Abstract
Background
One of the primary biomechanical factors influencing arterial health is their deformation across the cardiac cycle, or cyclic strain, which is often associated with arterial stiffness. Deleterious changes in the cardiovascular system, e.g., increased arterial stiffness, can remain undetected until the system is challenged, such as under a cardiac stressor like dobutamine.
Purpose
To quantify cyclic strain in mice at different locations along the arterial tree prior to and during dobutamine infusion, while evaluating the effects of sex and age.
Study Type
Control/cohort study.
Animal Model
Twenty C57BL/6 mice; male, female; ∼12 and 24 weeks of age; n = 5 per group.
Field Strength/Sequence
7T; CINE MRI with 12 frames, velocity compensation, and prospective cardiac gating.
Assessment
Prior to and during the infusion of dobutamine, Green–Lagrange circumferential cyclic strain was calculated from perimeter measurements derived from CINE data acquired at the carotid artery, suprarenal and infrarenal abdominal aorta, and iliac artery.
Statistical Tests
Analysis of variance (ANOVA) followed by post‐hoc tests was used to evaluate the influence of dobutamine, anatomical location, sex, and age.
Results
Heart rates did not differ between groups prior to or during dobutamine infusion (P = 0.87 and P = 0.08, respectively). Dobutamine increased cyclic strain in each group. Within a group, increases in strain were similar across arteries. At the suprarenal aorta, strain was reduced in older mice at baseline (young 27.6 > mature 19.3%, P = 0.01) and during dobutamine infusion (young 53.0 > mature 36.2%, P = 0.005). In the infrarenal aorta, the response (dobutamine – baseline) was reduced in older mice (young 21.9 > mature 13.5%, P = 0.04).
Data Conclusion
Dobutamine infusion increases circumferential cyclic strain throughout the arterial tree of mice. This effect is quantifiable using CINE MRI. The results demonstrate that strain prior to and during dobutamine is influenced by anatomical location, sex, and age.
Level of Evidence: 3
Technical Efficacy: Stage 2
J. Magn. Reson. Imaging 2019;49:69–80.
Keywords: CINE MRI, dobutamine, cyclic strain, mouse, artery
Cardiovascular (CV) disease is the leading cause of death in the United States1 and worldwide.2 Many CV diseases are associated with advancing age and are known to differ between the sexes with respect to risk factors,3 incidence rates,4 symptoms,5 and response to treatment.6 Compromised function of the CV system can go undetected until a challenge is presented, e.g., during exercise.
Clinically, exercise is used as a diagnostic technique in CV disease. Exercise stress tests are most commonly used to evaluate cardiac function and can predict all‐cause mortality.7 If a patient is unable to perform exercise, pharmacological surrogates such as dobutamine can be used instead.8 The response to these challenges is assessed by measuring vital signs, the electrical activity of the heart, and/or blood biomarkers. While useful, these initial measurements can lack sensitivity9 and do not provide spatially and temporally resolved information regarding blood vessel biomechanics that are known to play a pivotal role in vascular health and disease.
Cyclic strain examines vessel deformation across the cardiac cycle and is one of the primary biomechanical factors influencing the health of arteries.10, 11 Green–Lagrange circumferential cyclic strain (GL‐strain) is a localized functional measurement of the arterial wall and is often associated with stiffness.12 Increases in arterial stiffness, due to normal aging or the presence of disease, are known to adversely affect organ systems throughout the body.13, 14
Magnetic resonance imaging (MRI) has been used to noninvasively quantify GL‐strain in the aorta of mice.15 Previously, we and others have shown that reductions in strain can accompany the onset of pathology.16, 17 Loeber et al's work appears to be the sole investigation of how arterial cyclic strain might change with the administration of dobutamine.18 Dobutamine and MRI have been applied to murine models to investigate cardiac function19 and the venous system.20 With the latter work demonstrating that dobutamine has little impact on GL‐strain in veins, and since mice are the most common preclinical model used in the development of therapies and interventions, the purpose of this work was to quantify whether dobutamine could be used to increase GL‐strain along the arterial tree in vivo in murine models.
Materials and Methods
All experiments were performed with approval from the local Institutional Animal Care and Use Committee.
Mice
Adult C57BL/6 mice were used at two ages, 11–13 weeks old (n = 5 male and n = 5 female) and 22–24 weeks old (n = 5 male and n = 5 female). The former is an age range used in a variety of CV models,21 while the latter matches the age used in one of the most common murine models of abdominal aortic aneurysm (AAA).15, 16 In humans, these murine ages correspond to ∼20 years of age (young‐adult) and mid‐30s (mature‐adult), respectively.22 Animals were anesthetized during imaging using 1.5% isoflurane in 1 L/min of oxygen. Heart rate (HR) was monitored with two subcutaneous ECG needles in a lead II configuration, from right arm to left leg, and respiration was monitored using a small pneumatic pillow sensor (SA Instruments, Stony Brook, NY). Core body temperature was maintained at 37 ± 0.5°C using a rectal temperature probe and a proportional‐integral‐derivative (PID) controller (Labview, National Instruments, Austin, TX) interfaced with a commercially available system that circulated warm air through the bore of the magnet (SA Instruments).
Dobutamine Infusion
Aliquots of dobutamine (Hospira, Lake Forest, IL) were prepared with 0.5 μg/mL in 0.9% saline and injected via a tail vein catheter at a rate of 2 μL/min (Cole Parmer, Vernon Hills, IL). This corresponds to a dosage of ∼40 μg/kg murine body weight per minute, based on an average murine body weight of 25 g. As used clinically, the primary goal was to elevate HR equivalently across all subjects, independent of body weight (body weights: young male 25.3 ± 1.1 g; mature male 31.0 ± 2 g; young female 23.0 ± 0.9 g; mature female 25.0 ± 0.9 g). To verify that changes observed in the vasculature were not primarily due to increases in blood volume, the same infusion (volume, rate, and duration) and MRI data acquisition procedures were repeated with saline instead.
MRI
Imaging was performed at 7T field strength using a Direct Drive console (Agilent Technologies, Santa Clara, CA). Animals were imaged in the supine position using a 40‐mm diameter transmit‐receive RF volume coil (Agilent Technologies). Coronal and sagittal 2D gradient echo images were acquired ventral and perpendicular to the spine, respectively, to locate the kidneys and cervical curve as anatomical landmarks. Then, coronal maximum intensity projections derived from 3D gradient echo data were used to plan 2D CINE acquisitions perpendicular to each vessel of interest (Fig. 1A,B). Twelve CINE ECG‐gated images were acquired during a cardiac cycle, using a gradient echo pulse sequence with flow compensating gradients in the slice selection / flow direction (Fig. 1C). Imaging parameters were: repetition time / echo time (TR/TE) ∼110 (period of the cardiac cycle)/2.4 msec, field of view (FOV) 20 mm2, flip angle (α) 60°, slice thickness 1 mm, number of excitations (NEX) 6, and a data acquisition matrix of 2562 (78 × 78 μm in‐plane resolution) zero‐filled to 5122.
Figure 1.
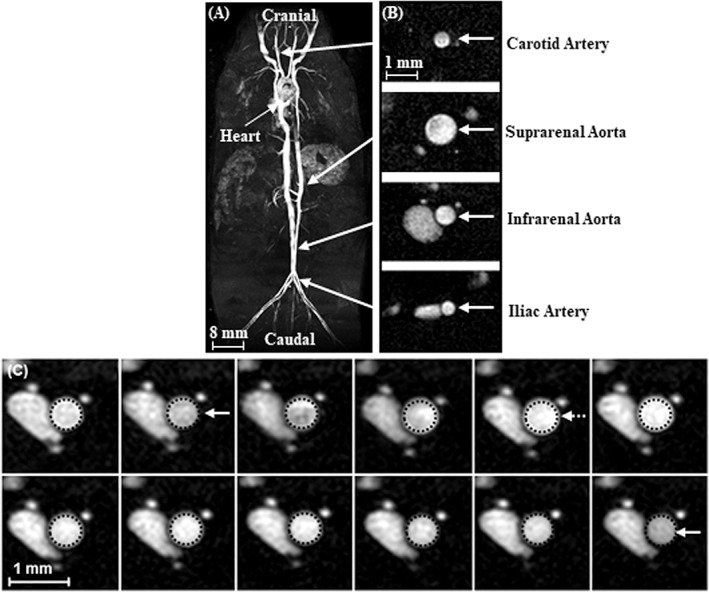
Representative data from the four anatomical locations investigated. A: Coronal maximum intensity projection from a multiple thin slab 3D acquisition. B: Single cross‐sectional CINE frame from each anatomical location studied (shown on the same scale). C: Twelve CINE frames from the infrarenal aorta of a young male mouse. Dotted circles represent the perimeter of the vessel at diastole and are shown in each image to visualize vessel expansion across the cardiac cycle. Solid arrows are shown for end‐diastole and the dotted arrow represents peak‐systole.
CINE images were acquired at four locations. Common carotid artery images were acquired rostral to the curvature of the cervical spine. Suprarenal abdominal aorta images were acquired above the renal arteries and the superior mesenteric artery (SMA). Infrarenal aorta images were acquired 5 mm above the iliac bifurcation (IB) to avoid gonadal branching vessels. Iliac artery images were acquired 3 mm below the IB. After acquiring baseline data from all four locations, dobutamine infusion was initiated. Once the HR reached an elevated steady rate, a second set of CINE images was acquired from the same four locations. The total imaging time for each animal was ∼110 minutes, with ∼40 minutes of dobutamine infusion during that time.
Cyclic strain and area of each vessel were quantified across the cardiac cycle using an in‐house script (MatLab 2016, MathWorks, Natick, MA). The 512 × 512 CINE images were smoothed using a narrow Gaussian filter, reducing random noise at the small cost of spatial resolution. The smoothed images were then upsampled to more easily assign a voxel as either belonging to the vessel or lying outside of it. This assignment was made using an intensity threshold of 50% of maximum signal intensity in a user‐defined region of interest (ROI) within the vessel lumen. Our automated boundary‐finding MatLab routine identified voxels in the vessel using an iterative search algorithm. With marked voxels belonging to the lumen, the vessel boundary was defined as the path through the outermost voxels and was displayed on top of the image. The user could then accept or correct the boundary depending on the automated boundary quality. The Cartesian coordinates of the finalized boundary were transformed to polar coordinates about the center of the vessel, and the resulting boundary curve was fitted to a six‐term Fourier series. The resulting fit parameterized the smoothly varying and continuous boundary for the vessel in each image. Using this fit, vessel perimeter and area were calculated by polar integration. This process was repeated for each individual CINE frame spanning the cardiac cycle.
Cyclic Strain and Average Area
GL circumferential cyclic strain was calculated as:
where P i is the perimeter in the given CINE frame (i = 1 – 12) and P dias is the minimum perimeter at end diastole. GL circumferential strain is proportional to changes in area when the expansion preserves the vessel's shape, for example, when a vessel boundary remains circular as it expands and recoils through the cardiac cycle. For such a shape‐preserving expansion, a strain value of 50% corresponds to a doubling of cross‐sectional area across the cardiac cycle. Maximum cyclic strain was calculated at baseline and during infusion of dobutamine, with the absolute change in cyclic strain between baseline and dobutamine used to assess response.
Secondary to cyclic strain, area averaged across the cardiac cycle was calculated at baseline and during dobutamine, to compare the vascular response to dobutamine to previously studied cardiac responses. To compare the response across groups and locations, the relative change in average area was calculated for each mouse to account for potential differences in the size of anatomical structures:
Statistical Analysis
All statistical analysis was performed using GraphPad Prism (v. 7, GraphPad Software, La Jolla, CA). Data were plotted and reported as mean ± standard error (SEM). Within each group, repeated‐measures analysis of variance (ANOVA) with Tukey's post‐hoc test was used to evaluate the influence of location on maximum cyclic strain (at baseline, during dobutamine, or the difference between dobutamine and baseline) or relative change in average area. Also within each group, two‐way ANOVA with a Bonferroni's post‐hoc test was used to evaluate the effect of dobutamine on maximum cyclic strain or average area. At a given anatomical location, two‐way ANOVA with a Bonferroni's post‐hoc test was used to determine the effects of sex and age on maximum cyclic strain (at baseline, during dobutamine, or difference between dobutamine and baseline) or the relative change in average area. Differences were considered statistically significant at P < 0.05.
Results
Heart Rates Did Not Differ Between Groups
At baseline, HR did not differ between groups (young male, 496 ± 29; mature male, 508 ± 20; young female, 496 ± 19; mature female, 516 ± 15 bpm; P = 0.87). Dobutamine resulted in an ∼18% increase in HR (baseline vs. dobutamine: 506 ± 10 vs. 597 ± 5 bpm, P < 0.0001). HR during dobutamine did not differ between groups (young male, 585 ± 10; mature male, 612 ± 12; young female, 581 ± 2; mature female, 601 ± 6 bpm; P = 0.08). The absolute difference in HR between dobutamine and baseline was not different between groups (young male, 89 ± 14; mature male, 104 ± 23; young female, 84 ± 18; mature female, 85 ± 14 bpm; P = 0.86).
Increases in Circumferential Cyclic Strain With Dobutamine
Cyclic strain waveforms acquired from the infrarenal aorta of all mice, at baseline and during dobutamine, are displayed in Fig. 2. The baseline and dobutamine maximum strain values for each location and group are plotted in Fig. 3. Saline infusion resulted in a small increase in maximum strain of the young male infrarenal aorta with an increase of only 3% due to saline, compared to an increase of 21% with dobutamine (data not shown).
Figure 2.
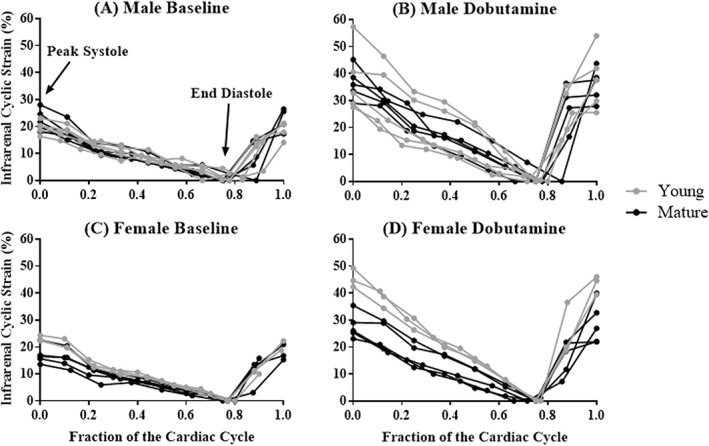
Green‐Lagrange circumferential cyclic strain of the infrarenal aorta across the cardiac cycle for male (A,B) and female (C,D) mice, before (left) and during (right) dobutamine infusion. Young animals are shown in gray (n = 5 male, n = 5 female) and mature animals are shown in black (n = 5 male, n = 5 female). Dobutamine infusion increased cyclic strain.
Figure 3.
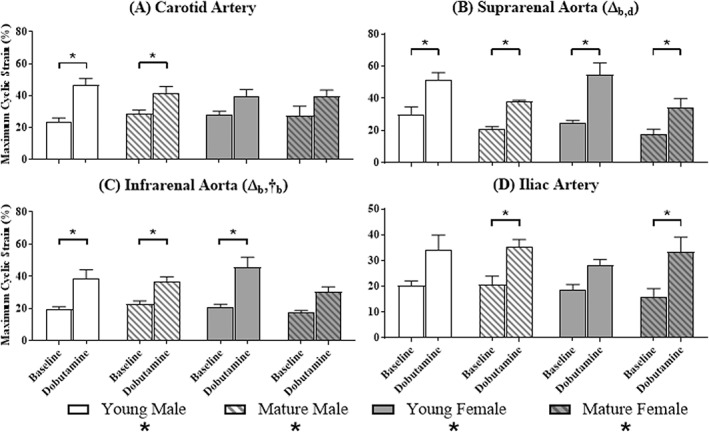
Maximum Green‐Lagrange circumferential cyclic strain at baseline and during the infusion of dobutamine. Baseline and dobutamine maximum cyclic strain are displayed side‐by‐side for each group at each of the four locations (n = 5 per group). Significant differences based on location (#) or dobutamine (*) within a group; and age (Δ) or sex (†) at a given location. Subscripts correspond to baseline (b) or dobutamine (d).
At baseline, maximum strain was independent of location except for an overall effect in young females, where it steadily decreased cranial‐to‐caudal (P = 0.03). Neither age nor sex affected baseline maximum strain at the carotid (P = 0.57; P = 0.66) or iliac (P = 0.68; P = 0.25) locations. Age, but not sex, had a significant effect on baseline maximum strain at the suprarenal location, with young mice having a higher baseline strain compared to mature mice (Fig. 3B: young 27.6 ± 2.6% > mature 19.3 ± 1.3%; P = 0.01). For baseline maximum strain at the infrarenal location, the effects of age and sex were not independent (Fig. 3C: young male 19.8 ± 1.1% < mature male 22.9 ± 1.9%; young female 21.0 ± 1.7% > mature female 17.4 ± 1.3%; P = 0.05).
Comparing baseline to dobutamine within each group, there was an overall effect on maximum strain values for all groups when dobutamine was administered. At the carotid artery, there were statistically significant differences between means within the groups of young (Fig. 3A, P = 0.001) and mature (P = 0.005) male mice. At the suprarenal aorta, there were differences between means within every group (Fig. 3B: young male, P = 0.002; mature male, P = 0.0003; young female, P < 0.0001; mature female, P = 0.03). At the infrarenal aorta, there were differences between means within the groups of male mice (Fig. 3C: young, P = 0.007; mature, P = 0.003) and young females (young, P = 0.0004; mature, P = 0.15). At the iliac location, there were differences between means within the groups of mature mice (Fig. 3D: male, P = 0.002; female, P = 0.03).
During dobutamine, maximum strain was independent of location within each group. Neither age nor sex affected dobutamine maximum strain at the carotid (P = 0.5; P = 0.3), infrarenal (P = 0.08; P = 0.9), or iliac locations (P = 0.5; P = 0.4). However, at the suprarenal location young mice had a higher dobutamine maximum strain compared to mature mice (Fig. 3B: young 53.0 ± 1.6% > mature 36.2 ± 1.6%; age, P = 0.005; sex, P = 0.9).
The difference in maximum strain (dobutamine – baseline) was calculated, as visualized in Fig. 4. The difference in maximum strain was independent of location within each group. Neither age nor sex affected the difference in maximum strain at the carotid (P = 0.30; P = 0.19), suprarenal (P = 0.08; P = 0.40), or iliac (P = 0.20; P = 0.99) locations. At the infrarenal location, young mice had a larger increase in strain compared to mature mice (Fig. 3C: young 21.9 ± 2.9% > mature 13.5 ± 0.6%; age, P = 0.04; sex, P = 0.55).
Figure 4.
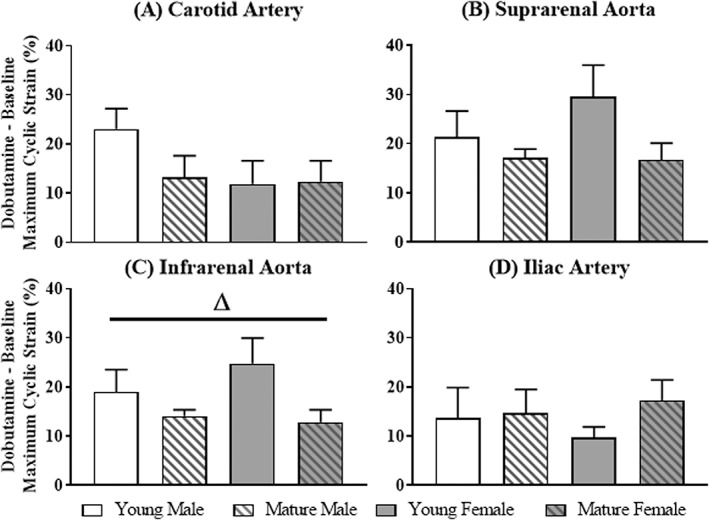
Change in maximum cyclic strain at the carotid, suprarenal, infrarenal, and iliac locations for each group. Significant differences based on age (Δ) or sex (†).
Decreases in Cross‐sectional Area With Dobutamine
Area waveforms acquired from the infrarenal aorta of all mice, at baseline and during dobutamine, are displayed in Fig. 5. The baseline and dobutamine average area values for each location and group are plotted in Fig. 6.
Figure 5.
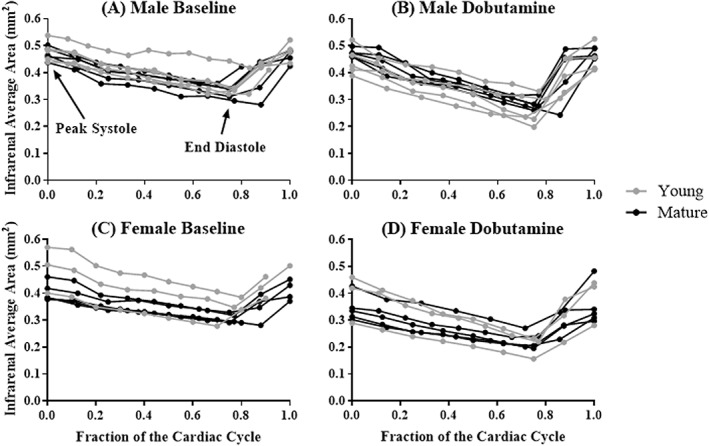
Cross‐sectional area of the infrarenal aorta across the cardiac cycle, for male (A,B) and female (C,D) mice, before (left) and during (right) dobutamine infusion. Young animals are shown in gray (n = 5 male, n = 5 female) and mature animals are shown in black (n = 5 male, n = 5 female). Dobutamine infusion decreased area.
Figure 6.
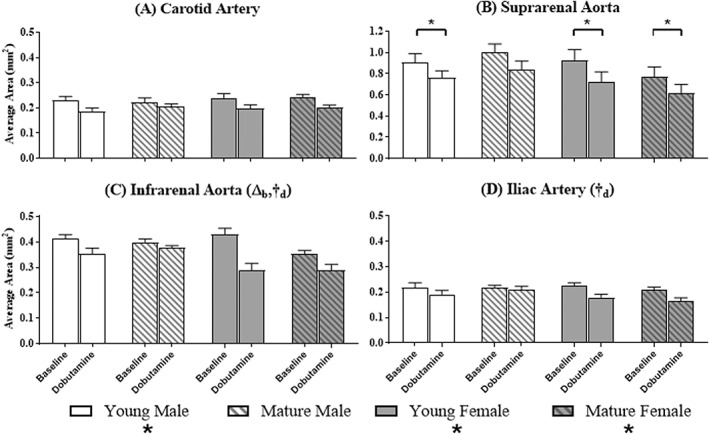
Average cross‐sectional area at baseline and during the infusion of dobutamine. Baseline and dobutamine average area are displayed side‐by‐side for each group at each of the four locations (n = 5 per group). Significant differences based on dobutamine (*) within a group; and age (Δ) or sex (†) at a given location. Subscripts correspond to baseline (b) or dobutamine (d).
At baseline, average area was independent of age and sex at the carotid (P = 0.8; P = 0.4), suprarenal (P = 0.7; P = 0.2), and iliac (P = 0.6; P = 0.9) locations. Age (P = 0.02), but not sex (P = 0.4), had a significant effect on baseline average area at the infrarenal location, with young mice having higher baseline average areas compared to mature mice (Fig. 6C: young 0.42 ± 0.009 mm2 > mature 0.38 ± 0.02 mm2; age, P = 0.02).
Comparing baseline to dobutamine within each group, except for mature males (P = 0.07), there was an overall effect on average area when dobutamine was administered (young male, P = 0.02; young female, P = 0.004; mature female, P = 0.02). At the suprarenal aorta, except for mature males, there were statistically significant differences between means (Fig. 5B: young male, P = 0.04; young female, P = 0.01; mature female, P = 0.05). End‐diastolic area decreased more than peak‐systolic area in all groups (data not shown; young male, P = 0.001; mature male, P = 0.0002; young female, P = 0.002; mature female, P = 0.04).
During dobutamine, neither age nor sex had an effect on average area at the carotid (P = 0.36, P = 0.81) and suprarenal locations (P = 0.86, P = 0.14; Fig. 6A,B). Sex, but not age (P = 0.6, P = 0.88), had a significant effect on dobutamine average area at the infrarenal and iliac locations, respectively, with male mice maintaining larger areas compared to female mice (infrarenal: Fig. 6C, male 0.36 ± 0.01 mm2 > female 0.29 ± 0.0002 mm2, P = 0.003; Iliac: Fig. 6D, male 0.20 ± 0.01 mm2 > female 0.17 ± 0.01 mm2, P = 0.05).
The relative change in average area was calculated, as visualized in Fig. 7. The relative change in average area was independent of location within each group. Neither age nor sex had an effect at the carotid (P = 0.1; P = 0.4) and suprarenal (P = 0.8; P = 0.052) locations. However, age and sex had a significant effect at the infrarenal location, with larger relative decreases in both young mice compared to mature mice (young, –0.24 ± 0.09; mature, –0.12 ± 0.06 mm2; P = 0.01) and females compared to males (male, –0.10 ± 0.04; female, –0.26 ± 0.07 mm2; P = 0.002). Additionally, sex had a significant effect on the relative change in average area at the iliac location, decreasing more in females compared to males (male, –0.08 ± 0.04; female, –0.21 ± 0.01 mm2; P = 0.0007).
Figure 7.
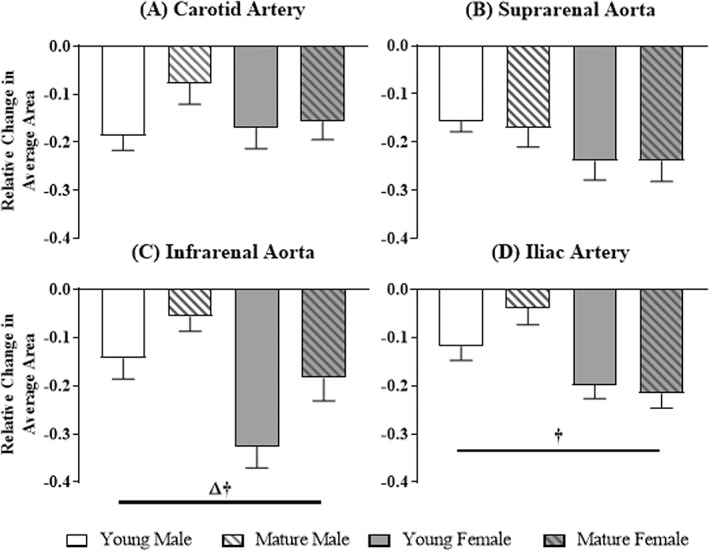
Relative change in average area at the carotid, suprarenal, infrarenal, and iliac locations for each group. Significant differences based on age (Δ) or sex (†).
To illustrate their relationship, change in maximum strain and relative change in average area are plotted together for each group in Fig. 8. In general, greater relative reductions in area coincided with greater increases in maximum strain. For male mice and the mature female group, the location with the largest relative decrease in area corresponded to the largest increase in maximum strain (young male, carotid artery; mature male, suprarenal aorta; mature female, iliac artery). In young female mice the largest relative reduction in area was at the infrarenal aorta, while the largest increase in maximum strain was at the suprarenal aorta.
Figure 8.
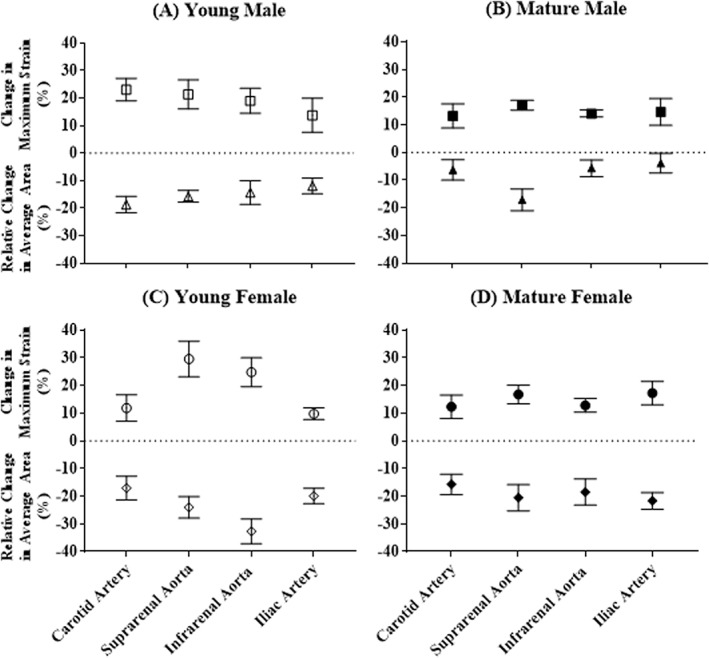
Comparison of change in maximum strain and relative change in average area due to the administration of dobutamine, for young males (A), mature males (B), young females (C), and mature females (D); (n = 5 per group). Typically, a larger relative reduction in area was matched with a larger increase in maximum strain.
Discussion
We have confirmed that dobutamine can be used to increase GL circumferential cyclic strain throughout the arterial tree of murine models. Furthermore, we quantified location‐, sex‐, and age‐dependent differences in strain at baseline and due to the administration of dobutamine. Using minimally invasive strategies, which are translatable to the human condition,23 we are able to provide innovative data relevant to location‐specific cardiovascular pathophysiology, address lack of sex diversity in preclinical investigations,24 and further highlight the need to consider age as an experimental variable in preclinical models of CV disease.21
Circumferential cyclic strain is emerging as a reproducible, localized metric of arterial wall biomechanics in small‐animal preclinical models. Here, our baseline maximum strain measurements in the carotid artery of young male mice match others' ultrasound data well (23.8% vs. ∼25–31%).17 Suprarenal and infrarenal baseline maximum strain measurements in mature males agree with previous MRI results acquired at a different field strength and laboratory (suprarenal: 20.7% vs. 19.1%; infrarenal: 22.9% vs. 23%).15 Young male data from the infrarenal aorta compared favorably with previous ultrasound data (19.8% vs. 15.7%).25 The observation from these studies that the infrarenal strain in young males is lower than mature males is pertinent to the baseline results discussed below. Lastly, our current results also agree with previous studies in mice which showed no difference in cyclic strain between the suprarenal and infrarenal aorta at rest.15
Interestingly, to date, murine results from the carotid artery are higher than values derived from human subjects (15% by CINE MRI at 3T).26 This will require further investigation but could be related to differences in posture between species (biped vs. quadruped), the number and composition of elastic lamellar units within the media, or pressure wave propagation.27 In comparison, the magnitude of strain in the healthy, adult, infrarenal aorta at rest has been shown to be invariant across species.25
Although the arteries investigated here spanned the length of the murine body, all are classified as large elastic arteries that act to dampen pulsatility in order to provide steady flow at the capillary bed. The location‐dependent strain results seen in young female mice at baseline may be indicative of an interaction between vessel length and reflected waves, as observed in humans.28 Otherwise, our findings show that elastic arteries along the arterial tree in mice, within the given sex and age ranges studied here, exhibit comparable strain values at baseline and during dobutamine. It will be important to determine whether this is true in humans because variations in cyclic strain along the arterial tree has implications for the integration of medical devices, vascular grafts, and tissue engineered blood vessels. Regarding the last, the application of cyclic strain has been shown to improve strength and vasoreactivity of tissue constructs.29 The higher strain values we observed during dobutamine could be used to evaluate if additional biomechanical stimuli further improve construct characteristics prior to implantation.
Based on recent work by Raaz et al showing that localized dysfunction along the aorta can have an impact on adjacent locations,30 age‐dependent baseline strain results observed at both locations in the aorta may be related. Mature mice of both sexes had significantly lower baseline strain at the suprarenal location. However, in the infrarenal aorta of mature male mice, strain levels were greater compared to their young counterparts. This contrasting effect is not observed in female mice, where mature animals had lower strain values compared to their young comparators. The results we see in the aorta are also in opposition to human data that shows earlier age‐related changes in vascular function in men, with results from women aligning with men only after menopause.31
The effect of dobutamine was consistent, increasing strain values overall in each group. This is in opposition to Loeber et al, who showed no increases in strain in the pulmonary artery and ascending aorta of a canine model. These differences may be due to different species or anatomical locations, a lower dose of dobutamine, or the use of invasive methods.18
Pairwise comparisons varied between dobutamine and baseline within each group. At the carotid location, male groups exhibiting a significant increase in maximum strain may reflect sex‐dependent differences in the control of the baroreflex system.32 Increases in maximum strain seen at the suprarenal aorta in every group could be explained by its position proximal to the kidneys, which are susceptible to microvascular injury if hemodynamic forces are poorly modulated.13 The fact that mature female mice were the only group not to demonstrate a significant increase in maximum strain at the infrarenal aorta is unexpected. However, human data regarding the properties of females arteries has described the influence of changes in blood flow demands and wave reflections due to impending reductions in reproductive potential.33 Increases in maximum strain at the iliac artery in mature but not young mice may again reflect adjacent counterbalancing effects relative to proximal locations in the aorta.30 However, in conjunction with the angle of the iliac artery, this location begins to challenge the resolution of our system, which can lead to greater variability in data (coefficient of variation averaged across the three conditions, baseline‐dobutamine‐change: carotid 39%, suprarenal 29%, infrarenal 27%, iliac 42%).
Two patterns of age‐dependent differences in the aorta of mature mice were observed when comparing maximum strain values during dobutamine and evaluating the vascular response as (dobutamine – baseline). At the suprarenal location, mature mice had significantly smaller maximum strain values during dobutamine, while the vascular response was not different between young and mature mice. This implies that the shift of the suprarenal aorta up the stress–stretch curve during the administration of dobutamine is not dependent on an animal's age. However, because vascular function is compromised to begin with (i.e., lower baseline strain in mature mice, discussed above), the functional state during dobutamine remains reduced compared to younger animals (i.e., lower dobutamine strain in mature mice). The second pattern seen in the infrarenal aorta included sex‐based differences at baseline (discussed above), no differences during dobutamine, and a reduced vascular response in mature groups. These data suggest that the mature infrarenal aorta is unable to mount a similar response compared to younger animals. Although exercise and dobutamine have similar and disparate effects (e.g., both increase cardiac output and decrease peripheral resistance, while exercise increases blood pressure and dobutamine decreases or does not change it), our results provide additional context regarding the use of physical activity to alter biomechanics of the vessel wall, in addition to hemodynamic conditions, in order to treat aortic disease.34
In contrast to the standard notion that aortic diameter increases with age in humans, the average cross‐sectional area at baseline was larger in the infrarenal aorta of young animals. However, some have suggested that this age‐dependent enlargement in humans may be specific to the upper percentiles of the population.35 Alternatively, bipedal posture may have a differential influence on geometry of the aging aorta.
Previous work evaluating the cardiac response to dobutamine in mice showed decreases in left ventricle end‐diastolic and peak‐systolic volumes.19 We show a downward shift in left ventricle volume is paralleled in the vasculature by a decrease in average arterial cross‐sectional area, with diastolic area decreasing more than systolic area. The fact that only mature male mice lacked an overall effect on area from the administration of dobutamine and also lacked a significant reduction in area at the suprarenal aorta, in particular, is intriguing given the greater propensity for male mice to develop angiotensin‐II‐induced suprarenal dissecting AAAs.36 Notably, this lack of response in mature males as measured by area is coupled with cyclic strain also being lower at the suprarenal aorta in mature mice, at baseline, and during the infusion of dobutamine.
During the infusion of dobutamine, average cross‐sectional areas at the infrarenal aorta and iliac artery were smaller in female mice. Similarly, the relative reduction in areas of the infrarenal aorta and iliac artery were greater in female mice than males. Thus, the vessels in male mice at these locations are left in an enlarged state. Additionally, the relative reduction in area at the infrarenal aorta was greater in young animals. This suggests that mature male mice may be exhibiting deleterious effects based on both sex and age, which is consistent with previously published results from mice37 and humans.31
Evaluating cross‐sectional area alone provides little sense of how deformation across the cardiac cycle is impacted. And, conversely, area data suggest possible reasons for reduced strain in the older murine aorta. With the administration of dobutamine, mature animals tend to have smaller decreases in area. Thus, on average, the vessel is left in an enlarged state and the resulting reduction in strain values demonstrates we are beginning to disrupt the vessel's ability to deform during the cardiac cycle in the same manner as young counterparts. Although other studies have not addressed the relationship between changes in strain and area directly, murine17, 37 and human data demonstrate a similar relationship between larger area and reduced strain that we observe with dobutamine. Geometric changes have long been a foci with respect to aortic disease.35 The integration of structural changes (area) with their subsequent impact on vessel wall function across the cardiac cycle (strain) could provide an improved metric.
With respect to limitations of the work presented here, changes in stroke volume or blood pressure may be occurring. However, when dobutamine is used clinically the goal is to reach 85% of a patient's age‐predicted maximal heart rate irrespective of blood pressure, although the latter is likely to be monitored. We confirmed that the clinically relevant cardiac response of increase in HR was similar between groups. The dose of dobutamine used here was chosen to match the maximum clinical dose used to achieve the desired HR increases8 and to allow for comparisons to previously documented outcomes in murine models.19 Due to differences in metabolic rates, we did not expect to reach the same fractional increase of HR in mice. The complex physiological response to the administration of a racemic mixture of dobutamine38 typically results in minimal or hypotensive effects in animal models19 and humans.8 The effects of anesthesia on measurements of cyclic strain also need to be considered. For example, isoflurane may reduce cyclic strain due to its vasodilatory effects (i.e., an enlarged vessel has a reduced ability to deform across the cardiac cycle). Therefore, we would hypothesize that performing these measurements in awake humans would result in either similar or greater effects. Differences between groups of mice that are ∼15–20 years apart in human years (murine ages selected to match common ages used in the field) is not surprising, given the acknowledged declines in performance per decade of life in humans.39 However, older cohorts should be evaluated in future studies, along with a continued focus on sex differences. Lastly, although voxels are anisotropic, 1‐mm slice thickness retains signal‐to‐noise while providing a localized measurement that minimizes partial voluming effects (e.g., due to curvature) by planning slices perpendicular to the vessel using 3D acquisitions (Fig. 1A, scale bar for reference).
In conclusion, just as the clinic has leveraged dobutamine to uncover deficits that may remain undetected at rest, the intent of this study was fulfilled by confirming that dobutamine could be used to increase GL‐strain throughout the arterial tree in mice, the most common preclinical animal model used as a proxy for the human condition. Novel findings include differences in strain based on location or sex. Combined with previous findings using reactive hyperemia and hyperthermia to challenge the older murine CV system,37, 40 our body of work clearly illustrates that, like the human condition,7 healthy mice are not immune to the challenges aging imposes. Therefore, just as sex diversity in preclinical studies has come to the forefront as a critical experimental parameter,24 so too should the age of mice when modeling CV diseases associated with aging in humans. Lastly, numerous biochemical pathways that are induced by cyclic strain have been identified.10, 11 However, the majority of this foundational work was performed and has remained in vitro. The methods presented here might be used to investigate hypothesized genetic and molecular factors involved in how changes in strain contribute to a healthy or diseased vascular system by using transgenic mice in conjunction with more specific reagents.
Conflict of Interest
The authors report no conflicts of interest.
Acknowledgments
Contract grant sponsor: National Institutes of Health (NIH); contract grant number: T32‐HL125242 (to A.C.C.); American Heart Association (AHA); contract grant number: 14SDG18220010 (to C.J.G.).
References
- 1. Mensah GA, Brown DW. An overview of cardiovascular disease burden in the United States. Health Aff (Millwood) 2007;26:38–48. [DOI] [PubMed] [Google Scholar]
- 2. WHO . Cardiovascular diseases (CVDs). Geneva, Switzerland: WHO; 2017. [Google Scholar]
- 3. Maas AHEM, Appelman YEA. Gender differences in coronary heart disease. Neth Heart J 2010;18:598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. CDC . Aortic aneurysm fact sheet abdominal aortic aneurysms other types of aneurysms risk factors for aortic aneurysm. Atlanta, GA: Centers for Disease Control; 2016. [Google Scholar]
- 5. Berg J, Bjorck L, Dudas K, Lappas G, Rosengren A. Symptoms of a first acute myocardial infarction in women and men. Gend Med 2009;6:454–462. [DOI] [PubMed] [Google Scholar]
- 6. Arshad A, Moss AJ, Foster E, et al. Cardiac resynchronization therapy is more effective in women than in men. J Am Coll Cardiol 2011;57:813–820. [DOI] [PubMed] [Google Scholar]
- 7. Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension 2001;37:1236–1241. [DOI] [PubMed] [Google Scholar]
- 8. Abram S, Arruda‐Olson AM, Scott CG, et al. Typical blood pressure response during dobutamine stress echocardiography of patients without known cardiovascular disease who have normal stress echocardiograms. Eur Hear J Cardiovasc Imaging 2016;17:557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aktas MK, Ozduran V, Pothier CE, Lang R, Lauer MS. Global risk scores and exercise testing for predicting all‐cause mortality in a preventive medicine program. JAMA 2004;292:1462–1468. [DOI] [PubMed] [Google Scholar]
- 10. Liu X, Peyton KJ, Durante W. Physiological cyclic strain promotes endothelial cell survival via the induction of heme oxygenase. Am J Physiol Heart Circ Physiol 2013;304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wanjare M, Agarwal N, Gerecht S. Biomechanical strain induces elastin and collagen production in human pluripotent stem cell‐derived vascular smooth muscle cells. Am J Physiol Cell Physiol 2015;309:C271–C281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Humphrey JD, Delange SL. An introduction to biomechanics: Solids and fluids, analysis and design. New York: Springer; 2004. [Google Scholar]
- 13. O'Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: Cause and logic of therapy. Hypertension 2005;46:200–204. [DOI] [PubMed] [Google Scholar]
- 14. Raaz U, Zöllner AM, Schellinger IN, et al. Segmental aortic stiffening contributes to experimental abdominal aortic aneurysm development. Circulation 2015;131:1783–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goergen CJ, Barr KN, Huynh DT, et al. In vivo quantification of murine aortic cyclic strain, motion, and curvature: Implications for abdominal aortic aneurysm growth. J Magn Reson Imaging 2010;32:847–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goergen CJ, Azuma J, Barr KN, et al. Influences of aortic motion and curvature on vessel expansion in murine experimental aneurysms. Arterioscler Thromb Vasc Biol 2011;31:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Favreau JT, Liu C, Yu P, et al. Acute reductions in mechanical wall strain precede the formation of intimal hyperplasia in a murine model of arterial occlusive disease. J Vasc Surg 2014;60:1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Loeber CP, Goldberg SJ, Marx GR, Carrier M, Emery RW. How much does aortic and pulmonary artery area vary during the cardiac cycle? Am Heart J 1987;113:95–100. [DOI] [PubMed] [Google Scholar]
- 19. Wiesmann F, Ruff J, Engelhardt S, et al. Dobutamine‐stress magnetic resonance microimaging in mice: Acute changes of cardiac geometry and function in normal and failing murine hearts. Circ Res 2001;88:563–569. [DOI] [PubMed] [Google Scholar]
- 20. Palmer OR, Chiu CB, Cao A, Scheven UM, Diaz JA, Greve JM. In vivo characterization of the murine venous system before and during dobutamine stimulation: Implications for preclinical models of venous disease. Ann Anat Anat Anzeiger 2017;214:43–52. [DOI] [PubMed] [Google Scholar]
- 21. Jackson SJ, Andrews N, Ball D, et al. Does age matter? The impact of rodent age on study outcomes. Lab Anim 2017;51:160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Flurkey KM, Currer J, Harrison DE. Chapter 20. Mouse models in aging research In: Mouse Biomedical Research. Amsterdam: Elsevier; 2007:637–672. [Google Scholar]
- 23. Morrison TM, Choi G, Zarins CK, Taylor CA. Circumferential and longitudinal cyclic strain of the human thoracic aorta: Age‐related changes. J Vasc Surg 2009;49:1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. NOT‐OD‐15‐102. Consideration of sex as a biological variable in NIH‐funded research. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-102.html
- 25. Goergen CJ, Johnson BL, Greve JM, Taylor CA, Zarins CK. Increased anterior abdominal aortic wall motion: Possible role in aneurysm pathogenesis and design of endovascular devices. J Endovasc Ther 2007;14:574–584. [DOI] [PubMed] [Google Scholar]
- 26. Lin AP, Bennett E, Wisk LE, Gharib M, Fraser SE, Wen H. Circumferential strain in the wall of the common carotid artery: Comparing displacement‐encoded and cine MRI in volunteers. Magn Reson Med 2008;60:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wagenseil JE, Mecham RP. Elastin in large artery stiffness and hypertension. J Cardiovasc Transl Res 2012;5:264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smulyan H, Marchais SJ, Pannier B, Guerin AP, Safar ME, London GM. Influence of body height on pulsatile arterial hemodynamic data. J Am Coll Cardiol 1998;31:1103–1109. [DOI] [PubMed] [Google Scholar]
- 29. Seliktar D, Nerem RM, Galis ZS. Mechanical strain‐stimulated remodeling of tissue‐engineered blood vessel constructs. Tissue Eng 2003;9:657–666. [DOI] [PubMed] [Google Scholar]
- 30. Raaz U, Zöllner AM, Schellinger IN, et al. Segmental aortic stiffening contributes to experimental abdominal aortic aneurysm development. Circulation 2015;131:1783–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Laogun AA, Gosling RG. In vivo arterial compliance in man. Clin Phys Physiol Meas 1982;3:201–212. [DOI] [PubMed] [Google Scholar]
- 32. Huxley VH. Sex and the cardiovascular system: The intriguing tale of how women and men regulate cardiovascular function differently. Adv Physiol Educ 2007;31:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smulyan H, Asmar RG, Rudnicki A, London GM, Safar ME. Comparative effects of aging in men and women on the properties of the arterial tree. J Am Coll Cardiol 2001;37:1374–1380. [DOI] [PubMed] [Google Scholar]
- 34. Myers J, McElrath M, Jaffe A, et al. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc 2014;46:2–9. [DOI] [PubMed] [Google Scholar]
- 35. Wilmink ABM, Quick CRG. Epidemiology and potential for prevention of abdominal aortic aneurysm. Br J Surg 1998;85:155–162. [DOI] [PubMed] [Google Scholar]
- 36. Henriques TA, Huang J, D'Souza SS, Daugherty A, Cassis LA. Orchidectomy, but not ovariectomy, regulates angiotensin II‐induced vascular diseases in apolipoprotein E‐deficient mice. Endocrinology 2004;145:3866–3872. [DOI] [PubMed] [Google Scholar]
- 37. Crouch AC, Manders AB, Cao AA, Scheven UM, Greve JM. Cross‐sectional area of the murine aorta linearly increases with increasing core body temperature. Int J Hyperth 2017;1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ruffolo RR. Review: The pharmacology of dobutamine. Am J Med Sci 1987;294:244–248. [DOI] [PubMed] [Google Scholar]
- 39. Ades PA, Toth MJ. Accelerated decline of aerobic fitness with healthy aging: What is the good news? Circulation 2005;112:624–626. [DOI] [PubMed] [Google Scholar]
- 40. Greve JM, Williams SP, Bernstein LJ, et al. Reactive hyperemia and BOLD MRI demonstrate that VEGF inhibition, age, and atherosclerosis adversely affect functional recovery in a murine model of peripheral artery disease. J Magn Reson Imaging 2008;28:996–1004. [DOI] [PubMed] [Google Scholar]


