Abstract
Aflatoxins are toxic metabolites of Aspergillus moulds and are widespread in the food supply, particularly in low‐ and middle‐income countries. Both in utero and infant exposure to aflatoxin B1 (AFB1) have been linked to poor child growth and development. The objective of this prospective cohort study was to investigate the association between maternal aflatoxin exposure during pregnancy and adverse birth outcomes, primarily lower birth weight, in a sample of 220 mother–infant pairs in Mukono district, Uganda. Maternal aflatoxin exposure was assessed by measuring the serum concentration of AFB1‐lysine (AFB‐Lys) adduct at 17.8 ± 3.5 (mean ± SD)‐week gestation using high‐performance liquid chromatography. Anthropometry and birth outcome characteristics were obtained within 48 hr of delivery. Associations between maternal aflatoxin exposure and birth outcomes were assessed using multivariable linear regression models adjusted for confounding factors. Median maternal AFB‐Lys level was 5.83 pg/mg albumin (range: 0.71–95.60 pg/mg albumin, interquartile range: 3.53–9.62 pg/mg albumin). In adjusted linear regression models, elevations in maternal AFB‐Lys levels were significantly associated with lower weight (adj‐β: 0.07; 95% CI: −0.13, −0.003; p = 0.040), lower weight‐for‐age z‐score (adj‐β: −0.16; 95% CI: −0.30, −0.01; p = 0.037), smaller head circumference (adj‐β: −0.26; 95% CI: −0.49, −0.02; p = 0.035), and lower head circumference‐for‐age z‐score (adj‐β: −0.23; 95% CI: −0.43, −0.03; p = 0.023) in infants at birth. Overall, our data suggest an association between maternal aflatoxin exposure during pregnancy and adverse birth outcomes, particularly lower birth weight and smaller head circumference, but further research is warranted.
Keywords: aflatoxin, aflatoxin B1‐lysine adduct, birth weight, head circumference, maternal exposure, pregnancy outcome
Key messages.
AFB‐Lys levels were detected in 100% of maternal serum samples, indicating widespread dietary exposure to AFB1 of the sample population.
Elevated maternal AFB‐Lys levels were significantly associated with lower infant birth weight, in addition to lower WAZ, smaller head circumference, and lower HCZ in infants at birth.
Initiatives to reduce aflatoxin exposure, especially targeted at women of reproductive age, may result in improved birth outcomes in LMICs.
1. INTRODUCTION
Worldwide, an estimated 15.5% of infants are born low birth weight (LBW),i.e., birth weight < 2,500 g, with the vast majority (>95%) of cases occurring in low‐ and middle‐income countries (LMICs; Wardlaw, 2004). Overall, birth weight is an indicator of maternal health and nutrition status as well as infants' short‐ and long‐term well‐being. Infants born LBW are at an increased risk of a number of short‐ and long‐term consequences, including neonatal mortality and morbidity, impaired immune function, childhood stunting, reduced cognitive development, and chronic diseases later in life (Wardlaw, 2004).
Aflatoxins are naturally occurring, toxic secondary metabolites of Aspergillus moulds, particularly A. flavus and A. parasiticus. They are widely prevalent in staple foods, such as maize, sorghum, and groundnuts, particularly in LMICs where poor harvest and storage practices leave food supplies vulnerable to contamination (Hell, Cardwell, Setamou, & Poehling, 2000; Kachapulula, Akello, Bandyopadhyay, & Cotty, 2017). About 4.5 billion people, mainly in LMICs, are at risk of chronic exposure to aflatoxins (Williams et al., 2004), which have been linked to a number of carcinogenic, teratogenic, and immunotoxic health effects, most notably liver cancer (Liu & Wu, 2010).
Aflatoxin B1 (AFB1), the most prevalent and toxic type of aflatoxin (Hussein & Brasel, 2001), has also been linked to poor growth and development (Gong et al., 2002; Gong et al., 2004; Shirima et al., 2015) and immune function impairment (Turner, Moore, Hall, Prentice, & Wild, 2003) in young children. Furthermore, AFB1 can cross the placental barrier during pregnancy (Denning, Allen, Wilkinson, & Morgan, 1990; Partanen et al., 2010), putting the fetus at risk of exposure. In a limited number of studies, maternal aflatoxin exposure during pregnancy has been linked to adverse birth outcomes (Abdulrazzaq, Osman, & Ibrahim, 2002; De Vries, Maxwell, & Hendrickse, 1989; Shuaib et al., 2010), particularly LBW, as well as continued poor growth during infancy and early childhood (Groopman et al., 2014; Turner et al., 2007).
Previous studies have shown that aflatoxin exposure is widespread in both the food supply (Kaaya & Kyamuhangire, 2006; Kitya, Bbosa, & Mulogo, 2010) and population (Asiki et al., 2014) in Uganda. The primary objective of this study was to investigate the association between maternal exposure to aflatoxin during pregnancy and subsequent infant birth weight in Mukono district, Uganda. Secondary outcomes of interest were infant length, weight‐for‐age z‐score (WAZ), weight‐for‐length z‐score (WLZ), length‐for‐age z‐score (LAZ), head circumference, head circumference‐for‐age z‐score (HCZ), and gestational age at birth. In this study, maternal aflatoxin exposure was measured at ~18‐week gestation using the serum concentration of the AFB1‐lysine (AFB‐Lys) adduct, which is an established biomarker of dietary aflatoxin exposure over the previous 2–3 months (Wild et al., 1992). We hypothesized that higher levels of AFB‐Lys in pregnant women would be associated with adverse birth outcomes, particularly lower infant weight at birth.
2. METHODS
2.1. Study site and population
This was a prospective cohort study conducted in Mukono district, Uganda, from February to November 2017. Women were initially enrolled during their first prenatal visit at Mukono Health Center IV (MHC IV). Women qualified for the study if they were between 18 and 45 years old, resided within 10 km of MHC IV, carried a singleton pregnancy, and planned to remain in Mukono district throughout their pregnancy. Women were excluded if they were <18 years old or >45 years old, HIV‐positive (verified via routine rapid HIV test conducted at first prenatal visit), severely malnourished (defined as body mass index [BMI] <16.0 kg/m2), severely anaemic (defined as Hb < 7 g/dL), or planned to move away from Mukono district prior to delivery.
Among 300 women screened, 258 met the inclusion criteria and were enrolled in the study. A sample size of 258 allowed for the detection of a relative risk of 2.0 within 50% of the true risk parameters, assuming 80% power, 0.05 significance, a 5% frequency of LBW, and 15% loss to follow up. Primary reasons for exclusion included multiple gestation and HIV infection. Of the enrolled 258 participants, 236 had a follow‐up visit conducted prior to delivery, 11 elected to drop out of the study, and 11 were considered lost to follow‐up as they moved away from Mukono district. Birth outcome data were collected within 48 hr for 232 infants, and 4 births were missed by the field team. Ten infants were stillborn, and two infants died before anthropometry measurements could be taken. Descriptive characteristics and birth outcome data were therefore analysed for 220 mothers and their infants.
The study was approved by the Tufts Health Sciences Institutional Review Board in Boston, Massachusetts; the Mengo Hospital Research Ethics Committee in Kampala, Uganda; and the Uganda National Council for Science and Technology in Kampala, Uganda. Written consent was obtained from all participants prior to enrolment.
2.2. Study parameters and measurement
Following enrolment, height (0.1‐cm precision; Infant/Child/Adult ShorrBoard, Shorr Production, Olney, MD, USA), weight (0.1‐kg precision; Seca 874, Hanover, MD, USA), mid‐upper arm circumference (MUAC; 0.1‐cm precision; tri‐coloured, nonstretch adult MUAC tape), and blood pressure (Omron 10 Series, Omron Healthcare, Kyoto, Japan) measurements were taken by the study nurse. All anthropometry measurements, including height, weight, and MUAC, were taken in triplicate and averaged. Maternal BMI was calculated using the formula BMI = kg/m2. Pulse pressure was calculated by taking the difference between the systolic and diastolic blood pressure.
Haemoglobin measurements were taken using a portable hemoglobinometer (HemoCue Hb 301; HemoCue, Inc., Brea, CA, USA). Estimated date of delivery was assessed from an obstetric ultrasound examination by a trained technician. A venous blood draw (BD Vacutainer, Becton Dickinson, Durham, NC, USA) was performed by the phlebotomist at MHC IV, and blood samples were centrifuged at MHC IV for 5 min at speed of 4,000 rpm to separate serum.
Finally, a questionnaire was administered by the study nurse that included questions related to demographics, prior pregnancies, health status, diet, food security (using the Household Food Insecurity Access Scale; Coates, Swindale, & Bilinsky, 2007), and water, hygiene, and sanitation practices. Diet was assessed using a diet diversity questionnaire that asked participants to respond yes/no to having eaten >50 specific foods in the previous 24 hr. Foods were selected based on their inclusion in the Ugandan Demographic and Health Survey, with minor modifications to account for the norms and preferences of the study site. Foods consumed by >10% of the participants are presented in Table S1. Responses were used to generate a Minimum Dietary Diversity for Women score, based on the number of food groups (0–10) consumed (Food and Agriculture Organization, 2016). Groups were considered (1) grains, white roots and tubers, and plantains; (2) pulses (beans, peas, and lentils); (3) nuts and seeds; (4) dairy; (5) meat, poultry, and fish; (6) eggs; (7) dark green leafy vegetables; (8) other vitamin A‐rich fruits and vegetables; (9) other vegetables; and (10) other fruits.
Infant anthropometry data, including length (0.1‐cm precision; Infant/Child/Adult ShorrBoard, Shorr Production, Olney, MD, USA), weight (0.1‐kg precision; Seca 874, Hanover, MD, USA), and head circumference (0.1‐cm precision; flexible measuring tape), were assessed within 48 hr of delivery. All anthropometry measurements were taken in triplicate and averaged. Head circumference was measured as the largest possible occipital‐frontal circumference.
2.3. Chemicals
AFB1 (> 98% purity), albumin determination reagent bromocreosol purple, and normal human serum were purchased from Sigma Aldrich Chemical Co. (St. Louis, MO, USA). Pronase (25 kU, Nuclease‐free) was purchased from Calbiochem (La Jolla, CA, USA). Protein assay dye reagent concentrate and protein standards were purchased from Bio‐Rad Laboratories Inc. (Hercules, CA, USA). Mixed mode solid phase extraction cartridges were purchased from the Waters Corp. (Milford, MA, USA). Authentic AFB‐Lys was synthesized as previously described (Sabbioni, Skipper, Büchi, & Tannenbaum, 1987). All other chemicals and solvents used were of highest grade commercially available.
2.4. Analysis of AFB‐Lys adduct levels
Midgestation maternal aflatoxin exposure was assessed using the serum AFB‐Lys adduct biomarker. Serum samples were transported on dry ice to the Wang laboratory at the University of Georgia, Athens, USA, and analysed with a high‐performance liquid chromatography (HPLC)‐fluorescence method. This included measurement of albumin and total protein concentrations for each sample, digestion with protease to release amino acids, concentration and purification of the AFB‐Lys adduct, and finally separation and quantification by HPLC (Qian et al., 2013a; Qian, Tang, Liu, & Wang, 2010).
Specifically, thawed serum samples were inactivated for possible infectious agents via heating at 56°C for 30 min, followed by measurement of albumin and total protein concentrations using modified procedures as previously described (Qian et al., 2013b). A portion of each sample (approximately 150 μL) was digested by pronase (pronase: total protein, 1:4, w: w) at 37°C for 3 hr to release AFB‐Lys. AFB‐Lys in digests were further extracted and purified by passing through a Waters MAX solid phase extraction cartridge, which was preprimed with methanol and equilibrated with water. The loaded cartridge was sequentially washed with 2 ml water, 1 ml 70% methanol, and 1 ml 1% ammonium hydroxide in methanol at a flow rate of 1 ml/min. Purified AFB‐Lys was eluted with 1 ml 2% formic acid in methanol. The eluent was vacuum‐dried with a Labconco Centrivap concentrator (Kansas City, MO, USA) and reconstituted for HPLC‐fluorescence detection.
The analysis of AFB‐Lys adduct was conducted in an Agilent 1200 HPLC‐fluorescence system (Santa Clara, CA, USA). The mobile phases consisted of buffer A (20 mM NH4H2PO4, pH 7.2) and buffer B (100% Methanol). The Zorbax Eclipse XDB‐C18 reverse phase column (5 micron, 4.6 × 250 mm) equipped with a guard column was used (Agilent, Santa Clara, CA, USA). Column temperature was maintained at 25°C during analysis, and a volume of 100 μL was injected at a flow rate of 1 ml/min. A gradient was generated to separate the AFB‐Lys adduct within 25 min of injection. Adduct was detected by fluorescence at maximum excitation and emission wavelengths of 405 and 470 nm, respectively. Calibration curves of authentic standard were generated weekly, and the standard AFB‐Lys was eluted at approximately 13.0 min. The limit of detection was 0.2 pg/mg albumin. The average recovery rate was 90%. The AFB‐Lys concentration was adjusted by albumin concentration.
Quality assurance and quality control procedures were maintained during analyses, which included simultaneous analysis of one authentic standard in every 10 samples and two quality control samples daily. Furthermore, following completion of the laboratory analysis, sets of three samples were selected and pooled into 11 intraday reproducibility samples, which were analysed twice on the same day by the same analyst, and 11 interday reproducibility samples, which were analysed on different days by different analysts, to demonstrate laboratory precision and sampling reproducibility.
2.5. Statistical analysis
All statistical analyses were performed using STATA 15 software (Stata Corps, College Station, TX, USA). Variables were first assessed for outliers and normality. Because of their skewed distribution, AFB‐Lys levels were natural log (ln) transformed prior to all analyses. Weight, length, and head circumference measurements were converted to z‐scores for WAZ, LAZ, WLZ, and HCZ using the World Health Organization standards. Outliers were defined as −6 > WAZ > +5, −5 > WLZ > +5, −6 > LAZ > +6, and −5 > HCZ > +5 based on the World Health Organization's recommendation for biologically implausible values and were excluded from analysis. (Group, 2006).
Enrolment characteristics for mothers were calculated and presented as mean ± SD. Pearson's correlation coefficients were calculated to assess the relationship between maternal characteristics and ln AFB‐Lys levels and between maternal characteristics and infant birth weight. T tests were used to compare maternal ln AFB‐Lys levels by foods consumed in the 24‐hr dietary recall.
Associations between ln maternal AFB‐Lys levels and infant birth characteristics were assessed using unadjusted and adjusted linear regression models. Covariates with a bivariate association with infant birth weight (p‐value < 0.10) were included in the adjusted models except in cases of collinearity with other covariates. For all adjusted models, the absence of multi‐collinearity was verified using variance inflation factor. For all analyses, p < 0.05 was considered statistically significant.
3. RESULTS
3.1. Maternal AFB‐Lys levels
All 220 maternal serum samples had detectable AFB‐Lys (pg/mg albumin) levels. The median maternal AFB‐Lys level was 5.83 pg/mg albumin (range: 0.71–95.60 pg/mg albumin, interquartile range: 3.53–9.62 pg/mg albumin). The arithmetic mean ± SD AFB‐Lys level was 8.87 ± 11.61 pg/mg albumin (95% CI: 7.33–10.41 pg/mg albumin), and the geometric mean AFB‐Lys level was 5.89 pg/mg albumin (95% CI: 5.25–6.60 pg/mg albumin). The arithmetic mean ± SD albumin level was 3.97 ± 0.45 g/dl (95% CI: 3.91–4.03 g/dl).
3.2. Maternal characteristics and their association with AFB‐Lys levels and birth outcomes
Characteristics of participating mothers and their correlation with both maternal AFB‐Lys levels and infant birth weight are presented in Table 1. At the time of enrolment, participants were ~24 years of age and ~18‐week gestation. In all cases, maternal characteristics were not significantly different between those who dropped out of the study or were lost to follow up and those that remained in the study.
Table 1.
Characteristics of 220 study mothers in Mukono district, Uganda, and their correlation with aflatoxin exposure (ln AFB‐Lys levels) and infant birth weight
| Mean ± SD | Maternal AFB‐Lys, pg/mg albumin | p‐value | Infant birth weight | p‐value | |
|---|---|---|---|---|---|
| Age, years | 23.9 ± 4.3 | −0.0413 | 0.5423 | 0.1729 | 0.0104 |
| Gestation at enrolment, weeks | 17.8 ± 3.5 | 0.1791 | 0.0078 | 0.0263 | 0.6987 |
| Weight, kg | 60.7 ± 9.8 | −0.0695 | 0.3048 | 0.3081 | 0.0000 |
| Height, cm | 158.5 ± 6.1 | 0.0305 | 0.6529 | 0.1883 | 0.0052 |
| MUAC, cm | 27.1 ± 3.4 | −0.1252 | 0.0637 | 0.2323 | 0.0005 |
| BMI, kg/m2 | 24.1 ± 3.5 | −0.0884 | 0.1913 | 0.2356 | 0.0004 |
| Systolic blood pressure, mmHg | 109.8 ± 11.3 | 0.0281 | 0.6782 | 0.0587 | 0.3870 |
| Diastolic blood pressure, mmHg | 72.7 ± 8.3 | −0.0222 | 0.7432 | −0.0605 | 0.3728 |
| Pulse pressure, mmHg | 37.1 ± 9.2 | 0.0546 | 0.4205 | 0.1270 | 0.0606 |
| Haemoglobin, g/dl | 11.9 ± 1.4 | 0.0170 | 0.8020 | 0.0030 | 0.9646 |
| Albumin, g/dl | 4.0 ± 0.5 | −0.0733 | 0.2792 | −0.0073 | 0.9139 |
| Diet diversity, MDD‐W score | 5.2 ± 1.7 | −0.1128 | 0.0951 | 0.0117 | 0.8629 |
| Education, years | 9.9 ± 2.9 | 0.1342 | 0.0467 | 0.1366 | 0.0435 |
| Household members | 3.5 ± 2.1 | −0.0416 | 0.5392 | 0.0727 | 0.2838 |
Note. AFB‐Lys, AFB1‐lysine adduct; BMI, body mass index; mmHg; millimetres of mercury; MDD‐W, minimum dietary diversity for women; MUAC, mid‐upper arm circumference; SD, standard deviation.
Maternal age (r = 0.1729, p = 0.0104), weight (r = 0.3081, p = 0.0000), height (r = 0.1883, p = 0.0052), MUAC (r = 0.2323, p = 0.0005), BMI (r = 0.2356, p = 0.0004), and years of education (r = 0.1366, p = 0.0435) were significantly associated with infant birth weight. Maternal ln AFB‐Lys levels were significantly associated with years of education (r = 0.1342; p = 0.0467), and nearly significantly associated with MUAC (r = −0.1252, p = 0.0637). In addition, maternal ln AFB‐Lys levels were significantly associated with gestational age at enrolment when the blood draw occurred (r = 0.179, p = 0.0078; Figure 1).
Figure 1.
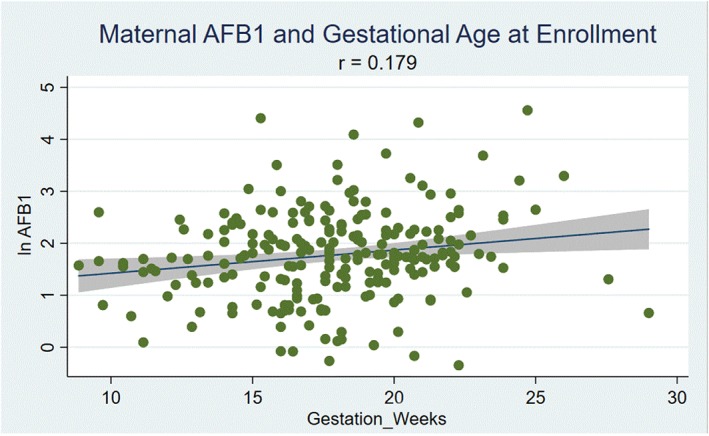
Correlation between maternal aflatoxin exposure (ln aflatoxin B1 [AFB1]‐lysine levels) and gestational age at enrolment (weeks) for 220 mother–infant pairs in Mukono district, Uganda (p = 0.0078)
Finally, differences in maternal ln AFB‐Lys levels by foods reportedly consumed in the 24‐h‐r recall are presented in Table S1. Maternal ln AFB‐Lys levels were significantly higher in those who reported eating cassava in the previous 24 hr compared with those that did not (1.94 ± 0.94 vs. 1.70 ± 0.82 pg/mg albumin, p = 0.0491). No association was observed between maternal ln AFB‐Lys levels and any other food.
3.3. Associations between maternal AFB‐Lys levels and birth outcomes
Among the 220 infants, 115 (52.3%) were female. Mean ± SD gestational age at birth was 39.7 ± 2.1 weeks. Mean ± SD weight and length at birth were 3.3 ± 0.5 kg and 48.1 ± 3.2 cm, respectively. Mean ± SD WLZ, WAZ, and LAZ were 0.47 ± 1.54, −0.10 ± 1.01, and −0.44 ± 1.07, respectively. Mean ± SD head circumference and HCZ were 35.2 ± 1.5 and 0.88 ± 1.19, respectively.
Table 2 shows the association between maternal AFB‐Lys levels and birth outcome characteristics. In unadjusted and adjusted models, controlling for maternal age, weight, pulse pressure, years of education, and gestational age at birth, maternal ln AFB‐Lys levels were significantly associated with lower birth weight (adj‐β: −0.07; 95% CI: −0.13, −0.003; p = 0.040) and lower WAZ (adj‐β: −0.16; 95% CI: −0.30, −0.01; p = 0.037) at birth.
Table 2.
Association between maternal aflatoxin exposure during pregnancy (ln AFB‐Lys levels) and birth characteristics for 220 mother‐infant pairs in Mukono district, Uganda, using unadjusted and adjusted linear regression modelsa
| Unadjusted model | Adjusted modela | |
|---|---|---|
| Weight, kg | −0.07 (−0.14, −0.002) p = 0.045 | −0.07 (−0.13, −0.003) p = 0.040 |
| Length, cm | −0.09 (−0.41, 0.24) p = 0.598 | −0.10 (−0.42, 0.22) p = 0.532 |
| Weight‐for‐age z‐score | −0.16 (−0.32, −0.006) p = 0.041 | −0.16 (−0.30, −0.01) p = 0.037 |
| Weight‐for‐length z‐score | −0.15 (−0.40, 0.10) p = 0.238 | −0.15 (−0.40, 0.11) p = 0.267 |
| Length‐for‐age z‐score | −0.06 (−0.23, 0.11) p = 0.444 | −0.07 (−0.24, 0.10) p = 0.406 |
| Head circumference, cm | −0.24 (−0.48, −0.005) p = 0.045 | −0.26 (−0.49, −0.02) p = 0.035 |
| Head circumference‐for‐age z‐score | −0.22 (−0.42, −0.02) p = 0.030 | −0.23 (−0.43, −0.03) p = 0.023 |
| Gestational age at birth, weeks | −0.11 (−0.44, 0.22) p = 0.526 | −0.07 (−0.41, 0.26) p = 0.663 |
Cells present β coefficient, 95% confidence interval, and p‐value.
Adjusted linear regression model controls for maternal age, weight, pulse pressure, and years of education in all models. Infant gestational age at birth was controlled for in all models except for when an outcome variable.
Additionally, in unadjusted and adjusted linear regression models with the same controls, maternal ln AFB‐Lys levels were significantly associated with smaller head circumference (adj‐β: ‐0.26; 95% CI: −0.49, −0.02; p = 0.035) and lower HCZ (adj‐β: ‐0.23; 95% CI: −0.43, −0.03; p = 0.023) at birth. No significant associations were observed between maternal ln AFB‐Lys levels and infant length, WLZ, LAZ, or gestational age at birth.
4. DISCUSSION
In this prospective cohort study conducted in Mukono district, Uganda, we examined the relationship between maternal aflatoxin (AFB1) exposure during pregnancy (i.e. AFB‐Lys levels measured at enrolment, or ~18‐week gestation) and adverse birth outcomes, primarily LBW. Our results showed that exposure to dietary aflatoxin during pregnancy is widespread in the population, with 100% of samples having detectable AFB‐Lys levels ranging from 0.71 to 95.60 pg/mg albumin. Although we cannot determine the main sources of dietary aflatoxin exposure from this study, we found that maternal ln AFB‐Lys levels were significantly higher in those who reported eating cassava in the previous 24 hr compared with those that did not. Although previous studies have demonstrated relatively high levels of aflatoxin in cassava in sub‐Saharan Africa (Kitya et al., 2010; Manjula, Hell, Fandohan, Abass, & Bandyopadhyay, 2009), we did not find significant differences in foods more commonly associated with aflatoxin contamination, such maize and groundnuts. Although noteworthy, the authors acknowledge the limitation of using a single 24‐hr dietary recall, which does not provide information on a typical diet at the individual level.
In both adjusted and unadjusted linear regression models, elevated maternal ln AFB‐Lys levels were significantly associated with lower birth weight, in addition to lower WAZ, smaller head circumference, and lower HCZ in infants at birth. According to our results, a 100% decrease in AFB1 exposure during pregnancy in this Ugandan population would result in infants born, on average, 70 g heavier and with 0.26 cm larger head circumference.
Although previous findings have demonstrated that aflatoxins are capable of crossing the placental barrier (Denning et al., 1990; Partanen et al., 2010) and that infant exposure is associated with poor growth outcomes (Gong et al., 2002; Gong et al., 2004; Turner et al., 2003), only a few other studies have examined the association between maternal aflatoxin exposure and adverse birth outcomes. In a prospective study of 201 women in the United Arab Emirates, aflatoxin levels measured in cord blood were significantly negatively associated with birth weight (p < 0.001; Abdulrazzaq et al., 2002). Furthermore, in a cross‐sectional study of 785 pregnant Ghanaian women, participants in the highest quartile of AFB1 exposure were more than twice as likely to have a LBW infant (OR: 2.09, 95% CI, 1.19–3.68; Shuaib et al., 2010). However, findings were inconsistent across studies. A study by Maxwell et al. reported no association between in utero aflatoxin exposure measured in cord blood samples and infant birth weight in a sample of 625 Nigerian infants (Maxwell, Familusi, Sodeinde, Chan, & Hendrickse, 1994). It is worth noting, however, that only 14.6% of serum samples in this study detected the presence of aflatoxin.
Additionally, although previous studies have established that AFB1 can cross the blood brain barrier (Qureshi et al., 2015, A. Oyelami, Maxwell, Adelusola, Aladekoma, & Oyelese, 1995), to our knowledge, this is the first human study to examine the association between maternal aflatoxin exposure during pregnancy and infant head circumference and HCZ at birth. We found a significant negative association, which is particularly noteworthy given the established association between infant head circumference and brain size (Cooke, Lucas, Yudkin, & Pryse‐Davies, 1977; H Bartholomeusz, Courchesne, & Karns, 2002) as well as cognitive ability later in life (Gale, O'callaghan, Bredow, & Martyn, 2006; Veena et al., 2010). Finally, few other studies have looked at the association between in utero aflatoxin exposure and infant length, head circumference, or gestational age at birth. The study by Shuaib et al. found no association between preterm birth (<37‐week gestation) and aflatoxin‐albumin biomarkers (Shuaib et al., 2010), which was consistent with our findings of no association.
Our findings that higher AFB‐Lys levels are positively associated with gestational age at enrolment are consistent with the findings from Castelino et al., which found a significant difference between aflatoxin‐albumin levels between early (<16 weeks) and later (>16 weeks) stages of pregnancy in the dry season in the Gambia (geometric mean: 34.5 vs. 41.8 pg/mg, p < 0.05; Castelino et al., 2014). Furthermore, they are also consistent with findings from animal models that show that pregnancy enhances the toxicological impact of AFB1 exposure. In one study, pregnant C57BL/6J mice given a single dose of AFB1 accumulated 2‐fold higher AFB 1‐N 7‐guanine DNA adducts in the liver compared with nonpregnant controls (Sriwattanapong et al., 2017).
AFB1 is metabolically activated to the toxic AFB1‐8, 9‐epoxide via various cytochrome P450 enzyme families (CYP1A2, CYP3A4, and CYP3A5; Guengerich et al., 1998). This aflatoxin‐epoxide is capable of binding to DNA, proteins, and other macromolecules, resulting in adduct formation as well as mutagenic and carcinogenic responses. In the case of some of these enzymes, pregnancy may increase their activity, causing more AFB1 to be metabolized and converted to aflatoxin‐epoxides (Tracy, Venkataramanan, Glover, & Caritis, 2005). Furthermore, the early presence of CYP3A7 in the foetal liver indicates that the fetus may be able to convert maternal transplacental AFB1 to AFB1‐8, 9‐epoxides as well (Doi, Patterson, & Gallagher, 2002; Hashimoto et al., 1995). Overall, these results suggest that pregnancy may be a window of high risk to aflatoxin exposure for pregnant women and their foetuses.
In conclusion, midgestation exposure to aflatoxin in pregnant women was significantly associated with lower birth weight, WAZ, head circumference, and HCZ in infants at birth in Uganda. Our findings suggest that interventions to reduce dietary exposure to aflatoxin may have positive effects on birth outcomes in LMICs. There were, however, several limitations to the study. AFB‐Lys levels were measured at only one point in pregnancy, and our relatively small sample size meant we were underpowered to determine associations between maternal EED biomarkers and less common adverse birth outcomes, such as spontaneous abortion and stillbirth. Moving forward, there is a need for larger, more robust studies that examine the relationship between maternal aflatoxin exposure and a diverse set of birth outcomes across different populations with high likelihoods of exposure.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
JML designed the research study, conducted the research, analysed the data, and wrote the paper; CPD, LMA, JKG, PW, and SG provided input into the study design and data analysis and contributed to the manuscript; NN and EA provided input into the study design and contributed to the manuscript; JW and KSX analysed samples and contributed to the manuscript; all authors read and approved the final manuscript.
Supporting information
Table S1: Differences in maternal aflatoxin exposure (ln AFB‐Lys levels) by foods consumed according to the 24‐hr dietary recall
ACKNOWLEDGMENTS
The authors would like to express special gratitude to the Nutrition Innovation Lab team based at Tufts University in Boston, MA, USA; the lab of Jia‐Sheng Wang at the University of Georgia in Athens, GA, USA; the team of enumerators and staff at MHC IV in Mukono, Uganda; and the study participants in Mukono, Uganda.
Lauer JM, Duggan CP, Ausman LM, et al. Maternal aflatoxin exposure during pregnancy and adverse birth outcomes in Uganda. Matern Child Nutr. 2019;15:e12701 10.1111/mcn.12701
REFERENCES
- Abdulrazzaq, Y. M. , Osman, N. , & Ibrahim, A. (2002). Fetal exposure to aflatoxins in the United Arab Emirates. Annals of Tropical Paediatrics, 22, 3–9. 10.1179/027249302125000094 [DOI] [PubMed] [Google Scholar]
- Asiki, G. , Seeley, J. , Srey, C. , Baisley, K. , Lightfoot, T. , Archileo, K. , … Routledge, M. N. (2014). A pilot study to evaluate aflatoxin exposure in a rural Ugandan population. Tropical Medicine & International Health, 19, 592–599. 10.1111/tmi.12283 [DOI] [PubMed] [Google Scholar]
- Castelino, J. M. , Dominguez‐Salas, P. , Routledge, M. N. , Prentice, A. M. , Moore, S. E. , Hennig, B. J. , … Gong, Y. Y. (2014). Seasonal and gestation‐stage associated differences in aflatoxin exposure in pregnant Gambian women. Tropical Medicine & International Health: TM & IH, 19, 348–354. 10.1111/tmi.12250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates, J. , Swindale, A. , & Bilinsky, P. (2007). Household Food Insecurity Access Scale (HFIAS) for measurement of food access: indicator guide (ed., Vol. 34). Washington, DC: Food and Nutrition Technical Assistance Project, Academy for Educational Development. [Google Scholar]
- Cooke, R. W. , Lucas, A. , Yudkin, P. L. , & Pryse‐Davies, J. (1977). Head circumference as an index of brain weight in the fetus and newborn. Early Human Development, 1, 145–149. 10.1016/0378-3782(77)90015-9 [DOI] [PubMed] [Google Scholar]
- De Vries, H. R. , Maxwell, S. M. , & Hendrickse, R. G. (1989). Foetal and neonatal exposure to aflatoxins. Acta Paediatrica Scandinavica, 78, 373–378. 10.1111/j.1651-2227.1989.tb11095.x [DOI] [PubMed] [Google Scholar]
- Denning, D. W. , Allen, R. , Wilkinson, A. P. , & Morgan, M. R. (1990). Transplacental transfer of aflatoxin in humans. Carcinogenesis, 11, 1033–1035. 10.1093/carcin/11.6.1033 [DOI] [PubMed] [Google Scholar]
- Doi, A. M. , Patterson, P. E. , & Gallagher, E. P. (2002). Variability in aflatoxin B(1)‐macromolecular binding and relationship to biotransformation enzyme expression in human prenatal and adult liver. Toxicology and Applied Pharmacology, 181, 48–59. 10.1006/taap.2002.9399 [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization, F (2016). Minimum dietary diversity for women: A guide for measurement. Rome: FAO. [Google Scholar]
- Gale, C. R. , O'callaghan, F. J. , Bredow, M. , & Martyn, C. N. (2006). The influence of head growth in fetal life, infancy, and childhood on intelligence at the ages of 4 and 8 years. Pediatrics, 118, 1486–1492. 10.1542/peds.2005-2629 [DOI] [PubMed] [Google Scholar]
- Gong, Y. , Hounsa, A. , Egal, S. , Turner, P. C. , Sutcliffe, A. E. , Hall, A. J. , … Wild, C. P. (2004). Postweaning exposure to aflatoxin results in impaired child growth: a longitudinal study in Benin, West Africa. Environmental Health Perspectives, 112, 1334–1338. 10.1289/ehp.6954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, Y. Y. , Cardwell, K. , Hounsa, A. , Egal, S. , Turner, P. C. , Hall, A. J. , & Wild, C. P. (2002). Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: cross sectional study. BMJ, 325, 20–21. 10.1136/bmj.325.7354.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groopman, J. D. , Egner, P. A. , Schulze, K. J. , Wu, L. S. , Merrill, R. , Mehra, S. , … Christian, P. (2014). Aflatoxin exposure during the first 1000 days of life in rural South Asia assessed by aflatoxin B(1)‐lysine albumin biomarkers. Food and Chemical Toxicology, 74, 184–189. 10.1016/j.fct.2014.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group, W. M. G. R. S (2006). WHO Child Growth Standards based on length/height, weight and age. Acta Paediatrica. Supplement, 450, 76–85. [DOI] [PubMed] [Google Scholar]
- Guengerich, F. P. , Johnson, W. W. , Shimada, T. , Ueng, Y.‐F. , Yamazaki, H. , & Langouët, S. (1998). Activation and detoxication of aflatoxin B1. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 402, 121–128. 10.1016/S0027-5107(97)00289-3 [DOI] [PubMed] [Google Scholar]
- H Bartholomeusz, H. , Courchesne, E. , & Karns, C. 2002. Relationship between head circumference and brain volume in healthy normal toddlers, children, and adults. [DOI] [PubMed]
- Hashimoto, H. , Nakagawa, T. , Yokoi, T. , Sawada, M. , Itoh, S. , & Kamataki, T. (1995). Fetus‐specific CYP3A7 and adult‐specific CYP3A4 expressed in Chinese hamster CHL cells have similar capacity to activate carcinogenic mycotoxins. Cancer Research, 55, 787–791. [PubMed] [Google Scholar]
- Hell, K. , Cardwell, K. F. , Setamou, M. , & Poehling, H. (2000). The influence of storage practices on aflatoxin contamination in maize in four agroecological zones of Benin, west Africa. Journal of Stored Products Research, 36, 365–382. 10.1016/S0022-474X(99)00056-9 [DOI] [PubMed] [Google Scholar]
- Hussein, H. S. , & Brasel, J. M. (2001). Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology, 167, 101–134. 10.1016/S0300-483X(01)00471-1 [DOI] [PubMed] [Google Scholar]
- Kaaya, A. N. , & Kyamuhangire, W. (2006). The effect of storage time and agroecological zone on mould incidence and aflatoxin contamination of maize from traders in Uganda. International Journal of Food Microbiology, 110, 217–223. 10.1016/j.ijfoodmicro.2006.04.004 [DOI] [PubMed] [Google Scholar]
- Kachapulula, P. W. , Akello, J. , Bandyopadhyay, R. , & Cotty, P. J. (2017). Aflatoxin contamination of groundnut and maize in Zambia: Observed and potential concentrations. Journal of Applied Microbiology, 122, 1471–1482. 10.1111/jam.13448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitya, D. , Bbosa, G. S. , & Mulogo, E. (2010). Aflatoxin levels in common foods of South Western Uganda: a risk factor to hepatocellular carcinoma. Eur J Cancer Care (Engl), 19, 516–521. 10.1111/j.1365-2354.2009.01087.x [DOI] [PubMed] [Google Scholar]
- Liu, Y. , & Wu, F. (2010). Global burden of aflatoxin‐induced hepatocellular carcinoma: A risk assessment. Environmental Health Perspectives, 118, 818–824. 10.1289/ehp.0901388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjula, K. , Hell, K. , Fandohan, P. , Abass, A. , & Bandyopadhyay, R. (2009). Aflatoxin and fumonisin contamination of cassava products and maize grain from markets in Tanzania and republic of the Congo. Toxin Reviews, 28, 63–69. 10.1080/15569540802462214 [DOI] [Google Scholar]
- Maxwell, S. M. , Familusi, J. B. , Sodeinde, O. , Chan, M. C. K. , & Hendrickse, R. G. (1994). Detection of naphthols and aflatoxins in Nigerian cord blood. Annals of Tropical Paediatrics, 14, 3–5. 10.1080/02724936.1994.11747684 [DOI] [PubMed] [Google Scholar]
- Oyelami, O. A. , Maxwell, S.M. , Adelusola, K.A. , Aladekoma, T. A. , & Oyelese, A.O. (1995). Aflatoxins in the autopsy brain tissue of children in Nigeria. [DOI] [PubMed]
- Partanen, H. A. , EL‐Nezami, H. S. , Leppanen, J. M. , Myllynen, P. K. , Woodhouse, H. J. , & Vahakangas, K. H. (2010). Aflatoxin B1 transfer and metabolism in human placenta. Toxicological Sciences, 113, 216–225. 10.1093/toxsci/kfp257 [DOI] [PubMed] [Google Scholar]
- Qian, G. , Tang, L. , Liu, W. , & Wang, J. (2010). Development of a non‐antibody method for rapid detection of serum aflatoxin B1‐lysine adduct. Toxicologist, 114, 248. [Google Scholar]
- Qian, G. , Tang, L. , Wang, F. , Guo, X. , Massey, M. E. , Williams, J. H. , … Wang, J.‐S. (2013a). Physiologically based toxicokinetics of serum aflatoxin B1‐lysine adduct in F344 rats. Toxicology, 303, 147–151. 10.1016/j.tox.2012.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, G. , Tang, L. , Wang, F. , Guo, X. , Massey, M. E. , Williams, J. H. , … Wang, J.‐S. (2013b). Physiologically based toxicokinetics of serum aflatoxin B(1)‐lysine adduct in F344 rats. Toxicology, 303, 147–151. 10.1016/j.tox.2012.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi, H. , Hamid, S. S. , Ali, S. S. , Anwar, J. , Siddiqui, A. A. , & Khan, N. A. (2015). Cytotoxic effects of aflatoxin B1 on human brain microvascular endothelial cells of the blood‐brain barrier. Medical Mycology, 53, 409–416. 10.1093/mmy/myv010 [DOI] [PubMed] [Google Scholar]
- Sabbioni, G. , Skipper, P. L. , Büchi, G. , & Tannenbaum, S. R. (1987). Isolation and characterization of the major serum albumin adduct formed by aflatoxin B 1 in vivo in rats. Carcinogenesis, 8, 819–824. 10.1093/carcin/8.6.819 [DOI] [PubMed] [Google Scholar]
- Shirima, C. P. , Kimanya, M. E. , Routledge, M. N. , Srey, C. , Kinabo, J. L. , Humpf, H. U. , … Gong, Y. Y. (2015). A prospective study of growth and biomarkers of exposure to aflatoxin and fumonisin during early childhood in Tanzania. Environmental Health Perspectives, 123, 173–178. 10.1289/ehp.1408097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuaib, F. M. , Jolly, P. E. , Ehiri, J. E. , Yatich, N. , Jiang, Y. , Funkhouser, E. , … Williams, J. H. (2010). Association between birth outcomes and aflatoxin B1 biomarker blood levels in pregnant women in Kumasi, Ghana. Tropical Medicine & International Health, 15, 160–167. 10.1111/j.1365-3156.2009.02435.x [DOI] [PubMed] [Google Scholar]
- Sriwattanapong, K. , Slocum, S. L. , Chawanthayatham, S. , Fedeles, B. I. , Egner, P. A. , Groopman, J. D. , … Essigmann, J. M. (2017). Editor's highlight: Pregnancy alters aflatoxin B1 metabolism and increases DNA damage in mouse liver. Toxicological Sciences, 160, 173–179. 10.1093/toxsci/kfx171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy, T. S. , Venkataramanan, R. , Glover, D. D. , & Caritis, S. N. (2005). Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A Activity) during pregnancy. American Journal of Obstetrics and Gynecology, 192, 633–639. 10.1016/j.ajog.2004.08.030 [DOI] [PubMed] [Google Scholar]
- Turner, P. C. , Collinson, A. C. , Cheung, Y. B. , Gong, Y. , Hall, A. J. , Prentice, A. M. , & Wild, C. P. (2007). Aflatoxin exposure in utero causes growth faltering in Gambian infants. International Journal of Epidemiology, 36, 1119–1125. 10.1093/ije/dym122 [DOI] [PubMed] [Google Scholar]
- Turner, P. C. , Moore, S. E. , Hall, A. J. , Prentice, A. M. , & Wild, C. P. (2003). Modification of immune function through exposure to dietary aflatoxin in Gambian children. Environmental Health Perspectives, 111, 217–220. 10.1289/ehp.5753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veena, S. R. , Krishnaveni, G. V. , Wills, A. K. , Kurpad, A. V. , Muthayya, S. , Hill, J. C. , … Srinivasan, K. (2010). Association of birthweight and head circumference at birth to cognitive performance in 9‐10 year old children in South India: Prospective birth cohort study. Pediatric Research, 67, 424–429. 10.1203/PDR.0b013e3181d00b45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw, T. M. (2004). Low birthweight: Country, regional and global estimates Unicef. [Google Scholar]
- Wild, C. P. , Hudson, G. J. , Sabbioni, G. , Chapot, B. , Hall, A. J. , Wogan, G. N. , … Groopman, J. D. (1992). Dietary intake of aflatoxins and the level of albumin‐bound aflatoxin in peripheral blood in The Gambia, West Africa. Cancer Epidemiology, Biomarkers & Prevention, 1, 229–234. [PubMed] [Google Scholar]
- Williams, J. H. , Phillips, T. D. , Jolly, P. E. , Stiles, J. K. , Jolly, C. M. , & Aggarwal, D. (2004). Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. The American Journal of Clinical Nutrition, 80, 1106–1122. 10.1093/ajcn/80.5.1106 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Differences in maternal aflatoxin exposure (ln AFB‐Lys levels) by foods consumed according to the 24‐hr dietary recall


