Abstract
Diastolic heart failure (DHF) is characterized by slow left ventricular (LV) relaxation, increased LV stiffness, interstitial deposition of collagen, and a modified extracellular matrix proteins. Among Europeans, the frequency of asymptomatic diastolic LV dysfunction (DD) is 25%. This constitutes a large pool of people at high risk of DHF. The goal of this review was to describe the discovery and the initial validation of new multidimensional urinary peptidomic biomarkers (UPB) indicative of DD, mainly consisting of collagen fragments, and to describe a roadmap for their introduction into clinical practice. The availability of new drugs creates a window of opportunity for mounting a randomized clinical trial consolidating the clinical applicability of UPB to screen for DD. If successfully completed, such trial will benefit ≈25% of all people older than 50 years and open a large market for a UPB diagnostic tool and the drug tested. Moreover, sequenced peptides making up UPB will generate novel insights in the pathophysiology of DD and facilitate personalized treatment of patients with DHF for whom prevention came too late. If proven cost‐effective, the clinical application of UPB will contribute to the sustainability of health care in aging population in epidemiologic transition.
Keywords: diastolic heart failure, diastolic left ventricular dysfunction, population science, urinary peptidomics
1. Introduction
Heart failure (HF) is a progressive condition that begins with risk factors for left ventricular (LV) dysfunction (e.g., hypertension), proceeds to asymptomatic changes in cardiac structure (e.g., LV hypertrophy) and function (e.g., impaired relaxation) and then evolves into clinically overt HF, disability, and death.1 The 5‐year mortality rate of symptomatic HF is ≈60%.2 Diastolic HF is characterized by slow LV relaxation, increased LV stiffness, increased interstitial deposition of collagen, and modified extracellular matrix proteins.3 Diastolic HF accounts for 40–50% of all HF cases and has a prognosis as ominous as systolic HF.3 In randomly recruited European population samples, the frequency of asymptomatic echocardiographically diagnosed diastolic LV dysfunction (early stage) is as high as 25%.4, 5 This constitutes a large pool of subjects at high risk of diastolic HF. The goal of this article is to describe the discovery and the validation of a new multidimensional urinary peptidomic biomarker, called HF1, and to describe a roadmap for its introduction in clinical practice.
2. Context of Use
2.1. The HF Epidemic
HF is a global pandemic and a major public health problem affecting over 37 million of individuals worldwide.6 It is a progressive condition that begins with risk factors for (LV) dysfunction (e.g., hypertension—stage A), proceeds to asymptomatic changes in cardiac structure (e.g., LV hypertrophy) and function (e.g., impaired relaxation—stage B), and then evolves into clinically overt HF (stages C and D), disability, and death.1 The key pathological mechanism leading to the worsening of the condition is LV remodeling, which is the combination of diverse processes (e.g., molecular, cellular), leading to changes in cardiac size, mass and function.1 Initially, the molecular and morphological changes in the heart related to LV remodeling are asymptomatic. However, over the course of time, LV remodeling leads to symptomatic HF, at which stage (C and D) management becomes increasingly difficult gravely affecting quality of life and causing huge health care costs because of repeated hospitalizations. Thus, to improve the management of HF, there should be a paradigm shift from treating established disease to the early diagnosis of clinically silent LV dysfunction. Strategies to detect and treat pathological events (i.e., LV remodeling) at an early stage with already established molecular/structural alterations yet with no signs of symptoms will be the most efficient.
The diagnosis of asymptomatic LV dysfunction is usually achieved through imaging techniques including echocardiography. However, the echocardiographic diagnosis requires costly equipment and highly trained observers, is time consuming, and is influenced by intra‐ and inter‐operator variability.7 Hence, there is a need for novel technologies that will enable the diagnosis of asymptomatic LV dysfunction and the early initiation of therapeutic interventions at a stage when the pathogenetic process is still reversible.
2.2. Urinary Peptidomic Technology
Urine is a stable bio‐fluid and contains an array of low‐molecular‐weight peptides that can be analyzed without additional manipulation, such as proteolysis. Under physiological conditions, about 70% of the urinary peptidome originates from the kidney and the urinary tract, while 30% is derived from plasma.8 Approximately 60% of the total mass of urinary peptides and proteins consist of collagen fragments.9 LV remodeling is characterized by alterations in the extracellular matrix (ECM) and particularly in collagen homeostasis. Urine is, therefore, a more suitable biological source than blood to identify collagen peptides, because their abundance in blood is low.10 The urinary peptidome does not undergo significant changes when urine is stored for 3 days at 4 °C11 or for 6 h at room temperature.12 For studies running over a long time period, urine can be stored for years at –20 °C without alteration of the peptidome.13 The urinary proteome is well characterized and reference standards are available.14
Urinary peptidomics based on CE–MS is a powerful technology to improve the management of chronic diseases. The CE–MS platform enables the separation of naturally occurring peptides in a single step, using a strong electrical field and subsequent detection using MS. It is a robust and operator‐independent technology, allowing the high‐resolution detection of several thousand peptides (0.8 ≤ 18 kDa) in a single 5‐mL urine sample. A detailed description of urine sample preparation, proteome analysis by CE–MS, data processing, and sequencing of the urinary peptides allowing the identification of parental proteins is beyond the scope of this article, but has been published before.14, 15 The implementation of CE–MS for the management of chronic kidney diseases is nearing and awaiting the results from the PRIORITY trial (Proteomic Prediction and Renin Angiotensin Aldosterone System Inhibition Prevention of Early Diabetic Nephropathy in Type‐2 Diabetic Patients with Normal Albumin Excretion).16 It aims to evaluate the benefit of urinary peptidomics in guiding therapeutic intervention in type‐2 diabetes mellitus patients.16
3. Development and Validation of HF Classifiers
The studies summarized below are embedded in the Flemish Study on Environment, Genes and Health Outcomes (FLEMENGHO)17, 18, 19, 20, 21 and Urinary Proteomics in Predicting Heart Transplantation Outcomes (uPROPHET).15, 22 FLEMENGHO is a family‐based population study, for which recruitment started in 1985. The initial participation rate was 78.0%. Participants were invited for follow‐up examinations, which included echocardiography. uPROPHET (study registration number, NCT03152422) is a proof‐of‐concept project sponsored by the European Research Council that should lead to the initial validation and clinical application of profiling of the urinary peptidome in recipients of allogenic heart transplant with the goal to help choosing treatment modalities with the greatest probability of achieving long‐term graft survival with high‐quality years added to the patients’ life. The Ethics Committee of the University of Leuven approved FLEMENGHO and uPROPHET. All participants provided informed written consent.
In FLEMENGHO, a single observer acquired the echocardiographic images and did the offline analysis according to current guidelines.23 In continuous analyses, lower e’ (early LV relaxation) and higher E/e’ (LV filling pressure) indicated worse diastolic LV function. Patients with diastolic LV dysfunction had an abnormally low age‐specific transmitral E/A ratio indicative of impaired relaxation or a mildly‐to‐moderately elevated LV filling pressure (E/e' > 8.5) with normal or decreased age‐specific E/A ratio.4 These age‐specific criteria, first established in a healthy reference sample of the FLEMENGHO study,4 were subsequently replicated in an independent European cohort.5 Previous publications describe the statistical workflow for biomarker discovery in the currently reviewed articles.15
3.1. Discovery of HF1
In a discovery case‐control study, 19 hypertensive patients with asymptomatic LV diastolic dysfunction and 19 healthy controls were compared.17 To classify the urinary polypeptides according to whether they were down‐ or upregulated in cases versus controls, the ratio (R) of ∑ (ln signal amplitude × frequency/number of participants) in controls to ∑ (ln signal amplitude × frequency/number of participants) in cases was calculated. Table 1 lists further details on the 85 polypeptides with different abundance between cases and controls, ordered by ascending R. The information provided includes polypeptide identifier (SQL number), mass, CE‐migration time, percentage of samples, in which the polypeptide could be detected, signal amplitude of the polypeptides, R and p values for comparison of the polypeptide signal amplitude distribution between cases and controls. With adjustment for multiple testing applied, three potential biomarkers (IDs 8725, 40737, and 61984) remained significantly different between cases and controls (p ≤ 0.02).
Table 1.
List of polypeptides included in the HF1 classifier
| Polypeptide | Cases | Controls | ||||||
|---|---|---|---|---|---|---|---|---|
| ID | Mass [Da] | CE Time [min] | Percentage [%] | MA | Percentage [%] | MA | R | p‐Value (unadjusted) |
| 81272 | 2211.98 | 33.23 | 0 | 0 | 0.42 | 2.67 | 0 | 1.99E–03 |
| 129821 | 3333.36 | 19.42 | 0 | 0 | 0.47 | 2.39 | 0 | 8.72E–04 |
| 8725 | 949.4 | 25.79 | 0.05 | 1.94 | 0.63 | 2.28 | 0.067 | 2.22E–04 |
| 123106 | 3130.43 | 30.82 | 0.05 | 1.98 | 0.47 | 2.63 | 0.080 | 2.57E–03 |
| 1577 | 840.41 | 23.17 | 0.05 | 1.65 | 0.47 | 1.85 | 0.095 | 3.29E–03 |
| 103493 | 2658.22 | 19.5 | 0.05 | 3.36 | 0.47 | 3.29 | 0.109 | 4.71E–03 |
| 44146 | 1518.6 | 19.37 | 0.11 | 1.91 | 0.58 | 2.49 | 0.145 | 1.33E–03 |
| 4845 | 900.27 | 43.66 | 0.16 | 1.55 | 0.63 | 2.44 | 0.161 | 1.33E–03 |
| 37610 | 1421.59 | 38.71 | 0.11 | 1.73 | 0.53 | 1.87 | 0.192 | 6.07E–03 |
| 83441 | 2248.97 | 33.69 | 0.11 | 3.45 | 0.53 | 3.56 | 0.201 | 4.88E–03 |
| 74703 | 2087.84 | 19.42 | 0.11 | 2.64 | 0.53 | 2.7 | 0.203 | 6.76E–03 |
| 101157 | 2616.16 | 28.39 | 0.11 | 1.97 | 0.53 | 1.98 | 0.206 | 6.76E–03 |
| 103022 | 2649.2 | 34.85 | 0.16 | 2.52 | 0.68 | 2.56 | 0.232 | 2.50E–03 |
| 57360 | 1734.66 | 19.9 | 0.16 | 2.2 | 0.58 | 2.24 | 0.271 | 1.03E–02 |
| 46091 | 1554.66 | 28.59 | 0.16 | 2.08 | 0.53 | 2.24 | 0.280 | 1.18E–02 |
| 32022 | 1319.58 | 20.89 | 0.21 | 1.99 | 0.58 | 2.21 | 0.326 | 1.57E–02 |
| 102269 | 2638.18 | 28.42 | 0.26 | 2.3 | 0.68 | 2.49 | 0.353 | 1.26E–02 |
| 82708 | 2235.04 | 34.17 | 0.32 | 2.57 | 0.84 | 2.68 | 0.365 | 2.53E–03 |
| 188895 | 11967.55 | 20.47 | 0.26 | 2.68 | 0.63 | 2.94 | 0.376 | 9.50E–03 |
| 98089 | 2559.18 | 19.41 | 0.32 | 2.97 | 0.84 | 3 | 0.377 | 3.76E–03 |
| 138143 | 3593.47 | 20.2 | 0.26 | 2.67 | 0.68 | 2.68 | 0.381 | 1.50E–02 |
| 167786 | 4771.07 | 20.2 | 0.37 | 2.74 | 0.79 | 3.13 | 0.410 | 4.34E–03 |
| 61984 | 1835.71 | 19.91 | 0.53 | 2.64 | 1 | 3.12 | 0.448 | 1.33E–04 |
| 46369 | 1560.7 | 29.79 | 0.32 | 2.78 | 0.68 | 2.84 | 0.461 | 2.27E–02 |
| 143947 | 3801.77 | 33.46 | 0.37 | 2.26 | 0.79 | 2.24 | 0.473 | 2.67E–02 |
| 39275 | 1445.62 | 37.36 | 0.47 | 2.59 | 0.79 | 2.96 | 0.521 | 4.87E–03 |
| 56493 | 1716.66 | 20.18 | 0.47 | 2.56 | 0.79 | 2.74 | 0.556 | 2.11E–02 |
| 41972 | 1478.61 | 39.3 | 0.53 | 2.75 | 0.84 | 2.95 | 0.588 | 3.16E–03 |
| 24168 | 1195.48 | 37.51 | 0.58 | 2.8 | 0.84 | 3.26 | 0.593 | 3.12E–03 |
| 107858 | 2751.34 | 29.23 | 0.63 | 2.36 | 0.89 | 2.69 | 0.621 | 3.00E–03 |
| 23356 | 1179.52 | 37.49 | 0.58 | 2.63 | 0.84 | 2.9 | 0.626 | 2.67E–02 |
| 97599 | 2547.99 | 21.44 | 0.58 | 2.59 | 0.89 | 2.66 | 0.635 | 3.15E–02 |
| 8695 | 949.22 | 34.33 | 0.53 | 2.46 | 0.68 | 3.01 | 0.637 | 2.78E–02 |
| 23697 | 1186.53 | 22.39 | 0.68 | 2.8 | 1 | 2.88 | 0.661 | 2.08E–02 |
| 36566 | 1401.38 | 36.56 | 0.58 | 2.77 | 0.74 | 3.27 | 0.664 | 8.74E–03 |
| 153832 | 4196.75 | 20.84 | 0.68 | 2.41 | 0.95 | 2.59 | 0.666 | 4.93E–03 |
| 26670 | 1235.56 | 26.65 | 0.63 | 3.02 | 0.84 | 3.3 | 0.686 | 1.08E–02 |
| 58050 | 1749.81 | 30.61 | 0.63 | 2.57 | 0.84 | 2.79 | 0.691 | 3.04E–02 |
| 28005 | 1255.48 | 35.77 | 0.68 | 3.08 | 0.84 | 3.4 | 0.733 | 3.19E–02 |
| 159396 | 4409.89 | 20 | 0.74 | 2.72 | 0.84 | 3.23 | 0.742 | 2.68E–02 |
| 69979 | 1996.79 | 20.98 | 0.79 | 2.86 | 0.95 | 3.17 | 0.750 | 8.53E–03 |
| 40737 | 1462.62 | 39.42 | 0.84 | 3.33 | 1 | 3.68 | 0.760 | 2.62E–04 |
| 65368 | 1901.82 | 43.83 | 0.79 | 3.17 | 0.89 | 3.61 | 0.779 | 1.52E–02 |
| 128086 | 3286.55 | 30.92 | 0.79 | 3.13 | 0.89 | 3.51 | 0.792 | 6.91E–04 |
| 73434 | 2067.82 | 20.62 | 0.84 | 3.1 | 1 | 3.28 | 0.794 | 1.42E–02 |
| 148086 | 3986.65 | 20.6 | 0.84 | 3.53 | 0.95 | 3.82 | 0.817 | 2.75E–03 |
| 108574 | 2764.21 | 42.63 | 0.79 | 3.56 | 0.89 | 3.85 | 0.821 | 2.43E–02 |
| 90344 | 2377.1 | 20.8 | 0.89 | 3.12 | 0.95 | 3.46 | 0.845 | 1.95E–02 |
| 36759 | 1405.61 | 39.04 | 0.89 | 2.94 | 0.95 | 3.18 | 0.866 | 1.02E–02 |
| 147541 | 3968.6 | 21.09 | 0.89 | 3.14 | 0.89 | 3.57 | 0.880 | 1.77E–03 |
| 28561 | 1265.59 | 27.09 | 0.89 | 3.36 | 0.89 | 3.79 | 0.887 | 1.10E–02 |
| 107460 | 2742.25 | 28.98 | 0.95 | 2.91 | 1 | 3.11 | 0.889 | 1.19E–02 |
| 32171 | 1321.59 | 28.37 | 0.95 | 4.07 | 1 | 4.27 | 0.906 | 1.82E–02 |
| 39322 | 1446.64 | 39.43 | 1 | 3.2 | 1 | 3.49 | 0.917 | 3.19E–02 |
| 35339 | 1378.61 | 28.82 | 1 | 3.36 | 1 | 3.53 | 0.952 | 1.54E–02 |
| 81196 | 2210.95 | 33.61 | 1 | 3.72 | 1 | 3.59 | 1.036 | 2.15E–02 |
| 41601 | 1469.67 | 23.69 | 1 | 3.72 | 1 | 3.56 | 1.045 | 2.33E–02 |
| 62866 | 1854.81 | 40.92 | 1 | 3.89 | 1 | 3.71 | 1.048 | 1.98E–02 |
| 99021 | 2570.19 | 42.56 | 1 | 3.88 | 1 | 3.7 | 1.049 | 1.19E–02 |
| 79136 | 2175 | 33.28 | 1 | 3.74 | 1 | 3.49 | 1.072 | 1.09E–02 |
| 50840 | 1623.73 | 24.12 | 0.95 | 4.17 | 0.95 | 3.86 | 1.080 | 9.77E–03 |
| 72533 | 2046.92 | 32.58 | 0.95 | 3.49 | 0.95 | 3.21 | 1.087 | 1.06E–02 |
| 57537 | 1737.78 | 23.73 | 1 | 4.02 | 0.95 | 3.82 | 1.108 | 2.15E–02 |
| 50212 | 1613.82 | 23.99 | 0.89 | 2.7 | 0.89 | 2.43 | 1.111 | 3.30E–02 |
| 60149 | 1794.8 | 23.92 | 1 | 3.72 | 0.95 | 3.47 | 1.128 | 6.20E–03 |
| 103198 | 2654.19 | 23.92 | 0.89 | 2.94 | 0.89 | 2.47 | 1.190 | 5.52E–03 |
| 104786 | 2679.2 | 23.53 | 1 | 3.58 | 0.89 | 3.34 | 1.204 | 7.89E–03 |
| 33135 | 1338.6 | 23.99 | 1 | 2.86 | 0.89 | 2.65 | 1.213 | 1.20E–02 |
| 73291 | 2064.92 | 24.46 | 0.84 | 2.75 | 0.79 | 2.37 | 1.234 | 3.25E–02 |
| 45021 | 1532.62 | 26.35 | 1 | 2.82 | 0.89 | 2.55 | 1.243 | 1.67E–02 |
| 99475 | 2577.25 | 24.67 | 0.95 | 2.78 | 0.89 | 2.38 | 1.247 | 6.05E–03 |
| 40294 | 1452.66 | 23.61 | 1 | 2.85 | 0.84 | 2.62 | 1.295 | 2.17E–03 |
| 35424 | 1380.64 | 23.83 | 0.95 | 2.79 | 0.79 | 2.56 | 1.311 | 7.17E–03 |
| 131294 | 3375.57 | 31.92 | 1 | 2.87 | 0.79 | 2.71 | 1.341 | 1.80E–02 |
| 111564 | 2841.26 | 24.54 | 0.89 | 3.21 | 0.79 | 2.67 | 1.354 | 4.98E–03 |
| 104195 | 2663.2 | 23.51 | 0.89 | 2.61 | 0.74 | 2.29 | 1.371 | 2.07E–02 |
| 28747 | 1268.57 | 27.25 | 1 | 3.44 | 0.74 | 3.32 | 1.400 | 1.01E–02 |
| 44802 | 1526.69 | 23.92 | 0.79 | 2.51 | 0.63 | 2.1 | 1.499 | 1.10E–02 |
| 113452 | 2889.35 | 24.08 | 0.89 | 2.47 | 0.58 | 2.29 | 1.655 | 7.34E–03 |
| 69681 | 1989.88 | 32.44 | 0.84 | 2.43 | 0.42 | 2.51 | 1.936 | 2.03E–02 |
| 55516 | 1696.72 | 23.95 | 0.79 | 2.54 | 0.42 | 2.39 | 1.999 | 1.59E–02 |
| 80360 | 2196.02 | 33.16 | 0.68 | 2.74 | 0.26 | 2.73 | 2.625 | 1.15E–02 |
| 82784 | 2236.98 | 27.14 | 0.63 | 2.28 | 0.21 | 2.31 | 2.961 | 1.29E–02 |
| 56806 | 1723.52 | 37.74 | 0.53 | 2.31 | 0.11 | 2.52 | 4.417 | 1.03E–02 |
| 129182 | 3320.51 | 24.25 | 0.47 | 2.07 | 0.05 | 2.1 | 9.266 | 4.71E–03 |
ID, polypeptide identifier (SQL number); %, percentage of samples, in which the polypeptide could be detected; MA, mean signal amplitude of the polypeptides. R was calculated as ∑ (ln signal amplitude × frequency/number of participants) in controls divided by ∑ (ln signal amplitude × frequency/number of participants) in cases. The polypeptides were ordered by ascending R. Reproduced with permission from ref. 18.
The 85 potential biomarkers were combined in a high‐dimensional classifier, which was applied in a blinded manner to an independent test set of 16 hypertensive patients with symptomatic HF and 16 healthy controls. Upon unblinding, the area under the receiver operating characteristic curve of the classifier was 0.84 (95% confidence interval [CI], 0.70 to 0.98; p = 0.001). Downregulated peptides included fragments of collagens type I and IV, whereas collagen type III fragments were upregulated. Among the downregulated peptides was a fragment of WW domain‐binding protein 11 (ID 61984; WBP11; p < 0.02). The WBP‐11 gene encodes a nuclear protein, which in cell nuclei colocalizes with mRNA splicing factors.24 In cardiomyocytes, WBP‐11 interacts with the 52‐amino acid integral membrane protein phospholamban (PP‐1) and thereby contributes to the regulation of the transmembrane Ca2+ flux via the Ca2+ pump (SERCA), which transports Ca2+ from the cytosol to the sarcoplasmic reticulum. Phosphorylation of PP‐1 by protein kinase A and dephosphorylation by WBP‐11, respectively, stimulates and inhibits SERCA.25 Downregulation of WBP‐11, as observed in patients with diastolic LV dysfunction, might enhance SERCA activity and impair electromechanical coupling in the heart.26
A second multidimensional urinary polypeptide marker, HF2, consists of 671 peptide fragments. To generate the HF2 classifier, all urinary proteomic datasets from cases available in the Mosaiques database9 were combined and compared with data from sex‐ and age‐matched controls. Cases were 98 patients with diastolic LV dysfunction recruited from FLEMENGHO17 (n = 35) or admitted to the hospital because of overt HF (n = 63).
3.2. A Proof‐of‐Concept Population Study
In a subsequent proof‐of‐concept population study,18 the cross‐sectional association of diastolic LV function with HF1 (Figure 1) and HF2 was evaluated. The analyses, involving 745 FLEMENGHO participants, were adjusted for sex, age, BMI, blood pressure, heart rate, LV mass index, and intake of medications. Association sizes were expressed per 1‐SD increment in the classifiers.18 HF1 was associated with 0.204 cm s–1 lower e’ peak velocity (95% CI, 0.057 to 0.351; p = 0.007) and 0.145 higher E/e’ ratio (95% CI, 0.023 to 0.268; p = 0.020), while HF2 was associated with a 0.174 higher E/e’ ratio (95% CI, 0.046 to 0.302; p = 0.008). According to published definitions,4, 5 67 (9.0%) participants had impaired LV relaxation and 96 (12.9%) had elevated LV filling pressure. The odds of impaired relaxation associated with HF1 was 1.38 (95% CI, 1.01 to 1.88; p = 0.043) and that of increased LV filling pressure associated with HF2 was 1.38 (95% CI, 1.00 to 1.90; p = 0.052).18
Figure 1.
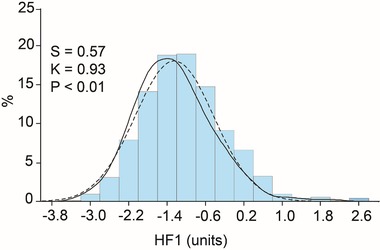
Distribution of the multidimensional urinary biomarker HF1 in 745 participants enrolled in the Flemish Study on Environment, Genes and Health Outcomes. The curves represent the fitted normal (full line) and kernel (dashed line) density plots. S and K are the coefficients of skewness and kurtosis, respectively. The p‐value is for the Kolmogorov–Smirnov test and indicates departure from normality.
In a further analysis of the same population sample,20 70 sequenced urinary peptides were detected in over 95% of participants. Serum measurements included carboxyterminal propeptide of procollagen type‐1 (PICP), as marker of collagen I synthesis, and tissue inhibitor of matrix metalloproteinase type 1 (TIMP‐1), an inhibitor of collagen‐degrading enzymes. In multivariable‐adjusted analyses with Bonferroni correction of the significance levels, effect sizes were expressed per 1‐SD in urinary collagen I or III fragments. In relation to collagen I fragments, e’ decreased by 0.183 cm s–1 (95% CI, 0.017 to 0.350; p = 0.025), whereas E/e’ increased by 0.210 (0.067 to 0.353; p = 0.0012). E/e’ decreased with urinary collagen III fragments by 0.168 (0.021 to 0.316; p = 0.018). Based on age‐specific echocardiographic criteria,4, 5 182 participants (23.3%) had subclinical diastolic LV dysfunction. Partial least squares discriminant analysis contrasting normal versus diastolic LV dysfunction confirmed the aforementioned associations with the urinary collagen I and III fragments. The circulating profibrotic biomarkers PICP and TIMP‐1 increased in relation to the urinary collagen I fragments (p < 0.0001), whereas these serum markers decreased with urinary collagen III (p ≤ 0.0006). Diastolic LV dysfunction was also associated with higher levels of TIMP‐1 (653 vs 696 ng mL–1; p = 0.013).20
In patients with hypertensive heart disease, there was a positive gradient and a direct correlation of the PICP and TIMP‐1 concentrations in blood sampled at the coronary sinus and the antecubital vein, whereas this was not the case in normotensive controls.27, 28 In hypertensive patients with HF but normal ejection fraction, elevated estimated capillary wedge pressure compared with normal LV filling pressure was associated with higher TIMP‐1 levels and a lower metalloproteinase‐1 to TIMP‐1 ratio, indicative of lower breakdown of collagen.29 In patients with hypertension with or without diastolic HF, circulating TIMP‐1 levels, but not metalloproteinases, were elevated compared to normotensive controls.30 The FLEMENGHO results moved the field forward by demonstrating that in a general population, diastolic LV dysfunction was associated with higher levels of TIMP‐1 and that plasma TIMP‐1 increased in relation to urinary collagen I fragments.20 By linking circulating TIMP‐1 to urinary collagen I fragments, the paper20 supported the hypothesis that an excess of TIMP‐1 inhibits collagen degradation, thereby promoting collagen deposition in the myocardium and diastolic LV dysfunction characterized by higher LV filling pressure.31 In short, this population study generalized previous studies in patients27, 28, 29, 30, 31 to randomly recruited people, in whom diastolic LV function ranged from normal to subclinical impairment, but did not encompass overt diastolic HF.20
3.3. Prediction of Cardiovascular Disease
The aforementioned proof‐of‐concept study18 suggested that the urinary peptidome carried prognostic information. Indeed, studies in patients with HF32, 33, 34 or hypertension35 demonstrated that high E/e’ independently predicted cardiac mortality and readmission to the hospital for HF32, 33, 34 or the risk of a cardiac event.35 In the three HF studies,32, 33, 34 the E/e’ ratio was the sole32 or a strong33, 34 predictor of the primary endpoint. Furthermore, a substudy to the Anglo‐Scandinavian Outcomes Trial35 involved 980 high‐risk hypertensive patients, free of cardiac disease at baseline and followed up for a median of 4.2 years. In multivariable‐adjusted Cox‐proportional hazards models, a 1‐unit rise in the E/e’ ratio was associated with a 17% increment in the risk of a cardiac event (95% CI, 1.05 to 1.29; p = 0.003).36
The above findings justified to verify in 791 FLEMENGHO participants whether the classifiers predicted cardiovascular complications.19 Over 6.1 years (median), 35 participants died and 63, 45, and 22 experienced fatal or nonfatal cardiovascular, cardiac, or coronary events, respectively. The incidence of fatal combined with nonfatal cardiovascular and cardiac endpoints, standardized for sex and age, increased across one‐third of the HF1 distribution (p ≤ 0.014). The multivariable‐adjusted hazard ratios (+1‐SD) were 1.30 (95% CI, 1.03 to 1.65; p = 0.029) and 1.39 (95% CI, 1.06 to 1.84; p = 0.018) for cardiovascular and cardiac events in relation to HF1. Prognostic accuracy significantly (p ≤ 0.006) improved by adding HF1 to Cox models already including the other baseline predictors. Thus, over a 6‐year period, the urinary proteome predicted cardiovascular and cardiac disease over and beyond classical risk factors, including the recently measured systolic blood pressure.19
3.4. A Study in Heart Transplant Recipients
Right heart pressures change significantly in the first month after heart transplantation as the allograft and the vasculature of the host adjust to the posttransplant condition.36 Right heart catheterization after this adaptive phase usually reveals smaller pressure changes unless rejection occurs. In a proof‐of‐concept study of 298 recipients of a heart transplant, the feasibility of using HF1 and HF2 to monitor right heart pressures was evaluated.22 Mean pressures in the right atrium (mRAP) and pulmonary artery (mPAP) and capillaries (mPCWP) were measured 7.6 years (median) after surgery. In multivariable models, mRAP and mPAP increased with HF2 (+0.42 and +0.62 mm Hg; p ≤ 0.035), but not with HF1. The adjusted odds ratio for having an elevated right heart pressure (mRAP, mPAP, or mPCWP, ≥10, ≥24, or ≥17 mm Hg, respectively) was 1.56 for HF2 (p ≤ 0.005). Adding HF2 per optimized threshold (≥0.15) increased both the integrated discrimination improvement37 (+1.92%; p = 0.023) and the net reclassification improvement37 (+30.3%; p = 0.010). The association of mRAP and mPAP with the urinary classifier HF2 is physiologically plausible, because fibrosis is a hallmark of graft malfunction. Of the urinary peptides with known amino‐acid sequence that were included in HF2, 68.9% were collagen fragments.38 Thus, monitoring right heart pressures in recipients of a heart transplant was feasible, although further refinement and validation of this biomarker in this particular indication is required.22
3.5. A Proposal for the Clinical Application of HF1
Hypertension, obesity, and older age are major risk factors for diastolic LV dysfunction, but easily applicable screening tools in people at risk are lacking. Circulating levels of natriuretic peptides in patients with diastolic LV dysfunction or even diastolic HF are often normal.4, 5, 39 This justified the search for novel biomarkers specific for diastolic LV dysfunction at an early stage long before irreversible cardiac damage sets in. The relation of the echocardiographically assessed diastolic LV function (2009 to 2013) with HF1 measured ≈5 years earlier (2005 to 2010) was assessed in 645 FLEMENGHO participants.21 In multivariable‐adjusted analyses, per 1‐SD increment in HF1, e’ was –0.193 cm s–1 lower (95% CI, –0.352 to –0.033; p = 0.018) and E/e’ 0.174 units higher (95% CI, 0.005 to 0.342; p = 0.043). Of 645 participants, 179 (27.8%) had diastolic LV dysfunction at follow‐up. For a 1‐SD increment in HF1, the adjusted odds ratio was 1.37 (95% CI, 1.07 to 1.76; p = 0.013). The integrated discrimination improvement37 (+1.14%) and the net reclassification improvement37 (+31.7%) of the optimized HF1 threshold (–0.350) in discriminating normal from abnormal diastolic LV function at follow‐up over and beyond other risk factors were significant (p ≤ 0.024).21
While the diagnosis of diastolic HF remains challenging in a hospital environment, this is even more the case for asymptomatic diastolic LV dysfunction at the point of entry in health care. Echocardiography is the diagnostic approach recommended by guidelines, but requires highly skilled observers and costly equipment and is impossible to implement on a large scale. Hence, screening by means of biomarkers in primary care is an option to be favored. Figure 2 proposes how HF1 might be applied in clinical practice in asymptomatic high‐risk individuals.21 In the presence of clinical risk factors for diastolic LV dysfunction, in particular the combination of older age, overweight or abdominal obesity, and hypertension (n = 162; 25.1% of the study population21), HF1 might be used as a screening tool. If the value is less than –0.350, managing risk factors over a 5‐year time span is the intervention to be recommended. In contrast, if HF1 is –0.350 or higher, a second test might inform the health care provider whether continuing managing risk factors for 5 years is sufficient or whether the patient should be referred for echocardiography. An added benefit is that HF1 predicts worsening of renal function38 and as summarized above the 5‐year incidence of cardiovascular and cardiac events.19 In our study,21 in line with previous publications,4, 5, 39 N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) did not add to the prediction of diastolic LV dysfunction over and beyond classical risk factors. Moreover, HF1 in the presence of NT‐proBNP fully retained its prognostic value.21
Figure 2.
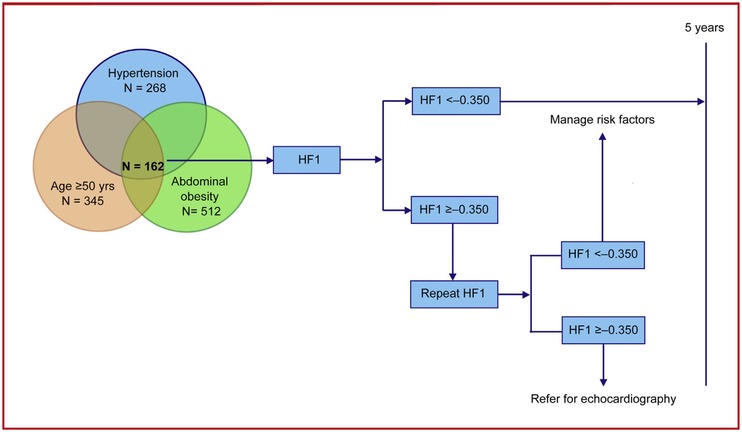
Proposal for the clinical application of HF1 over a 5‐year horizon.
3.6. Challenges Toward the Clinical Application of HF1
The readiness level of the CE–MS technology required for measurement HF1 and HF2 is TRL 9—actual system proven in environment. However, one major hurdle on the way to clinical implementation of the proposed biomarkers is the lack of randomized clinical trials showing cost‐effectiveness. While this is true in the context of subclinical left ventricular dysfunction, application of CKD273 has been proven cost‐effective for the early detection of chronic kidney diseases.40 Indeed, in patients with high‐risk of developing diabetic nephropathy, CKD273‐based screening was cost effective compared with screening based on microalbuminuria.40 Along different lines, another potential limitation of the HF1 and HF2 studies is the lack of a direct proof that the urinary peptides making up these multidimensional biomarkers originate from the heart. We addressed this issue in a previous study, in which we demonstrated consistency between circulating and urinary biomarkers of collagen turnover.20 Sequencing of the urinary peptide fragments20 allowed us to translate previous observations in endomyocardial biopsies28, 41, 42, 43 to people randomly recruited from the general population, in whom diastolic LV function ranged from normal to subclinical dysfunction, but did not encompass overt diastolic heart failure. Furthermore, a recent study comparing the plasma and urine peptidome demonstrated that serum peptides do pass the glomerular sieve and appear in the urine.10
4. Other Urinary Proteomic Studies in HF
To review additional urinary peptidomics studies in the context of HF, a literature search using Web of Science and key words including “urine* AND proteome*” and “heart failure” was performed, which identified 37 articles. However, only few bared some relevance to the issues currently discussed. Among the studies identified by the PubMed search were those already summarized in this paper. Other studies were excluded, because they did not deal with HF, because they were not studies of humans, or because they were reviews or conference documents. In a case‐control study comparing HF patients with individuals with cardiovascular risk factors, the biomarker insulin like growth factor binding protein 2 was identified in urine using CE–MS and validated using western blot and ELISA in a total of 336 patients.44 The biomarker was demonstrated to perform better than NT‐proBNP.44 The lack of studies exploiting the information contained in urine in the management of HF might be ascribed to a lack of awareness of the potential of urinary proteomics.
5. Conclusions and Perspective
Both HF1 and HF2 are associated with diastolic LV dysfunction18, 20, 21 and cardiovascular prognosis.19 From a clinical point of view, a biomarker allowing screening for the early asymptomatic stage of a condition that affects over 25% of randomly selected people4, 5 and has a progression rate of 10% over 5 years21 should have precedence over a biomarker that sides with later symptomatic disease. Along these lines, HF1 is a biomarker of early asymptomatic diastolic LV dysfunction derived in a case‐control study only involving patients with subclinical LV dysfunction,17 whereas HF2 is a marker of more advanced disease, which was derived by including also cases with overt HF.22 However, introduction of any biomarkers into clinical practice requires a randomized clinical trial to prove its diagnostic performance, its utility as a guide for the initiation of treatment and its cost‐effectiveness.
We propose a trial of HF1 (Figure 3) as marker of asymptomatic diastolic LV dysfunction with a design similar to that of the PRIORITY study,16 which aims to substantiate the clinical value of multidimensional urinary CKD273 in the early stage of chronic kidney disease.45, 46 In a trial of HF1, patients with three or more risk factors for diastolic LV dysfunction in addition to age (≥50 years) will be stratified according to their urinary HF1 level. Patents at low risk (HF1 < –0.35) will be observed without intervention other than usual care, while those at high risk (HF1 ≥ ‐0.35) will be randomized to usual care or usual care plus an intervention. The treatment or observational period will be 3 years. The primary endpoint will be the development of echocardiographically confirmed diastolic LV dysfunction. Comparison of the observational arm with the control group in the clinical trial allows proving the diagnostic and prognostic performance of HF1; comparison of control and intervention groups in the randomized trial allows proving the efficacy and cost‐effectiveness of HF1‐guided early pharmacological intervention. Drugs to be considered include a selective aldosterone receptor antagonist (finerenone),47 a selective inhibitor of the sinoatrial pacemaker If current (ivabradine),48 an angiotensin receptor neprilysin inhibitor (sacubitril/valsartan),49 or an orally active soluble guanylate‐cyclase stimulator (vericiguat).50 In the intervention arm, the experimental drug will be administered on top of usual treatment, as done in many contemporary trials, although—should sacubitril/valsartan be the experimental drug—dual inhibition of the renin–angiotensin system as defined in current guidelines51 must be avoided and the angiotensin receptor neprilisyn inhibitor will replace previous treatment with an angiotensin‐converting enzyme inhibitor or an angiotensin receptor blocker. Sample size calculations were informed by data collected within the framework of FLEMENGHO.21 The 5‐year risk of developing diastolic LV dysfunction was 30% among older participants (≥50 years) with overweight, hypertension, and urinary HF1 levels of –0.35 or higher. Among patients with the same risk profile, but with urinary HF1 levels below –0.35, the 5‐year rate of diastolic LV dysfunction was 15%. Sample size calculations for the randomized trial assumed a 30% decrease in the incidence of diastolic LV dysfunction in patients receiving a specific pharmacological intervention compared with the control group. With the accrual rate set at 2 years and with the α‐level and power of the log‐rank test set at 0.05 and 0.80, respectively, testing a two‐sided hypothesis over 3 years of follow‐up will require 1160 participants (580 per group). With the α‐level and power set similarly, 580 patients enrolled in the low‐risk observational group should be sufficient for the comparison of the prognostic accuracy of HF1 with the high‐risk patients randomized to the control arm in the trial.
Figure 3.
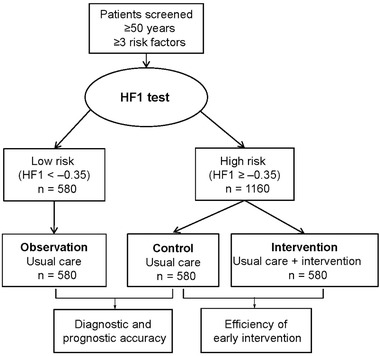
Proposed design of a randomized clinical trial combined with an observational study.
The studies summarized in this review highlight the potential of urinary peptidomics in the screening for silent diastolic LV dysfunction at a stage when prevention of irreversible cardiac dysfunction is still possible. Further validation of HF1 should pave the way for its introduction in clinical practice, in particular at the point of entry in health care, i.e., primary practice, where the need of a noninvasive screening tool for early diastolic LV dysfunction is of the greatest importance. All what is needed to measure HF1 is a 5‐mL mid‐morning urine sample. The availability of new drugs creates a window of opportunity for mounting a randomized clinical trial pursuing this objective. If successfully concluded, such trial will benefit an estimated 25% of all people older than 50 years at risk of diastolic LV dysfunction, thereby providing a large potential market for a diagnostic test and any drug tested. The sequenced peptides making up HF1, by identification of the parental proteins, will provide novel insights in the pathophysiology of diastolic LV dysfunction and facilitate personalized treatment of patients with diastolic HF for whom prevention came too late. Finally, if proven cost effective, the clinical application of HF1 will contribute to the sustainability of health care in aging population in epidemiologic transition.52
Conflict of Interest
E.N.‐K. is an employee of Mosaiques‐Diagnostics GmbH, Hannover, Germany. The other authors declare no conflict of interest.
Acknowledgements
The European Union (HEALTH‐F7‐305507 HOMAGE) and the European Research Council (Advanced Researcher grant 2011‐294713‐EPLORE and Proof‐of‐Concept grant 713601‐uPROPHET), the European Research Area Net for Cardiovascular Diseases (JTC2017‐046‐PROACT), and the Research Foundation Flanders, Ministry of the Flemish Community, Brussels, Belgium (G.0881.13) currently support the Research Unit Hypertension and Cardiovascular Research. These sponsors had no role in the preparation of this report.
Zhang Z., Nkuipou‐Kenfack E., Staessen J. A., Urinary Peptidomic Biomarker for Personalized Prevention and Treatment of Diastolic Left Ventricular Dysfunction. Prot. Clin. Appl. 2019, 13, 1800174 10.1002/prca.201800174
References
- 1. Hunt A. S., Abraham W. T., Chin M. H., Feldman A. M., Francis G. S., Ganiats T. G., Jessup M., Konstam M. A., Mancini D. M., Mich K., Oates J. A., Rahko P. S., Silver M. A., Warner Stevenson L., Yancy C. W., Antman E. M., Smith Jr S. C., Adams C. D., Anderson J. L., Faxon D. P., Fuster V., Halperin J. L., Hiratzka L. F., Jacobs A. K., Nishimura R., Ornato J. P., Page R. L., Riegel B.. Circulation 2005, 112, e154. [DOI] [PubMed] [Google Scholar]
- 2. Paulus W. J., Tschöpe C., Sanderson J. E., Rusconi C., Flachskampf F. A., Rademakers F. E., Marino P., Smiseth O. A., De Keulenaer G., Leite‐Moreira A. F., Borbély A., Edes I., Handoko M. L., Heymans S., Pezzali N., Pieske B., Dickstein K., Fraser A. G., Brutsaert D. L.. Eur. Heart J. 2007, 28, 2539. [DOI] [PubMed] [Google Scholar]
- 3. Borlaug B. A., Paulus W. J.. Eur. Heart J. 2011, 32, 670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuznetsova T., Herbots L., López B., Jin Y., Richart T., Thijs L., González A., Herregods M. C., Fagard R. H., Díez J., Staessen J. A.. Circ. Heart Fail. 2009, 2, 105. [DOI] [PubMed] [Google Scholar]
- 5. Kloch‐Badelek M., Kuznetsova T., Sakiewicz T. W., Tikhonoff V., Ryabikov A., González A., Loster M., Thijs L., Jin Y., Malyutina S., Stolarz‐Skrzypek K., Casiglia E., Díez J., Narkiewicz K., Kawecka‐Jaszcz K., Staessen J. A., The European Project on Genes in Hypertension (EPOGH) Investigators . Cardiovasc. Ultrasound 2012, 10, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ziaeian B., Fonarow G. C.. Nat. Rev. Cardiol. 2016, 3, 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cole G. D., Dhutia N. M., Shun‐Shin M. J., Willson K., Harrison J., Raphael C. E., Zolgharni M., Mayet J., Francis D. P.. Int. J. Cardiovasc. Imaging 2015, 31, 1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pieper R., Gatlin C. L., McGrath A. M., Makusky A. J., Mondal M., Seonarain M., Field E., Schatz C. R., Estock M. A., Ahmed N., Anderson N. G., Steiner S.. Proteomics 2004, 4, 1159. [DOI] [PubMed] [Google Scholar]
- 9. Coon J. J., Zürbig P., Dakna M., Dominiczak A. F., Decramer S., Fliser D., Frommberger M., Golovko I., Good D. M., Herget‐Rosenthal S., Jankowski J., Julian B. A., Kellmann M., Kolch W., Massy Z., Novak J., Rossing K., Schanstra J. P., Schiffer E., Theodorescu D., Vanholder R., Weissinger E. M., Mischak H., Schmitt‐Kopplin P.. Proteomics Clin. Appl. 2008, 2, 964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Magalhães P., Pontillo C., Pejchinovski M., Siwy J., Krochmal M., Makridakis M., Carrick E., Klein J., Mullen W., Jankowski J., Vlahou A., Mischak H., Schanstra J. P., Zürbig P., Pape L.. Proteomics Clin. Appl. 2018, 12, e1700163. [DOI] [PubMed] [Google Scholar]
- 11. Schaub S., Wilkins J., Weiler T., Sangster K., Rush D., Nickerson P.. Kidney Intern. 2004, 65, 323. [DOI] [PubMed] [Google Scholar]
- 12. Theodorescu D., Wittke S., Ross M. M., Walden M., Conaway M., Just I., Mischak H., Frierson H. F.. Lancet Oncol. 2006, 7, 230. [DOI] [PubMed] [Google Scholar]
- 13. Fliser D., Novak J., Thongboonkerd V., Argilés A., Jankowski V., Girolami M. A., Jankowski J., Mischak H.. J. Am. Soc. Nephrol. 2007, 18, 1057. [DOI] [PubMed] [Google Scholar]
- 14. Mischak H., Kolch W., Aivalotis M., Bouyssié D., Court M., Dihazi G. H., Franke J., Garin J., Gonzalez de Peredo A., Iphöfer A., Jänsch L., Lacroix C., Makridakis M., Masselon C., Metzger J., Monsarrat B., Mrug M., Norling M., Novak J., Pich A., Pitt A., Bongcam‐Rudolff E., Siwy J., Suzuki H., Thongboonkerd V., Wang L. S., Zoidakis J., Zürbig P., Schanstra J. P., Vlahou A.. Proteomics Clin. Appl. 2010, 4, 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jantos‐Siwy J., Schiffer E., Brand K., Schumann G., Rossing K., Delles C., Mischak H., Metzger J.. J. Proteome Res. 2009, 8, 268. [DOI] [PubMed] [Google Scholar]
- 16. Huang Q. F., Trenson S., Zhang Z. Y., Yang W. Y., Van Aelst L., Nkuipou‐Kenfack E., Wei F. F., Mujaj B., Thijs L., Ciarka A., Zoidakis J., Droogné W., Vlahou A., Janssens S., Vanhaecke J., Van Cleemput J., Staessen J. A.. PLoS One 2017, 12, e0184443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lindhardt M., Persson F., Currie G., Pontillo C., Beige J., Delles C., von der Leyen H., Mischak H., Navis G., Noutsou M., Ortiz A., Ruggenenti P. L., Rychlik I., Spasovski G., Rossing P.. Br. Med. J. Open 2016, 6, e010310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuznetsova T., Mischak H., Mullen W., Staessen J. A.. Eur. Heart J. 2012, 33, 2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Z. Y., Staessen J. A., Thijs L., Gu Y., Liu Y., Jacobs L., Koeck T., Zürbig P., Mischak H., Kuznetsova T.. Int. J. Cardiol. 2014, 176, 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang Z. Y., Thijs L., Petit T., Petit T., Gu Y. M., Jacobs L., Yang W. Y., Liu Y. P., Koeck T., Zürbig P., Jin Y., Verhamme P., Voigt J. U., Kuznetsova T., Mischak H., Staessen J. A.. Hypertension 2015, 66, 52. [DOI] [PubMed] [Google Scholar]
- 21. Zhang Z. Y., Ravassa S., Yang W. Y., Petit T., Pejchinovski M., Zürbig P., López B., Wei F. F., Pontillo C., Thijs L., Jacobs L., González A., Koeck T., Delles C., Voigt J. U., Verhamme P., Kuznetsova T., Díez J., Mischak H., Staessen J. A.. PLoS One 2016, 11, e0167582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Z. Y., Nkuipou‐Kenfack E., Yang W. Y., Wei F. F., Cauwenberghs N., Thijs L., Huang Q. F., Feng Y. M., Schanstra J. P., Kuznetsova T., Voigt J. U., Verhamme P., Mischak H., Staessen J. A.. J. Am. Soc. Hypertens 2018, 12, 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang Q. F., Trenson S., Zhang Z. Y., Van Keer J., Van Aelst L. N. L., Yang W. Y., Nkuipou‐Kenfack E., Thijs L., Wei F. F., Mujaj B., Ciarka A., Droogné W., Vanhaecke J., Janssens S., Van Cleemput J., Mischak H., Staessen J. A.. Transplant Direct 2018, 4, e346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gottdiener J. S., Bednarz J., Devereux R., Gardin J., Klein A., Manning W. J., Morehead A., Kitzman D., Oh J., Quinones M., Schiller N. B., Stein J. H., Weissman N. J.. J. Am. Soc. Echocardiogr. 2004, 17, 1086. [DOI] [PubMed] [Google Scholar]
- 25. Llorian M., Beullens M., Andrés I., Ortiz J. M., Bollen M.. Biochem. J. 2004, 378, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neumann J.. Basic Res. Cardiol. 2002, 97, I91. [DOI] [PubMed] [Google Scholar]
- 27. Pfeiffer E. R., Tangney J. R., Omens J. H., McCulloch A. D.. J. Biomech. Eng. 2014, 136, 0210071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Querejeta R., López B., González A., Sánchez E., Laeman M., Martínez Ubago J. L., Díez J.. Circulation 2004, 110, 1263. [DOI] [PubMed] [Google Scholar]
- 29. López B., González A., Querejeta R., Larman M., Díez J.. J. Am. Coll. Cardiol. 2006, 48, 89. [DOI] [PubMed] [Google Scholar]
- 30. González A., López B., Querejeta R., Zubillaga E., Echeverría T., Díez J.. Hypertension 2010, 55, 1418. [DOI] [PubMed] [Google Scholar]
- 31. Zile M. R., Baicu C. F., Ikonomidis J. S., Stroud R. E., Nietert P. J., Bradshaw A. D., Slater R., Palmer B. M., Van Buren P., Meyer M., Redfield M. M., Bull D. A., Granzier H. L., LeWinter M. M.. Circulation 2015, 131, 1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. López B., González A., Ravassa S., Beaumont J., Moreno M. U., San José G., Querejeta R., Díez J.. J. Am. Coll. Cardiol. 2015, 65, 2449. [DOI] [PubMed] [Google Scholar]
- 33. Acil T., Wichter T., Stypmann J., Janssen F., Paul M., Grude M., Scheld H. H., Breithardt G., Bruch C., Int. J. Cardiol. 2005, 103, 175. [DOI] [PubMed] [Google Scholar]
- 34. Dokainish H., Zoghbi W. A., Lakkis N. M., Ambriz E., Patel R., Quinones M. A., Nagueh S. E.. J. Am. Coll. Cardiol. 2005, 45, 1223. [DOI] [PubMed] [Google Scholar]
- 35. Olson J. M., Samad B. A., Alam M.. Am. J. Cardiol. 2008, 102, 722. [DOI] [PubMed] [Google Scholar]
- 36. Sharp A. S., Tapp R. J., Thom S. A., Francis D. P., Highes A. D., Stanton A. V., Zambanini A., O'Brien E., Chaturvedi N., Lyons S., Byrd S., Poulter N. R., Server P. S., Mayet J.. Eur. Heart J. 2010, 31, 747. [DOI] [PubMed] [Google Scholar]
- 37. Bedanova H., Orban M., Vrsansky D., Spinarova L., Hude P., Krejci J., Ondrasek J., Nemec P.. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2013, 157, 35. [DOI] [PubMed] [Google Scholar]
- 38. Pencina M. J., D'Agostino Sr R. B., D'Agostino Jr R. B., Vasan R. S.. Stat. Med. 2008, 27, 157. [DOI] [PubMed] [Google Scholar]
- 39. Gu Y. M., Thijs L., Liu Y. P., Zhang Z. Y., Jacobs L., Koeck T., Zürbig P., Lichtinghagen R., Brand K., Kuznetsova T., Olivi L., Verhamme P., Delles C., Mischak H., Staessen J. A.. Nephrol. Dial. Transplant. 2014, 29, 2260. [DOI] [PubMed] [Google Scholar]
- 40. Redfield M. M.. N. Engl. J. Med. 2016, 375, 1868. [DOI] [PubMed] [Google Scholar]
- 41. Cristelis E., Vlahou A., Stel V. S., Morton R. C.. Nephrol. Dial. Transplant. 2018, 33, 441. [DOI] [PubMed] [Google Scholar]
- 42. Lapiere M. C., Nusgens B., Pierard G. E.. Connect. Tissue Res. 1977, 1977, 21. [DOI] [PubMed] [Google Scholar]
- 43. Kasner M., Westermann D., Lopez B., Gaub R., Escher F., Küh U., Schultheiss H. P., Tschöpe C.. J. Am. Coll. Cardiol. 2011, 57, 977. [DOI] [PubMed] [Google Scholar]
- 44. Aoki T., Fukumoto Y., Sugimura K., Oikawa M., Satoh K., Nakano M., Nakayama M., Shimokawa H.. Circ. J. 2011, 75, 2605. [DOI] [PubMed] [Google Scholar]
- 45. Berry M., Galinier M., Delmas C., Fournier P., Desmoulin F., Turkeih A., Mischak H., Mullen W., Barutaut M., Eurlings L. W., van Wijk S., Rocca H. P. B. L., Caubere C., J., Butler , Roncalli J., Evaristi M. F., Cohen‐Solal A., Seronde M. F., Escamilla R., Ferrières J., Koukoui F., Smih F., Rouet P.. IJC Metab. Endocr. 2015, 6, 5. [Google Scholar]
- 46. Pontillo C., Zhang Z. Y., Schanstra J. P., Jacobs L., Zürbig P., Thijs L., Ramírez‐Torres A., Heerspink H. J. L., lindhardt M., Klein R., Orchard T., Porta M., Bilous R. W., Charturvedi N., Rossing P., Vlahou A., Schepers E., Glorieux G., Mullen W., Delles C., Verhamme P., Vanholder R., Staessen J. A., Mischak H., Jankowski J.. Kidney Int. Rep. 2017, 2, 1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nkuipou‐Kenfack E., Zürbig P., Mischak H.. Proteomics Clin. Appl. 2017, 11, 201600104. [DOI] [PubMed] [Google Scholar]
- 48. Grune J., Beyhoff N., Smeir E., Chudek R., Blumrich A., Ban Z., Brix S., Betz I. R., Schupp M., Foryst‐Ludwig A., Klopfleisch R., Stawowy P., Houtman R., Kolkhof P., Kintscher U.. Hypertension 2018, 71, 599. [DOI] [PubMed] [Google Scholar]
- 49. Swedberg K., Komajda M., Böhm M., Borer J. S., Ford I., Dubost‐Brama A., Lerebours G., Tavazzi L.. Lancet 2010, 376, 875. [DOI] [PubMed] [Google Scholar]
- 50. McMurray J. J., Packer M., Desai A. S., Gong J., Lefkowitz M. P., Rizkala A. R., Rouleau J. L., Shi V. C., Solomon S. D., Swedberg K., Zile M. R.. N. Engl. J. Med. 2014, 371, 993.25176015 [Google Scholar]
- 51. Pieske B., Maggioni A. P., Lam C. S. P., Pieske‐Kraigher E., Filippatos G., Butler J., Ponikowski P., Shah S. J., Solomon S. D., Scalise A. V., Mueller K., Roessig L., Gheorghiade M.. Eur. Heart J. 2017, 38, 1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Williams B., Mancia G., Spiering W., Agabiti Rosei E., Azizi M., Burnier M., Clement D. L., Coca A., de Simone G., Dominiczak A., Kahan T., Mahfoud F., Redon J., Ruilope L., Zanchetti A., Kerins M., Kjeldsen S. E., Kreutz R., Laurent S., Lip G. Y. H., McManus R., Narkiewicz K., Ruschitzka F., Schmieder R., Shlyakhto E., Tsioufis C., Aboyans V., Desormais I., ESC Scientific Document Group. Eur. Heart J. 2018, 39, 3021.30165516 [Google Scholar]
- 53. GBD 2013 DALYs and HALE Collaborators . Lancet 2017, 386, 2145. [Google Scholar]


