Abstract
Oesophageal squamous cell carcinoma (ESCC) has markedly high incidence rates in Kenya and much of East Africa, with a dire prognosis and poorly understood aetiology. Consumption of hot beverages—a probable carcinogen to humans—is associated with increased ESCC risk in other settings and is habitually practiced in Kenya. We conducted a case–control study in Eldoret, western Kenya between August 2013 and March 2018. Cases were patients with endoscopically confirmed oesophageal cancer whose histology did not rule out ESCC. Age and sex‐matched controls were hospital visitors and hospital out and in‐patients excluding those with digestive diseases. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated for self‐reported drinking temperatures; consumption frequency; mouth burning frequency and hot porridge consumption using logistic regression models adjusted for potential confounders. Drinking temperature association with tumour sub‐location was also investigated. The study included 430 cases and 440 controls. Drinkers of ‘very hot’ and ‘hot’ beverages (>95% tea) had a 3.7 (95% CI: 2.1–6.5) and 1.4‐fold (1.0–2.0) ESCC risk, respectively compared to ‘warm’ drinkers. This trend was consistent in males, females, never and ever alcohol/tobacco and was stronger over than under age 50 years. The tumour sub‐location distribution (upper/middle/lower oesophagus) did not differ by reported drinking temperature. Our study is the first comprehensive investigation in this setting to‐date to observe a link between hot beverage consumption and ESCC in East Africa. These findings provide further evidence for the role of this potentially modifiable risk factor in ESCC aetiology.
Keywords: oesophageal cancer, hot beverages, Africa, aetiology
Short abstract
What's new?
Oesophageal squamous cell carcinoma (ESCC) has markedly high incidence rates in Kenya and much of East Africa, with a dire prognosis and poorly understood aetiology. Hot beverage consumption – a probable carcinogen to humans – is associated with increased ESCC risk in other settings. In this first comprehensive case‐control study to investigate the role of hot beverages in esophageal cancer in Kenya and East Africa, the authors find overall risks of 3.7 and 1.4‐fold for drinking “very hot” and “hot” beverages, respectively, compared to “warm” These findings will contribute to local and global prevention efforts by addressing this potentially modifiable risk factor.
Abbreviations
- ASR
age‐standardised rate
- CI
confidence interval
- EC
oesophageal cancer
- ESCC
oesophageal squamous cell carcinoma
- IARC
International Agency for Research on Cancer
- MTRH
Moi Teaching and Referral Hospital
- OR
odds ratio
Introduction
Oesophageal cancer (EC) is the 8th commonest cancer and the 6th highest cause of cancer deaths worldwide,1 with some of the most striking contrasts in incidence of all cancers; both between and within countries. In addition to the Asian high incidence belt,2 an East African corridor has been documented,3 albeit less well studied, for more than 50 years.4 In Kenya, EC—predominantly oesophageal squamous cell carcinoma (ESCC)—is the third most common cancer in both sexes.1 Age‐standardised incidence rates (ASR) were estimated at 20.5 and 15.1 in men and women, respectively in 2012.1 The East African EC burden is also characterised by an unusually high number of young cases.5 Given the dire prognosis of EC in this and other settings, primary prevention through risk factor identification is a priority, but a scarcity of comprehensive etiological studies has persisted in Kenya and the wider East African region to‐date.
A recent review3 of putative EC risk factors concluded that many factors established elsewhere (e.g. alcohol and tobacco consumption) are also prevalent in African populations. This includes the consumption of very hot beverages—evaluated in relation to EC as ‘probably carcinogenic to humans’ (Group 2A) by the International Agency for Research on Cancer (IARC) in 20166, 7 and suspected since as early as 1939 of increasing EC risk.8 Compelling evidence for IARC's evaluation came from case–control studies, such as those conducted in the ESCC hotspot in Iran's Golestan Province,9 where an eightfold risk for drinking very hot compared to warm tea was found; and in South America, where an increased risk was found in relation very hot maté consumption.10 Since the evaluation, further evidence has emerged from a Chinese prospective cohort11 in which the hazard ratio for men who drank ‘burning hot’ tea compared to less than weekly drinkers was 1.55 and an interaction with alcohol was found: a hazard ratio of 5 when in combination with >15 g/day of alcohol and compared to less than weekly drinkers and consumers of <15 g/day of alcohol.11
Hot beverage consumption may be of particular relevance to the East African EC burden due to widespread habitual consumption. In Kenya, a traditional African tea is prepared in a 1:1 mixture of boiled water and full‐fat milk and served from pots on hot coals or thermal flasks. In a cross‐sectional study12 of measured beverage temperatures conducted in the high‐ESCC incidence community encircling Tanzania's Mount Kilimanjaro, the mean temperature at first sip—72 °C—was among the highest yet to be recorded worldwide. Participants recalled first consuming hot beverages at a mean age of 2 years and slower cooling rates were observed for milky tea. In a small (n = 159 cases; 159 controls) case–control study13 previously conducted in Eldoret, Kenya, an odds ratio of 12.78 was reported for ‘preferring’ hot drinks before disease onset.
In the present article we examined associations of self‐reported hot beverage temperatures and drinking habits with ESCC from the western Kenya ESCC African Prevention Research (ESCCAPE) case–control study.14 We additionally investigated the effect of drinking temperatures on the anatomical sub‐locations (i.e. the upper, lower or middle portion) of ESCC tumours—not previously investigated in relation to this risk factor.
Materials and Methods
Ethics approval and consent to participate
The study was approved by the Institutional Research and Ethics Committee of Moi University (000921) and the IARC Ethics Committee (IEC 14–15). Written informed consent was sought after a full explanation of the study to participants, who received no payment for their involvement, but for cases the 1,000 Kenyan shillings fee for histological results was funded.
Study design
An EC case–control study was conducted at the Moi Teaching and Referral Hospital (MTRH) in the town of Eldoret in western Kenya.14 The hospital's catchment area covers >10 counties and extends 150 km to the Kenya‐Uganda border. The study was conducted in two funding phases, with negligible protocol changes for the present analyses: a pilot phase (August 2013–September 2014) and a main study phase (October 2015–March 2018). The case definition was patients aged ≥18 years presenting with suspected incident first primary EC at the MTRH endoscopy unit where a pinch biopsy was taken during oesophagogastroduodenoscopy and whose histology subsequently confirmed (90%) or did not rule out ESCC (10%, e.g. tumour visualised at endoscopy but biopsy specimen was insufficient for evaluation). During endoscopy, the sub‐locations of visualised tumours were recorded (main study phase only) in centimetres (distance from incisors) and categorised as follows: cervical (proximal) oesophagus (15–19 cm); upper thoracic (20–24 cm); middle thoracic (25–29 cm); lower thoracic (30–39 cm); oesophageal‐gastro junction (40+ cm).
Controls were selected from adults aged ≥18 years who also made the journey to MTRH, i.e. 20% were hospital visitors and 80% hospital in and out‐patients, recruited from MTRH out‐patients, surgery, medical, orthopaedic and ophthalmology wards. Patients were not eligible if attending for digestive disease or cancer, or their hospital stay extended more than three nights—the latter to prevent normal dietary habits from changing sufficiently and being reflected in short term biospecimens. Controls were frequency matched by age and sex to the observed distribution of cases and approached at random during long waiting times during which they could complete the study.
Recruitment of at least 400 cases and 400 controls was targeted; enabling 80% power to detect odds ratios of 1.5 for exposures with 30% prevalence. Participation rates were 96% and 92% in cases and controls, respectively. Population representativeness of controls was examined by comparing their sociodemographic characteristics with those of indirectly age and sex standardised data from the 2014 Kenya Demographic Health Survey (KDHS).15
Questionnaire and exposure assessment
Consenting participants completed a face‐to‐face interview in their appropriate vernacular with trained interviewers, provided biospecimens and had anthropometric measurements taken. Data were immediately entered into a tablet with a preloaded Open Data Kit questionnaire. Across both study phases, participants were asked which hot beverage they consume most frequently (tea; coffee or other); the temperature at which they consume it (very hot; hot; warm; cold) and the number of cups/glasses per day (typically 200–300 ml serving sizes). In the main study phase, participants were additionally asked to estimate the frequency that they burn their mouths when drinking hot beverages (often; sometimes; never; do not know) as this has previously shown to be correlated with drinking temperatures;12 whether they consume hot porridge and the temperature at which they consume it (very hot; hot; warm; cold). Extensive data were collected on confounders and potential effect modifiers. For the present analyses, we included age, sex, ethnicity, education, family history of oesophageal cancer, alcohol consumption, tobacco use (smoking and smokeless)—the exact definitions of which are provided in results.
Statistical analysis
Measures of the exposure of interest—hot beverage consumption, were: drinking temperature (very hot; hot; warm/cold), number of cups/servings per day (1; 2; 3; 4+); frequency of mouth burning (often; sometimes; never/not known) and whether hot porridge is consumed (yes; no). Odds ratios (ORs) and their 95% confidence intervals (CI) of EC associated with each exposure were estimated using logistic regression models. Reference categories with a robust number of controls were selected. In Model 1 (minimal adjustment), ORs were first estimated with adjustment for interviewer, phase (pilot/main, where applicable) and the design factors of sex (binary) and age (continuous) to account for small imbalances in age/sex frequency matching. Thereafter, Model 2 (full adjustment) was further adjusted for family history of EC (yes; no; unknown), education level (none; primary; secondary), alcohol and tobacco usage (ever/never), tobacco usage (smokes/chews per day) and grams of ethanol per day (0; 1–19; 20–49; 50–99; 100+). Missing data on any of these confounders were rare (<2%), and were included using a missing category. Chi‐squared tests were used to investigate associations of possible confounders with beverage drinking temperatures, as well as associations of drinking temperatures with tumour anatomic sub‐locations. The study was not sufficiently powered to investigate interactions, such as that observed between hot beverages and alcohol consumption;11 however, we did stratify by ever tobacco/alcohol use. Statistical analyses and graphics were generated using R version 3.4.3 (base package) with the RStudio GUI.
Results
Participant and exposure characteristics
After the exclusion of 36 oesophageal adenocarcinomas and one each of Kaposi sarcoma, leiomyoma and papilloma, 430 cases and 440 controls were included. Their characteristics are shown in Table 1. The majority of participants were of Kalenjin (55%) or Luhya (22%) ethnicity and of Christian faith. A male: female ratio of 1.94 was observed among cases (66% male), with a mean age at diagnosis of 59 years and 7% below age 40. Comparable distributions of religion and education between controls and KDHS data have been presented previously for our study population.16 Geocoded residential locations of cases and controls occupied a comparable spatial extent—the median distance to MTRH was 54 km and 47 km, respectively.
Table 1.
Demographic and exposure characteristics of cases and controls
| Characteristics | Cases n= (column %) | Controls n= (column %) |
|---|---|---|
| Study phase1 | ||
| Pilot | 143 (33) | 155 (35) |
| Main | 287 (67) | 285 (65) |
| Sex2 | ||
| Male | 282 (66) | 272 (62) |
| Female | 148 (34) | 168 (38) |
| Age2 (years) at diagnosis/interview | ||
| Mean (SD) | 59 (14) | 57 (15) |
| IQR | 50–69 | 45–68 |
| Ethnic group | ||
| Kalenjin | 247 (58) | 233 (53) |
| Luhya | 100 (23) | 95 (22) |
| Other | 83 (19) | 112 (25) |
| Religion | ||
| Protestant | 244 (57) | 282 (64) |
| Catholic | 155 (36) | 140 (32) |
| Muslim | 2 (<1) | 4 (<1) |
| None | 27 (6) | 12 (3) |
| Other/No answer | 2 (<1) | 2 (<1) |
| Education (score) | ||
| None (1) | 104 (24) | 99 (23) |
| Some primary (2) | 163 (38) | 110 (25) |
| Complete primary (3) | 85 (20) | 91 (21) |
| Some (4)/ Complete secondary (5) | 59 (14) | 85 (19) |
| Technical college (6)/ University (7) | 19 (4) | 54 (12) |
| Mean (SD) education score | 2.5 (1.4) | 3.0 (1.7) |
| EC family history | ||
| Yes | 30 (7) | 21 (5) |
| No | 381 (89) | 406 (92) |
| Not known | 19 (4) | 13 (3) |
| Most frequently consumed beverage | ||
| Tea | 423 (98) | 419 (95) |
| Coffee | 2 (<1) | 2 (<1) |
| Other: | 5 (1) | 19 (4) |
| Porridge | 4 | 14 |
| Cocoa/Drinking chocolate | 1 | 1 |
| Milk | 0 | 1 |
| Soya | 0 | 3 |
| Drinking temperature (of most frequent beverage) | ||
| Very hot | 91 (21) | 30 (7) |
| Hot | 252 (59) | 298 (68) |
| Warm | 87 (20) | 112 (25) |
| Number consumed (cups/servings/day)3 | ||
| Mean (SD) | 3 (1.7) | 3 (1.5) |
| Range | 1–12 | 1–12 |
| Frequency of mouth burning from hot beverage4 | ||
| Often | 8 (3) | 9 (3) |
| Sometimes | 159 (55) | 137 (48) |
| Never | 110 (38) | 129 (46) |
| Not known | 10 (4) | 9 (3) |
| Whether hot porridge consumed4 | ||
| Yes | 175 (61) | 196 (69) |
| No | 112 (39) | 87 (31) |
| Not known | 0 | 2 (<1) |
Values refer to both phases unless otherwise stated;
Frequency‐matched design factors;
approximately 250 ml for most people (see methods);
Asked to main phase participants only.
A mean consumption frequency of three servings per day (i.e. ∼750 ml/day) was found for respondents’ preferred beverages; of which tea was the most frequently consumed beverage by cases (98%) and controls (95%). Hot porridge was additionally consumed by a majority of participants in the main study phase (61% in cases and 69% in controls), but data on porridge consumption temperatures were incomplete. Mouth burning was reported to occur at least sometimes in >50% of main phase study participants. Among controls (both study phases), the prevalence of very hot, hot and warm drinking temperature was 7%, 68% and 25%, respectively. A higher prevalence of hot drinkers was found in men (p = 0.03) and lower prevalences of very hot and hot drinkers were found among female ever tobacco users (p < 0.01). Drinking temperatures did not differ by any other of the potential confounders investigated (Table 2).
Table 2.
Beverage drinking temperatures in controls, overall and by potential confounding factors
| Category | Beverage temperature distribution, n (row %1) | p Value2 | ||
|---|---|---|---|---|
| Very hot | Hot | Warm | ||
| All | 30 (7) | 298 (68) | 112 (25) | ‐ |
| Sex | ||||
| Men | 15 (6) | 197 (72) | 60 (22) | 0.03 |
| Women | 15 (9) | 101 (60) | 52 (31) | |
| Age (years) at interview | ||||
| 18‐ < 40 | 7 (13) | 37 (67) | 11 (20) | 0.45 |
| 40‐ < 50 | 7 (9) | 53 (66) | 20 (25) | |
| 50‐ < 60 | 6 (5) | 81 (72) | 26 (23) | |
| 60‐ < 70 | 7 (7) | 62 (63) | 29 (30) | |
| 70+ | 3 (3) | 65 (69) | 26 (28) | |
| Ethnicity | ||||
| Kalenjin | 19 (8) | 151 (65) | 63 (27) | 0.11 |
| Luhya | 3 (3) | 75 (79) | 17 (18) | |
| Other | 8 (7) | 72 (64) | 32 (29) | |
| Education | ||||
| None | 3 (3) | 71 (72) | 25 (25) | 0.43 |
| Some primary | 15 (8) | 132 (65) | 55 (27) | |
| Secondary or higher | 12 (9) | 95 (68) | 32 (23) | |
| EC family history | ||||
| Yes | 2 (10) | 16 (76) | 3 (14) | 0.45 |
| No | 26 (6) | 274 (68) | 106 (26) | |
| Tobacco use (men) | ||||
| Ever | 9 (8) | 74 (67) | 27 (25) | 0.17 |
| Never | 6 (4) | 123 (76) | 33 (20) | |
| Tobacco use (women) | ||||
| Ever | 2 (7) | 10 (37) | 15 (56) | <0.01 |
| Never | 13 (9) | 91 (65) | 37 (26) | |
| Alcohol consumption (men) | ||||
| Ever | 7 (5) | 105 (71) | 36 (24) | 0.54 |
| Never | 8 (7) | 92 (74) | 24 (19) | |
| Alcohol consumption (women) | ||||
| Ever | 2 (5) | 23 (53) | 18 (42) | 0.15 |
| Never | 13 (10) | 78 (63) | 34 (27) | |
Percentages are of non‐missing values. Data were complete except for ES family history (13 missing responses) and education (1 missing response).
p Values from chi‐squared tests.
Relative risks
Odds ratios (ORs) of ESCC associated with various hot beverage exposure metrics are shown in Table 3. In the minimally adjusted model, ‘very hot’ and ‘hot’ drinkers (of most frequently consumed beverage) had a 3.17‐ and 1.17‐fold increased ESCC risk, respectively compared to ‘warm’ drinkers. These ORs increased to 3.66 and 1.40 in the fully adjusted model, due to the adjustment for the confounding effect of lower drinking temperatures among female smokers. Sub‐group analyses were conducted by different demographic and exposure groups and ORs and 95% CIs are plotted in Figure 1. Overall, the same trend was observed across all participant sub‐groups, but ORs for ‘very hot’ were notably higher in over 50s (5.68; 95% CI: 2.68, 12.10) compared to under 50s (1.49; 95% CI: 0.54, 4.16). No significant difference in consumption frequency was observed between under and over 50s (chi‐squared—p = 0.22).
Table 3.
Odds ratios (OR) and 95% confidence intervals (CI) for the association of hot beverage consumption factors (temperature, frequency of consumption, frequency of mouth burning and porridge consumption) with oesophageal cancer risk in Kenya
| Model 1—minimal adjustment1 | Model 2—full adjustment2 | |||
|---|---|---|---|---|
| Exposure variable | Categories/unit | Number of cases/controls | OR (95% CI) | OR (95% CI) |
| Drinking temperature (of most frequent beverage) | Warm | 87/112 | 1 | 1 |
| Hot | 252/298 | 1.17 (0.83, 1.64) | 1.40 (0.97, 2.03) | |
| Very hot | 91/30 | 3.17 (1.91, 5.37) | 3.66 (2.10, 6.50) | |
| Number consumed (cups or servings/day) | All | |||
| 1 | 31/40 | 0.84 (0.49, 1.43) | 0.82 (0.45, 1.45) | |
| 2 | 167/153 | 1 | 1 | |
| 3 | 135/139 | 0.98 (0.70, 1.38) | 0.95 (0.66, 1.37) | |
| 4+ | 97/108 | 1.03 (0.71, 1.49) | 1.00 (0.67, 1.49) | |
| Very hot/hot drinkers | ||||
| 1 | 19/28 | 0.76 (0.39, 1.47) | 0.73 (0.35, 1.49) | |
| 2 | 133/115 | 1 | 1 | |
| 3 | 113/105 | 1.05 (0.71, 1.53) | 1.05 (0.69, 1.59) | |
| 4+ | 78/80 | 1.06 (0.70, 1.62) | 1.05 (0.67, 1.67) | |
| Warm drinkers | ||||
| 1 | 12/12 | 1.27 (0.48, 3.37) | 1.41 (0.49, 4.15) | |
| 2 | 34/38 | 1 | 1 | |
| 3 | 22/34 | 0.80 (0.39, 1.65) | 0.75 (0.33, 1.71) | |
| 4+ | 19/28 | 0.97 (0.44, 2.12) | 1.02 (0.43, 2.45) | |
| Frequency of mouth burning from hot beverage3 | Never/not known | 120/138 | 1 | 1 |
| Sometimes | 159/137 | 1.24 (0.88, 1.76) | 1.19 (0.80, 1.76) | |
| Often | 8/9 | 0.99 (0.36, 2.72) | 0.51 (0.16, 1.66) | |
| Whether hot porridge consumed3 | No | 112/87 | 1 | 1 |
| Yes | 175/196 | 0.66 (0.46, 0.94) | 0.62 (0.41, 0.91) | |
Minimal adjustment: age (continuous); sex; study phase; interviewer (all binary).
Full adjustment: additionally adjusted for tobacco and alcohol consumption (ever/never); estimated grams of ethanol per day; family history of EC; education level (all categorical) and number of daily tobacco smokes/chews (continuous).
Available for main study participants only.
Figure 1.
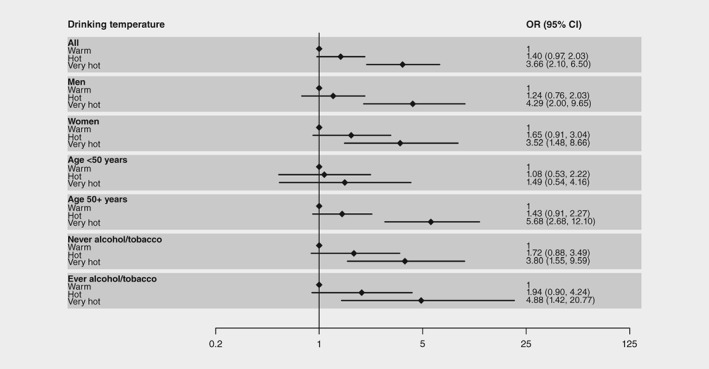
Odds ratios (OR) and 95% confidence intervals (CI) for the association of self‐reported drinking temperatures with oesophageal cancer risk in Kenya—overall and by subgroup.
Aside from self‐reported drinking temperatures, no other hot beverage exposure metrics yielded statistically significant positive associations with ESCC (Table 3). For consumption frequency, compared to drinking 2+ servings per day, we found a decreased OR for drinking <2 servings per day, which was more evident when the analysis was restricted to very hot/hot drinkers (OR: 0.73; 95% CI: 0.35, 1.49), though the confidence interval was wide. The OR for ‘sometimes’ burning the mouth when drinking was 1.24 (0.88, 1.76) compared to never/not known, but no increased OR was seen for the few participants who reported ‘often’ burning the mouth. A significant inverse association was found for consuming hot porridge compared to not (OR: 0.62; 95% CI: 0.41, 0.91).
Sensitivity analyses
We conducted a sensitivity analysis for associations of self‐reported drinking temperatures with ESCC risk and the positive association was consistently present across study phases and control participant type (data not shown). However, we observed a potential interview bias, as ORs were of different magnitude between the two interviewers. Upon further inspection of the data and discussions with interviewers, we attributed these differences to difficulties experienced by participants when self‐reporting beverage temperatures, namely distinguishing between ‘very hot’ and ‘hot’ temperatures. Interviewers needed to elaborate the question further to obtain answers and, in doing so, adopted different probing techniques, thus investigating slightly different aspects of drinking habits. One interviewer elaborated to ask whether tea was drank straight from the jiko (stove) and their ORs were 8.49 (95% CI: 3.88, 19.49) and 1.97 (95% CI: 1.06, 3.73). The other interviewer elaborated to ask whether tea was sipped slowly or gulped and their ORs were 1.79 (95% CI: 0.63, 5.03) and 1.27 (95% CI: 0.78, 2.09) for ‘very hot’ and ‘hot’, respectively. No systematic differences were detected in other sections of the interview and the age and sex distributions of participants did not significantly differ between interviewers (p = 0.21 and 0.76 respectively).
Tumour sub‐location in relation to drinking temperature
Tumour sub‐locations within the oesophagus were investigated by drinking temperature (available for 250 main phase study cases). The following frequencies of tumours at each sub‐location were determined: cervical (proximal) oesophagus: n = 10; upper thoracic: n = 40; middle thoracic: n = 82; lower thoracic: n = 110; oesophageal‐gastro junction n = 8. Drinking temperatures in relation to sub‐locations (broadly grouped into upper; middle and lower tumours) are shown in Table 4 and no relationship was observed (p = 0.91). Performing the analysis separately by interviewer did not change these findings.
Table 4.
Beverage drinking temperatures by tumour sub‐location for main phase oesophageal cancer cases
| Tumour location | Beverage temperature distribution, n (row %) | p Valuea | ||
|---|---|---|---|---|
| Very hot | Hot | Warm | ||
| Upper (0–24 cm) | 10 (20) | 31 (62) | 9 (18) | 0.91 |
| Middle (25–29 cm) | 22 (27) | 44 (54) | 16 (19) | |
| Lower (30+ cm) | 33 (28) | 68 (58) | 17 (14) | |
| Missing | 10 (27) | 21 (57) | 6 (16) | |
p Values from chi‐squared tests.
Discussion
This paper reports on outcomes from the first comprehensive case–control study to assess the consumption of hot beverages in relation to ESCC risk in Kenya. Overall, we found progressively increased ESCC risks associated with drinking ‘hot’ and ‘very hot’ beverages (predominantly tea) relative to ‘warm’ beverages. This trend held true across subgroups of age, sex and tobacco/alcohol consumption. However, uncertainty remains over the magnitude of risk due to difficulties in participant self‐recall and drinking temperature categorisation. There was no evidence that the thermal injury‐ESCC risk differed by ESCC anatomical sub‐location.
Our findings are consistent with numerous previous investigations17 into the role of hot beverage consumption and ESCC risk, such as a case–control study conducted in Iran9 and a cohort study in China.11 While habitual hot tea consumption is widespread in Kenya and other parts of East Africa,12 the volumes reported in the present study, a mean of ~750 ml/day, were considerably lower than those typically found in Iran,9 where hot beverages may play a larger role in the local burden. Nevertheless, the findings provide further evidence that hot beverage consumption likely contributes to ESCC risk, including in East African populations.
Our study was subject to several commonly cited limitations. Firstly, as with all questionnaire‐oriented case–control study designs, recall bias may have influenced outcomes. Assessments of hot beverage consumption may be particularly susceptible to recall bias due to the subjective nature of what is perceived as ‘very hot’, ‘hot’ or ‘warm’, which may vary between individuals even at the same temperature and possibly also due to the pre‐perceived risk intuitively associated with this exposure. Similarly, the retrospective exposure assessment may have led to difficulties in the recall of past exposures, particularly for cases who no longer drink hot beverages due to dysphagia. Self‐reported drinking temperature classifications, such as those used in the present study, have also been questioned previously and were a limitation cited in the IARC Monograph carcinogenicity evaluation of very hot beverages.6 The non‐objective nature of this exposure assessment was also of particular concern in the present study, potentially resulting in uncertainty in the magnitude but not direction of the association. Difficulty in distinguishing more than two exposure categories, i.e. the difference between ‘very hot’ and ‘hot’, led to different exposure profiles between interviewers, unless this was due to chance. This reiterates a considerable limitation in this method of exposure assessment. Other question variations should be included in future studies, such as asking participants to estimate how long they wait before drinking, with shorter waiting times having been associated with increased ECCC risk in a previous case–control study.9
In considering whether our findings are biologically plausible, we note the apparent lack of association in under 50 year olds, though that stratum had few participants (107 cases and 135 controls). A defining characteristic of the African EC burden is the relatively high number of young (i.e. <50 years) cases and we have observed an early onset of hot beverage consumption in both a previous cross‐sectional study of measured tea temperatures in Tanzania (mean 2 years at first drinking)12 and in similar ongoing fieldwork in western Kenya. However, in the latter study, we observed a practice called ‘poesha’, whereby a child's hot beverage is poured back and forth between two cups in long streams to cool the liquid before it is drunk. The abovementioned findings suggest that the onset of exposure to thermal injury may occur later in life in this setting; an exposure duration not sufficient to increase ESCC risk at young ages, but further studies will be needed to address these questions. Furthermore, larger pooled studies will be required to address whether hot tea is a stronger risk factor in drinkers, as found in a Chinese cohort,11 given that a large contribution to the Kenyan ESCC burden is attributable to alcohol, namely consumption of locally produced beer ‘busaa’ and spirits such as ‘changaa’ in men. Investigating the possible interaction of hot beverages with other carcinogens is also warranted.
The present findings have profound implications for public health and cancer prevention in Kenya, the wider East African region and beyond. They are particularly pertinent in high‐risk ESCC populations where hot beverage/food consumption is habitual and widespread, e.g. where tea is consumed to stay warm at high altitude in Rift Valley communities;3 and daily consumers of hot porridge—‘genfo’—a dietary staple in Ethiopia.18 Hot beverage consumption exemplifies a risk factor that is potentially modifiable in the community through effective communications strategies aimed at encouraging people to wait for a sufficient time for their beverages to cool. What constitutes ‘sufficient’ is a subject of future research as a required element of any prevention strategy is to define ‘sufficiently cool’ to avoid harm, while at the same time remaining sufficiently hot to maintain the benefits of keeping warm.
In conclusion, our study provides further evidence of the role of hot beverage consumption—a potentially modifiable risk factor—in ESCC from the first comprehensive analytical study to be conducted in the East African EC corridor. Further important research avenues will be to seek mechanistic evidence of hot beverage carcinogenicity and develop objective exposure assessment protocols for use in case–control studies.
Acknowledgements
This work was supported by the International Agency for Research on Cancer (IARC), including an IARC post‐doctoral fellowship to D. Middleton partially supported by the European Commission FP7 Marie Curie Actions – People – Co‐funding of regional, national and international programs (COFUND). The authors are grateful to all study participants.
Contributor Information
Daniel RS Middleton, Email: middletond@fellows.iarc.fr.
Diana Menya, Email: dianamenya@gmail.com.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–E86. [DOI] [PubMed] [Google Scholar]
- 2. Lin Y, Totsuka Y, He Y, et al. Epidemiology of esophageal cancer in Japan and China. J Epidemiol 2013;23:233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McCormack VA, Menya D, Munishi MO, et al. Informing etiologic research priorities for squamous cell esophageal cancer in Africa: a review of setting‐specific exposures to known and putative risk factors. Int J Cancer 2016;140:259–71. doi: 10.1002/ijc.30292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGlashan ND. Oesophageal cancer and alcoholic spirits in Central Africa. Gut 1969;10:643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dawsey SP, Tonui S, Parker RK, et al. Esophageal cancer in young people: a case series of 109 cases and review of the literature. PLoS One 2010;5:e14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Loomis D, Guyton KZ, Grosse Y, et al. Carcinogenicity of drinking coffee, mate, and very hot beverages. Lancet Oncol 2016;17:877–8. [DOI] [PubMed] [Google Scholar]
- 7. IARC . Drinking Coffee, Mate, and Very Hot Beverages / IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (2016: Lyon, France) (IARC monographs on the evaluation of carcinogenic risks to humans ; volume 116). Lyon, France: International Agency for Research on Cancer, 2018. [PubMed] [Google Scholar]
- 8. Watson W. Cancer of the esophagus: some etiological considerations. Am J Roentgenol 1939;14:420–4. [Google Scholar]
- 9. Islami F, Pourshams A, Nasrollahzadeh D, et al. Tea drinking habits and oesophageal cancer in a high risk area in northern Iran: population based case‐control study. BMJ 2009;338:b929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lubin JH, De SE, Abnet CC, et al. Mate drinking and esophageal squamous cell carcinoma in South America: pooled results from two large multicenter case‐control studies. Cancer Epidemiol Biomarkers Prev 2014;23:107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yu C, Tang H, Guo Y, et al. Effect of hot tea consumption and its interactions with alcohol and tobacco use on the risk for esophageal cancer. Ann Intern Med 2018;168:489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Munishi MO, Hanisch R, Mapunda O, et al. Africa's oesophageal cancer corridor: do hot beverages contribute? Cancer Causes Control 2015;26:1477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patel K, Wakhisi J, Mining S, et al. Esophageal cancer, the topmost cancer at MTRH in the Rift Valley, Kenya, and its potential risk factors. ISRN Oncol 2013;2013:503249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. D Menya NK, Oduor M, Maina SK, et al. Cancer Epidemiology fieldwork in a resource‐limited setting: experience in the ESCCAPE esophageal cancer case‐control study in Western Kenya. Cancer Epidemiol 2018;57:45–52. doi: 10.1016/j.canep.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenya National Bureau of Statistics, Ministry of Health/Kenya, National AIDS Control Council/Kenya, Kenya Medical Research Institute, Population NCf, Development/Kenya. Kenya Demographic and Health Survey 2014. Rockville, MD, USA; 2015.
- 16. D Menya NK, Oduor M, Maina SK, et al. Traditional and commercial alcohols and esophageal cancer risk in Kenya. Int J Cancer 2018. doi: 10.1002/ijc.31804. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Islami F, Boffetta P, Ren JS, et al. High‐temperature beverages and foods and esophageal cancer risk‐‐a systematic review. Int J Cancer 2009;125:491–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leon ME, Assefa M, Kassa E, et al. Qat use and esophageal cancer in Ethiopia: a pilot case‐control study. PLoS One 2017;12:e0178911. [DOI] [PMC free article] [PubMed] [Google Scholar]


