Abstract
The diagnosis of biliary atresia (BA) remains a clinical challenge because affected infants have signs, symptoms, and serum liver biochemistry that are also seen in those with other causes of neonatal cholestasis (non‐BA). However, an early diagnosis and prompt surgical treatment are required to improve clinical outcome. Recently, the relative abundance of serum matrix metalloproteinase‐7 (MMP‐7) was suggested to have discriminatory features for infants with BA. To test the hypothesis that elevated serum concentration of MMP‐7 is highly diagnostic for BA, we determined the normal serum concentration of MMP‐7 in healthy control infants, and then in 135 consecutive infants being evaluated for cholestasis. The median concentration for MMP‐7 was 2.86 ng/mL (interquartile range, IQR: 1.32‐5.32) in normal controls, 11.47 ng/mL (IQR: 8.54‐24.55) for non‐BA, and 121.1 ng/mL (IQR: 85.42‐224.4) for BA (P < 0.0001). The area under the curve of MMP‐7 for the diagnosis of BA was 0.9900 with a cutoff value of 52.85 ng/mL; the diagnostic sensitivity and specificity were 98.67% and 95.00%, respectively, with a negative predictive value of 98.28%. Conclusion: Serum MMP‐7 assay has high sensitivity and specificity to differentiate BA from other neonatal cholestasis, and may be a reliable biomarker for BA.
Abbreviations
- APRi
AST to platelet ratio index
- AUC
area under curve
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BA
biliary atresia
- CI
confidence interval
- GGT
gamma glutamyl transpeptidase
- IQR
interquartile range
- MMP‐7
matrix metalloproteinase‐7
- NC
normal control
- NNM
number needed to misdiagnose
Biliary atresia (BA) is a destructive cholangiopathy with rapid progression to end‐stage cirrhosis if not diagnosed early in life and treated with hepatoportoenterostomy (also known as the Kasai procedure).1, 2, 3 Among all clinical and laboratory features, the age at hepatoportoenterostomy is most consistently correlated with clinical outcome, with younger patients being more likely to have improved biliary drainage and better survival with the native liver at 2 years of age.4, 5 However, the early diagnosis of BA in clinical practice is challenging due to an overlap of signs and symptoms with infants with other causes of cholestasis.
When evaluating neonates with cholestasis, clinicians routinely use an array of laboratory studies to assess the degree of cholestasis, hepatocellular injury and function, followed by a screen for other causes of intrahepatic cholestasis (e.g., alpha‐1‐antitrypsin level and Pi phenotype in serum), anatomical abnormalities by ultrasound, hepatobiliary scintigraphy, and liver biopsy. Depending on the available expertise, endoscopic retrograde cholangiopancreatography and other tests may be pursued. Among these tests, liver biopsy has the best positive predictive value, estimated in one study at 90.7%,6 but is associated with considerable morbidity. Therefore, the discovery of novel biomarkers of disease has the potential to simplify the diagnostic algorithm and shorten the time for surgical treatment.
Analyzing the serum of cholestatic infants with aptamer‐based proteomics, investigators recently reported high relative levels of matrix metalloproteinase‐7 (MMP‐7) in infants with BA.7 MMP‐7, a protease involved in intercellular signaling through breakdown of extracellular matrix, has been linked to liver fibrosis in children with BA8, 9, 10 and experimentally implicated in pathogenesis of bile duct epithelial injury.7 Based on these studies, we hypothesized that elevated serum concentration of MMP‐7 is highly diagnostic for BA. To test this hypothesis, we quantified serum MMP‐7 in 135 consecutive infants with cholestasis and in 54 age‐matched normal controls (NCs). After establishing normal concentration in controls, we found that serum MMP‐7 at 52.85 ng/mL differentiates BA from other causes of cholestasis (non‐BA) with a sensitivity of 98.67%, specificity of 95.00%, and negative predictive value of 98.28%.
Methods
Study Design and Population
Infants evaluated for cholestasis (defined as serum direct bilirubin above 17 µmol/L) at three major pediatric centers in the city of Wuhan, China, were consecutively enrolled in an observational study between October 2016 and April 2018. A separate cohort of normal subjects included infants and adults without evidence of liver disease. Blood samples for MMP‐7 measurements were obtained from cholestatic subjects at the time of the first visit, prior to ascertainment of the diagnosis. The evaluation of cholestasis included standard liver biochemistry, ultrasound, screen for gene mutation (when blood DNA was available), and a liver biopsy (when indicated). Subjects without a specific diagnosis and biopsies with obstructive features (increased number of bile duct profiles) underwent intraoperative cholangiography. The diagnosis of BA was made by the presence of fibrosing obstruction of extrahepatic biliary remnants excised after intraoperative cholangiography. The exclusion of BA was based on at least one of the following criteria: (1) cholangiography showing a patent biliary tree, (2) etiology confirmed by genetic testing, and (3) recovery of cholestasis as shown by the normalization of serum bilirubin and/or the normal color of stools on follow‐up visits. Informed, written consent to participate in this research was obtained from the parent for subjects aged less than 2 years, and from adult controls themselves. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in the approval by the institutional review board and ethics committee at Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Biochemical Studies and MMP‐7 Expression
Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, direct bilirubin, gamma glutamyl transpeptidase (GGT), and platelet counts were determined in clinical laboratories as part of the evaluation for all cholestatic patients. The AST to platelet ratio index (APRi score) was calculated as reported previously.11
Serum concentration of MMP‐7 was measured using an enzyme‐linked immunosorbent assay, according to the manufacturer’s protocol (Cloud‐Clone Corp., Wuhan, China). At the tissue level, MMP‐7 was detected by immunohistochemistry in tissue sections from paraffin‐embedded liver biopsies from BA and non‐BA subjects using an anti‐MMP‐7 antibody (Abcam, Cambridge, United Kingdom; dilution of 1:2000); control liver tissue was obtained from the margin of a resected hepatic hemangioma in an infant. The secondary amplification and signal detection of the immunohistochemical staining used the HRP AffiniPure Goat Anti‐Rabbit IgG kit (EarthOx, San Francisco, CA). Localization of positive signal was compared in each group under direct microscope examination. In these sections, the degree of hepatic fibrosis was assessed by the Metavir scoring system.
Statistical Analysis
Unpaired t test was used to analyze basic characteristics of the study subjects. For the analysis of serum MMP‐7, we first tested for normal distribution using both the D’Agostino & Pearson omnibus normality test and the Shapiro‐Wilk normality test. As the data failed these tests, we applied a nonparametric test (Mann‐Whitney U test). Measurement of serum MMP‐7 is presented with medians and interquartile ranges (IQRs). We calculated the area under the receiver operating characteristic curves (AUCs), and the optimal cutoff values of serum MMP‐7 were determined by optimizing sensitivity and specificity. Linear regression tests were performed to assess the correlation between serum MMP‐7 and other biochemical parameters. We also calculated the number needed to misdiagnose (NNM, defined as the number of subjects correctly assigned a diagnosis before an erroneous diagnosis is made) for both MMP‐7 and GGT.7, 12 Statistical calculations were performed using GraphPad Prism 6 software (GraphPad, La Jolla, CA).
Results
Study Subjects
We enrolled 135 infants less than 6 months of age with cholestasis: 75 subjects with BA and 60 with non‐BA cholestasis, with a median age of 54 and 59.5 days, respectively. We also enrolled 54 age‐matched NCs (median age of 53.5 days) who had blood samples drawn as part of evaluation/care for non‐liver diseases (Supporting Table S1) as well as an older healthy cohort of 6 months to 2 years of age (n = 8) and adults (n = 10, ages 25‐54 years old) (Fig. 1). Clinical, biochemical, genetic, and radiologic data of the 60 subjects in the non‐BA group showed that the largest group (n = 26 or 43.3%) had idiopathic cholestasis, followed by cytomegalovirus hepatitis (n = 16 or 26.7%; defined by IgM+ serum), choledochal cyst (n = 4 or 6.7%), Alagille syndrome (n = 3 or 5%), citrin deficiency (n = 3), and others (Table 1 and Supporting Table S2). Follow‐up data showed resolution of cholestasis in all subjects, except in the following patients who showed persistent or recurring cholestasis: 1 infant who died of multi‐organ failure (with heterozygous CFTR mutation), 1 with patent extrahepatic bile ducts by cholangiogram (later diagnosed with Alagille syndrome), 1 with heterozygous mutation in NPHP3 who had persistent cholestasis in the presence of colored stools, 1 with Dubin‐Johnson syndrome who had recurring cholestasis, 1 with choledochal cyst who suffered from recurring cholestasis postoperatively, and 1 with Niemann‐Pick disease who was lost to follow‐up. Comparison of all subjects in the BA and non‐BA groups revealed higher serum levels of direct bilirubin, AST, and GGT in BA (P < 0.01; Table 2).
Figure 1.
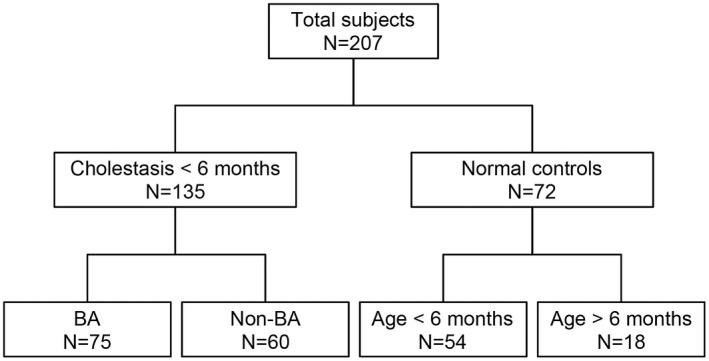
Flow chart of subject enrollment. A total of 207 infants were enrolled in this study, among whom 135 infants younger than 6 months of age were consecutively enrolled at the time of evaluation for conjugated hyperbilirubinemia: 75 were found to have BA and 60 had other diseases (non‐BA). The remaining 72 subjects were controls without liver disease (NC), among whom 54 were younger than 6 months, and 18 subjects were older.
Table 1.
Etiological Diagnosis of Patients Classified as non‐BA
| Diagnosis | Case No. | Percent (%) |
|---|---|---|
| Idiopathic cholestasis | 26 | 43.3 |
| Cytomegalovirus hepatitis | 16 | 26.7 |
| Choledochal cyst | 4 | 6.7 |
| Alagille syndrome | 3 | 5.0 |
| Citrin deficiency | 3 | 5.0 |
| Cholelithiasis | 1 | 1.7 |
| Dubin‐Johnson | 1 | 1.7 |
| Giant hemangioma | 1 | 1.7 |
| Hemangioendothelioma | 1 | 1.7 |
| Niemann‐Pick disease | 1 | 1.7 |
| Syphilis hepatitis | 1 | 1.7 |
| Sepsis‐associated cholestasis | 1 | 1.7 |
| Parenteral nutrition–associated liver disease | 1 | 1.7 |
| TOTAL | 60 | 100 |
Table 2.
Demographics and Laboratory Values of Study Subjects
| NC (54) | Non‐BA (60) | BA (75) | P * | |
|---|---|---|---|---|
| Age, median (IQR), days | 53.5 (35.5‐66.0) | 59.5 (46.3‐76.8) | 54 (43‐67) | 0.56 |
| Sex, n (%) | ||||
| Male | 31 (57.4) | 35 (58.3) | 34 (45.3) | 0.17 |
| Female | 23 (42.6) | 25 (41.7) | 41 (54.7) | |
| TB, † μmol/L | 13.8 (9.4‐37.0) | 155.0 (119.7‐203.8) | 182.6 (145.5‐224.5) | 0.014 |
| DB, † μmol/L | 4.7 (3.0‐8.5) | 95.4 (57.8‐130.8) | 124.8 (95.5‐156.0) | < 0.001 |
| ALT, † U/L | 19.5 (13.0‐26.0) | 81.5 (43.0‐120.8) | 128.0 (86.0‐219.0) | 0.080 |
| AST, † U/L | 30.0 (22.8‐41.0) | 123.0 (82.2‐183.0) | 214.0 (136.0‐297.0) | 0.004 |
| GGT, † U/L | 53.0 (32.5‐88.8) | 169.0 (113.3‐343.0) | 425.0 (193.0‐799.0) | < 0.001 |
P value: Between BA and Non‐BA cholestasis. Sex was tested using Fisher’s exact test. All other rows were tested using Student t test.
Median (IQR).
Abbreviations: DB, direct bilirubin; TB, total bilirubin.
Serum MMP‐7 Concentration in NC, BA, and Non‐BA
The serum levels of MMP‐7 in NCs had a median of 2.86 ng/mL (IQR: 1.32‐5.32), which was similar to older subjects in the control group (Fig. 2A). Although several subjects in the non‐BA cohort had MMP‐7 levels within the normal range, the median concentration was higher than age‐matched controls at 11.47 ng/mL (IQR: 8.54‐24.55; P < 0.0001). In contrast, MMP‐7 in BA subjects had an average 10‐fold increase above non‐BA, with a median of 121.1 ng/mL (IQR: 85.42‐224.4; Fig. 2A). Plotting individual concentrations according to the age of subjects showed that the levels did not vary significantly in the first 6 months in NCs (r = 0.1667, P = 0.2283), trended upward in non‐BA (r = 0.2257, P = 0.0829), and reached significance in BA (r = 0.5358, P < 0.0001; Fig. 2B). These analyses showed that subjects with BA have high levels of MMP‐7, with minimal overlap with non‐BA.
Figure 2.
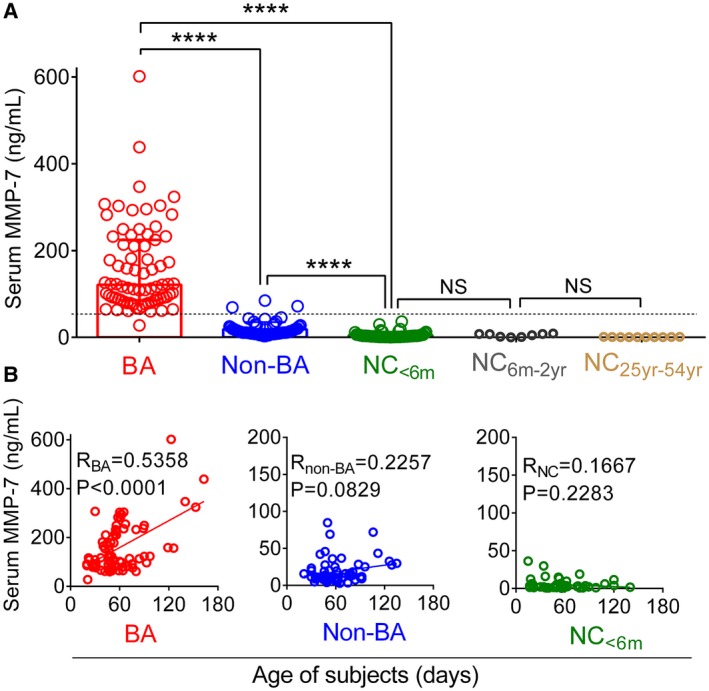
Serum MMP‐7 concentration in BA, non‐BA, and NC. (A) Dots represent MMP‐7 concentration for individual subjects and cohorts (and different ages for NCs). Boxes and whiskers represent median and interquartile range. (B) Serum MMP‐7 plotted against age of subjects. The r and P value are calculated by linear regression model; ****P < 0.0001.
Performance of Serum MMP‐7 as a Biomarker for BA Diagnosis
To investigate the predictive value of serum MMP‐7 concentration for BA, we calculated the AUC and compared with that of serum GGT based on previous reports that GGT is high in infants with BA.13, 14, 15 The AUC for MMP‐7 in BA was 0.9900 (95% confidence interval [CI]: 0.9786‐1.000; P < 0.0001; Fig. 3A), whereas the AUC for GGT was 0.7186 (95% CI: 0.6321‐0.8050; P < 0.0001; Fig. 3B). The sensitivity and specificity for GGT were also comparatively lower at 64.00% and 71.67%, respectively, at a cutoff value 314 U/L. In contrast, analyses of optimal threshold cutoff value of MMP‐7 identified 52.85 ng/mL for BA, with sensitivity of 98.67% and specificity of 95.00% (Supporting Table S3), and produced a negative predictive value of 98.28%. Based on the correlation of increasing levels of serum MMP‐7 with age as shown in Fig. 2B and on the reported clinical benefit of diagnosing biliary atresia before 60 days of age, we performed a subanalysis of the data focused on subjects younger than 60 days, which included 48 BA infants, 34 non‐BA, and 37 NCs. The diagnostic performance for this younger subgroup was similar to the entire cohort (all subjects under 180 days), with sensitivity of 97.92% and specificity of 94.12% at an identical optimal cutoff of 52.85 ng/mL, and an AUC of 0.9859 (Supporting Fig. S1 and Supporting Table S4). These results point to a similar performance of serum MMP‐7 for younger subjects.
Figure 3.
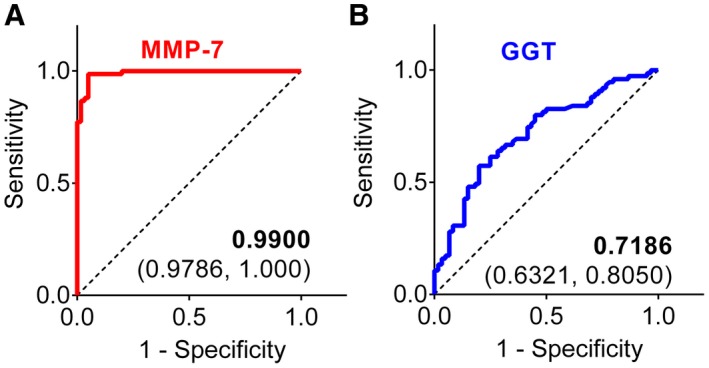
Discriminatory features for MMP‐7 and GGT. AUC for serum MMP‐7 (A) and GGT (B) in differentiating BA from non‐BA cholestasis in logistic regression analysis. The AUC value is shown with the 95% CI.
Finally, to directly compare the diagnostic performance of MMP‐7 with GGT, we calculated the number needed to misdiagnose for both markers.7, 12 The NNM for MMP‐7 was 25 (meaning that 1 of 25 subjects is misdiagnosed) and 3 for GGT (1 of 3 is misdiagnosed). These data demonstrated a superiority of MMP‐7 over GGT in identifying infants with BA.
Relationship of MMP‐7 with Liver Biochemistry
Based on the limited knowledge of the biological function of MMP‐7 and how it relates to standard liver function tests, we next investigated its correlation with serum markers of liver injury and cholestasis, and with APRi as an indicator of liver fibrosis. MMP‐7 correlated only with GGT (r = 0.27, P = 0.0223), but not with total or direct bilirubin, ALT, AST, or APRi (Fig. 4). Based on the correlation with GGT, we developed a composite model combining MMP‐7 and GGT, which produced an AUC of 0.9898 (95% CI: 0.9784‐1.0000; P < 0.0001; Fig. 5A), with sensitivity of 99.00% and specificity of 95.00%, with 3 of the non‐BA subjects being visually identified at the lower levels for BA (Fig. 5B). The NNM for the composite MMP‐7 + GGT was 25 (1 of 25 subjects is misdiagnosed). Collectively, these data suggested that adding GGT to serum MMP‐7 levels does not substantially increase the diagnostic accuracy for BA (compared with MMP‐7 alone).
Figure 4.
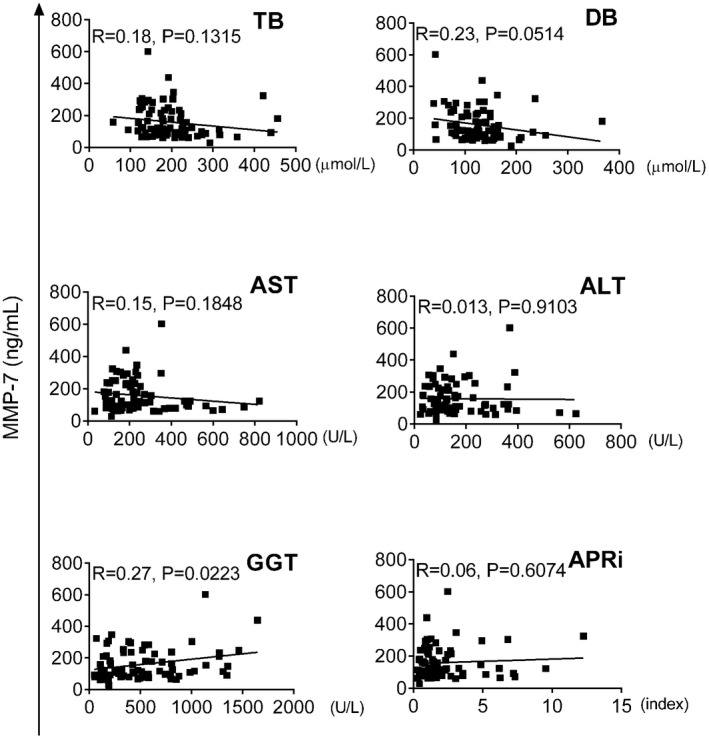
Correlations of serum MMP‐7 with liver biochemical parameters and APRi. Serum MMP‐7 plotted against total bilirubin (TB), direct bilirubin (DB), AST, ALT, GGT, and the APRi score, calculated as ([AST/upper limit of normal AST]/platelet count)*100.
Figure 5.
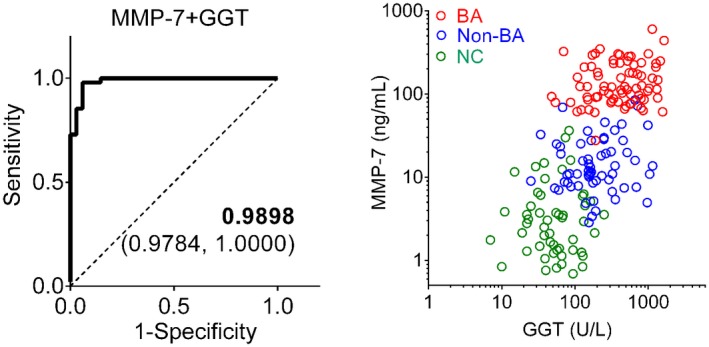
Performance of combined MMP‐7 and GGT in identifying subjects with BA. (A) AUC for combined MMP‐7 and GGT in differentiating BA from non‐BA in logistic regression analysis. The AUC value is shown with the 95% CI. (B) MMP‐7 plotted against GGT for each subject in BA (red), non‐BA (blue), and NC (green).
Hepatic MMP‐7 Expression
Using immunostaining to determine the cellular source of MMP‐7 expression in diseased livers at the time of diagnosis,7, 8, 9, 10, 16 we found the protein expressed primarily in intrahepatic cholangiocytes of BA (n = 10), with signal also present in few nonparenchymal cells (Supporting Fig. S2A). In correlative analyses of this small cohort, there was no significant correlation between the serum concentration of MMP‐7 with its immunostaining intensity in the liver or with the histological fibrosis score (Supporting Fig. S2B,C).
Discussion
We found that high serum concentrations of MMP‐7 identified all but 1 subject with BA, with an average increase of 10‐fold above non‐BA. There was minimal overlap of individual MMP‐7 concentrations between the two cohorts; in contrast, the concentrations for most non‐BA subjects were within the range of age‐matched NCs. At a cutoff value of 52.85 ng/mL, serum MMP‐7 had 98.67% sensitivity for BA and incorrectly assigned a BA diagnosis in only 3 of 60 cholestatic infants, which represents a specificity of 95.00%. These findings support a notably high discriminatory value of MMP‐7 for BA.
The argument for the use of MMP‐7 as a diagnostic test for BA is supported by its high sensitivity, specificity, and negative predictive value, but its potential use in the clinical setting requires a comparison with other available tests. Among them, GGT has been reported to be the best available test, in which a cutoff value at 250‐303 IU/L has been reported to have a sensitivity of 82.8%‐83.3% and specificity of 70.6%‐81.6%.7, 14, 15 In our current study, the availability of both GGT and MMP‐7 for all subjects enabled a direct comparison of the two biomarkers, which showed a superior performance for MMP‐7 with much better AUC, sensitivity, specificity, and number needed to misdiagnose. A similarly improved performance for MMP‐7 was also seen in previous cohorts when the abundance of MMP‐7 was estimated using signal intensity produced by the aptamer‐based technology of SOMAscan (Somalogic Inc., Boulder, CO).7 In that report, MMP‐7 had an AUC of 0.97 (versus 0.9 for GGT), sensitivity of 97% (versus 83% for GGT), and specificity of 91% (versus 81% for GGT). In our current study, using both MMP‐7 and GGT in a composite model did not substantially improve AUC, sensitivity, or specificity. The superiority of MMP‐7 over GGT as a single biomarker may be best demonstrated by the number needed to misdiagnose, which uses the estimated prevalence of biliary atresia of approximately 25% among cholestatic infants in clinical practice, calculated at 25 for MMP‐7 and 3 for GGT, and still 25 for the MMP‐7 + GGT combined model.
The quantification of MMP‐7 concentrations in serum samples using an antibody‐based assay allowed for the determination of normal values for control infants less than 6 months of age. Furthermore, serum MMP‐7 was also tested in healthy subjects of other age groups, including 6 months to 2 years and adults aged 25‐54 years old, which showed no substantial age‐based changes. Previous publications reported similar concentration ranges in normal subjects.17, 18 Based on these findings, the trend observed in our analysis that MMP‐7 correlates with increasing age in BA and non‐BA subjects suggests that the increase may relate to the duration or severity of biliary injury. However, these possibilities require further testing in future studies with larger cohort sizes in each age group. The results reported herein will also allow for comparison with future studies seeking to further validate our findings in other races and ethnic backgrounds. Different than the U.S.‐based population of the earlier study of MMP‐7 as a diagnostic biomarker for BA,7 the use of an entirely Chinese cohort points to a consistent finding across different populations.
The shared clinical and biochemical features between BA and inherited syndromes of intrahepatic cholestasis make it challenging to identity those infants with BA. Based on our data, serum MMP‐7 may be helpful in facilitating the diagnosis of BA; however, because these syndromes make up less than 30% of our non‐BA cohort, further studies are needed to test the diagnostic performance of serum MMP‐7 to differentiate BA from rare cholestatic liver diseases such as Alagille syndrome, alpha‐1‐antitrypsin deficiency, citrin deficiency, and ductal plate malformation. Serum MMP‐7 was previously shown to have a modest correlation with hepatic fibrosis in older patients with BA,10 but our preliminary analysis does not support such correlation in young infants at the time of diagnosis. We recognize that the single time point (at diagnosis) and the immunostaining of only 10 patients limit the strength of our correlative analyses. Thus, the potential value of MMP‐7 as a biomarker of fibrosis requires a longitudinal study that measures serum MMP‐7 concentrations in different stages of progression of liver disease in children with BA.
In summary, our findings of distinctly high concentrations of serum MMP‐7 in BA subjects identify its role as a biomarker of disease, with a real potential to diagnose the disease in a timely fashion. In clinical practice, the ability of practitioners to identify cholestatic infants unlikely to benefit from a liver biopsy or intraoperative cholangiogram has the potential to minimize the morbidity associated with these procedures and decrease health care cost. In this regard, MMP‐7 performed really well by its negative predictive value of 98.28%. Based on the evidence presented herein, the serum concentration of MMP‐7 emerges as a biomarker that substantially simplifies diagnostic algorithms of infants with cholestasis by using a blood test that is highly predictive of BA. The use of serum MMP‐7 in clinical practice does not negate the potential use of serum GGT as an additional biomarker of BA, although our data suggest that it does not substantially increase the diagnostic accuracy. Thus, we propose that the early acquisition of the serum MMP‐7 level will identify patients more likely to benefit from the timely indication for an intraoperative cholangiogram and potential hepatoportoenterostomy.
Potential conflict of interest
Nothing to report.
Supporting information
Acknowledgment
We thank Dr. Zhenhua Luo for his advice on the statistical analyses.
Supported by the National Science foundation of China (NSFC‐30973137, NSFC‐81700501) and the National Institutes of Health (DK‐64008, DK‐83781, and DK‐78392) (to J.A.B.).
*These authors contributed equally to this work.
Contributor Information
Jorge A. Bezerra, Email: jorge.bezerra@cchmc.org.
Shao‐tao Tang, Email: tshaotao83@hust.edu.cn.
References
Author names in bold designate shared co‐first authorship.
- 1. Shneider BL, Moore J, Kerkar N, Magee JC, Ye W, Karpen SJ, et al. Initial assessment of the infant with neonatal cholestasis‐Is this biliary atresia? PLoS One 2017;12:e0176275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feldman AG, Mack CL. Biliary atresia: clinical lessons learned. J Pediatr Gastroenterol Nutr 2015;61:167‐175. [DOI] [PubMed] [Google Scholar]
- 3. Sundaram SS, Mack CL, Feldman AG, Sokol RJ. Biliary atresia: indications and timing of liver transplantation and optimization of pretransplant care. Liver Transpl 2017;23:96‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin JS, Chen SC, Lu CL, Lee HC, Yeung CY, Chan WT. Reduction of the ages at diagnosis and operation of biliary atresia in Taiwan: a 15‐year population‐based cohort study. World J Gastroenterol 2015;21:13080‐13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Serinet MO, Wildhaber BE, Broue P, Lachaux A, Sarles J, Jacquemin E, et al. Impact of age at Kasai operation on its results in late childhood and adolescence: a rational basis for biliary atresia screening. Pediatrics 2009;123:1280‐1286. [DOI] [PubMed] [Google Scholar]
- 6. Russo P, Magee JC, Boitnott J, Bove KE, Raghunathan T, Finegold M, et al. Design and validation of the biliary atresia research consortium histologic assessment system for cholestasis in infancy. Clin Gastroenterol Hepatol 2011;9:357‐362, e352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lertudomphonwanit C, Mourya R, Fei L, Zhang Y, Gutta S, Yang L, et al. Large‐scale proteomics identifies MMP‐7 as a sentinel of epithelial injury and of biliary atresia. Sci Transl Med 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hsieh CS, Chuang JH, Huang CC, Chou MH, Wu CL, Lee SY, et al. Evaluation of matrix metalloproteinases and their endogenous tissue inhibitors in biliary atresia‐associated liver fibrosis. J Pediatr Surg 2005;40:1568‐1573. [DOI] [PubMed] [Google Scholar]
- 9. Huang CC, Chuang JH, Chou MH, Wu CL, Chen CM, Wang CC, et al. Matrilysin (MMP‐7) is a major matrix metalloproteinase upregulated in biliary atresia‐associated liver fibrosis. Mod Pathol 2005;18:941‐950. [DOI] [PubMed] [Google Scholar]
- 10. Kerola A, Lampela H, Lohi J, Heikkila P, Mutanen A, Hagstrom J, et al. Increased MMP‐7 expression in biliary epithelium and serum underpins native liver fibrosis after successful portoenterostomy in biliary atresia. J Pathol Clin Res 2016;2:187‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grieve A, Makin E, Davenport M. Aspartate aminotransferase‐to‐platelet ratio index (APRi) in infants with biliary atresia: prognostic value at presentation. J Pediatr Surg 2013;48:789‐795. [DOI] [PubMed] [Google Scholar]
- 12. Habibzadeh F, Yadollahie M. Number needed to misdiagnose: a measure of diagnostic test effectiveness. Epidemiology 2013;24:170. [DOI] [PubMed] [Google Scholar]
- 13. Robie DK, Overfelt SR, Xie L. Differentiating biliary atresia from other causes of cholestatic jaundice. Am Surg 2014;80:827‐831. [PMC free article] [PubMed] [Google Scholar]
- 14. Rendon‐Macias ME, Villasis‐Keever MA, Castaneda‐Mucino G, Sandoval‐Mex AM. Improvement in accuracy of gamma‐glutamyl transferase for differential diagnosis of biliary atresia by correlation with age. Turk J Pediatr 2008;50:253‐259. [PubMed] [Google Scholar]
- 15. Chen X, Dong R, Shen Z, Yan W, Zheng S. Value of gamma‐glutamyl transpeptidase for diagnosis of biliary atresia by correlation with age. J Pediatr Gastroenterol Nutr 2016;63:370‐373. [DOI] [PubMed] [Google Scholar]
- 16. Hung TM, Chang SC, Yu WH, Wang YW, Huang C, Lu SC, et al. A novel nonsynonymous variant of matrix metalloproteinase‐7 confers risk of liver cirrhosis. Hepatology 2009;50:1184‐1193. [DOI] [PubMed] [Google Scholar]
- 17. Bauer Y, White ES, de Bernard S, Cornelisse P, Leconte I, Morganti A, et al. MMP‐7 is a predictive biomarker of disease progression in patients with idiopathic pulmonary fibrosis. ERJ Open Res 2017;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roderfeld M, Rath T, Schulz R, Seeger W, Tschuschner A, Graf J, et al. Serum matrix metalloproteinases in adult CF patients: relation to pulmonary exacerbation. J Cyst Fibros 2009;8:338‐347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


