we are also fighting a battle against the Dark Side of unproven stem cell therapies (Box 2).
Figure 2.
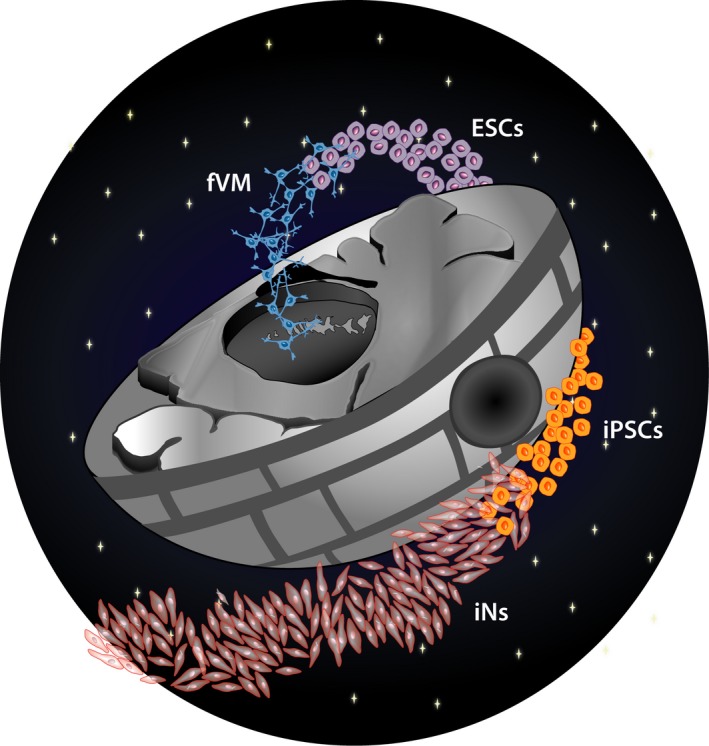
Schematic drawing illustrating the evolution of the different sources of cells used for replacement therapy in PD, starting with dopaminergic (DA) progenitors originating from fetal ventral midbrain (fVM), moving on to human pluripotent stem cells (ESCs/iPSCs), and eventually to the development of DA progenitor directly reprogrammed from somatic cells such as skin fibroblasts
Box 2. Rogue One—unproven therapies.
1.
Just like any good story, there are a number of spin‐offs. The most important one to mention here is unproven stem cells therapies. Patients are desperate for new treatments and there are a number of negligent actors that are more than happy to prey on that hope and sell “stem cell”‐based treatments. For a hefty sum of money, you can buy health, beauty, and youth. In fact, a stem cell therapy for PD can be as cheap as 10,000–30,000 USD. The snag of course is that what is for sale is not a proven stem cell therapy, but rather ineffective cells and potentially dangerous treatments. The stem cell field is fighting hard against unproven therapies (Barker et al., 2017; Murdoch, Zarzeczny, & Caulfield, 2018). While patients are anguished by the long time it takes to bring new therapies to the clinic—this must be done in a considered way in order to bring safe and efficacious therapies to patients. The long, evidence‐based road is the only way to win this battle. Tom was very active in this war against unproven therapies and now the rest of us have to join forces and carry on that important work.
4.2. The last Jedi—cellular reprogramming
Stem cells certainly have great potential to generate cells for transplantation, but there is no good story without an unexpected twist. In the latest Star Wars movie, new and previously unknown Jedi(s) suddenly appear and give the impression that the future looks more promising than ever. In a similar way, cellular reprogramming has revolutionized medical sciences by allowing large‐scale studies of patient‐specific cells including those of the nervous system, and with that, a new and previously unconsidered source of cells for therapy is emerging.
Cell reprogramming was first reported by Sir John Gurdon in the early 1960s and revealed the multipotency of somatic cells by generating tadpoles from nuclei isolated from the frog intestine (Gurdon, 1962). Decades later, this became a hot topic when it was linked to the cloning of Dolly the sheep, followed by the revolutionary findings by Shinya Yamanaka who discovered that human skin cells can be reprogrammed into induced pluripotent stem cells (iPSCs), simply by delivering a small number of transcription factors (Takahashi, Okita, Nakagawa, & Yamanaka, 2007). Of high relevance and important for the stem cell transplantation field is that iPSCs seem to carry the same differentiation potential as ESCs, and therefore can be used in a similar manner to generate authentic and functional DA neurons for transplantation (Kikuchi et al., 2017; Kriks et al., 2011). In addition, iPSCs have the benefit that they can be generated from any individual, which opens up the possibility of developing patient‐specific or human leukocyte antigen (HLA)‐matched personalized medicine (Taylor, Peacock, Chaudhry, Bradley, & Bolton, 2012). Emerging studies shows that in fact MHC matching improves cell viability and function of iPSC‐derived DA neurons in nonhuman primates (Morizane et al., 2017).
Reprogramming to pluripotency is based on changing the existing transcriptional program of a cell to match that of a pluripotent cells that can subsequently be differentiated into specialized cells. It is now clear that cells do not need to transition via a pluripotent stage but that one somatic cell type can be directly reprogrammed into another specialized cell, including neurons, which are called induced neurons (iNs) (Masserdotti, Gascón, & Götz, 2016; Pfisterer et al., 2011; Vierbuchen et al., 2010). Both iPSCs and iNs have been used to generate cells from fetal and adult individuals, including patients with neurodegenerative disease. The cells generated display characteristics of bonafide mesencephalic DA neurons, such as shared marker protein expression, matching gene expression profiles, and ability for DA release and uptake (Caiazzo et al., 2011; Kikuchi et al., 2017; Pfisterer et al., 2011; Soldner et al., 2009). While currently, convincing evidence for functional recovery in transplantation studies of PD models using iNs are scarce (Dell'Anno et al., 2014; Pereira et al., 2014; Torper et al., 2013), the benefits of using directly reprogrammed cells over fetal or pluripotent cell sources in the future are numerous. First, fibroblasts for generating iNs can easily be acquired from skin biopsies, thereby avoiding any ethical concerns in terms of fetal and embryonic tissue (López‐León, Outeiro, & Goya, 2017). Secondly, as the fibroblasts can be obtained from individual patients, it is also possible to generate cells with matching immune profiles thereby reducing the risk of graft rejection. These first two benefits are shared with iPSCs, but a major additional benefit exclusive to iN cells is that direct reprogramming avoids the pluripotent stage and directly generates postmitotic cells (Marro et al., 2011), thereby the risk of transplants containing proliferating cells which could develop tumors is minimized. While much work remains to be done before directly reprogrammed neurons can be considered for any clinical applications, rapid developments in this field are setting direct reprogramming on a fast track for a promising future. A list of advantages and disadvantages for each source of cells is provided in Table 1.
Table 1.
Advantages and disadvantages of the different cellular approaches for transplantation in Parkinson's disease (PD)
| Fetal VM progenitors | ES cells | iPS cells | Induced neurons | |
|---|---|---|---|---|
| Advantages |
|
|
|
|
| Disadvantages |
|
|
|
|
However, as with any new Jedi, the reprogrammed cells can be tempted by the Dark Side—and before such cells can be used clinically as a source of therapeutic neurons, it is important to ascertain their safety and efficacy in preclinical studies, and also to ensure that they are fully and irreversibly reprogrammed, not showing any disease‐related pathology (Figure 3).
Figure 3.

Schematic representation reflecting the struggle of developing safe and efficient protocols that prevent the reprogrammed cell product to revert back to its original state. An additional concern with autologous therapy is to exclude the expression of disease‐related pathology in the converted neuron
5. CONCLUSION
As many of us impatiently await the release of Star Wars Episode IX in December 2019, many of us are also impatiently awaiting the wide‐scale application of safe and efficacious cell‐based therapies. In both cases, only time will tell the outcome, but we dare to predict that the Force will be with us.
CONFLICTS OF INTEREST
MP is the owner of Parmar Cells AB and co‐inventor of the U.S. patent application 15/093927 owned by Biolamina AB and EP17181588 owned by Miltenyi Biotec.
AUTHOR CONTRIBUTIONS
MP conceptualized the theme of the review and wrote the manuscript. OT participated to the concept and the writing. JDO participated to the concept, the writing, and made the figures.
Supporting information
ACKNOWLEDGEMENTS
We especially thank Lyndsey Taylor Isaacs for generously sharing Tom's script and images that served as inspiration for the format of this review. We also thank Drs Deirdre Hoban and Shane Grealish for helpful discussions and edits of the text. MP receives funding from the New York Stem Cell Foundation, the European Research Council, the Swedish Research Council, the Strategic Research Area Multipark at Lund University Multipark, the Swedish Parkinson Foundation and the Swedish Brain Foundation. MP is a New York Stem Cell foundation Robertson Investigator.
Parmar M, Torper O, Drouin‐Ouellet J. Cell‐based therapy for Parkinson's disease: A journey through decades toward the light side of the Force. Eur J Neurosci. 2019;49:463–471. 10.1111/ejn.14109
Edited by Paul Bolam. Reviewed by Roger Barker (University of Cambridge); Patrik Brundin (Van Andel Institute)
All peer review communications can be found with the online version of the article.
DATA ACCESSIBILITY
This review does not contain any primary data.
REFERENCES
- Allen, G. S. , Burns, R. S. , Tulipan, N. B. , & Parker, R. A. (1989). Adrenal medullary transplantation to the caudate nucleus in Parkinson's disease. Initial clinical results in 18 patients. Archives of Neurology, 46, 487–491. 10.1001/archneur.1989.00520410021016 [DOI] [PubMed] [Google Scholar]
- Bakay, R. A. , Raiser, C. D. , Stover, N. P. , Subramanian, T. , Cornfeldt, M. L. , Schweikert, A. W. , … Watts, R. (2004). Implantation of Spheramine in advanced Parkinson's disease (PD). Frontiers in Bioscience, 9, 592–602. 10.2741/1217 [DOI] [PubMed] [Google Scholar]
- Barker, R. A. , Barrett, J. , Mason, S. L. , & Bjorklund, A. (2013). Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson's disease. Lancet Neurology, 12, 84–91. 10.1016/S1474-4422(12)70295-8 [DOI] [PubMed] [Google Scholar]
- Barker, R. A. , Drouin‐Ouellet, J. , & Parmar, M. (2015). Cell‐based therapies for Parkinson disease—Past insights and future potential. Nature Reviews. Neurology, 11, 492–503. 10.1038/nrneurol.2015.123 [DOI] [PubMed] [Google Scholar]
- Barker, R. A. , Parmar, M. , Studer, L. , & Takahashi, J. (2017). Human trials of stem cell‐derived dopamine neurons for Parkinson's disease: Dawn of a new era. Cell Stem Cell, 21, 569–573. 10.1016/j.stem.2017.09.014 [DOI] [PubMed] [Google Scholar]
- Barker, R. A. , Studer, L. , Cattaneo, E. , Takahashi, J. , & G‐Force PD consortium . (2015). G‐Force PD: A global initiative in coordinating stem cell‐based dopamine treatments for Parkinson's disease. NPJ Parkinsons Disease, 1, 15017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund, A. , Dunnett, S. B. , Stenevi, U. , Lewis, M. E. , & Iversen, S. D. (1980). Reinnervation of the denervated striatum by substantia nigra transplants: Functional consequences as revealed by pharmacological and sensorimotor testing. Brain Research, 199, 307–333. 10.1016/0006-8993(80)90692-7 [DOI] [PubMed] [Google Scholar]
- Bjorklund, A. , & Stenevi, U. (1977). Reformation of the severed septohippocampal cholinergic pathway in the adult rat by transplanted septal neurons. Cell and Tissue Research, 185, 289–302. [DOI] [PubMed] [Google Scholar]
- Bjorklund, A. , & Stenevi, U. (1979). Reconstruction of the nigrostriatal dopamine pathway by intracerebral nigral transplants. Brain Research, 177, 555–560. 10.1016/0006-8993(79)90472-4 [DOI] [PubMed] [Google Scholar]
- Bjorklund, A. , Stenevi, U. , Dunnett, S. B. , & Iversen, S. D. (1981). Functional reactivation of the deafferented neostriatum by nigral transplants. Nature, 289, 497–499. 10.1038/289497a0 [DOI] [PubMed] [Google Scholar]
- Björklund, A. , Stenevi, U. , & Svendgaard, N. (1976). Growth of transplanted monoaminergic neurones into the adult hippocampus along the perforant path. Nature, 262, 787–790. 10.1038/262787a0 [DOI] [PubMed] [Google Scholar]
- Brundin, P. , Nilsson, O. G. , Gage, F. H. , & Björklund, A. (1985). Cyclosporin A increases survival of cross‐species intrastriatal grafts of embryonic dopamine‐containing neurons. Experimental Brain Research, 60, 204–208. [DOI] [PubMed] [Google Scholar]
- Brundin, P. , Pogarell, O. , Hagell, P. , Piccini, P. , Widner, H. , Schrag, A. , … Lindvall, O. (2000). Bilateral caudate and putamen grafts of embryonic mesencephalic tissue treated with lazaroids in Parkinson's disease. Brain, 123, 1380–1390. 10.1093/brain/123.7.1380 [DOI] [PubMed] [Google Scholar]
- Caiazzo, M. , Dell'Anno, M. T. , Dvoretskova, E. , Lazarevic, D. , Taverna, S. , Leo, D. , … Broccoli, V. (2011). Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature, 476, 224–227. [DOI] [PubMed] [Google Scholar]
- Chen, Y. , Xiong, M. , Dong, Y. , Haberman, A. , Cao, J. , Liu, H. , … Zhang, S. C. (2016). Chemical control of grafted human PSC‐derived neurons in a mouse model of Parkinson's disease. Cell Stem Cell, 18, 817–826. 10.1016/j.stem.2016.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Anno, M. T. , Caiazzo, M. , Leo, D. , Dvoretskova, E. , Medrihan, L. , Colasante, G. , … Broccoli, V. (2014). Remote control of induced dopaminergic neurons in parkinsonian rats. Journal of Clinical Investigation, 124, 3215–3229. 10.1172/jci74664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed, C. R. , Breeze, R. E. , Rosenberg, N. L. , Schneck, S. A. , Kriek, E. , Qi, J. X. , … Ansari, A. A. (1992). Survival of implanted fetal dopamine cells and neurologic improvement 12 to 46 months after transplantation for Parkinson's disease. New England Journal of Medicine, 327, 1549–1555. 10.1056/nejm199211263272202 [DOI] [PubMed] [Google Scholar]
- Freed, C. R. , Greene, P. E. , Breeze, R. E. , Tsai, W. Y. , DuMouchel, W. , Kao, R. , … Fahn, S. (2001). Transplantation of embryonic dopamine neurons for severe Parkinson's disease. New England Journal of Medicine, 344, 710–719. 10.1056/NEJM200103083441002 [DOI] [PubMed] [Google Scholar]
- Freeman, T. B. , Olanow, C. W. , Hauser, R. A. , Nauert, G. M. , Smith, D. A. , Borlongan, C. V. , … Gauger, L. L. (1995). Bilateral fetal nigral transplantation into the postcommissural putamen in Parkinson's disease. Annals of Neurology, 38, 379–388. 10.1002/ana.410380307 [DOI] [PubMed] [Google Scholar]
- Goetz, C. G. , Olanow, C. W. , Koller, W. C. , Penn, R. D. , Cahill, D. , Morantz, R. , … Shannon, K. M. (1989). Multicenter study of autologous adrenal medullary transplantation to the corpus striatum in patients with advanced Parkinson's disease. New England Journal of Medicine, 320, 337–341. 10.1056/NEJM198902093200601 [DOI] [PubMed] [Google Scholar]
- Grealish, S. , Diguet, E. , Kirkeby, A. , Mattsson, B. , Heuer, A. , Bramoulle, Y. , … Parmar, M. (2014). Human ESC‐derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson's disease. Cell Stem Cell, 15, 653–665. 10.1016/j.stem.2014.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grealish, S. , Heuer, A. , Cardoso, T. , Kirkeby, A. , Jönsson, M. , Johansson, J. , … Parmar, M. (2015). Monosynaptic tracing using modified rabies virus reveals early and extensive circuit integration of human embryonic stem cell‐derived neurons. Stem Cell Reports, 4(6), 975–983. 10.1016/j.stemcr.2015.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon, J. B. (1962). The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. Journal of Embryology and Experimental Morphology, 10, 622–640. [PubMed] [Google Scholar]
- Kefalopoulou, Z. , Politis, M. , Piccini, P. , Mencacci, N. , Bhatia, K. , Jahanshahi, M. , … Foltynie, T. (2014). Long‐term clinical outcome of fetal cell transplantation for Parkinson disease: Two case reports. JAMA Neurology, 71, 83–87. 10.1001/jamaneurol.2013.4749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi, T. , Morizane, A. , Doi, D. , Magotani, H. , Onoe, H. , Hayashi, T. , … Takahashi, J. (2017). Human iPS cell‐derived dopaminergic neurons function in a primate Parkinson's disease model. Nature, 548, 592–596. 10.1038/nature23664 [DOI] [PubMed] [Google Scholar]
- Kirkeby, A. , Grealish, S. , Wolf, D. A. , Nelander, J. , Wood, J. , Lundblad, M. , … Parmar, M. (2012). Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Reports, 1, 703–714. 10.1016/j.celrep.2012.04.009 [DOI] [PubMed] [Google Scholar]
- Kirkeby, A. , Nolbrant, S. , Tiklova, K. , Heuer, A. , Kee, N. , Cardoso, T. , … Parmar, M. (2017). Predictive markers guide differentiation to improve graft outcome in clinical translation of hESC‐Based therapy for Parkinson's disease. Cell Stem Cell, 20, 135–148. 10.1016/j.stem.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkeby, A. , Parmar, M. , & Barker, R. A. (2017). Strategies for bringing stem cell‐derived dopamine neurons to the clinic: A European approach (STEM‐PD). Progress in Brain Research, 230, 165–190. 10.1016/bs.pbr.2016.11.011 [DOI] [PubMed] [Google Scholar]
- Kriks, S. , Shim, J. W. , Piao, J. , Ganat, Y. M. , Wakeman, D. R. , Xie, Z. , … Studer, L. (2011). Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature, 480, 547–551. 10.1038/nature10648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall, O. , Brundin, P. , Widner, H. , Rehncrona, S. , Gustavii, B. , Frackowiak, R. , … Bjorklund, A. (1990). Grafts of fetal dopamine neurons survive and improve motor function in Parkinson's disease. Science, 247, 574–577. 10.1126/science.2105529 [DOI] [PubMed] [Google Scholar]
- Lindvall, O. , Rehncrona, S. , Brundin, P. , Gustavii, B. , Astedt, B. , Widner, H. , … Olson, L. (1989). Human fetal dopamine neurons grafted into the striatum in two patients with severe Parkinson's disease. A detailed account of methodology and a 6‐month follow‐up. Archives of Neurology, 46, 615–631. 10.1001/archneur.1989.00520420033021 [DOI] [PubMed] [Google Scholar]
- Lindvall, O. , Sawle, G. , Widner, H. , Rothwell, J. C. , Bjorklund, A. , Brooks, D. , … Rehncrona, S. (1994). Evidence for long‐term survival and function of dopaminergic grafts in progressive Parkinson's disease. Annals of Neurology, 35, 172–180. 10.1002/ana.410350208 [DOI] [PubMed] [Google Scholar]
- López‐León, M. , Outeiro, T. F. , & Goya, R. G. (2017). Cell reprogramming: Therapeutic potential and the promise of rejuvenation for the aging brain. Ageing Research Reviews, 40, 168–181. 10.1016/j.arr.2017.09.002 [DOI] [PubMed] [Google Scholar]
- Ma, Y. , Tang, C. , Chaly, T. , Greene, P. , Breeze, R. , Fahn, S. , … Eidelberg, D. (2010). Dopamine cell implantation in Parkinson's disease: Long‐term clinical and (18)F‐FDOPA PET outcomes. Journal of Nuclear Medicine, 51, 7–15. 10.2967/jnumed.109.066811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marro, S. , Pang, Z. P. , Yang, N. , Tsai, M. C. , Qu, K. , Chang, H. Y. , … Wernig, M. (2011). Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell, 9, 374–382. 10.1016/j.stem.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masserdotti, G. , Gascón, S. , & Götz, M. (2016). Direct neuronal reprogramming: Learning from and for development. Development, 143, 2494–2510. 10.1242/dev.092163 [DOI] [PubMed] [Google Scholar]
- Mendez, I. , Dagher, A. , Hong, M. , Gaudet, P. , Weerasinghe, S. , McAlister, V. , … Robertson, H. (2002). Simultaneous intrastriatal and intranigral fetal dopaminergic grafts in patients with Parkinson disease: A pilot study. Report of three cases. Journal of Neurosurgery, 96, 589–596. [DOI] [PubMed] [Google Scholar]
- Mendez, I. , Dagher, A. , Hong, M. , Hebb, A. , Gaudet, P. , Law, A. , … Robertson, H. (2000). Enhancement of survival of stored dopaminergic cells and promotion of graft survival by exposure of human fetal nigral tissue to glial cell line–derived neurotrophic factor in patients with Parkinson's disease. Report of two cases and technical considerations. Journal of Neurosurgery, 92, 863–869. 10.3171/jns.2000.92.5.0863 [DOI] [PubMed] [Google Scholar]
- Morizane, A. , Kikuchi, T. , Hayashi, T. , Mizuma, H. , Takara, S. , Doi, H. , … Takahashi, J. (2017). MHC matching improves engraftment of iPSC‐derived neurons in non‐human primates. Nature Communications, 8, 385 10.1038/s41467-017-00926-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch, B. , Zarzeczny, A. , & Caulfield, T. (2018). Exploiting science? A systematic analysis of complementary and alternative medicine clinic websites’ marketing of stem cell therapies. British Medical Journal Open, 8, e019414 10.1136/bmjopen-2017-019414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolbrant, S. , Heuer, A. , Parmar, M. , & Kirkeby, A. (2017). Generation of high‐purity human ventral midbrain dopaminergic progenitors for in vitro maturation and intracerebral transplantation. Nature Protocols, 12, 1962–1979. 10.1038/nprot.2017.078 [DOI] [PubMed] [Google Scholar]
- Olanow, C. W. , Goetz, C. G. , Kordower, J. H. , Stoessl, A. J. , Sossi, V. , Brin, M. F. , … Freeman, T. B. (2003). A double‐blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Annals of Neurology, 54, 403–414. 10.1002/(ISSN)1531-8249 [DOI] [PubMed] [Google Scholar]
- Olanow, C. W. , Koller, W. , Goetz, C. G. , Stebbins, G. T. , Cahill, D. W. , Gauger, L. L. , … Witt, T. (1990). Autologous transplantation of adrenal medulla in Parkinson's disease 18‐month results. Archives of Neurology, 47, 1286–1289. [DOI] [PubMed] [Google Scholar]
- Pereira, M. , Pfisterer, U. , Rylander, D. , Torper, O. , Lau, S. , Lundblad, M. , … Parmar, M. (2014). Highly efficient generation of induced neurons from human fibroblasts that survive transplantation into the adult rat brain. Scientific Reports, 4, 6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfisterer, U. , Kirkeby, A. , Torper, O. , Wood, J. , Nelander, J. , Dufour, A. , … Parmar, M. (2011). Direct conversion of human fibroblasts to dopaminergic neurons. Proceedings of the National Academy of Sciences of the United States of America, 108, 10343–10348. 10.1073/pnas.1105135108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccini, P. , Brooks, D. J. , Bjorklund, A. , Gunn, R. N. , Grasby, P. M. , Rimoldi, O. , … Lindvall, O. (1999). Dopamine release from nigral transplants visualized in vivo in a Parkinson's patient. Nature Neuroscience, 2, 1137–1140. 10.1038/16060 [DOI] [PubMed] [Google Scholar]
- Politis, M. , Oertel, W. H. , Wu, K. , Quinn, N. P. , Pogarell, O. , Brooks, D. J. , … Piccini, P. (2011). Graft‐induced dyskinesias in Parkinson's disease: High striatal serotonin/dopamine transporter ratio. Movement Disorders, 26, 1997–2003. 10.1002/mds.23743 [DOI] [PubMed] [Google Scholar]
- Redmond, D. E. J. , Robbins, R. J. , Naftolin, F. , Marek, K. L. , Vollmer, T. L. , Leranth, C. , … Bunney, B. S. (1993). Cellular replacement of dopamine deficit in Parkinson's disease using human fetal mesencephalic tissue: Preliminary results in four patients. Research Publications ‐ Association for Research in Nervous and Mental Disease, 71, 325–359. [PubMed] [Google Scholar]
- Soldner, F. , Hockemeyer, D. , Beard, C. , Gao, Q. , Bell, G. W. , Cook, E. G. , … Jaenisch, R. (2009). Parkinson's disease patient‐derived induced pluripotent stem cells free of viral reprogramming factors. Cell, 136, 964–977. 10.1016/j.cell.2009.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbeck, J. A. , Choi, S. J. , Mrejeru, A. , Ganat, Y. , Deisseroth, K. , Sulzer, D. , … Studer, L. (2015). Optogenetics enables functional analysis of human embryonic stem cell‐derived grafts in a Parkinson's disease model. Nature Biotechnology, 33, 204–209. 10.1038/nbt.3124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenevi, U. , Björklund, A. , & Svendgaard, N. A. (1976). Transplantation of central and peripheral monoamine neurons to the adult rat brain: Techniques and conditions for survival. Brain Research, 114, 1–20. 10.1016/0006-8993(76)91003-9 [DOI] [PubMed] [Google Scholar]
- Stover, N. P. , Bakay, R. A. , Subramanian, T. , Raiser, C. D. , Cornfeldt, M. L. , Schweikert, A. W. , … Watts, R. L. (2005). Intrastriatal implantation of human retinal pigment epithelial cells attached to microcarriers in advanced Parkinson disease. Archives of Neurology, 62, 1833–1837. 10.1001/archneur.62.12.1833 [DOI] [PubMed] [Google Scholar]
- Studer, L. (2017). Strategies for bringing stem cell‐derived dopamine neurons to the clinic‐The NYSTEM trial. Progress in Brain Research, 230, 191–212. 10.1016/bs.pbr.2017.02.008 [DOI] [PubMed] [Google Scholar]
- Takahashi, K. , Okita, K. , Nakagawa, M. , & Yamanaka, S. (2007). Induction of pluripotent stem cells from fibroblast cultures. Nature Protocols, 2, 3081–3089. 10.1038/nprot.2007.418 [DOI] [PubMed] [Google Scholar]
- Taylor, C. J. , Peacock, S. , Chaudhry, A. N. , Bradley, J. A. , & Bolton, E. M. (2012). Generating an iPSC bank for HLA‐matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell, 11, 147–152. 10.1016/j.stem.2012.07.014 [DOI] [PubMed] [Google Scholar]
- Thomson, J. A. , Itskovitz‐Eldor, J. , Shapiro, S. S. , Waknitz, M. A. , Swiergiel, J. J. , Marshall, V. S. , & Jones, J. M. (1998). Embryonic stem cell lines derived from human blastocysts. Science, 282, 1145–1147. 10.1126/science.282.5391.1145 [DOI] [PubMed] [Google Scholar]
- Torper, O. , Pfisterer, U. , Wolf, D. A. , Pereira, M. , Lau, S. , Jakobsson, J. , … Parmar, M. (2013). Generation of induced neurons via direct conversion in vivo. Proceedings of the National Academy of Sciences of the United States of America, 110, 7038–7043. 10.1073/pnas.1303829110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen, T. , Ostermeier, A. , Pang, Z. P. , Kokubu, Y. , Sudhof, T. C. , & Wernig, M. (2010). Direct conversion of fibroblasts to functional neurons by defined factors. Nature, 463, 1035–1041. 10.1038/nature08797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts, R. L. , Raiser, C. D. , Stover, N. P. , Cornfeldt, M. L. , Schweikert, A. W. , Allen, R. C. , … Bakay, R. A. (2003). Stereotaxic intrastriatal implantation of human retinal pigment epithelial (hRPE) cells attached to gelatin microcarriers: A potential new cell therapy for Parkinson's disease. Journal of Neural Transmission. Supplementum (65), 215–227. 10.1007/978-3-7091-0643-3_14 [DOI] [PubMed] [Google Scholar]
- Wenning, G. K. , Odin, P. , Morrish, P. , Rehncrona, S. , Widner, H. , Brundin, P. , … Lindvall, O. (1997). Short‐ and long‐term survival and function of unilateral intrastriatal dopaminergic grafts in Parkinson's disease. Annals of Neurology, 42, 95–107. 10.1002/ana.410420115 [DOI] [PubMed] [Google Scholar]
- Widner, H. , Tetrud, J. , Rehncrona, S. , Snow, B. , Brundin, P. , Gustavii, B. , … Langston, J. W. (1992). Bilateral fetal mesencephalic grafting in two patients with parkinsonism induced by 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP). New England Journal of Medicine, 327, 1556–1563. 10.1056/NEJM199211263272203 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This review does not contain any primary data.


