Abstract
In urothelial bladder cancer (UBC), risk stratification remains an important unmet need. Limitless self‐renewal, governed by TERT expression and telomerase activation, is crucial for cancer progression. Thus, telomerase activation through the interplay of mutations (TERTpMut) and epigenetic alterations in the TERT promoter may provide further insight into UBC behavior. Here, we investigated the combined effect of TERTpMut and the TERT Hypermethylated Oncological Region (THOR) status on telomerase activation and patient outcome in a UBC international cohort (n = 237). We verified that TERTpMut were frequent (76.8%) and present in all stages and grades of UBC. Hypermethylation of THOR was associated with higher TERT expression and higher‐risk disease in nonmuscle invasive bladder cancers (NMIBC). TERTpMut alone predicted disease recurrence (HR: 3.18, 95%CI 1.84 to 5.51, p < 0.0001) but not progression in NMIBC. Combined THORhigh/TERTpMut increased the risk of disease recurrence (HR 5.12, p < 0.0001) and progression (HR 3.92, p = 0.025). Increased THOR hypermethylation doubled the risk of stage progression of both TERTpwt and TERTpMut NMIBC. These results highlight that both mechanisms are common and coexist in bladder cancer and while TERTpMut is an early event in bladder carcinogenesis THOR hypermethylation is a dynamic process that contributes to disease progression. While the absence of alterations comprises an extremely indolent phenotype, the combined genetic and epigenetic alterations of TERT bring additional prognostic value in NMIBC and provide a novel insight into telomere biology in cancer.
Keywords: urothelial bladder cancer, telomerase, TERT promoter methylation, TERT promoter mutations, recurrence, progression
Short abstract
What's new?
Telomerase reverse transcriptase (TERT) activation is central to cancer cell immortalization. It acts, however, through relatively unknown mechanisms. In urothelial bladder cancer (UBC) in particular, TERT activation can occur in the presence or absence of mutation, raising questions about alternative activation mechanisms. Our study shows that hypermethylation of the TERT promoter (THOR) plays a key part in UBC, being a dynamic and progressive process, with hypermethylation levels increasing with bladder cancer severity. Moreover, both hypermethylation and TERT promoter mutation contributed to increased telomerase expression. The findings provide insight into telomere biology in UBC and may be applicable to other tumors.
Abbreviations
- HG
high‐grade tumors
- LG
low‐grade tumors
- MIBC
muscle invasive bladder cancer
- Mut
mutant
- NMIBC
nonmuscle invasive bladder cancer
- TERT
telomerase reverse transcriptase
- TERTpMut
TERT promoter mutations
- THOR
TERT hypermethylated oncological region
- THORhigh
THOR hypermethylated
- THORlow
THOR hypomethylated (or nonmethylated)
- UBC
urothelial bladder cancer
- Wild
Wild type
Introduction
Urothelial bladder cancer (UBC) poses significant burden as it is responsible for 123,051 deaths annually and at any given time there are more than 500,000 UBC patients in the USA alone.1, 2 UBC is a remarkably heterogeneous disease, including nonmuscle invasive (NMIBC) and muscle invasive disease (MIBC). Pathological tumor stage and grade drive prognostic predictions and ultimately, therapy recommendations. However, pathology alone is often insufficient to predict individual outcomes. Grade and stage are crude measures and many patients are over‐ or undertreated as a result. Unlike other cancer types, few molecular markers currently guide UBC management.3, 4
At diagnosis the majority of UBC (75%) are NMIBC (Ta, T1), mostly low grade (LG). LG tumors are rarely lethal but recur locally with variable and unpredictable rates.5, 6 Muscle‐invasive bladder cancers (MIBC – T2, T3, T4), on the other hand, almost universally high‐grade (HG), can be lethal and associated with worse clinical outcomes.5, 6 A subset of NMIBC are HG and destined to progress to life‐threatening MIBC. There is an unmet need to improve the prediction of those patients with NMIBC at risk of progression to MIBC. The stakes are high as NMIBC can be managed with conservative therapy whereas MIBC requires either the removal of the bladder (cystectomy) or chemo‐radiation.7
Telomerase, the enzyme complex responsible for maintaining telomere length and genome integrity is responsible for cellular immortalization (a hallmark of cancer).8, 9, 10, 11 Telomerase activity is upregulated in 85–90% of all cancers.12, 13 Mutations in the promoter of the catalytic subunit of the enzyme, termed telomerase reverse transcriptase (TERT) are frequently observed in several cancers and drive telomere maintenance.14, 15, 16 In UBC, TERT promoter mutations (C to T transitions at chr5:1,295,228 and chr5:1,295,250) are more common than any other genetic alterations and can lead to increased TERT expression and telomerase activity.17, 18, 19, 20 Importantly, TERT promoter mutations are associated with worse clinical outcome in most studies further highlighting the role of telomerase activation in tumor progression and recurrence.18, 19, 20, 21, 22, 23, 24, 25, 26, 27 Noteworthy, not all TERTp Mut tumors display telomerase activation and non‐TERTp Mut UBC may express TERT suggesting that the presence of additional mechanisms are necessary for telomerase activation in UBC.28
Our group and others have identified a parallel epigenetic control of telomerase activation in cancer. Specifically, a region located upstream of the core mutation area, within the TERT promoter, termed THOR (TERT Hypermethylated Oncological Region) is hypermethylated in many TERT‐expressing cancers, is associated with telomerase activation and predicts clinical outcomes in multiple tumor types.14, 29, 30 We therefore hypothesized that dual mechanisms activate telomerase in UBC. Here, we studied the interaction and contribution of both genetic (TERTp Mut) and epigenetic (THOR) TERT promoter alterations to telomerase activation and prognosis in UBC, using a multi‐institutional cohort.
Material and Methods
Open access data
The Cancer Genome Atlas (TCGA) Research Network (http://cancergenome.nih.gov) database for UBC was analyzed. A single, probe located within THOR (cg11625005) was used for methylation analysis (Illumina Infinium 450 k array). TERT expression data were evaluated from the gene expression dataset (polyA+IlluminaHiSeq) (details in Supporting Information).
Patients
Patients’ selection and pathological characteristics are presented in Supporting Information Table S1. All patients underwent surgery (either transurethral bladder resection or radical cystectomy) and followed for a median period of 107.4 months (IQR: 32.4–266.8 months). 331 bladder tissue samples, from 331 patients (237 UBC and 94 normal urothelium) were collected and analyzed for THOR methylation, TERT promoter mutations and a subgroup for hTERT expression. Survival data was collected for the 237 patients with UBC. On 10 of these patients, an additional tumor at recurrence/progression was analyzed.
Molecular analysis of the TERT promoter
Sanger Sequencing was used to determined TERT promoter mutation status. Samples were considered mutant (TERTpMut) if any of the mutations (1,295,228 G > A or 1,295,250 G > A) were present (Supporting Information Table S2). Quantitative sodium bisulfite pyrosequencing was performed for THOR as previously described.30 TERT expression was performed with the QX200 Droplet Digital PCR system (see Supporting Information for details).
Statistical analysis
SAS version 9.4 was used for statistical analyses. THOR was initially evaluated as a continuous value to determine its association with normal and malignant urothelial bladder tissue and to further interrogate its association with stage, grade and high and low risk disease. For the prognostic model we dichotomized into high‐ and low‐THOR‐methylation groups by receiver operating characteristic (ROC) analysis. Clinical outcomes for the TERT promoter mutations and THOR methylation were determined by Kaplan–Meier Survival curves. Cox Proportional Hazards (CPH) models were used to assess univariate and multivariate significance (details Supporting Information).
Results
TERT promoter mutations are early and frequent events in UBC
TERT promoter mutations (TERTpMut) were highly prevalent (76.8%, n = 182). The predominant alteration was g.1,295,228 C > T which accounted for 90.1% of all mutations. No mutations were found in normal urothelium (Table 1).
Table 1.
Summary of TERT promoter methylation (THOR) and TERT promoter mutation status
| THOR Methylation | ||||
|---|---|---|---|---|
| THORlow | 110 | 46.4% | ||
| THORhigh | 127 | 53.6% | ||
| Stage | THORlow | THORhigh | ||
| Ta | 45 | 43.7% | 58 | 56.3% |
| T1 | 49 | 51% | 47 | 49% |
| ≥ T2 | 16 | 42.1% | 22 | 57.9% |
| Total | 110 | 46.4% | 127 | 53.6% |
| Grade | THORlow | THORhigh | ||
| Low Grade | 76 | 56.3% | 59 | 43.5% |
| High Grade | 28 | 27.5% | 74 | 72.5% |
| Total | 104 | 43.9% | 133 | 56.1% |
| THOR Methylation | ||||
| TERTpMutStatus | ||||
| TERTpWt | 55 | 23.2% | ||
| TERTpMut | 182 | 76.8% | ||
| TERT promoter mutations (per mutation) | ||||
| C228T | 164 | 90.1% | ||
| C250T | 18 | 9.9% | ||
| C228T/C250T | 0 | 0% | ||
| Total | 182 | 100% | ||
| THOR Methylation | Wt (n/%) | Mutant (n/%) | ||
| Ta | 28 | 27.2% | 75 | 72.8% |
| T1 | 23 | 24.0% | 73 | 76.0% |
| ≥ T2 | 4 | 10.5% | 34 | 89.5% |
| Total | 55 | 23.2% | 182 | 76.8% |
| THOR Methylation | Wt (n/%) | Mutant (n/%) | ||
| Low Grade | 36 | 26.7% | 99 | 73.4% |
| High Grade | 19 | 18.6% | 83 | 81.4% |
| Total | 55 | 23.2% | 182 | 76.8% |
THORlow = THOR hypomethylated; THORhigh = THOR hypermethylated; TERTpMutStatus = TERT promoter mutation status; TERTpMut = TERT promoter mutation; TERTpWt = TERT promoter wild type.
Frequency of TERT promoter mutations (wild type and mutant) and THOR methylation (hypomethylated and hypermethylated) according to stage and grade disease.
TERTpMut were identified in all stages and grades of UBC [detected in 69.3% of NMIBC (n = 135/199) and 73.4% of low‐grade lesions]. TERTpMut were identified in all metastatic (tumor positive) pelvic lymph nodes as well as in their corresponding primary tumor. Interestingly, TERTpMut were also identified in some tumor negative lymph nodes (Supporting Information Table S3).
Consistent with previous studies, some of these TERTpMut UBC did not exhibit high TERT expression even when compared to UBC with a wild type promoter (TERTpWt) (Supporting Information Fig. S1A, S1C).18
These data support an early oncogenic role of TERTpMut and the possibility that other mechanisms may also upregulate TERT expression in UBC.
THOR hypermethylation is a dynamic process in UBC tumorigenesis
In order to evaluate the extent of THOR methylation and its effect on TERT expression in UBC we analyzed a representative CG site within THOR (cg11625005) in a cohort of MIBC from the TCGA (n = 433). When compared to normal urothelium, MIBC had significantly higher methylation at THOR and higher TERT mRNA levels (Supporting Information Fig. S2A, p < 0.0001; Supporting Information Fig.S2B, p < 0.0001). THOR hypermethylated (THORhigh) was associated with considerably higher TERT mRNA levels in malignant tissue (Supporting Information Fig. S2C, p < 0.0001) further supporting the role of TERT promoter methylation in TERT transcriptional activation.
To directly test this observation we assayed multiple representative CG sites from THOR in normal and UBC samples from our multi‐institutional cohort. THOR was hypermethylated (THORhigh) in 127 UBC (53.6%, Table 1) and significantly hypermethylated in tumors compared to benign histology in nonmatched samples (Fig. 1 a, p < 0.0001). Additionally, paired samples from the same surgical specimen revealed that THOR methylation is 2 times higher in the tumor region than the corresponding normal urothelium (Fig. 1 b).
Figure 1.
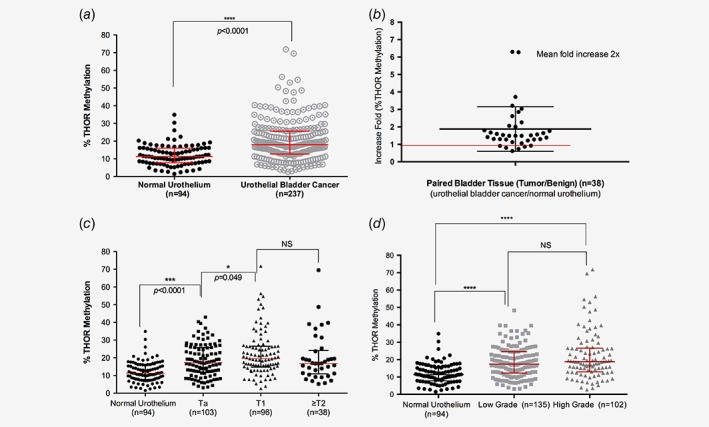
THOR methylation in urothelial bladder cancer. (a) THOR methylation status in normal urothelium and tumor tissue in the entire cohort (****, p < 0.0001) (b) The ratio of tumor/normal tissue in paired samples from the same patient (n = 38). Note a mean of twofold increase in THOR methylation status in the malignant tissue. (c) THOR methylation status in different tumor stages. (d) THOR methylation status within tumor grades, low grade has significantly higher THOR methylation than normal urothelium (****, p < 0.0001). NS = nonsignificant. Error bars represent median and Interquartile range (IQR). [Color figure can be viewed at wileyonlinelibrary.com]
Since UBC are stratified by invasiveness (T stage) and cellular morphology (grade) as predictors of tumor progression, we also tested THOR ability to distinguish stages and grades. THOR methylation was significantly higher in tumor tissue, even when comparing superficial lesions (Ta) with normal tissue (Fig. 1 c, p < 0.0001). THOR methylation levels are slightly higher in T1 disease than in Ta (Fig. 1 c, p = 0.049) but do not reach the same significant difference verified between normal urothelium and Ta disease. Similarly, THOR methylation demonstrated a progressive pattern from normal urothelium to low‐grade tumors and maintaining the same trend in high‐grade tumors (Fig. 1 d). Clinically, THOR exhibited higher hypermethylation in high‐risk (T1 and HG) when compared to low risk tumors (Supporting Information Fig. S3, p = 0.034).31
To further explore the changes in THOR over time, we assessed THOR methylation in UBC harvested from consecutive surgeries (at both the time of the initial diagnosis and the first recurrence or progression). A significant increase in THOR methylation levels was observed in tumors with stage progression (p = 0.018, mean increase fold of 1.76), but not in nonprogressive ones (p = 0.88) suggesting that THOR is hypermethylated in tumors harboring TERT promoter mutations (Supporting Information Table S4).
Finally, analysis of TERT expression revealed that higher levels of THOR methylation are related to higher levels of expression (Supporting Information Fig. S1B, p = 0.049). The highest TERT expression is observed when both alterations (methylation and mutations) are present (Supporting Information Fig. S1C).
Overall, these data suggest that THOR methylation increases progressively during the earlier stages of UBC and both TERT promoter mutations and hypermethylation contribute to increased telomerase expression.
Prognostic value of TERT promoter alterations in NMIBC
As expected, in these selected cohorts, pathological grade was predictive of progression (n = 199, p = 0.02) (Supporting Information Fig. S4).
As THOR methylation increases with disease stage while TERTpMut is an early event, we assessed the value of TERT promoter alterations as markers of recurrence and disease progression in NMIBC.
Consistent with previous reports, patients harboring TERTpMut had a significantly higher risk of recurrence (HR: 3.18, 95% CI 1.8–5.5; p < 0.0001; Table 2) with significantly decreased median disease free survival (Log rank p < 0.0001; Fig. 2 a).17, 18, 25 However, TERTpMut status did not reach statistical significance with respect to the risk of progression to invasive disease (p = 0.052) (Fig. 2 b, Table 2).17, 18
Table 2.
Univariate Cox proportional hazards regression analysis of time for disease recurrence and disease progression in NMIBC (n = 199)
| Disease Recurrence | Disease Progression | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | Chi Sq | P | HR | 95% CI | Chi Sq | P | |
| TERTpMutationStatus | ||||||||
| TERTpWt (n = 51) | Ref | Ref | ||||||
| TERTpMut (n = 148) | 3.18 | 1.8 to 5.5 | 17.16 | <0.0001 | 2.36 | 0.99 to 5.60 | 3.82 | 0.052 |
| THORMethy | ||||||||
| THORlow (n = 105) | Ref | Ref | ||||||
| THORhigh (n = 94) | 1.50 | 1.0 to 2.2 | 4.37 | 0.03 | 1.81 | 0.97 to 3.35 | 3.52 | 0.057 |
| THORMethy/TERTpMutStatus | ||||||||
| THORlow/TERTpWild (n = 35) | Ref | Ref | ||||||
| THORlow/TERTpMut
+ THORhigh/TERTpWild
(n = 85) |
4.53 | 2.0 to 10 | 13.86 | 0.0002 | 2.64 | 0.77 to 9.1 | 2.38 | 0.123 |
| THORhigh/TERTpMut
(n = 79) |
5.12 | 2.3 to 11.3 | 16.33 | <0.0001 | 3.92 | 1.2 to 13.0 | 4.97 | 0.025 |
HR = Hazard ratio; CI: confidence interval; Chi Sq = Chi Squared.
TERTpMutStatus = TERT promoter mutation status (for the studied mutations); TERTpWt = TERT promoter wild type; TERTpMut = TERT promoter mutant; THORMethy = THOR methylation status; THORhigh = THOR hypermethylated; THORlow = THOR hypomethylated.
Figure 2.
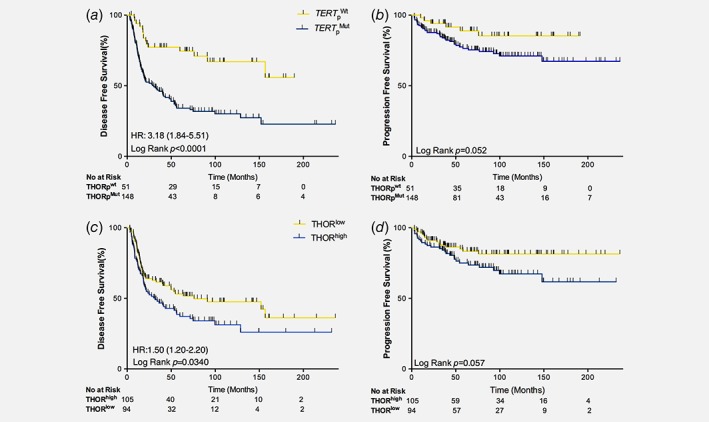
Survival estimates for patients with NMIBC stratified by TERT promoter alterations. (a) Disease free and (b) progression free survival for patients with NMIBC stratified by TERT promoter mutation status. (c) Disease free and (d) progression free survival for patients with NMIBC stratified by TERT promoter methylation status. THORlow = THOR hypomethylated, THORhigh = THOR hypermethylated. TERTpWt = TERT promoter wild type (for the two studied mutations); TERTpMut = TERT promoter mutant. Yellow = TERTpWt and THORlow; Blue = TERTpMut and THORhigh.
As for TERTpMut, THORhigh NMIBC recurred more frequently (HR: 1.5, 95% CI 1.02–2.20; p = 0.03; Table 2), with decreased median disease‐free survival (76 months vs. 31.7 months) (Log rank p = 0.034; Fig. 2 c). Also, THORhigh did not reach significance for the risk of progression (Log rank p = 0.059; Fig. 2 d, Table 2) in NMIBC.
Combined TERT promoter alterations predict disease progression in NMIBC
We then analyzed the combined prognostic impact of both TERT promoter alterations in NMIBC. We first evaluated the association of TERT promoter alterations and clinical outcomes according to grade and stage of NMIBC. THORhigh/TERT p Mut was the most common phenotype in NMIBC which recurred or progressed independently of stage (Supporting Information Figs. S5 and S6). The absence of any alteration (THORlow/TERT p Wt) was associated with a more indolent phenotype even in HG NMIBC (Supporting Information Fig. S6).
Concomitant THOR hypermethylation and mutations (THORhigh/TERT p Mut) were associated with increased risk of disease recurrence (HR: 5.12; 95% CI 2.23–11.32, Table 2) with less than 30% disease free survival (DFS) at 10 years. In contrast, the absence of both alterations (THORlow/TERT p Wt) was associated with 80% DFS at 10 years (Fig. 3 a, Log rank p < 0.0001). The presence of either TERT promoter alterations (TERTpMut or THORhigh) increased the risk of disease recurrence with a worse disease free survival (30.8% and 45% at 10 years, respectively; Fig. 3 a).
Figure 3.
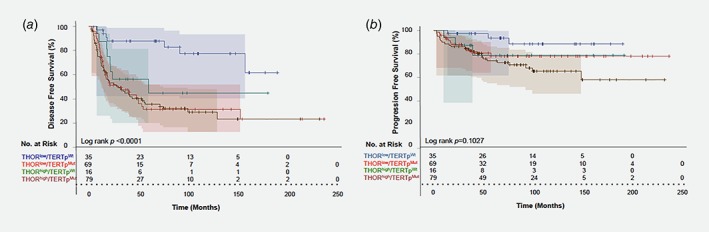
Survival analysis of combined TERT promoter alterations in patients with NMIBC. Kaplan–Meier analysis for (a) disease free and (b) progression free survival stratified by combined TERT promoter mutations and THOR methylation status in nonmuscle invasive bladder cancer patients. THORlow/TERTpWt = blue; THORlow/TERTpMut = light red; THORhigh/TERTpWt = green; THORhigh/TERTpMut = dark red.
The presence of either TERT promoter alterations conferred a 4.53‐fold increased risk of recurrence (95% CI 2.05–10.01; p = 0.0002) but not for progression (p = 0.14). Combined THORhigh /TERT p Mut was a risk factor for both recurrence (HR: 5.4; CI 95% 2.42–12.04; p < 0.0001) and progression (HR: 4.01; CI 95% 1.19–13.5; p = 0.024; Table 2). Furthermore, THORlow/TERT p Wt NMIBC patients had a 91.4% progression free survival (PFS) at 10 years of follow‐up, significantly better than THORhigh/TERT p Mut (66.4% PFS; Log rank p = 0.019; Fig. 3 b). When adjusting for stage (T1 vs. Ta), grade (HG vs. LG), gender and age, THORhigh/TERT p Mut also displays a trend toward significance (HR: 3.32, CI 95% 0.99–11.16; p = 0.05) (Supporting Information Table S5).
We then tested the effect of continuous increase in THOR methylation to enhance the predictive ability of disease progression in NMIBC, adjusting for TERT promoter mutations status. THOR levels affected disease progression in both TERTpMut and TERTpWt phenotypes. Increased THOR methylation from 10% (normal) to 50%, more than doubled the risk of disease progression independently of TERT promoter mutation status (Fig. 4).
Figure 4.
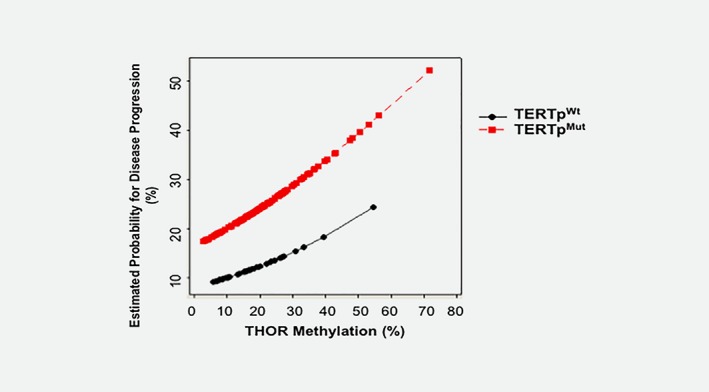
Estimated probability of disease progression based on THOR methylation levels. Risk of progression is estimated by any TERT promoter mutation status (wild type or mutant) as a result of increased THOR methylation. Red‐TERT promoter mutant NMIBC, Black‐TERTpWt NMIBC.
Discussion
Our study is the first to assess concomitant TERT promoter alterations in UBC. Our results suggest a temporal and cooperative association between TERT promoter mutations and methylation, impacting telomere biology and clinical outcomes.
TERT expression is upregulated in 85–90% of tumors via multiple molecular mechanisms including somatic mutations, TERT amplifications, TERT structural variants and epigenetic modifications through TERT promoter methylation.14 Our data reveal that 76.8% of UBC cancers harbor TERTpMut and 53.6% have THORhigh, and 24.6% (n = 49/199) of LG NMIBC acquired both alterations. The kinetics and interaction of these alterations in UBC, and the resulting effect on TERT expression are still unknown and important to decipher.
The observation that TERTpMut are found ubiquitously across all stages and grades of UBC, even in low stage and grade lesions with low TERT expression, suggests that TERTpMut is an early event necessary but insufficient by itself to drive disease progression. In fact other studies also verified that TERT promoter mutant UBC might express low mRNA levels.18 Similar data exist in other tumors where TERT promoter mutations are common. For example, in gliomas, TERTpMut are detected in lower grade lesions where TERT expression and self‐renewal are low.32, 33
TERTpMut is necessary but not sufficient to maintain telomere length or telomerase upregulation.28 In TERTpMut tumors additional alterations are likely required to upregulate telomerase and promote tumor progression.
We previously showed that THOR hypermethylation is a dynamic process during gliomagenesis and prostate cancer progression.29, 30 In UBC, TERTpMut is an early event while THOR hypermethylation is associated with disease progression and increased TERT expression. This pattern has been observed in other cancer types.14, 29, 30
A weakness of the WHO 2004/2016 pathological classification of NMIBC is that it gives almost no prognostic information in T1 patients as nearly all are classified as HG.34 In our study, TERT promoter alterations add significant value as prognostic biomarkers. For example, T1 THORlow/TERTpWt tumors, including HG lesions, carry a risk of progression of less than 10%, in sharp contrast with the 52% of progression for THORhigh/TERTpMut. If confirmed in prospective studies, this may help guide management when choosing conservative vs. aggressive therapy for these patients. Given the high recurrence rate in NMIBC, the identification of patients with very low potential for disease recurrence and progression might change the costly and invasive follow‐up protocols in favor of individualized strategies.
In contrast, THORhigh/TERTpMut significantly increased the chance of NMIBC recurrence and is a risk factor for disease progression across stages and grades. Furthermore, for the different TERT promoter mutation status, continuous THOR hypermethylation increases the risk of disease progression, reinforcing the dynamic and crucial role of THOR methylation in bladder cancer tumorigenesis. Further supporting the role of THOR methylation, tumors exhibited higher THOR methylation at the time of stage progression.
Together, our findings support the hypothesis that TERTpMut are early triggers in tumorigenesis which require cooperation with other events including THOR hypermethylation to ensure telomerase activation, immortality and disease progression.
The exact mechanism of telomerase activation by promoter hypermethylation remains under investigation. One possible explanation is that methylation leads to three‐dimensional changes in local chromatin architecture resulting in increased transcription.35 Alternatively, methylation of this distal part of the promoter might also prevent the binding of transcriptional repressors, which in turn enables TERT expression.36 These mechanisms ought to be explored further as they may result in the development of novel targeted therapies. Demethylating agents have shown some encouraging results in cancers where THOR is hypermethylated, and may have a role in decreasing the risk of progression of NMIBC.37
Our study has limitations due to its retrospective nature, patient selection, absence of centralized pathology review (although we chose specifically the HG vs. LG 2004/16 WHO classification less prone to inter‐observer variability).34 Importantly, one should interpret the univariate and multivariate analysis with caution as Ta ant T1 tumors are treated differently. Furthermore, since large numbers of tumors are necessary for survival analysis in NMIBC, in our multivariate analysis THORHigh/TERTpMut only showed a trend toward significance as a risk factor for disease progression. Future studies with higher number of NMIBC tumors are needed to empower and statically validate our preliminary findings. Similarly, the limited numbers of patients with muscle invasive disease prevented a meaningful evaluation of this subgroup. However, since most of the prognostic role of TERT activation through THOR hypermethylation is observed during early stages of tumor development in multiple cancers, and NMIBC was the focus of the present study, our results suggest a similar process in UBC carcinogenesis.
In summary, our study further supports the role of epigenetic control of the TERT promoter by THOR hypermethylation as a dynamic and progressive process in carcinogenesis, including UBC. The concomitant evaluation of TERT promoter mutation‐methylation in NMIBC has identified a group with more indolent outcome, independently from grade or stage. Additional prospective studies should confirm that THORlow/TERTpWt tumors have a reduced risk of recurrence and progression to invasive disease. This should be explored in other tumor types known for harboring TERT promoter mutations and telomerase upregulation. A better understanding of the interplay between these two tumor‐specific TERT activating mechanisms might improve clinical management in UBC and other TERT‐dependent cancers.
Supporting information
Appendix S1: Supporting Information
Ethics approval and consent to participate: Research Ethics Board of each Institution involved approved our study.
Conflict of Interest: The authors do not have any conflicts of interest.
Any views, opinions, findings and conclusions expressed in our study are those solely of the authors.
Contributor Information
Pedro Castelo‐Branco, Email: pjbranco@ualg.pt.
Uri Tabori, Email: uri.tabori@sickkids.ca.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- 2.SEER Cancer Stat Facts: Bladder Cancer [Internet]. 2014. Available from: http://seer.cancer.gov/statfacts/html/urinb.html.
- 3. Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer 2015;15:25–41. [DOI] [PubMed] [Google Scholar]
- 4. Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006;49:466–5. discussion 75–7. [DOI] [PubMed] [Google Scholar]
- 5. Epstein JI, Amin MB, Reuter VR, et al. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder consensus conference committee. Am J Surg Pathol 1998;22:1435–48. [DOI] [PubMed] [Google Scholar]
- 6. Humphrey PA, Moch H, Cubilla AL, et al. The 2016 WHO classification of Tumours of the urinary system and male genital organs‐part B: prostate and bladder Tumours. Eur Urol 2016;70:106–19. [DOI] [PubMed] [Google Scholar]
- 7. Kamat AM, Hahn NM, Efstathiou JA, et al. Bladder cancer. Lancet 2016;388:2796–810. [DOI] [PubMed] [Google Scholar]
- 8. Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 1985;43(2 Pt 1):405–13. [DOI] [PubMed] [Google Scholar]
- 9. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 10. Greider CW. Telomerase activity, cell proliferation, and cancer. Proc Natl Acad Sci USA 1998;95:90–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science 1994;266:2011–5. [DOI] [PubMed] [Google Scholar]
- 12. Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer 1997;33:787–91. [DOI] [PubMed] [Google Scholar]
- 13. Holt SE, Wright WE, Shay JW. Multiple pathways for the regulation of telomerase activity. Eur J Cancer 1997;33:761–6. [DOI] [PubMed] [Google Scholar]
- 14. Barthel FP, Wei W, Tang M, et al. Systematic analysis of telomere length and somatic alterations in 31 cancer types. Nat Genet 2017;49:349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vinagre J, Almeida A, Populo H, et al. Frequency of TERT promoter mutations in human cancers. Nat Commun 2013;4:2185. [DOI] [PubMed] [Google Scholar]
- 16. Huang DS, Wang Z, He XJ, et al. Recurrent TERT promoter mutations identified in a large‐scale study of multiple tumour types are associated with increased TERT expression and telomerase activation. Eur J Cancer 2015;51:969–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kinde I, Munari E, Faraj SF, et al. TERT promoter mutations occur early in urothelial neoplasia and are biomarkers of early disease and disease recurrence in urine. Cancer Res 2013;73:7162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Allory Y, Beukers W, Sagrera A, et al. Telomerase reverse transcriptase promoter mutations in bladder cancer: high frequency across stages, detection in urine, and lack of association with outcome. Eur Urol 2014;65:360–6. [DOI] [PubMed] [Google Scholar]
- 19. Hurst CD, Platt FM, Knowles MA. Comprehensive mutation analysis of the TERT promoter in bladder cancer and detection of mutations in voided urine. Eur Urol 2014;65:367–9. [DOI] [PubMed] [Google Scholar]
- 20. Wu S, Huang P, Li C, et al. Telomerase reverse transcriptase gene promoter mutations help discern the origin of urogenital tumors: a genomic and molecular study. Eur Urol 2014;65:274–7. [DOI] [PubMed] [Google Scholar]
- 21. Rachakonda PS, Hosen I, de Verdier PJ, et al. TERT promoter mutations in bladder cancer affect patient survival and disease recurrence through modification by a common polymorphism. Proc Natl Acad Sci USA 2013;110:17426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hosen I, Rachakonda PS, Heidenreich B, et al. Mutations in TERT promoter and FGFR3 and telomere length in bladder cancer. Int J Cancer 2015;137:1621–9. [DOI] [PubMed] [Google Scholar]
- 23. Bell RJ, Rube HT, Kreig A, et al. Cancer. The transcription factor GABP selectively binds and activates the mutant TERT promoter in cancer. Science 2015;348:1036–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang FW, Hodis E, Xu MJ, et al. Highly recurrent TERT promoter mutations in human melanoma. Science 2013;339:957–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Borah S, Xi L, Zaug AJ, et al. Cancer. TERT promoter mutations and telomerase reactivation in urothelial cancer. Science 2015;347:1006–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Horn S, Figl A, Rachakonda PS, et al. TERT promoter mutations in familial and sporadic melanoma. Science 2013;339:959–61. [DOI] [PubMed] [Google Scholar]
- 27. Descotes F, Kara N, Decaussin‐Petrucci M, et al. Non‐invasive prediction of recurrence in bladder cancer by detecting somatic TERT promoter mutations in urine. Br J Cancer 2017;117:583–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chiba K, Lorbeer FK, Shain AH, et al. Mutations in the promoter of the telomerase gene TERT contribute to tumorigenesis by a two‐step mechanism. Science 2017;357:1416–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Castelo‐Branco P, Leao R, Lipman T, et al. A cancer specific hypermethylation signature of the TERT promoter predicts biochemical relapse in prostate cancer: a retrospective cohort study. Oncotarget 2016;7:57726–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Castelo‐Branco P, Choufani S, Mack S, et al. Methylation of the TERT promoter and risk stratification of childhood brain tumours: an integrative genomic and molecular study. Lancet Oncol 2013;14:534–42. [DOI] [PubMed] [Google Scholar]
- 31. Babjuk M, Bohle A, Burger M, et al. EAU guidelines on non‐muscle‐invasive Urothelial carcinoma of the bladder: update 2016. Eur Urol 2017;71:447–61. [DOI] [PubMed] [Google Scholar]
- 32. Ceccarelli M, Barthel FP, Malta TM, et al. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse Glioma. Cell 2016;164:550–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tabori U, Vukovic B, Zielenska M, et al. The role of telomere maintenance in the spontaneous growth arrest of pediatric low‐grade gliomas. Neoplasia 2006;8:136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Soukup V, Capoun O, Cohen D, et al. Prognostic performance and reproducibility of the 1973 and 2004/2016 World Health Organization grading classification Systems in non‐muscle‐invasive Bladder Cancer: a European Association of Urology non‐muscle invasive bladder cancer guidelines panel systematic review. Eur Urol 2017;72:801–13. [DOI] [PubMed] [Google Scholar]
- 35. Bert SA, Robinson MD, Strbenac D, et al. Regional activation of the cancer genome by long‐range epigenetic remodeling. Cancer Cell 2013;23:9–22. [DOI] [PubMed] [Google Scholar]
- 36. Renaud S, Loukinov D, Abdullaev Z, et al. Dual role of DNA methylation inside and outside of CTCF‐binding regions in the transcriptional regulation of the telomerase hTERT gene. Nucleic Acids Res 2007;35:1245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mack SC, Witt H, Piro RM, et al. Epigenomic alterations define lethal CIMP‐positive ependymomas of infancy. Nature 2014;506:445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


