Abstract
Background
Tuberculosis (TB) kills millions of people every year. CD4 and CD8 T cells are critical in the immune response against TB. T cells expressing both CD4 and CD8 (CD4CD8 T cells) are functionally active and have not been examined in the context of TB.
Methods
We examine peripheral blood mononuclear cells (PBMC) and bronchoalveolar lavage cells (BAL) and lung granulomas from 28 cynomolgus macaques during Mycobacterium tuberculosis (Mtb) infection.
Results
CD4CD8 T cells increase in frequency during early Mtb infection in PBMC and BAL from pre‐infection. Peripheral, airway, and lung granuloma CD4CD8 T cells have distinct patterns and greater cytokine production than CD4 or CD8 T cells.
Conclusion
Our data suggest that CD4CD8 T cells transient the blood and airways early during infection to reach the granulomas where they are involved directly in the host response to Mtb.
Keywords: CD4CD8, mycobacteria, T cells, tuberculosis
1. INTRODUCTION
In 2017, 10.0 million new cases of tuberculosis (TB) were diagnosed worldwide, with an estimated death of 1.6 million people.1 The host immune response plays a critical role in the outcome of Mycobacterium tuberculosis (Mtb) infection. Both CD4 and CD8 T cells play important roles in the immunological responses to Mtb through the production of pro‐inflammatory cytokines (IFN‐γ, IL‐2, TNF, and IL‐17), anti‐inflammatory cytokine (IL‐10), and various cytolytic functions2, 3, 4, 5, 6, 7 among other mechanisms. Important roles in the CD4 T cell response to Mtb include production of IFN‐γ as well as production of TNF to optimize macrophage intracellular killing.8, 9, 10 CD4 T cells also stimulate the development of cytolytic CD8 T cell functions important for Mtb killing.11, 12 Cytolytic CD8 T cells (and CD4 T cells) can produce multiple cytolytic markers (eg granzyme B, granulysin, perforin), which can directly and indirectly kill Mtb‐infected macrophages.13, 14, 15 CD8 and CD4 T cells have been studied extensively within the TB literature; however, T cells expressing both CD4 and CD8 (CD4CD8 T cells) have not, leaving a gap in knowledge.
Although CD4 and CD8 T cells account for the majority of the CD3 T cell population, CD4CD8 T cells are present within blood16, 17, 18, 19, 20 and other sites and play functional roles in a variety of infections.17, 21, 22, 23, 24, 25, 26 In humans, approximately 1%‐6% of blood CD3 T cells are CD4CD8 T cells.16, 17, 18, 19, 20 Despite being low in frequency, CD4CD8 T cells display cytolytic and suppressive functions16, 18, 19, 27 (reviewed in28, 29). CD4CD8 T cells stimulated with anti‐CD3 or PHA, proliferate and kill target cells through granzyme B, and/or perforin‐dependent20, 27 and other unspecified mechanisms.17 They also express CD107a and produce TNF, IL‐4, IL‐2, IL‐10, IL‐17, and IFN‐γ20, 25, 30 when stimulated with anti‐CD3. CD4CD8 T cells functionally respond to HIV,17, 21, 22 SHIV,23 Mycobacterium leprae, 24 and various cancers.19, 20, 30, 31 CD4CD8 T cells from adults with rheumatoid arthritis produce more IFN‐γ than peripheral CD4 and CD8 T cells stimulated with mitogens.32 Taken together, CD4CD8 T cells express both pro‐inflammatory and anti‐inflammatory cytokines production and are able to exhibit cytolytic function. However, they have not been examined in the context of TB.
Here, we sought to characterize these CD4CD8 T cells during the course of Mtb infection in the blood, airways, and lung granulomas using a cynomolgus macaque model of TB. We show that CD4CD8 T cells increase in frequency in both blood and the airways during early Mtb infection and within lung granulomas with Mtb‐specific responses that are similar but distinct in pattern from conventional single‐expressing CD4 and CD8 T cells.
2. METHODS
2.1. Animals
Adult (>4 years of age) cynomolgus macaques (Macaca fascicularis) were screened for other co‐morbidities (eg parasites, SIV) before infection (Valley Biosystems, Sacramento, CA). Animals were infected with low‐dose (~25 CFU per monkey) Mtb (Erdman strain) via bronchoscopic instillation to the lower lung lobe and housed in Biosafety Level 3 (BSL‐3) primate facility.2, 33, 34 Infection was confirmed as previously described.34 Animals were declared as having active disease (ie repeated presence of Mtb growth in bronchoalveolar lavage [BAL] or gastric aspirate, elevated sedimentation rate [ESR], and/or clinical signs of active TB after infection) or latent infection (ie no clinical signs of active TB, normal ESR, and lack of persistent Mtb growth by 6 months post‐infection) based on previously published criteria.34 Twenty‐four monkeys were included in the PBMC intracellular cytokine (ICS) analysis, 28 monkeys were included in the BAL profile, and eight monkeys were included in the lung granuloma profile. Due to restrictions on animal procedures or low cell counts, some time points use fewer monkeys. Immune data for eight of these animals were previously published2, 35 but without CD4CD8 T cell analysis. All animal protocols and procedures were approved by the University of Pittsburgh's Institutional Animal Care and Use Committee that adheres to the national guidelines established in the Animal Welfare Act and Guide for the Care and Use of Laboratory Animals as mandated by the U. S. Public Health Service Policy.
2.2. Procedures
Cells from the BAL and PBMCs were obtained for analysis prior to Mtb infection, 4 weeks, 12 weeks post‐infection, and at time of clinical diagnosis (latent or active TB) as previously described.36, 37 Isolated cells were stimulated with ESAT6‐CFP10 peptide pools (10 μg/mL of every peptide) or incubated with only media (RPMI+10%hAB). PBMC incubated with or without the peptide pools for 1 hour in media, followed by the addition of Brefeldin A (Golgiplug: BD biosciences) for 5 hours at 37°C with 5% CO2. ESAT6‐CFP10 responses within PBMC T cells are presented after subtracting responses from PBMC incubated with media. BAL and granuloma cells were isolated and stimulated for 4 hours with peptide pools in the presence of Brefeldin A. When used, CD107a antibody was added prior to incubation. A minimum of 5 × 105 BAL or PBMC cells were incubated with each stimulation condition and acquired.
A total of 29 granulomas were used for analysis from eight macaques (2‐5 granulomas per animal) using the same ICS protocol as above for BAL. The range of cells analyzed from granulomas included CD4 (median = 116, IQR25%‐75% = 80‐347), CD8 (median = 135, IQR25%‐75% = 78‐304), and CD4CD8 (median = 111, IQR25%‐75% = 74‐260). T cells were in the estimated range of a previously published threshold.2 Granuloma‐specific T cell responses were only available from clinically latent animals as animals with active TB were dedicated for other studies and immune data were not available.
2.3. Flow cytometry
Flow cytometry was performed on PBMC, BAL, and granuloma cells after stimulation and stained with a combination of antibodies (Table S1), as previously described.2, 12 PBMC were stained for 2 panels: cytokines (CD3, CD4, CD8, IFN‐γ, TNF, IL‐2, IL‐10, and IL‐17) or cytolytic markers (CD107a, granzyme B, granulysin, perforin). BAL cells were stained for CD3, CD4, CD8, IFN‐γ, TNF, IL‐2, IL‐10, and IL‐17. Granuloma cells were stained for CD3, CD4, CD8, IFN‐γ, TNF, IL‐2, IL‐10, IL‐17, and granzyme B. Data acquisition was performed using an LSR II (BD) and analyzed using FlowJo Software v.9.7 (Treestar Inc, Ashland, OR). Gating strategies used for analysis are presented using ESAT6‐CFP10‐stimulated PBMC (Figure S1). To eliminate the possibility that CD4CD8 T cell population is a result of fluorophore spillover, the population was confirmed using combinations of fluorophores that are excited by different lasers (Figure S1).
2.4. Statistical analysis
Data were tested for normality with the Shapiro‐Wilk test and were not consistently normally distributed, so all statistics utilized were non‐parametric analyses (JMP Pro 12.1.0 statistical software). Kruskal‐Wallis test with Dunn's multiple comparison test was used to compare changes in cell type, cytokine production, and cytolytic marker expression over time within BAL and PBMC (GraphPad Prism 7.0d statistical software). Wilcoxon method (with unadjusted P‐values) was used to compare frequencies of CD4, CD8, and CD4CD8 T cells in PBMC and BAL and granulomas within the same time points (JMP Pro 12.1.0 statistical software). Statistical significance was set at P ≤ 0.05. All P values P ≤ 0.1 are displayed to represent statistical trends.
3. RESULTS
To characterize the dynamics in CD4CD8 T cells during the course of Mtb infection, the frequency and function of CD4CD8 T cells in PBMC and BAL were examined before M. tuberculosis infection, 4 weeks, 12 weeks post‐infection, and at the time of latent infection diagnosis (at 6 months after infection) and active TB diagnosis (typically before 6 months after infection) (Figure 1). CD4CD8 T cell frequencies doubled from pre‐infection levels at 4 weeks post‐Mtb infection (Figure 1) in both PBMC and BAL cells. At 4 weeks post‐Mtb infection, an increased production of Mtb‐specific TNF and granzyme B was observed in PBMC CD4CD8 T cells compared to pre‐infection levels (Figure 1). Similarly, CD4CD8 T cells in the airways increased significantly at 4 weeks post‐Mtb infection compared to pre‐infection levels. Higher frequencies of TNF producing airway CD4CD8 T cells were also seen at 4 weeks after Mtb infection but then decreased to pre‐infection levels during the remaining infection period.
Figure 1.
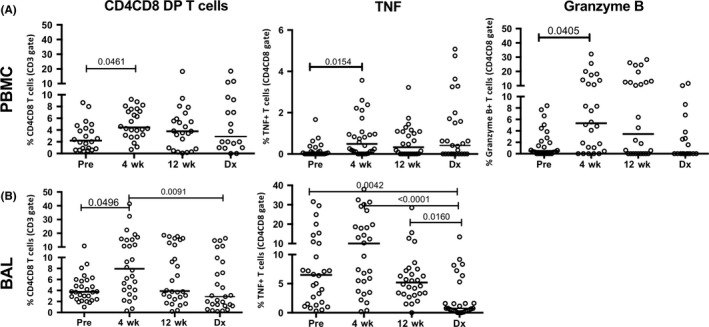
Frequency and function of CD4CD8 T cells in PBMC and BAL during macaque Mycobacterium tuberculosis (Mtb) infection. A, Increased frequencies of CD4CD8 T cells in PBMCs are seen at 4 wks after infection (from baseline) with an increase in Mtb‐specific production of TNF and granzyme B observed among CD4CD8 T cells. Cytokine production in ESAT6‐CFP10‐stimulated PBMC was subtracted from cytokine production by PBMC incubated with media. (B) An increased frequency of CD4CD8 T cells from bronchoalveolar lavage (BAL) is observed soon after Mtb infection with increased Mtb‐specific TNF production. Samples were obtained before infection (“Pre”), at 4 (“4 wk”) and 12 (“12 wk”) weeks after Mtb infection, and at the time in which animals met the clinical definition of active or latent infection (“Dx”). Twenty‐four and 28 monkeys were included in the PBMC and BAL analyses, respectively. Kruskal‐Wallis tests with Dunn's multiple comparison post test were used to determine significance with P‐values as indicated
To distinguish PBMC and BAL CD4CD8 T cells from CD4 or CD8 T cells, we compared the frequency and activity of each cell type within the same time point during infection (Tables 1 and 2). In blood, a significantly smaller frequency of CD4CD8 T cells was observed at all time points compared to CD4 and CD8 T cells. Within PBMC, CD4CD8 T cells are able to produce both cytolytic markers and Th1 cytokines during acute TB. At 4 weeks post‐Mtb infection, Mtb‐specific CD4CD8 T cells in PBMC express a trend of higher CD107a than CD4 T cells, and significantly more granzyme B and perforin than CD4 T cells (Table 1) but not CD8 T cells. In contrast, a higher proportion of perpiheral CD4CD8 T cells producing Mtb‐specific IFN‐γ and TNF were observed at 4 weeks after infection compared to CD8 T cells (Table 1). Taken together, peripheral CD4CD8 T cells appear to have higher cytolytic potential during acute Mtb infection than CD4 T cells but more Mtb‐specific cytokine producing properties than CD8 T cells.
Table 1.
Frequency of T cells from peripheral blood mononuclear cells (PBMC) producing antigen‐specific cytokines during Mycobacterium tuberculosis infection in non‐human primates (n = 28)
| PBMC | Weeks post‐Mtb infection | CD4 | CD4CD8 | CD8 | P value (CD4 vs CD4CD8) | P value (CD8 vs CD4CD8) |
|---|---|---|---|---|---|---|
| Median [IQR 25%, 75%] | Median [25%, 75%] | Median [25%, 75%] | ||||
| T cell % | 0 | 52.9 [40.0‐58.4] | 2.2 [0.77‐4.4] | 35.6 [30.1‐41.6] | <0.0001 | <0.0001 |
| 4 wk | 46.7 [36.6‐54.9] | 4.4 [3.1‐7.5] | 40.0 [31.9‐47.0] | <0.0001 | <0.0001 | |
| 12 wk | 45.4 [36.5‐54.3] | 3.8 [0.80‐5.9] | 43.3 [34.3‐51.5] | <0.0001 | <0.0001 | |
| Dx | 52.5 [40.0‐58.8] | 3.9 [1.2‐8.1] | 38.3 [31.1‐45.8] | <0.0001 | <0.0001 | |
| CD107a % | 0 | 0.05 [0‐0.2] | 0.081 [0‐0.50] | 0.1 [0.02‐0.42] | ||
| 4 wk | 0.015 [0‐0.56] | 0.1 [0‐0.43] | 0.25 [0.07‐0.42] | 0.0570 | ||
| 12 wk | 0.028 [0‐0.10] | 0.10 [0‐0.20] | 0.11 [0.01‐0.44] | |||
| Dx | 0 [0‐0.12] | 0.19 [0‐1.3] | 0 [0‐0.2] | 0.0722 | ||
| Granulysin % | 0 | 0.08 [0‐0.45] | 0.4 [0‐1.5] | 0.4 [0.04‐1.6] | ||
| 4 wk | 0.2 [0‐0.56] | 0.7 [0‐1.9] | 0.9 [0.3‐1.3] | |||
| 12 wk | 0.1 [0‐0.42] | 0.3 [0‐0.56] | 0.4 [0‐1.0] | |||
| Dx | 0.067 [0‐1.1] | 0 [0‐1.4] | 0.065 [0‐3.2] | |||
| Granzyme B % | 0 | 0.14 [0‐1.4] | 0.49 [0‐4.6] | 0.24 [0‐3.5] | ||
| 4 wk | 0.8 [0.1‐2.9] | 5.2 [0.9‐13.9] | 2.1 [0.2‐15.2] | 0.0207 | ||
| 12 wk | 3.0 [0.03‐5.2] | 3.4 [0‐12.7] | 1.4 [0‐18.1] | |||
| Dx | 0 [0‐4.0] | 0 [0‐4.5] | 0.3 [0‐4.01] | |||
| Perforin % | 0 | 0 [0‐0.05] | 0.19 [0‐0.97] | 0.3 [0.01‐1.4] | 0.0288 | |
| 4 wk | 0.06 [0.02‐0.45] | 1.04 [0.5‐2.1] | 0.69 [0.3‐2.4] | 0.0434 | ||
| 12 wk | 0.02 [0‐0.09] | 0.1 [0‐0.42] | 0.23 [0.1‐0.51] | |||
| Dx | 0 [0‐0.2] | 0 [0‐0.47] | 0.023 [0‐0.83] | |||
| IL10% | 0 | 0.01 [0‐0.2] | 0.40 [0‐1.3] | 0.01 [0‐0.1] | ||
| 4 wk | 0.7 [0‐3.5] | 0 [0‐0.9] | 0 [0‐0.5] | |||
| 12 wk | 0.012 [0‐3.5] | 0 [0‐0.8] | 0.11 [0‐0.3] | |||
| Dx | 0.52 [0‐1.3] | 0.29 [0.01‐1.6] | 0.53 [0‐4.4] | |||
| IFN‐γ % | 0 | 0.03 [0‐0.13] | 0 [0‐0.72] | 0.03 [0‐0.14] | ||
| 4 wk | 0.15 [0.02‐0.34] | 0.25 [0‐0.75] | 0.05 [0‐0.16] | 0.0401 | ||
| 12 wk | 0.10 [0.03‐0.30] | 0.07 [0‐0.40] | 0.06 [0.01‐0.14] | |||
| Dx | 0.02 [0‐1.3] | 0.07 [0‐5.0] | 0 [0‐1.4] | |||
| IL2% | 0 | 0.093 [0‐0.15] | 0.27 [0‐0.53] | 0.11 [0.02‐0.20] | ||
| 4 wk | 0.13 [0.04‐0.32] | 0.002 [0‐1.5] | 0.11 [0.02‐0.36] | |||
| 12 wk | 0.09 [0.04‐0.34] | 0.42 [0‐0.76] | 0.083 [0‐0.27] | |||
| Dx | 1.2 [0.2‐2.9] | 0 [0‐0.01] | 0.1 [0‐3.7] | <0.0001 | 0.0011 | |
| TNF % | 0 | 0.045 [0‐0.12] | 0.015 [0‐0.18] | 0.037 [0‐0.08] | ||
| 4 wk | 0.36 [0.06‐0.47] | 0.49 [0.11‐1.04] | 0.12 [0‐0.33] | 0.0196 | ||
| 12 wk | 0.20 [0.01‐0.40] | 0.33 [0‐1.1] | 0.16 [0.02‐0.3] | |||
| Dx | 0.47 [0.02‐1.2] | 0.41 [0‐1.7] | 0 [0‐0.84] | 0.0998 | ||
| IL17% | 0 | 0 [0‐0.08] | 0 [0‐0] | 0 [0‐0.17] | ||
| 4 wk | 0.10 [0‐0.76] | 0.13 [0‐1.6] | 0.015 [0‐0.24] | |||
| 12 wk | 0.014 [0‐0.22] | 0.03 [0‐0.58] | 0.0043 [0‐0.2] | |||
| Dx | 0.76 [0.15‐2.8] | 1 [0‐3.5] | 0.018 [0‐1.9] |
CD4, CD4CD8, and CD8 T cells within the T cell gate (CD3+) are shown in the top row. Percentages of cells expressing each cytolytic marker or cytokine are represented for CD4, CD4CD8, or CD8 T cells. ESAT6/CFP10 stimulated cells were subtracted from media incubated cells. Analysis performed across each cell type within each time point using the (unadjusted P‐values shown in last column).
Dx, time that clinical outcome is declared (active disease or latent infection); Wk, weeks post‐infection.
Significance is P ≤ 0.05.
Table 2.
Frequency of T cells from bronchoalveolar lavage cells producing cytokines during Mycobacterium tuberculosis infection in non‐human primates (n = 28). CD4, CD4CD8, and CD8 T cells within the T cell gate (CD3+) are shown in the top row
| BAL | Weeks post‐Mtb infection | CD4 | CD4CD8 | CD8 | P value (CD4 vs CD4CD8) | P value (CD8 vs CD4CD8) |
|---|---|---|---|---|---|---|
| Median [25%, 75%] | Median [25%, 75%] | Median [25%, 75%] | ||||
| T cell % | 0 | 36.8 [31.4‐45.6] | 4.0 [2.5‐6.2] | 39.7 [31.3‐46.1] | <0.0001 | <0.0001 |
| 4 wk | 30.5 [22.4‐42.0] | 8.0 [4.1‐15.3] | 42 [40.5‐49.6] | <0.0001 | <0.0001 | |
| 12 wk | 37.4 [23.0‐45.2] | 3.9 [2.5‐12.6] | 44.6 [39.9‐52.5] | <0.0001 | <0.0001 | |
| Dx | 33.4 [26.6‐44.3] | 2.9 [1.2‐9.2] | 50.2 [40.8‐56.6] | <0.0001 | <0.0001 | |
| IFN‐γ % | 0 | 0.75 [0.4‐2.2] | 6.5 [0.6‐16.0] | 2.1 [0.7‐5.5] | 0.0027 | 0.0232 |
| 4 wk | 2.9 [1.1‐9.4] | 5.3 [2.8‐9.6] | 1.4 [0.5‐2.5] | 0.0753 | <0.0001 | |
| 12 wk | 2.9 [0.98‐6.3] | 3.4 [1.4‐5.6] | 0.85 [0.5‐2.2] | 0.0001 | ||
| Dx | 1.45 [0.5‐4.1] | 1.2 [0.3‐2.8] | 0.3 [0.1‐0.6] | 0.0126 | ||
| IL‐10% | 0 | 2.3 [0.9‐7.3] | 8.1 [3.3‐13.6] | 2.5 [1.1‐3.7] | 0.0192 | 0.0020 |
| 4 wk | 1 [0.2‐3.7] | 6.1 [2.2‐13.2] | 1.1 [0.38‐4.3] | 0.0284 | 0.0365 | |
| 12 wk | 1.9 [0.6‐5.4] | 9.7 [4.9‐19.2] | 2.4 [0.5‐13.6] | 0.0015 | ||
| Dx | 0.66 [0.2‐3.2] | 7.1 [0‐13.6] | 1.7 [0.05‐6.5] | |||
| IL‐17% | 0 | 1.4 [0.7‐3.1] | 5.6 [2.6‐14.6] | 1.25 [0.6‐2.6] | <0.0001 | <0.0001 |
| 4 wk | 2.4 [1.2‐7.9] | 5.3 [2.4‐10.4] | 0.95 [0.5‐3.7] | 0.0464 | 0.0016 | |
| 12 wk | 0.8 [0.3‐2.6] | 2.8 [1.6‐7.7] | 0.3 [0.1‐0.58] | 0.0008 | <0.0001 | |
| Dx | 0.34 [0‐1.9] | 4.76 [1.7‐9.9] | 0.35 [0.1‐0.65] | <0.0001 | <0.0001 | |
| IL‐2% | 0 | 1.3 [0.8‐5.5] | 5.1 [2.3‐9.6] | 1.3 [0.8‐2.6] | 0.0046 | 0.0001 |
| 4 wk | 3.6 [1.5‐5.2] | 4.7 [3.3‐7.0] | 1.6 [0.9‐2.1] | 0.0003 | ||
| 12 wk | 2.3 [1.4‐3.4] | 3.4 [2.4‐5.1] | 0.8 [0.5‐1.7] | 0.0460 | <0.0001 | |
| Dx | 2.1 [1.0‐3.2] | 2.4 [0.9‐5.8] | 1.1 [0.2‐1.7] | 0.0236 | ||
| TNF % | 0 | 1.5 [0.9‐4] | 6.5 [1.6‐10.7] | 2.8 [1.5‐7.8] | <0.0051 | |
| 4 wk | 6.8 [3‐12.3] | 10.0 [4.1‐19.4] | 2.6 [0.9‐9.2] | 0.0018 | ||
| 12 wk | 5.1 [2.5‐8.1] | 5.2 [3.2‐7.4] | 2 [1.0‐3.0] | 0.0001 | ||
| Dx | 1.8 [0.2‐5.2] | 0.76 [0.4‐5.3] | 1.2 [0.2‐3.2] |
Percentages of cells expressing each cytokine are represented for CD4, CD4CD8, or CD8 T cells from cells stimulated with ESAT6/CFP10. Analysis performed on each pair of cell types at each time point using the Wilcoxon method (unadjusted P‐values shown in last column).
Dx, time that clinical outcome is declared (active disease or latent infection); Wk, weeks post‐infection.
Significance is P ≤ 0.05.
Similar to blood, the frequency of CD4CD8 T cells in the airway was significantly lower compared to CD4 and CD8 T cells. In contrast to blood, airway CD4CD8 T cells expressed significantly greater IFN‐γ, IL‐10, IL‐17, IL‐2, and TNF than CD4 and CD8 T cells prior to Mtb infection suggesting that these cells may play a non‐specific role but could contribute to the immune response in the airways. At 4 weeks post‐Mtb infection, CD4CD8 T cells produced more IL‐17 and IL‐10 compared to both CD4 and CD8 T cell and greater Th1 cytokines (IFN‐γ, TNF, and IL‐2) than CD8 T cells (Table 2). Over the duration of the infection, CD4CD8 T cells were more likely to produce more IFN‐γ and IL‐2 than CD8 T cells. Interestingly, IL‐17 production within CD4CD8 T cells was higher than both CD4 and CD8 T cells. At the time of diagnoses, macaques that developed active TB maintained a lower frequency of BAL CD4CD8 T cells compared to those with controlled latent Mtb infection (Figure S2) though no differences in cytokine production were observed. These data suggest that CD4CD8 T cells have overlapping functions with both CD4 and CD8 T cells, but that their function in the airway compartment may be quite different than the blood.
Lung granulomas, a collection of localized immune cells (eg lymphocytes, macrophages, neutrophils), are the hallmark of Mtb infection and play a critical role in direct pathogen‐host interaction. T cell responses in the granuloma are known to play a key role in outcome; therefore, we sought to characterize the frequency and functionality of CD4CD8 T cells in granulomas (Figure 2) and compare them to single positive CD4 and CD8 T cells. CD4CD8 T cell accounted for a median of 11% of CD3 T cells in the granuloma, and this was significantly less than the 36.2% CD4 T cells and 25% CD8 T cells (Figure 2A). A significantly lower frequency of CD4CD8 T cells expressed granzyme B compared to both CD4 and CD8 T cells (Figure 2A). The proportion of CD4CD8 T cells producing IL‐10 was greater than CD8 T cells but similar to CD4 T cells. In contrast, the proportion of TNF producing CD4CD8 T cells was significantly higher than CD4 T cells and more similar to CD8 T cells. No differences were observed in their production of IFN‐γ, IL‐2, or IL‐17 compared to conventional CD4 and CD8 T These data demonstrate that CD4CD8 T cells have both distinct and similar functions from CD4 and CD8 T cells within individual granulomas.
Figure 2.
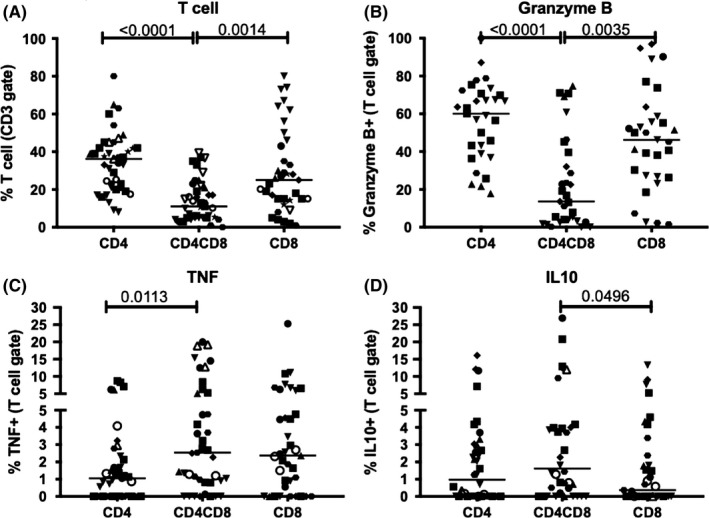
CD4CD8 T cells are distinct from CD4 and CD8 T cells in both frequency and function within lung granulomas of M. tuberculosis‐infected macaques. A) CD4CD8 T cells are lower in frequency than CD4 and CD8 T cells. B) CD4CD8 T cells produce less granzyme B than CD4 and CD8 T cells. C and D) Differences in TNF and IL‐10 production in CD4CD8 T cells compared to CD4 and CD8 T cells. Each data point represents an individual lung granulomas. Different symbols represent individual macaques. Kruskal‐Wallis test with Dunn's multiple comparison (post hoc), P‐values shown
In summary, CD4CD8 T cells are increased in frequency during early Mtb infection in the blood and airways and can be found in granulomas with distinct Mtb‐specific functions from single‐expressing CD4 and CD8 T cells.
4. DISCUSSION
This study aimed to characterize the frequency and function of CD4CD8 T cells during Mtb infection in PBMC, BAL, and lung granulomas and determine if they are functionally distinct from CD4 and CD8 T cells. CD4CD8 T cells are traditionally regarded as existing during a time of cellular differentiation toward CD4 or CD8 T cells. However, extrathymic CD4CD8 T cells located within the blood and numerous tissues have been reported27, 28, 32, 38, 39 and more than 96% of blood CD4CD8 T cells express alpha‐beta TCR.32
We determined that CD4CD8 T cells represent a minority population of T cells within PBMC (median ranges pre‐ and 4 week post‐infection of 2.2%‐4.4%), BAL (median ranges pre‐ and 4 weeks post‐infection of 2.9%‐8.0%), and lung granulomas (median 11%). In blood, Mtb‐specific CD4CD8 T cells express more cytolytic markers (CD107a, granzyme B, and perforin) than CD4 T cells but not CD8 T cells and more Th1 cytokines (TNF and IFN‐γ) than CD8 T cells that was most robust at 4 weeks after Mtb infection compared to baseline. The pattern of greater cytolytic function among CD4CD8 T cells is consistent with reports from others.27 The early increase in TNF production is consistent with the acute peripheral CD4 and CD8 T cell cytokine responses during this period and was associated with animals that developed active TB compared to latent infection suggesting that the responses were likely associated with TB pathology. While their proportions were small, CD4CD8 T cells in the BAL produced Th1 (IFN‐γ and IL‐2) and Th17 (IL‐17) cytokines more robustly than either the CD8 or CD4 T cells before and during the course of infection. There was a significant increase in the frequency of CD4CD8 T cells at 4 weeks post‐Mtb infection with greater production of Th1 cytokines than CD8 T cells. At this time point, CD4CD8 T cells also produced more IL‐10 than mono‐expressing CD4 or CD8 T cells. Taken together, these data suggest that these cells have both pro‐inflammatory (Th1) and anti‐inflammatory (IL‐10) roles that are important in the immune response to Mtb infection.
Within the granuloma, CD4CD8 T cells were present in higher frequencies than BAL and PBMC. CD4CD8 T cells express more IL10 than CD8 T cells (similar to the BAL cells) and TNF than CD4 T cells and produce similar amounts of IFN‐γ, IL2, IL17, and IL4 as CD4 and CD8 T cells. The transient increase in frequency of CD4CD8 T cells in both PBMC and BAL during early Mtb infection and the observed higher frequency of CD4CD8 T cells in the granuloma would suggest that these cells are being recruited during early infection and transiting the blood and airways to the granuloma, where the host‐pathogen response is most critical.
In conclusion, we have identified a small population of T cells that express both CD4 and CD8 receptors that have both cytokine and cytotoxic capacities. Their responses during Mtb infection are similar in property but different in characteristic than mono‐expressing CD4 or CD8 T cells. Furthermore, their role in the host immune response is likely dependent on their inherent immunologic compartments (eg blood vs airway vs granulomas), whose characteristics differ during the course of Mtb infection. Even within an individual anatomic compartment, these cells are heterogeneous and able to produce both pro‐inflammatory (Th1 and Th17 driven response) and anti‐inflammatory (IL‐10) cytokines that are important to the balance of host immune responses in tuberculosis. Further characterization of their activation and suppression signals would be of interest for future studies. Importantly, this dual expressing T cell population is often combined with either CD4 or CD8 T cells populations but as we have shown, their function is different from mono‐expression CD4 or CD8 T cells. Therefore, we recommend that CD4CD8 T cells should be separately analyzed from single‐expressing CD4 and CD8 T cells in immunologic studies.
CONFLICTS OF INTEREST
The authors do not have any conflicts of interest associated with this manuscript.
AUTHOR CONTRIBUTION
Conception and design: CRD, PLL; Analysis and interpretation: CRD, PLL; Drafting the manuscript for important intellectual content: CRD, TR, AJM, HPG, PLL, PM; Experimentation: CRD, TB, TR, AJM, HPG.
Supporting information
ACKNOWLEDGMENTS
We thank our dedicated veterinarians Drs. Edwin Klein and Christopher Janssen in performing necropsies; Charles Scanga for coordinating the studies; Mark Rodgers, Catherine Cochran, Carolyn Bigbee, Brianne Stein, Chelsea Chedrick, Cassaundra Updike, Paul Johnston, Melanie O'Malley, Jaime Tomko, and Dan Fillmore. We would also like to thank JoAnne L Flynn (J.L.F) for her critical evaluation of the manuscript and use of samples. This study was funded by the Bill and Melinda Gates Foundation (J.L.F, P.L.L.) and the NIH (AI0 AI111871‐P.L.L.).
Diedrich CR, Gideon HP, Rutledge T, et al. CD4CD8 Double Positive T cell responses during Mycobacterium tuberculosis infection in cynomolgus macaques. J Med Primatol. 2019;48:82–89. 10.1111/jmp.12399
REFERENCES
- 1. World Health Organization . Global Tuberculosis Report 2018. Geneva: World Health Publications; 2017. [Google Scholar]
- 2. Gideon HP, Phuah J, Myers AJ, et al. Variability in tuberculosis granuloma T cell responses exists, but a balance of pro‐ and anti‐inflammatory cytokines is associated with sterilization. Lewinsohn DM, ed. PLoS Pathog. 2015;11(1):e1004603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lin PL, Myers A, Smith L, et al. Tumor necrosis factor neutralization results in disseminated disease in acute and latent Mycobacterium tuberculosis infection with normal granuloma structure in a cynomolgus macaque model. Arthritis Rheum. 2010;62(2):340‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaushal D, Foreman TW, Gautam US, et al. Mucosal vaccination with attenuated Mycobacterium tuberculosis induces strong central memory responses and protects against tuberculosis. Nat Commun. 2015;6:8533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mattila JT, Maiello P, Sun T, Via LE, Flynn JL. Granzyme B‐expressing neutrophils correlate with bacteria load in granulomas from Mycobacterium tuberculosis‐infected cynomolgus macaques. Cell Microbiol. 2015;17(8):n/a‐n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andersson J, Samarina A, Fink J, Rahman S, Grundström S. Impaired expression of perforin and granulysin in CD8+ T cells at the site of infection in human chronic pulmonary tuberculosis. Infect Immun. 2007;75(11):5210‐5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kumar MM, Raja A. Cytotoxicity responses to selected ESAT‐6 and CFP‐10 peptides in tuberculosis. Cell Immunol. 2010;265(2):146‐155. [DOI] [PubMed] [Google Scholar]
- 8. Barry SM, Lipman MC, Bannister B, Johnson MA, Janossy G. Purified protein derivative‐activated type 1 cytokine‐producing CD4(+) T lymphocytes in the lung: a characteristic feature of active pulmonary and nonpulmonary tuberculosis. J Infect Dis. 2003;187(2):243‐250. [DOI] [PubMed] [Google Scholar]
- 9. Gallegos AM, van Heijst JWJ, Samstein M, Su X, Pamer EG, Glickman MS. A gamma interferon independent mechanism of CD4 T cell mediated control of M. tuberculosis infection in vivo. Ramakrishnan L, ed. PLoS Pathog. 2011;7(5):e1002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sakai S, Mayer‐Barber KD, Barber DL. ScienceDirectDefining features of protective CD4 T cell responses to Mycobacterium tuberculosis . Curr Opin Immunol. 2014;29:137‐142. 10.1016/j.coi.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Serbina NV, Lazarevic V, Flynn JL. CD4(+) T cells are required for the development of cytotoxic CD8(+) T cells during Mycobacterium tuberculosis infection. J Immunol. 2001;167(12):6991‐7000. [DOI] [PubMed] [Google Scholar]
- 12. Lin PL, Rutledge T, Green AM, et al. CD4 T cell depletion exacerbates acute Mycobacterium tuberculosis while reactivation of latent infection is dependent on severity of tissue depletion in cynomolgus macaques. AIDS Res Hum Retroviruses. 2012;28(12):1693‐1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin PL, Flynn JL. CD8 T cells and Mycobacterium tuberculosis infection. Semin Immunopathol. 2015;37(3):239‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Canaday DH, Wilkinson RJ, Li Q, Harding CV, Silver RF, Boom WH. CD4(+) and CD8(+) T cells kill intracellular Mycobacterium tuberculosis by a perforin and Fas/Fas ligand‐independent mechanism. J Immunol. 2001;167(5):2734‐2742. [DOI] [PubMed] [Google Scholar]
- 15. Stenger S, Hanson DA, Teitelbaum R, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282(5386):121‐125. [DOI] [PubMed] [Google Scholar]
- 16. Kay NE, Bone N, Hupke M, Dalmasso AP. Expansion of a lymphocyte population co‐expressing T4 (CD4) and T8 (CD8) antigens in the peripheral blood of a normal adult male. Blood. 1990;75(10):2024‐2029. [PubMed] [Google Scholar]
- 17. Kitchen SG, Jones NR, LaForge S, et al. CD4 on CD8(+) T cells directly enhances effector function and is a target for HIV infection. Proc Natl Acad Sci USA. 2004;101(23):8727‐8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee WW, Nam KH, Terao K, Yoshikawa Y. Possible role of genetic factor(s) on age‐related increase of peripheral CD4(+)CD8(+) double positive T cells in cynomolgus monkeys. Exp Anim. 2003;52(4):309‐316. [DOI] [PubMed] [Google Scholar]
- 19. Desfrancois J, Derre L, Corvaisier M, et al. Increased frequency of nonconventional double positive CD4CD8 alpha beta T cells in human breast pleural effusions. Int J Cancer. 2009;125(2):374‐380. 10.1002/ijc.24366. [DOI] [PubMed] [Google Scholar]
- 20. Parrot T, Allard M, Oger R, et al. IL‐9 promotes the survival and function of human melanoma‐infiltrating CD4(+) CD8(+) double‐positive T cells. Eur J Immunol. 2016;46(7):1770‐1782. [DOI] [PubMed] [Google Scholar]
- 21. Hughes GJ, Cochrane A, Leenc C, Morris S, Bell JE, Simmonds P. HIV‐1‐infected CD8+CD4+ T cells decay in vivo at a similar rate to infected CD4 T cells during HAART. Aids. 2008;22(1):57‐65. [DOI] [PubMed] [Google Scholar]
- 22. Weiss L, Roux A, Garcia S, et al. Persistent expansion, in a human immunodeficiency virus‐infected person, of V beta‐restricted CD4+CD8+ T lymphocytes that express cytotoxicity‐associated molecules and are committed to produce interferon‐gamma and tumor necrosis factor‐alpha. J Infect Dis. 1998;178(4):1158‐1162. [DOI] [PubMed] [Google Scholar]
- 23. Miyake A, Ibuki K, Suzuki H, et al. Early virological events in various tissues of newborn monkeys after intrarectal infection with pathogenic simian human immunodeficiency virus. J Med Primatol. 2005;34(5–6):294‐302. 10.1111/j.1600-0684.2005.00127.x. [DOI] [PubMed] [Google Scholar]
- 24. Ottenhoff TH, Elferink DG, Klatser PR, de Vries RR. Cloned suppressor T cells from a lepromatous leprosy patient suppress Mycobacterium leprae reactive helper T cells. Nature. 1986;322(6078):462‐464. [DOI] [PubMed] [Google Scholar]
- 25. Sarrabayrouse G, Bossard C, Chauvin J‐M, et al. CD4CD8aa lymphocytes, a novel human regulatory T cell subset induced by colonic bacteria and deficient in patients with inflammatory bowel disease. Marrack P, ed. PLoS Biol. 2014;12(4):e1001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sarrabayrouse G, Alameddine J, Altare F, Jotereau F. Microbiota‐specific CD4CD8 alpha alpha Tregs: role in intestinal immune homeostasis and implications for IBD. Front Immunol. 2015;6:522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nam K, Akari H, Terao K, Shibata H, Kawamura S, Yoshikawa Y. Peripheral blood extrathymic CD4(+)CD8(+) T cells with high cytotoxic activity are from the same lineage as CD4(+)CD8(‐) T cells in cynomolgus monkeys. Int Immunol. 2000;12(7):1095‐1103. [DOI] [PubMed] [Google Scholar]
- 28. Overgaard NH, Jung J‐W, Steptoe RJ, Wells JW. CD4(+)/CD8(+) double‐positive T cells: more than just a developmental stage? J Leukoc Biol. 2015;97(1):31‐38. [DOI] [PubMed] [Google Scholar]
- 29. Zuckermann FA. Extrathymic CD4/CD8 double positive T cells. Vet Immunol Immunopathol. 1999;72(1–2):55‐66. [DOI] [PubMed] [Google Scholar]
- 30. Desfrancois J, Moreau‐Aubry A, Vignard V, et al. Double positive CD4CD8 alpha beta T cells: a new tumor‐reactive population in human melanomas. Lowenstein PR, ed. PLoS ONE. 2010;5(1):e8437 10.1371/journal.pone.0008437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Altomare E, Fallarini S, Biaggi G, Gattoni E, Botta M, Lombardi G. Increased frequency of circulating invariant natural killer T cells in malignant pleural mesothelioma patients. Cancer Biol Ther. 2012;13(9):702‐711. [DOI] [PubMed] [Google Scholar]
- 32. Quandt D, Rothe K, Scholz R, Baerwald CW, Wagner U. Peripheral CD4CD8 double positive T cells with a distinct helper cytokine profile are increased in rheumatoid arthritis. Frey O, ed. PLoS ONE. 2014;9(3):e93293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin PL, Ford CB, Coleman MT, et al. Sterilization of granulomas is common in active and latent tuberculosis despite within‐host variability in bacterial killing. Nat Med. 2014;20(1):75‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin PL, Rodgers M, Smith L, et al. Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect Immun. 2009;77(10):4631‐4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marino S, Gideon HP, Gong C, et al. Computational and empirical studies predict Mycobacterium tuberculosis‐specific T cells as a biomarker for infection outcome. Beauchemin CAA, ed. PLoS Comput Biol. 2016;12(4):e1004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Capuano SV, Croix DA, Pawar S, et al. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect Immun. 2003;71(10):5831‐5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lin PL, Pawar S, Myers A, et al. Early events in Mycobacterium tuberculosis infection in cynomolgus macaques. Infect Immun. 2006;74(7):3790‐3803. 10.1128/IAI.00064-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nam KH, Akari H, Terao K, Ohto H, Itagaki S, Yoshikawa Y. Age‐dependent remodeling of peripheral blood CD4+CD8+ T lymphocytes in cynomolgus monkeys. Dev Comp Immunol. 1998;22(2):239‐248. [DOI] [PubMed] [Google Scholar]
- 39. Akari H, Terao K, Murayama Y, Nam KH, Yoshikawa Y. Peripheral blood CD4+CD8+ lymphocytes in cynomolgus monkeys are of resting memory T lineage. Int Immunol. 1997;9(4):591‐597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


