Abstract
There are no studies of oral health in relation to esophageal cancer in Africa, or of Eastern Africa's endemic dental fluorosis, an irreversible enamel hypo‐mineralization due to early‐life excessive fluoride intake. During 2014–18, we conducted a case–control study of squamous cell esophageal cancer in Eldoret, western Kenya. Odds ratios (AORs (95% confidence intervals)) were adjusted for design factors, tobacco, alcohol, ethnicity, education, oral hygiene and missing/decayed teeth. Esophageal cancer cases (N = 430) had poorer oral health and hygiene than controls (N = 440). Compared to no dental fluorosis, moderate/severe fluorosis, which affected 44% of cases, had a crude OR of 20.8 (11.6, 37.4) and on full adjustment was associated with 9.4‐fold (4.6, 19.1) increased risk, whilst mild fluorosis (43% of cases) had an AOR of 2.3 (1.3, 4.0). The prevalence of oral leukoplakia and tooth loss/decay increased with fluorosis severity, and increased cancer risks associated with moderate/severe fluorosis were particularly strong in individuals with more tooth loss/decay. Using a mswaki stick (AOR = 1.7 (1.0, 2.9)) rather than a commercial tooth brush and infrequent tooth brushing also independently increased risk. Geographic variations showed that areas of high esophageal cancer incidence and those of high groundwater fluoride levels have remarkably similar locations across Eastern Africa. In conclusion, poor oral health in combination with, or as a result of, high‐altitude susceptibility to hydro‐geologically influenced dental fluorosis may underlie the striking co‐location of Africa's esophageal cancer corridor with the Rift Valley. The findings call for heightened research into primary prevention opportunities of this highly fatal but common cancer.
Keywords: Africa, cancer, esophageal cancer, dental fluorosis, oral health
Short abstract
What's new?
To date, there are no comprehensive studies of oral health and esophageal cancer in Eastern Africa, nor of this area's endemic dental fluorosis, an irreversible enamel hypo‐mineralization due to early‐life excessive fluoride intake. Here, the authors found that moderate/severe fluorosis is associated with a ten‐fold increased cancer risk in Eastern Africa, particularly if tooth loss or decay is co‐present. The finding is striking because of the remarkable co‐location of Africa's esophageal cancer corridor with areas of high groundwater fluoride. Identification of the causal mechanisms will be critical to primary prevention of this common cancer.
Abbreviations
- EC
esophageal cancer
- ESCC
esophageal squamous cell carcinoma
- ESCCAPE
ESCC African PrEvention research
- MTRH
Moi Teaching and Referral Hospital, Eldoret, Kenya
Introduction
Esophageal cancer (EC), predominantly esophageal squamous cell carcinoma (ESCC), is the third most common cause of cancer death in Eastern Africa.1 The hypothesized etiological model for this African EC corridor involves the presence of an unknown but highly spatially‐patterned factor conveying substantial risk, on top of which lifestyle factors act.2 In Kenya alcohol contributes to ESCC but to a lesser extent than in western countries.3 Among other potential contributors, poor oral health has not been examined comprehensively in Africa, but beyond the continent studies have found that different aspects of poor oral health status are associated with increased ESCC risk. In Asia (6 studies4, 5, 6, 7, 8, 9), the US,10 a European‐Latin American study11 and in a single small Kenyan study,12 each study found that at least one of periodontal disease, poor oral hygiene and/or tooth loss and decay were positively associated with ESCC risk. The magnitude of increased ESCC risk were of the order of 1.2‐fold for oral leukoplakia,9 1.5‐ to 2.5‐fold for extremes of missing teeth, and 2‐ to 3‐fold of lack or regular oral hygiene. Public oral health status in Kenya is of good teeth retention to old age and a low though increasing prevalence of dental caries.13, 14 However, oral hygiene is poor, the Arak tree stick (mswaki) is used instead of a commercial tooth brush, and both periodontitis and oral cancer's precursor oral leukoplakia (17% prevalence) are common.15, 16, 17
In the present study, we investigated the aforementioned oral health indicators with risk of ESCC in Kenya. In addition to these measures of oral health, this setting's endemic dental fluorosis enabled its first‐ever investigation in relation to ESCC.18 Dental fluorosis is an irreversible hypomineralization of the tooth enamel and in Kenya occurs predominantly due to early‐life excessive intake of fluoride which occurs naturally in water originating from aquifers in the high fluorine volcanic rocks of the African Rift Valley. WHO's maximum fluoride concentration of 1.5 mg/L is exceeded in non‐piped water supplies in parts of Kenya19 and the resulting dental fluorosis provides, via visual inspection for opacities and mottling of enamel, an easily assessed long‐term exposure biomarker in a cancer case–control study.
Methods
Participant recruitment
The western Kenyan ESCCAPE (ESCC African PrEvention research) case–control study consisted of pilot (August 2013–September 2014) and main study recruitment phases (October 2015–April 2018). The detailed fieldwork experience is published.20 Cases were patients aged ≥18 years who underwent endoscopy at Moi Teaching and Referral Hospital (MTRH), Eldoret, where an esophageal tumor was visualized and a biopsy taken with a morphological examination that confirmed ESCC (90%) or did not rule it out. Age and sex frequency‐matched controls were hospital visitors (main study only, 30% of controls) and hospital in‐patients (38% of controls), hospital out‐patients (32%), excluding patients with digestive diseases or cancer, or those with more than 3 nights stay in MTRH. Participation rates were 96% in cases and 92% in controls.
Oral health and other exposure variables
Participants completed an interview‐administered questionnaire and provided biospecimens. In both study phases, frequency of tooth brushing (daily, 2–6 times/week, once/week or never) was asked. In the main phase only, we also asked about type of toothbrush (commercial, mswaki stick or other) and interviewers were trained by the study dentist (CK) to additionally assess: (i) the count of Decayed, Missing and Filled teeth, which sum to the DMFT index (range 0 best to 32 worst); (ii) oral leukoplakia, i.e. a white or gray patch which could not be wiped away with a cotton gauze, which is a reversible condition21; (iii) dental fluorosis in 4 categories corresponding to the Thylstrup‐Fejerskov index (TFI) scores of, ‘normal’: no dental fluorosis (TFI 0), ‘mild’: opacities visible (TFI 1–4), ‘moderate’: small/medium pits (TFI 5–7) and ‘severe’: large pits with loss of enamel (TFI 8–9) (Supporting Information Fig. S1). Critical exposure ages for development of dental fluorosis are from 1 to 7 years of age during permanent dentition development when excessive fluoride exposure disrupts enamel mineralization, with dental fluorosis externally evident, visually, upon eruption of these permanent teeth. We also asked about water source, as ESCC risk has been linked to consumption of non‐piped water,22, 23 and in Kenya surface waters have some of the highest fluoride levels worldwide.24
For the present investigation, socioeconomic factors (job, education and amenities), age, ethnicity, alcohol (by type), tobacco (smoking and smokeless), hot beverages, household air pollution, and family history of ESCC represent potential confounders or effect modifiers. Place of birth and of current residence were obtained, and, with the aid of databases of all Kenyan places (www.geonames.org) and their corresponding GPS coordinates, current residence was geocoded to investigate the potential of spatial clustering of dental fluorosis and of selection bias. Information on HIV and khat use were asked, but were not adjusted for as their prevalence was low and similar in cases (6.6% for HIV, 2.0% for khat) and controls (8.2% and 2.7% respectively).
We also visually examined maps of EC incidence rates across Africa in relation to those for risk of groundwater fluoride levels exceeding 1.5 mg/L, i.e. levels at which dental fluorosis occurs, using IARC GLOBOCAN 2018 and International Groundwater Resources Assessment Centre data respectively.1, 25
Statistical analysis
Using logistic regression models for ESCC, we estimated odds ratios (ORs) and their 95% confidence intervals (CI) associated with oral health indicators and water source. First a basic model (model 1) was fitted, with adjustment for design factors: interviewer, phase (pilot/main), gender and age (continuous). Model 2 was further adjusted for potential confounders, predominantly lifestyle factors (ethnicity, alcohol, tobacco, family history, hot beverage drinking, education) using variables listed in Table 2. Model 3 was further adjusted for indicators of a dental hygiene/dental caries pathway, i.e. for DMFT score, tooth brush type and brushing frequency ‐ these full‐adjusted ORs are reported in the abstract. We then examined two further pathways, by building on model 3 and additionally adjusting either for leukoplakia (model 4) – representing a possible intermediate ‐ or dental fluorosis (model 5). Stratified analyses by interviewer and control type were conducted to investigate potential biases, and by participant characteristics to explore potential effect modifiers. Statistical analyses were conducted in Stata version 14.0 and mapping in QGIS.
Results
Oral health in controls
A total of 430 cases and 440 controls participated from overlapping catchment areas (Fig. 1 a), of which 287 cases and 285 controls, i.e. those in the main study, had a full oral health examination. Cases were predominantly men, from Kalenjin and Luhya ethnic groups and had low educational level (Table 1). In controls, the prevalence of infrequent tooth brushing (i.e. less than daily, 41%), using a mswaki stick (28%) and missing teeth were higher in lower social and older age groups, whereas tooth decay and oral leukoplakia (26%) were linked to alcohol and tobacco use (Supporting Information Table S1). Eight percent of controls had moderate/severe dental fluorosis, and any degree of fluorosis was more common in the less educated, alcohol/tobacco users, and having a spring or river as the main water source, but fluorosis status did not differ by gender or age.
Figure 1.
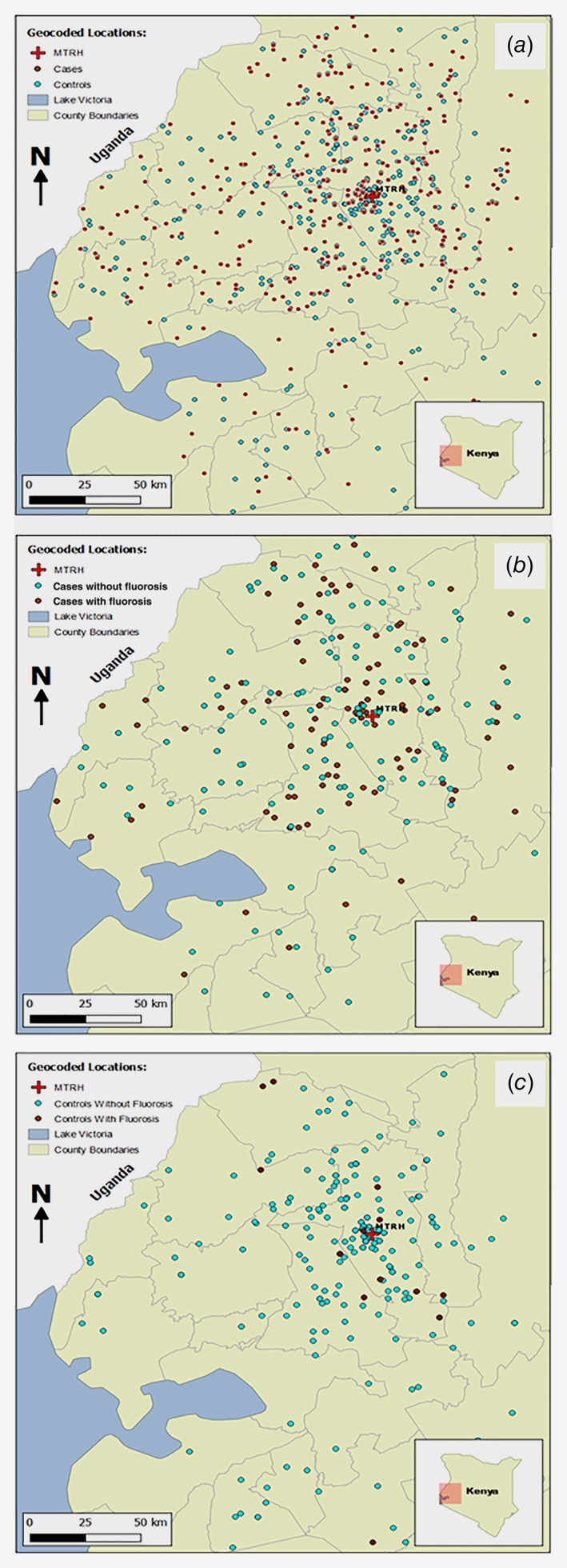
Residential location of participants by (a) case and control status, (b) moderate/severe dental fluorosis status, in cases; (c) moderate/severe dental fluorosis status, in controls.
Table 1.
Demographic and oral health characteristics of cases and controls
| N (%) among 430 cases (143 pilot + 287 main) | N (%) among 440 controls (155 pilot + 285 main) | |||
|---|---|---|---|---|
| N non‐missing (N missing)1 | N (% of non‐missing) | |||
| Pilot and main study phases | ||||
| Sex 2 | 870 (0) | Male | 282 (66) | 272 (62) |
| Female | 148 (34) | 168 (38) | ||
| Age 2 (years) at diagnosis/interview | 870 (0) | Mean (SD) | 59.5 (13.5) | 57.0 (15.2) |
| IQR | 50–69 | 45–68 | ||
| Ethnic group | 870 (0) | Kalenjin | 247 (57) | 233 (53) |
| Luhya | 100 (23) | 95 (22) | ||
| Luo/Kikuyu/Kisii/Other | 83 (19) | 112 (25) | ||
| Education (score) | 869 (1) | None | 104 (24) | 99 (23) |
| Some or all primary | 248 (58) | 201 (46) | ||
| Secondary or higher | 78 (18) | 139 (32) | ||
| No. children | 870 (0) | Mean (SD) | 6.2 (3.6) | 5.9 (3.4) |
| Occupation | 870 (0) | Farmer | 285 (66) | 233 (53) |
| Other | 145 (34) | 206 (47) | ||
| Frequency of tooth brushing | 870 (0) | Daily | 187 (43) | 260 (59) |
| 2–6 times per week | 119 (28) | 132 (30) | ||
| Once per week | 78 (18) | 26 (6) | ||
| Never | 46 (11) | 22 (5) | ||
| Main study phase only | ||||
| Type of tooth brush among brushers | 516 (0) | Commercial | 115 (45) | 193 (72) |
| Stick (mswaki) / other3 | 139 (55) | 76 (28) | ||
| Number of decayed teeth | 572 (0) | 0 | 73 (25) | 183 (64) |
| 1–2 | 83 (29) | 65 (23) | ||
| ≥3 | 131 (46) | 37 (13) | ||
| Number of missing teeth | 572 (0) | 0–1 | 72 (25) | 102 (36) |
| 2–3 | 76 (27) | 93 (33) | ||
| 4–5 | 52 (18) | 35 (12) | ||
| ≥6 | 87 (30) | 55 (19) | ||
| DMFT index4 | 572 (0) | Median (IQR) | 7 (3–15) | 3 (1–6) |
| Oral leukoplakia | 572 (0) | No | 66 (23) | 210 (74) |
| Yes5 | 221 (77) | 75 (26) | ||
| Dental fluorosis index | 569 (3) | None | 36 (13) | 133 (47) |
| Mild | 122 (43) | 129 (45) | ||
| Moderate | 78 (27) | 15 (5) | ||
| Severe | 49 (17) | 7 (2) | ||
| Water source | 572 (0) | Piped | 34 (11) | 64 (23) |
| Rain | 8 (3) | 3 (1) | ||
| Borehole | 35 (12) | 68 (24) | ||
| Well | 97 (34) | 89 (31) | ||
| Spring/ river6 | 113 (39) | 61 (22) | ||
Missing not by design. Maximum numbers 870 total (298 pilot + 572 main).
Frequency‐matched design factors.
Other tooth brushing implement = 7 cases and 2 controls used hand/handkerchief.
DMFT = sum of decayed + missing + filled teeth (range 0 = best to 21 = worst in this sample).
Over 90% on the tongue, the remainder on the cheek.
Two thirds is spring, one‐third river.
Oral hygiene, DMFT and esophageal cancer risk
Table 2 presents ORs for each of the oral health indicators. Tooth brushing once per week or less, and, independently, using a mswaki stick to brush teeth were associated with 2 to 3‐fold increases in risk (Model 1). These ORs were slightly attenuated upon adjustment for lifestyle confounders (Model 2), and, after adjustment for the DMFT score and oral leukoplakia or dental fluorosis, weekly vs. daily tooth brushing (OR 2.3 (95% CI 1.0 to 5.5)) and mswaki stick use (OR 1.7 (1.0 to 3.0) remained associated with risk. Lifestyle and oral hygiene adjusted (model 3) ORs were 8.8 (95% CI: 4.9 to 15.8) for ≥3 compared to no decayed teeth, 1.8 (1.0 to 3.3) for ≥6 missing teeth (vs. none) and, for the combined index, 5.7 (3.0 to 10.9) for a DMFT count of ≥8 vs. 0. The DMFT OR was strongly attenuated upon adjustment for, separately, leukoplakia (OR 3.7, model 4) or dental fluorosis (OR 3.0, model 5), but remained a significant independent risk factor.
Table 2.
Odds ratios for esophageal cancer associated with oral health indicators and water source: case–control study in western Kenya
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |||
|---|---|---|---|---|---|---|---|
| Adjustment | No. cases/controls | Design factors (age, gender, interviewer, phase) | Model 1 + lifestyle confounders1 | Model 2+ tooth brushing frequency + brush type +DMFT (not for lost/decayed teeth) | Model 3 + leukoplakia | Model 3 + dental fluorosis | |
| Odds ratio (95% CI) | |||||||
| Tooth brushing frequency | Daily | 187/260 | 1 | 1 | 1 | 1 | 1 |
| 2–6 times per week | 119/132 | 1.0 (0.8, 1.5) | 0.9 (0.6, 1.3) | 0.8 (0.5, 1.3) | 0.8 (0.5, 1.4) | 0.7 (0.4, 1.3) | |
| Once per week | 78/26 | 3.4 (2.0, 5.5) | 2.5 (1.4, 4.3) | 3.0 (1.1, 6.9) | 2.4 (1.0, 5.7) | 2.3 (1.0, 5.5) | |
| Never | 46/22 | 2.3 (1.3, 4.1) | 1.5 (0.8, 2.9) | 2.4 (1.1, 5.6) | 1.8 (0.8, 4.3) | 2.5 (1.0, 6.0) | |
| Tooth brush type | Commercial | 115/193 | 1 | 1 | 1 | 1 | 1 |
| Stick (197 + 3 other) | 133/75 | 2.9 (2.0, 4.2) | 2.2 (1.4, 3.5) | 1.7 (1.0, 2.9) | 1.7 (1.0, 2.8) | 1.7 (1.0, 3.0) | |
| Never brushes teeth | 46/22 | 3.6 (1.9, 6.8) | 2.7 (1.3, 5.7) | NA | NA | NA | |
| No. missing teeth | 0–1 | 72/102 | 1 | 1 | 1 | 1 | 1 |
| 2–3 | 76/93 | 1.1 (0.7, 1.8) | 1.2 (0.7, 2.0) | 1.2 (0.7, 2.0) | 1.1 (0.6, 2.0) | 1.2 (0.7, 2.1) | |
| 4–5 | 52/35 | 2.1 (1.1, 3.7) | 2.2 (1.1, 4.2) | 2.1 (1.1, 4.1) | 1.6 (0.8, 3.3) | 1.7 (0.8, 3.6) | |
| ≥6 | 87/55 | 2.3 (1.4, 3.9) | 2.0 (1.1, 3.5) | 1.8 (1.0, 3.3) | 1.2 (0.6, 2.4) | 1.3 (0.7, 2.5) | |
| No. decayed teeth | 0 | 73/183 | 1 | 1 | 1 | 1 | 1 |
| 1–2 | 32/65 | 3.3 (2.1, 5.1) | 2.8 (1.7, 4.8) | 2.7 (1.6, 4.7) | 2.1 (1.2, 3.7) | 1.9 (1.1, 3.4) | |
| ≥3 | 131/37 | 12.9 (7.8, 21.5) | 9.4 (4.6, 16.6) | 8.8 (4.9, 15.8) | 5.6 (3.0, 10.4) | 4.4 (2.3, 8.3) | |
| DMFT2 count | 0–1 | 39/82 | 1 | 1 | 1 | 1 | 1 |
| 2–3 | 41/81 | 1.1 (0.6, 1.8) | 1.3 (0.7, 2.5) | 1.1 (0.6, 2.2) | 1.2 (0.6, 2.4) | 1.0 (0.5, 2.1) | |
| 4–7 | 74/68 | 2.4 (1.4, 4.1) | 2.0 (1.1, 3.7) | 2.0 (1.0, 3.7) | 1.6 (0.8, 3.2) | 1.6 (0.8, 3.0) | |
| ≥8 | 133/54 | 7.3 (4.2, 12.6) | 6.4 (3.4, 12.1) | 5.7 (3.0, 10.9) | 3.7 (1.8, 7.4) | 3.0 (1.5, 6.1) | |
| Dental fluorosis | Normal | 36/133 | 1 | 1 | 1 | 1 | ‐ |
| Mild | 122/129 | 3.3 (2.1, 5.1) | 2.9 (1.7, 4.9) | 2.3 (1.3, 4.0) | 1.7 (0.9, 3.0) | ‐ | |
| Moderate/severe | 127/22 | 20.8 (11.6, 37.4) | 14.7 (7.6, 28.6) | 9.4 (4.6, 19.1) | 4.9 (2.3, 10.5) | ‐ | |
| Oral leukoplakia | No | 66/210 | 1 | 1 | 1 | ‐ | 1 |
| Yes | 221/75 | 9.7 (6.5, 14.3) | 6.9 (4.4, 10.9) | 4.9 (3.0, 7.8) | ‐ | 3.1 (1.8, 5.3) | |
| Water source | Piped/rain | 42/67 | 1 | 1 | 1 | 1 | 1 |
| Borehole | 35/68 | 0.8 (0.4, 1.4) | 0.6 (0.3, 1.3) | 0.6 (0.3, 1.4) | 0.5 (0.2, 1.2) | 0.7 (0.3, 1.6) | |
| Well | 97/89 | 1.6 (1.0, 2.7) | 1.6 (0.9, 3.0) | 1.7 (0.9, 3.2) | 1.5 (0.7, 3.0) | 1.9 (0.9, 3.8) | |
| Spring/river | 113/61 | 2.9 (1.8, 4.8) | 3.0 (1.6, 5.5) | 2.9 (1.5, 5.7) | 2.4 (1.1, 4.8) | 3.1 (1.5, 6.5) | |
Model 2: Categories: gender (M/F), ethnicity (6 groups), alcohol+tobacco (combined ever/never status), alcohol intensity (ethanol grams/day categories), very hot / hot/ warm beverage drinking, family history of EC, and continuous: age, education score (1 to 7).
DMFT = Sum of number of Decayed + Missing + Filled Teeth (range 0 = best to 21 = worst in this sample).
Dental fluorosis, leukoplakia and esophageal cancer risk
Moderate/severe dental fluorosis was present in 44% of cases compared to 8% of controls, whereas 13% of cases and 47% of controls had no fluorosis. The resulting Model 1 OR was 21 (95% CI: 12 to 37), 15 (8 to 29) with lifestyle adjustment (model 2) and 9.4 (4.6 to 19.1) upon further adjustment for DMFT score and oral hygiene (Model 3, Table 2), the latter attenuation was due to poorer oral hygiene and higher DMFT score in those with fluorosis. Notably, an association between dental fluorosis and both tooth decay and tooth loss was present in controls (p < 0.001 for both): the prevalence of 3 or more decayed teeth was 7% (9/133) in fluorosis‐free controls, 17% (22/129) in controls with mild fluorosis and 27% (6/22) in those with moderate/severe fluorosis. Similarly, the prevalence of 6 or more missing teeth was 14% (18/133) in fluorosis‐free, 20% (26/129) in mild and 45% (10/22) in moderate/severe fluorosis‐affected controls.
Oral leukoplakia was more common in cases (77%) than controls (26%), with an OR of 9.7 (6.5 to 14.3), which was attenuated to 4.9 upon adjustment for lifestyle factors, DMFT count and oral hygiene. However, because oral leukoplakia was measured at interview/diagnosis, it may mark a late effect on the same biological pathway as dental fluorosis, which also had a large OR but has an early‐life critical exposure period. Notably, leukoplakia prevalence by fluorosis status shown in Figure 2 shows their strong positive correlation, including in controls.
Figure 2.
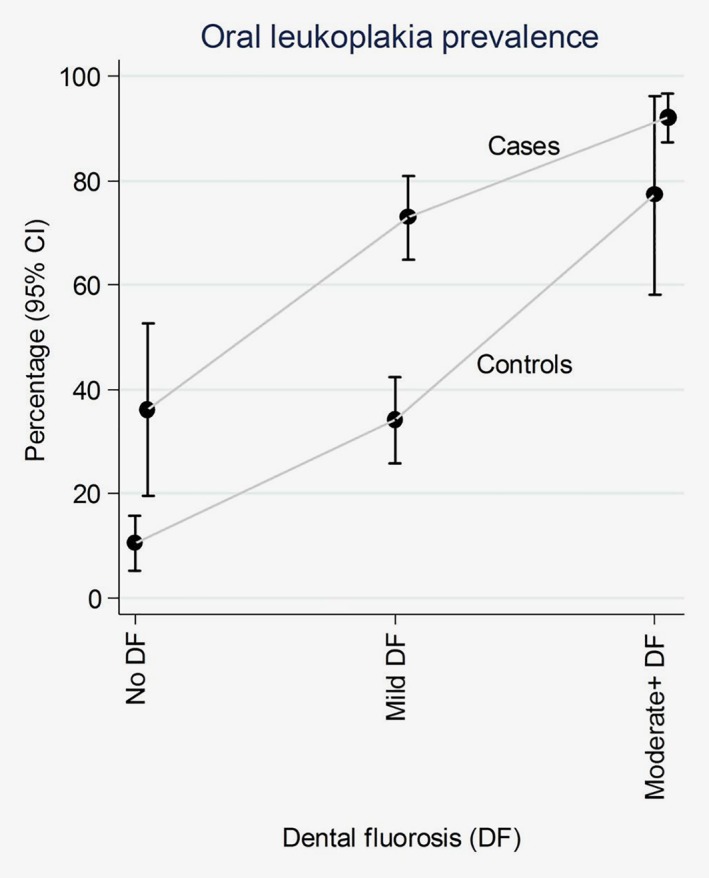
Prevalence (%) of oral leukoplakia according to dental fluorosis severity, by case–control status. Moderate+ DF = moderate/severe dental fluorosis.
Associations of fluorosis and, separately, leukoplakia with ESCC risk stratified by participant or design factors are shown in Figure 3. ORs remained remarkably strong and of similar magnitude by age, sex, alcohol, tobacco use, oral hygiene, interviewer and type of control. Some confidence intervals for mild fluorosis included 1 and statistical power to detect interactions was low. The only exception to this consistency was in analyses stratified by DMFT count, as follows. Increased ESCC risks associated with dental fluorosis were much stronger in people who also had more decayed or missing teeth, which formed the majority of the fluorosis‐affected individuals. In contrast, among the subgroup with no or few decayed or missing teeth, dental fluorosis was rarer and its association with ESCC risk was weak and non‐significant, i.e. the model 3 OR for moderate/severe fluorosis was 9.4 (4.6 to 19.1) overall, 2.5 (0.6, 10.1) if DMFT < 4 and 27.1 (9.6, 76.7) among those with a DMFT count of at least 4.
Figure 3.

Odds ratio for esophageal cancer associated with dental fluorosis and oral leukoplakia, overall and by subgroup or design factor, adjusted for lifestyle factors, DMFT count (Decayed+Missing+Filled tooth count) and oral hygiene (Model 3).
Finally, because of hydro‐geochemical and thus spatial influences on the risk of dental fluorosis, selection bias could have influenced results if cases and controls originated from different areas. Figures 1 b and 1c illustrate that fluorosis‐affected and fluorosis‐unaffected cases, as well as controls, originated from across the entire catchment area. Analyses excluding participants residing in the county of the diagnostic hospital did not differ (not shown).
Water source
After adjusting for lifestyle factors, DMFT and oral hygiene habits (Model 3), compared to using rain or piped water, using a well and stream/river were associated with 1.7‐fold (95% CI: 0.9 to 3.2) and 2.9‐fold (1.5 to 5.7) increased risks, whereas ESCC risks did not differ between consumers of piped and borehole water as the main water source (Table 3). ORs slightly strengthened on adjustment for dental fluorosis. Results stratified by time lived at current address did not differ, but we did not collect information on duration with current water source.
Africa‐wide perspective
Upon visual examination (Fig. 4), there is a close co‐location of African countries with high EC incidence rates and those with medium and high probabilities of high levels of fluoride in groundwater. Notably, increased risks of both problems occur throughout the African Rift Valley. The exception to this is the ESCC hotspot in the Eastern Cape area of South Africa, which does not have an excess fluoride problem, whereas excess fluoride areas in West Africa are not known to be high ESCC risk areas, though population densities are low and cancer registration data with high spatial resolution are lacking in affected areas.
Figure 4.
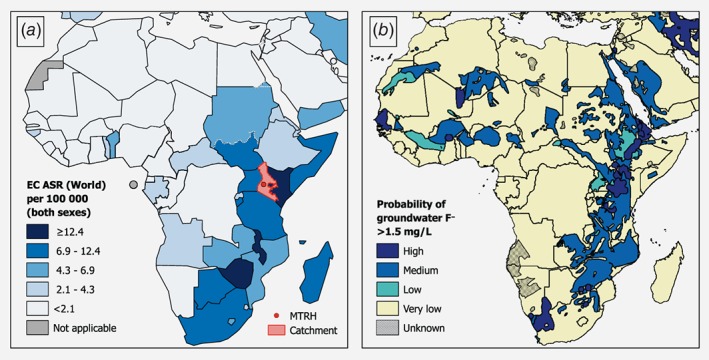
(a) Age‐standardized incidence rates (ASR) of esophageal cancer (EC) in Africa, both sexes (Globocan 20181), indicating counties included in our the present study's catchment area; and (b) Risk of groundwater fluoride levels over 1.5 mg/L across Africa, reproduced with permission.25
Discussion
Principle findings
For the first time in Africa, we assessed associations of multiple measures of poor oral health and risk of esophageal squamous cell cancer (ESCC). In Kenya, a remarkably strong (9.4‐fold) relative risk was found for the presence of moderate/severe dental fluorosis, with some evidence that the association was even stronger and restricted to individuals who also had tooth loss or decay. Independently, ESCC risk increased with poor oral hygiene including use of the mswaki stick to brush and using well or surface water for drinking water ‐ risk factors that were concentrated in lower social groups. These associations were consistent across population groups with ill‐understood ESCC risks, i.e. the young, in women and in alcohol/tobacco abstainers. Further, there is a remarkable overlap between the African high ESCC incidence corridor along the African Rift Valley and areas of high groundwater fluoride levels. Together, these findings represent a significant step forward to focus future research efforts for the prevention of this common fatal cancer in Eastern Africa.2
Comparison with other studies
Similar to our findings, ESCC has been linked to poor oral hygiene and tooth decay in Iran, China and Kashmir4, 5, 6 and edentulous EC patients were noted in South Africa.26 However population‐level DMFT counts were lower in Kenya than in other high risk populations (3 versus 28 in Iran4), possibly due to relatively improved oral hygiene and lower sugar intake in our study. Similar to our findings, 3 to 4‐fold increased ESCC risks with non‐piped water have been observed, but whereas in other studies they were often considered as indicators of low socioeconomic position or of an unspecified constituent, in the present study it likely represents risks through a dental fluorosis pathway. For oral leukoplakia, although the prevalence in this study's controls was only slightly higher than in Kenyan population surveys (26% in our study's controls versus 17%17) and lower than in very high risk populations (43% and 11% in men and women in the Linxian General Population Trial9), our study's leukoplakia‐ESCC relative risks were much larger (OR ~5) than in the latter cohort (OR 1.3), a difference that points to an influence of reverse‐causality or a late‐effect symptom in our study. The lack of temporality of this reversible trait excludes the predictive utility of this OR, nevertheless examination of the joint‐fluorosis‐leukoplakia status was informative to explore mechanistic pathways, as discussed below.
Plausibility
Poor oral health as indicated by dental loss, decay, periodontal disease and poor oral hygiene, is a consistent feature of high ESCC risk populations, for which the proposed biological pathways involve alteration of the oral microbiome to a milieu with pro‐carcinogenic properties or carcinogenic by‐products, e.g. through production of acetaldehyde, reduction of nitrates to nitrites and their conversion to nitrosamines, or increased bacterial species to produce reactive oxygen species among which are esophageal carcinogens.27 Alternative pathways concern reduced mastication leading to physical damage to the esophagus. Chronic inflammation may also be involved, not only for ESCC but also for other upper GI cancers and liver cancer.5
In the present study, the ESCC‐dental fluorosis association, which appeared to be concentrated in individuals who also had tooth decay or tooth loss, is unlikely to be due to chance or bias, given the strength of association, its consistency across strata including interviewer and control type, and the non‐complexity of exposure assessment for an irreversible exposure whose presence precedes cancer by decades. Instead, in its association with ESCC, we consider here whether dental fluorosis may be a proxy for exposures on different, not necessarily mutually exclusive, mechanistic pathways as follows. First, in this risk association, dental fluorosis could have been a proxy for fluoride, with this early‐life exposure marker indicating life‐long fluoride ingestion, causing EC through oxidative stress, disruption of salivary glutathione defenses, or a direct chemical effect on the esophageal epithelium. However this pathway is very unlikely due to the absence of a dental fluorosis–ESCC association in people with little tooth loss or decay. Further, there is no external evidence of fluoride carcinogenicity in humans. In 1987 the IARC monograph program evaluated inorganic fluoride in drinking water as “not classifiable as to its carcinogenicity to humans (Group 3)”28 and since then, associations with the primary site of interest osteosarcoma were null.29 Second, dental fluorosis may be a proxy for life‐long intake of water with naturally high fluoride levels, but the relevant ESCC carcinogenic effect comes from non‐fluoride constituents or properties of this water, such as its alkalinity, akin to the ESCC link to salt tea in Kashmir.30 Indeed, there are often chemicals co‐present in high‐fluoride water such as, further north in the Rift Valley, calcium, aluminum, copper and rubidium in Ethiopia.31 Third, we consider that the striking correlation between the severity of dental fluorosis and prevalence of oral leukoplakia, tooth loss and decay, which – of most relevance—was present in controls, suggests that a common oral cavity chronic irritation or oral microbiome alteration is most likely implicated. This pathway is further supported by the restriction of the dental fluorosis‐ESCC association to people who also had tooth loss or decay, and, albeit with limited power, the absence of such an association if tooth loss or decay was absent. It is plausible that the very steep increased risk associated with moderate/severe fluorosis in conjunction with tooth decay or loss, is because dental pits, being present from the time of dentition eruption, form a life‐long pro‐carcinogenic ecological niche in the oral cavity e.g. for bacterial communities, such as Porphyromonas gingivalis nominally associated with ESCC in US cohorts.32 Under this hypothesis, whilst the window of susceptibility to dental fluorosis is in the first ~6 years of life, in terms of exposures relevant to ESCC risk, such exposures commence after this period, i.e. upon eruption of the permanent dentition. The presence of dental pits since early in life thus represents prolonged exposure and as such, even in the extraordinarily young ESCC patients unique to this setting, by age 25 or 35 an individual with severe fluorosis has had almost 20 and 30 years, respectively, with dental pits and possibly also with a pro‐carcinogenic oral milieu.
Strengths and weaknesses
This is the first ESCC case–control study in Eastern Africa to include a wide range of potential risk factors/confounders and multiple measures of oral health. Histological confirmation reduced outcome misclassification and selection bias was reduced through a non‐capital city setting and inclusion of different types of controls, as discussed in a detailed evaluation of the pilot phase.20 The irreversibility of dental fluorosis rules out the possibility of reverse causality, however interviewer‐bias was possible but reduced due to professional training. Future studies should consider exposure validation by independent experts, inclusion of periodontal disease, using photographs and the granular 10‐point TFI range. The study also lacked a separate count of missing teeth due to accidents, rites of passage or other cultural practices, and a life‐time history of water source. Finally, the sample size was limited for a finer examination of effect modifiers. Regarding the spatial comparison of EC and fluoride across Africa, this is a simple visual examination, which lacks accuracy and granularity for cancer incidence rates.
Wider implications of the findings in Kenya and Africa
Dental fluorosis, a hypomineralization of the dental enamel, is prevalent in Kenya due to the combination of excessive fluoride intakes at ages 1–7 years during permanent dentition development and increased susceptibility to fluorosis at high altitudes where increased urinary pH reduces the kidney's excretion of fluoride (Eldoret is at 2100 m).33, 34 Fluoride is ingested during water intake, but is also present in staples grown or extracted from this fluoride‐rich ecosystem, including tea, trona (magadi), whole fish, spinach and maize. However, whilst the problem of high fluoride levels in Kenyan ground and surface water is well known and widespread, it is not ubiquitous.19 Fluoride levels can vary considerably on a small‐scale according to the locations of volcanic fissures, thus explaining the absence of clustering of affected participants and reducing the possibility that findings are due to an unmeasured confounder.
The findings clearly point to the potential of preventing ESCC in Kenya. From young ages, education of the importance of oral hygiene, and ensuring access to modern oral hygiene methods should be considered as part of Kenya's cancer control plan and would benefit ESCC, oral cancer and oral health. Such efforts need to ensure inclusion of socially disadvantaged population groups, in whom ESCC risks associated with reliance on non‐piped water, poor oral hygiene and alcohol and tobacco use are concentrated. Finally, the findings need to be considered in light of the proposed etiological model of a strong spatially‐patterned risk factor(s) underlying the African EC corridor. Excessive groundwater fluoride is a highly spatially‐correlated attribute prevalent in and near Africa's Rift Valley – including the high ESCC risks areas of Kilimanjaro Tanzania, the Ethiopian Rift, and parts of Malawi. In these areas, fluoride levels are among the world's highest. Compared to the WHO recommended levels of 0.5–1.0 mg/L and a maximum safe limit of 1.5 mg/L fluoride, a Kenyan survey of 1,282 nationwide samples found that 62% had fluoride concentrations above 1 mg/L, and 20% were above 5 mg/L. Thus, in light of the high levels of this unique exposure, the contribution, consistency and mechanisms of the dental fluorosis‐ESCC link need to be evaluated across Eastern Africa. The public health importance cannot be overstated. In 2020 alone, across this region 23,000 people will be newly diagnosed with ESCC, and 21,000 will die from this cancer.35 For prevention of this burden, in addition to the improvements in oral health, additional research will be needed to address how to break the oral health and dental fluorosis‐ESCC link, whether through improved oral hygiene and health, acceleration of de‐fluoridation efforts now underway across the region, improved access to piped water supplies or other means. In conclusion, given the magnitude of the association of dental fluorosis with esophageal cancer risk and the unique presence of this exposure circumstance in Africa's Rift Valley esophageal cancer corridor, research on oral microbiome or other biological mechanisms that explain this strong link is warranted.
Ethical approval
The study was approved by the Institutional Research and Ethics Committee of Moi University, Eldoret, Kenya (no. 000921) and the International Agency for Research on Cancer (IARC) Ethics Committee (IEC14‐15). Written informed consent was obtained from all participants.
Author contributions
DM, VMcC and JS were involved in the study concept, design and management. SKM and VMcC conducted statistical analyses and wrote the first draft of the study. All co‐authors contributed to the analysis of the data and interpretation of results.
Supporting information
Supplementary Figure 1 Pictures of dental fluorosis scores utilized. A Normal, no dental fluorosis. B. Mild opacities, but no pits. C. Small or medium pits. D. Large pits with loss of enamel
Supplementary Table 1: Prevalence of oral hygiene habits and oral health status in controls, by sociodemographic factors.
Acknowledgements
We express our thanks to the participants in this case–control study for their support and time. This work was supported by the International Agency for Research on Cancer (IARC), NIH/NCI grant R21CA191965, an IARC‐UICC Development Fellowship to SKM and an IARC post‐doctoral fellowship to DM partially supported by the European Commission FP7 Marie Curie Actions – People – Co‐funding of regional, national and international programs (COFUND).
Conflict of interest: The authors declare no completing interests.
Contributor Information
Diana Menya, Email: dianamenya@gmail.com.
Valerie A. McCormack, Email: mccormackv@iarc.fr.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. McCormack VA, Menya D, Munishi MO, et al. Informing etiologic research priorities for squamous cell esophageal cancer in Africa: a review of setting‐specific exposures to known and putative risk factors. Int J Cancer 2017;140:259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Menya D, Kigen N, Oduor M, et al. Traditional and commercial alcohols and esophageal cancer risk in Kenya. Int J Cancer 2019;144:459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abnet CC, Kamangar F, Islami F, et al. Tooth loss and lack of regular oral hygiene are associated with higher risk of esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 2008;17:3062–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abnet CC, Qiao YL, Mark SD, et al. Prospective study of tooth loss and incident esophageal and gastric cancers in China. Cancer Causes Control 2001;12:847–54. [DOI] [PubMed] [Google Scholar]
- 6. Dar NA, Islami F, Bhat GA, et al. Poor oral hygiene and risk of esophageal squamous cell carcinoma in Kashmir. Br J Cancer 2013;109:1367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen X, Yuan Z, Lu M, et al. Poor oral health is associated with an increased risk of esophageal squamous cell carcinoma ‐ a population‐based case‐control study in China. Int J Cancer 2017;140:626–35. [DOI] [PubMed] [Google Scholar]
- 8. Sato F, Oze I, Kawakita D, et al. Inverse association between toothbrushing and upper aerodigestive tract cancer risk in a Japanese population. Head Neck 2011;33:1628–37. [DOI] [PubMed] [Google Scholar]
- 9. Liang H, Yang Z, Wang JB, et al. Association between oral leukoplakia and risk of upper gastrointestinal cancer death: a follow‐up study of the Linxian general population trial. Thorac Cancer 2017;8:642–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Michaud DS, Liu Y, Meyer M, et al. Periodontal disease, tooth loss, and cancer risk in male health professionals: a prospective cohort study. Lancet Oncol 2008;9:550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guha N, Boffetta P, Wunsch Filho V, et al. Oral health and risk of squamous cell carcinoma of the head and neck and esophagus: results of two multicentric case‐control studies. Am J Epidemiol 2007;166:1159–73. [DOI] [PubMed] [Google Scholar]
- 12. Patel K, Wakhisi J, Mining S, et al. Esophageal cancer, the topmost cancer at MTRH in the Rift Valley, Kenya, and its potential risk factors. ISRN Oncol 2013;2013:503249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manji F, Baelum V, Fejerskov O. Tooth mortality in an adult rural population in Kenya. J Dent Res 1988;67:496–500. [DOI] [PubMed] [Google Scholar]
- 14. Macigo FG, James RM, Ogunbodede E, et al. Sugar consumption and dental caries experience in Kenya. Int Dent J 2016;66:158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baelum V, Fejerskov O, Manji F. Periodontal diseases in adult Kenyans. J Clin Periodontol 1988;15:445–52. [DOI] [PubMed] [Google Scholar]
- 16. Macigo FG, Gathece LW, Guthua SW, et al. Oral hygiene practices and risk of oral leukoplakia. East Afr Med J 2006;83:73–8. [DOI] [PubMed] [Google Scholar]
- 17. Macigo FG, Mwaniki DL, Guthua SW. Prevalence of oral mucosal lesions in a Kenyan population with special reference to oral leukoplakia. East Afr Med J 1995;72:778–82. [PubMed] [Google Scholar]
- 18. Manji F, Baelum V, Fejerskov O. Dental fluorosis in an area of Kenya with 2 ppm fluoride in the drinking water. J Dent Res 1986;65:659–62. [DOI] [PubMed] [Google Scholar]
- 19. Nair KR, Manji F, Gitonga JN. The occurrence and distribution of fluoride in groundwaters of Kenya. East Afr Med J 1984;61:503–12. [PubMed] [Google Scholar]
- 20. Menya D, Oduor M, Kigen N, et al. Cancer epidemiology fieldwork in a resource‐limited setting: experience from the western Kenya ESCCAPE esophageal cancer case‐control pilot study. Cancer Epidemiol 2018;57:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martin GC, Brown JP, Eifler CW, et al. Oral leukoplakia status six weeks after cessation of smokeless tobacco use. J Am Dent Assoc 1999;130:945–54. [DOI] [PubMed] [Google Scholar]
- 22. Golozar A, Etemadi A, Kamangar F, et al. Food preparation methods, drinking water source, and esophageal squamous cell carcinoma in the high‐risk area of Golestan, Northeast Iran. Eur J Cancer Prev 2016;25:123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tran GD, Sun XD, Abnet CC, et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer 2005;113:456–63. [DOI] [PubMed] [Google Scholar]
- 24. Malago J, Makoba E, Muzuka ANN. Fluoride levels in surface and groundwater in Africa: a review. Am J Water Sci Eng 2017;3:1–17. [Google Scholar]
- 25. Brunt R, Vasak L, Griffioen J. Fluoride in groundwater: probability of occurrence of excessive concentration on a global scale. The Netherlands: International Groundwater Resources Assessment Centre, 2004. [Google Scholar]
- 26. Loots E, Sartorius B, Madiba TE, et al. Oesophageal squamous cell cancer in a South African tertiary hospital: a risk factor and presentation analysis. S Afr J Surg 2017;55:42–6. [PubMed] [Google Scholar]
- 27. Abnet CC, Qiao YL, Dawsey SM, et al. Tooth loss is associated with increased risk of total death and death from upper gastrointestinal cancer, heart disease, and stroke in a Chinese population‐based cohort. Int J Epidemiol 2005;34:467–74. [DOI] [PubMed] [Google Scholar]
- 28. Monographs I. IARC Monographs. Overall evaluations of carcinogenicity: an updating of IARC Monographs Volumes 1 to 42. IARC: Lyon, France, 1987. [PubMed] [Google Scholar]
- 29. Blakey K, Feltbower RG, Parslow RC, et al. Is fluoride a risk factor for bone cancer? Small area analysis of osteosarcoma and Ewing sarcoma diagnosed among 0‐49‐year‐olds in Great Britain, 1980‐2005. Int J Epidemiol 2014;43:224–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dar NA, Bhat GA, Shah IA, et al. Salt tea consumption and esophageal cancer: a possible role of alkaline beverages in esophageal carcinogenesis. Int J Cancer 2015;136:E704–10. [DOI] [PubMed] [Google Scholar]
- 31. Kravchenko J, Rango T, Akeshevich I, et al. The effect of non‐fluoride factors on risk of dental fluorosis: evidence from rural populations of the Main Ethiopian Rift. Sci Total Environ 2014;488:595–606. [DOI] [PubMed] [Google Scholar]
- 32. Peters BA, Wu J, Pei Z, et al. Oral microbiome composition reflects prospective risk for esophageal cancers. Cancer Res 2017;77:6777–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Manji F, Baelum V, Fejerskov O. Fluoride, altitude and dental fluorosis. Caries Res 1986;20:473–80. [DOI] [PubMed] [Google Scholar]
- 34. Whitford GM. Determinants and mechanisms of enamel fluorosis. Ciba Found Symp 1997;205:226–41. discussion 41‐5. [DOI] [PubMed] [Google Scholar]
- 35. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. 2013. http://globocan.iarc.fr.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Pictures of dental fluorosis scores utilized. A Normal, no dental fluorosis. B. Mild opacities, but no pits. C. Small or medium pits. D. Large pits with loss of enamel
Supplementary Table 1: Prevalence of oral hygiene habits and oral health status in controls, by sociodemographic factors.


