Abstract
Background: Iron deficiency and iron deficiency anemia have been shown to have negative effects on aspects of perception, attention, and memory.
Objective: The purpose of this investigation was to assess the extent to which increases in dietary iron consumption are related to improvements in behavioral measures of perceptual, attentional, and mnemonic function.
Methods: Women were selected from a randomized, double-blind, controlled food-fortification trial involving ad libitum consumption of either a double-fortified salt (DFS) containing 47 mg potassium iodate/kg and 3.3 mg microencapsulated ferrous fumarate/g (1.1 mg elemental Fe/g) or a control iodized salt. Participants' blood iron status (primary outcomes) and cognitive functioning (secondary outcomes) were assessed at baseline and after 10 mo at endline. The study was performed on a tea plantation in the Darjeeling district of India. Participants (n = 126; 66% iron deficient and 49% anemic at baseline) were otherwise healthy women of reproductive age, 18–55 y.
Results: Significant improvements were documented for iron status and for perceptual, attentional, and mnemonic function in the DFS group (percentage of variance accounted for: 16.5%) compared with the control group. In addition, the amount of change in perceptual and cognitive performance was significantly (P < 0.05) related to the amount of change in blood iron markers (mean percentage of variance accounted for: 16.0%) and baseline concentrations of blood iron markers (mean percentage of variance accounted for: 25.0%). Overall, there was evidence that the strongest effects of change in iron status were obtained for perceptual and low-level attentional function.
Conclusion: DFS produced measurable and significant improvements in the perceptual, attentional, and mnemonic performance of Indian female tea pickers of reproductive age. This trial was registered at clinicaltrials.gov as NCT01032005.
Keywords: iron deficiency, perception, memory, cognition, nutritional neuroscience, fortification
Introduction
Iron deficiency (ID) and ID anemia (IDA) are among the most prevalent nutritional deficiencies in the world (1). The effects of ID, with and without concurrent anemia, include declines in physical performance and productivity (2, 3). In addition, there is increasing evidence that ID is related to declines in perceptual and cognitive performance, including declines in behavioral (4–6) and neurophysiological (7, 8) measures of perception and cognition. There is also accumulating evidence that repletion of body iron concentrations can lead to measurable improvements in cognitive functioning, including improvements for women of reproductive age (4, 5).
At the population level, there is a range of possibilities for accomplishing such changes. Food fortification has been a standard approach for many years (9). One formulation of double-fortified salt (DFS), which has been promoted for use in India, contains 1000 ppm encapsulated ferrous fumarate and 47 ppm potassium iodate in a stable form that prevents iodine instability. Early studies of the efficacy of DFS suggest its potential in reducing the prevalence of ID and IDA (10). The efficacy of DFS in improving hemoglobin has been assessed in studies performed in India and Africa (10–12); in both cases, consumption of DFS produced a significant increase in hemoglobin relative to consumption of an iodized-only salt. The DFS used in the present study utilized microencapsulated ferrous fumarate and was previously tested in an efficacy study in Indian schoolchildren (10). It was considered acceptable by participants and was effective in increasing serum ferritin (sFt) and total-body iron (BdFe), and in lowering the prevalence of anemia in the iron-fortified group compared with the control group. The efficacy of the DFS used in the present study was demonstrated in a population of female tea pickers who showed significant increases in sFt and BdFe over 7–10 mo (13).
If such an intervention can produce changes in systemic measures of iron status, it is possible that it may also produce beneficial changes in brain states, leading to changes in behavioral measures of perception, attention, and memory. The present study tests this hypothesis in the context of a larger study of the efficacy of DFS in improving iron status in female laborers of reproductive age in India (13). We predicted that women with ID or IDA who consumed DFS, relative to those who consumed a control iodized salt, would show improvements in blood measurements of iron status (primary outcomes) and in measurements of perceptual and cognitive functioning (secondary outcomes). Furthermore, we predicted that the magnitude of the improvements in perceptual and cognitive functioning would be systematically related to the level of change in the blood iron markers and inversely related to the baseline levels of the blood iron markers.
Methods
Participants
Participants were 126 healthy female tea pickers, not pregnant, aged 18–55 y, belonging to either Nepali or Adivasi ethnic groups. Participants were drawn from a larger efficacy study (13) conducted from June 2009 to August 2010 at the Panighatta Tea Estate, Darjeeling District, West Bengal, India. See the study by Haas et al. (13) for a complete description of that study. All participants were experienced, full-time tea pickers and lived on the tea estate. Blood hemoglobin concentrations at baseline were used to classify women as either anemic (hemoglobin < 120 g/L) or nonanemic (hemoglobin ≥ 120 g/L), and inflammation-adjusted sFt concentrations were used to classify women as iron deficient (<12 μg/L), depleted (12 ≤ sFt < 20 μg/L), or sufficient (≥20 μg/L). Anthelmintic treatment (200 mg albendazole) was administered to all eligible participants 4 wk before and 4 mo after the initial baseline blood collection to eliminate parasitic worm infections, which can be exacerbated by and blunt the response to iron treatment. Women were randomly assigned into either the DFS group or the control group that used only iodized salt. Participants and all study personnel were blind as to the assignment to treatment group.
Study design
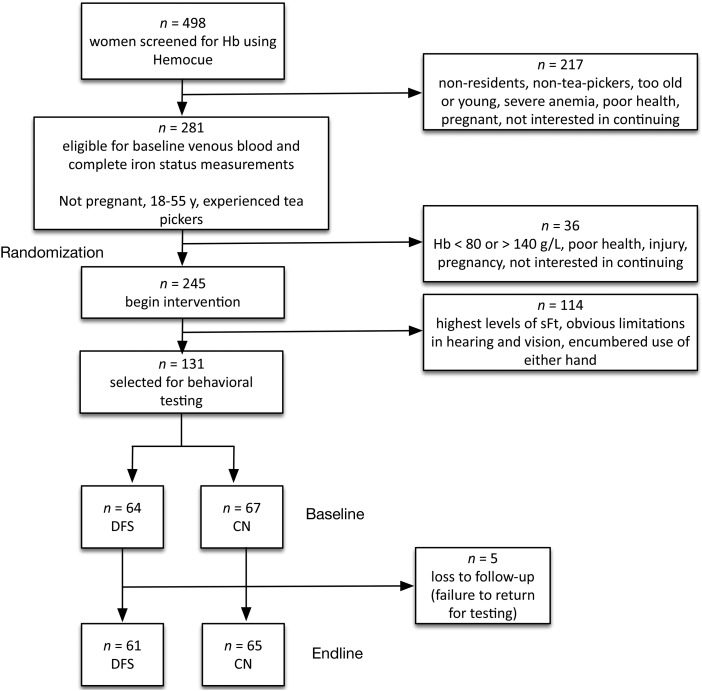
The overall study design was a randomized, double-blind, controlled food-fortification trial. A total of 498 women were voluntarily screened for eligibility, and 253 (57%) of these were ruled ineligible for a variety of reasons (age, pregnancy, health, residence), a rate of exclusion that is comparable to recent laboratory investigations (6). Thus, 245 were initially enrolled in the intervention (Figure 1). Based on minimum sample-size estimates, assuming 1 − β = 0.90, α = 0.05, and estimated variances based on published intervention studies (4), we selected a total of 131 women with the lowest values of baseline sFt and hemoglobin to participate in the behavioral testing, with 64 of these women randomly assigned to the DFS group and 67 assigned to the control group. After random assignment, 5 women were lost to follow-up, leaving a total of 126 women who provided data for both baseline and endline.
FIGURE 1.
Study design and sample selection. Hemocue (Radiometer America, Inc.). Hb, hemoglobin; CN, control group; DFS, double-fortified salt group; sFt, serum ferritin.
Venous blood collections were scheduled over a 5- to 6-wk period at both baseline and endline. Salt distribution began on 15 October 2009 and finished on 31 August 2010. Participants consumed either the DFS or the control salt for between 7.5 and 9.0 mo, depending on the date of the endline blood sampling. Blood was collected on the tea estate by trained phlebotomists from the Super Religare Laboratory. Written informed consent was obtained from all participants, and the study was approved by the institutional review boards of Cornell University, The Pennsylvania State University, McGill University, and the ethics committee of the Child in Need Institute (India). This trial was registered at clinicaltrials.gov as NCT01032005.
Salt
The treatment group received DFS that contained 47 mg I (provided as potassium iodate)/kg salt and 1106 mg elemental Fe (provided as microencapsulated ferrous fumarate)/kg salt. All salt was provided without charge to participants, and consumption was ad libitum. Additional details regarding the composition and distribution of the iodized salt and DFS can be found in the studies by Baxter and Zlotkin (14) and Haas et al. (13).
Blood analyses
Hemoglobin from whole blood and sFt, soluble transferrin receptor (sTfR), C-reactive protein (CRP), α-1 acid glycoprotein (AGP), vitamin B-12, and folate from serum were measured via a venous blood sample at baseline and endline. Blood hemoglobin concentration, hematocrit, and mean corpuscular volume were analyzed with a Coulter Counter (Beckman), and sFt and CRP were measured by chemiluminescent immunoassay on the Immulite 2000. Serum samples were analyzed for sTfR by ELISA (BioVendor), folate and vitamin B-12 on the Immulite 2000, and AGP by radial immunodiffusion (Kent Laboratories). Because the equation to compute BdFe reported by Thurnham et al. (15) used the sTfR value determined by the Ramco ELISA kit (Ramco Laboratories), we converted the BioVendor-derived sTfR to the Ramco-adjusted sTfR value with a regression equation derived from 35 random duplicate samples (13).
To adjust sFt for inflammation, reflected in elevated CRP and AGP values, the correction factors developed by Thurnham et al. (15) were applied. Analysis of the sFt and BdFe response to the intervention, as well as their relation to the behavioral variables, was performed by using both adjusted and unadjusted values and found to not differ. Therefore, only analyses that used unadjusted sFt and BdFe values are reported.
Perceptual and cognitive tasks
The 6 perceptual and cognitive tasks administered at baseline and endline were selected based on 3 criteria. First, we sought to assess the perceptual and cognitive processes that were involved in the performance of the participants' job as tea pickers. Second, we focused on measurements that tap the use of cortical systems and circuits that have documented dependency on iron status, in either the human or animal literatures. Third, we selected tasks that have been used extensively in the human experimental literature, to allow for checks on external validity. In fact, 3 of our tasks—simple reaction time (RT), contrast threshold, and temporal threshold—have been in continuous use in the experimental literature since the middle of the 19th century (16). All tasks were computer based, included a set of standardized explanatory instructions and practice trials, and were administered by local research assistants trained to common performance criteria.
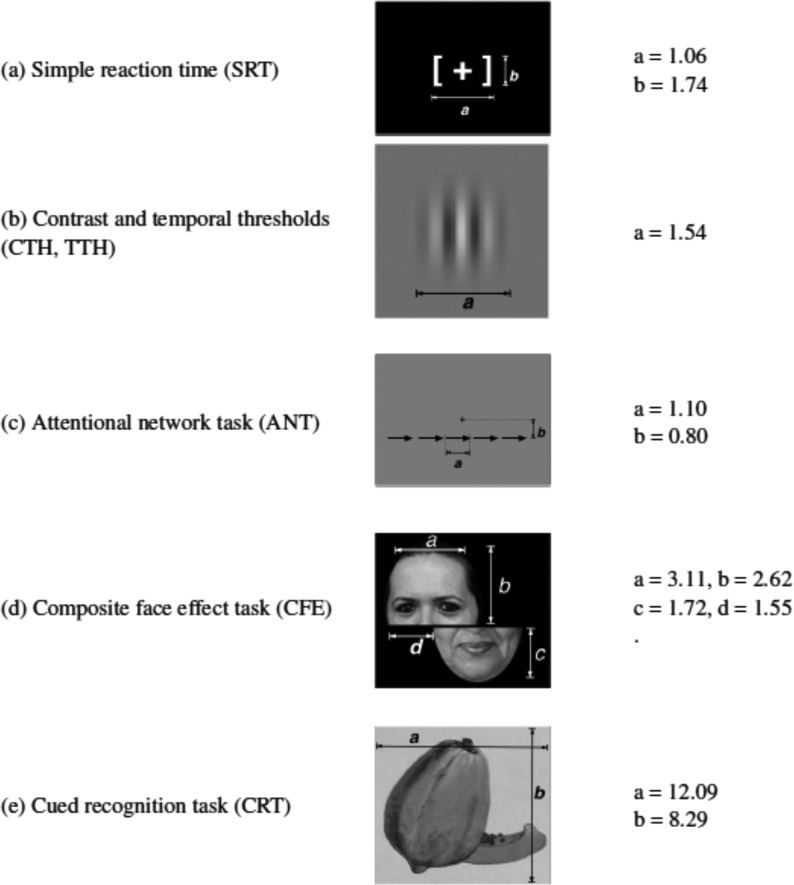
All the tasks were developed and programmed by MJW using public-domain software (17), which allowed for highly accurate timing of stimulus displays and behavioral responses (±1 ms), and all programs and stimuli are freely available on request. Example stimuli for each of the tasks are presented in Figure 2. The tasks were run in the order presented in Figure 2, and the average time to complete all of the tasks was 60–90 min. The dependent variables from each of the tasks are listed in Table 1. Brief descriptions of the tasks are provided here, with procedural details presented in the online Supplemental Material.
FIGURE 2.
Examples of the stimuli used in each of the 5 tasks (a–e). Dimensions are given in degrees of visual angle, assuming an unconstrained viewing distance of 70 cm. ANT, attentional network task; CFE, composite face effect task; CRT, cued recognition task; CTH, contrast threshold task; SRT, simple reaction time task; TTH, temporal threshold task.
TABLE 1.
Dependent variables for each of the perceptual and cognitive tasks and the direction of change indicative of improvement1
| Task/domain | Variable/change in value | Description |
|---|---|---|
| SRT/perception | Median RT/↓ | Median RT for correct responses |
| CTH/perception | HR/↑ | Proportion of correct responses to stimuli with nonzero contrast |
| FAR/↑ | Proportion of incorrect responses to stimuli with zero contrast | |
| d′/↑ | Sensitivity to the presence of contrast, d′ = Z−1(HR) − Z−1(FAR) | |
| c/? | Propensity to report the presence of contrast, independent of the state of the stimulus, c = −0.5 × [Z−1(HR) + Z−1(FAR)] | |
| τ/↓ | Lowest amount of contrast (in percentage of contrast) required for the observer to correctly report the presence of contrast | |
| TTH/perception | HR/↑ | Proportion of correct responses to stimuli with nonzero exposure durations |
| FAR/↓ | Proportion of incorrect responses to stimuli with zero exposure duration | |
| d′/↑ | Sensitivity to the presence of stimuli with nonzero exposure duration, calculated as above | |
| c/? | Propensity to report the presence of stimuli, independent of the exposure duration, calculated as above | |
| τ/↓ | Shortest exposure duration (in ms) required for the observer to correctly report the presence of the stimulus | |
| ANT/attention | RT, 0 cues/↓ | RT0: Median RT (in ms) for correct responses to stimuli with 0 cues |
| RT, 2 cues/↓ | RT2: Median RT (in ms) for correct responses to stimuli with 2 cues | |
| Alerting/↑ | RT0 − RT2 | |
| RT, center cues/↓ | RTc: Median RT (in ms) for correct responses to stimuli with center cues | |
| RT, spatial cues/↓ | RTs: Median RT (in ms) for correct responses to stimuli with spatial cues | |
| Orienting/↑ | RTc − RTs | |
| RT, consistent flankers/↓ | RTn: Median RT (in ms) for correct responses to stimuli with consistent flankers | |
| RT, inconsistent flankers/↓ | RTi: Median RT (in ms) for correct responses to stimuli with inconsistent flankers | |
| Conflict/↓ | RTn − RTi | |
| CFE/attention, memory | RT/↑ | Median RTs, interaction contrast (in ms): (FA − FM) − (UA − UM) |
| HR/↑ | Proportion of trials on which person 1 was cued that were judged to be person 1, interaction contrast: (FM − FA) − (UM − UA) | |
| FAR/↑ | Proportion of trials on which person 2 was cued that were judged to be person 1, interaction contrast: (FA − FM) − (UA − UM) | |
| d′/↑ | Sensitivity to the presence of person 1, calculated as above, interaction contrast: (FM − FA) − (UA − UM) | |
| c/? | Bias to respond person 1 independent of the cued identity, calculated as above, interaction contrast: (FM − FA) − (UM − UA) | |
| CRT/memory | RT, 4 cues/↑ | Median RT for correct responses to stimuli presented with all 4 quadrants visible |
| HR, 4 cues/↑ | Proportion of old items with all 4 quadrants visible correctly identified as old | |
| FAR, 4 cues/↓ | Proportion of new items with all 4 quadrants visible incorrectly identified as old | |
| d′, 4 cues/↑ | Sensitivity to the presence of old items with all 4 quadrants visible, calculated as above | |
| c, 4 cues/↑ | Propensity to identify a stimulus with all 4 quadrants visible as old, independent of the state of the stimulus, calculated as above | |
| PCC/↑ | Percentage of change in capacity, based on the proportionality estimated β obtained from the proportional hazards model, (eβ − 1) × 100 |
A, aligned; ANT, attentional network task; c, bias; CFE, composite face effect task; CRT, cued recognition task; CTH, contrast threshold task; d′, sensitivity; F, familiar; FAR, false-alarm rate; HR, hit rate; M, misaligned; PCC, proportional change in capacity; RT, response time; SRT, simple reaction time task; TTH, temporal threshold task; U, unfamiliar; Z−1, inverse normal transformation; τ, threshold; ↑, increase in the value of the variable indicates improvement; ↓, decrease in the value of the variable indicates improvement; ?, neither increase nor decrease in the value of the variable indicates improvement.
The simple RT task (SRT) provides an estimate of the simplest possible behavioral response to a visual stimulus, and the task required the participant to press a button in response to the onset of the stimulus (a simple string of characters). The task has limited attentional and mnemonic demands. The contrast threshold task (CTH) provides an estimate of the minimum amount of physical contrast (the physical difference between light and dark areas of an image) necessary for an individual to reliably perceive that contrast. Stimuli were presented at contrast amounts ranging from 0% (no stimulus) to 80%, running from an amount well below to well above what would be expected for normal adult vision (18, 19), and for each trial the participants pressed a button to indicate whether they could perceive the stimulus. The CTH was included because the task of picking tea leaves is dependent on the ability to first locate leaves and then determine that they are of the appropriate size and color among all the leaves on the tea bushes. This can be done by first finding the edges of the leaves, which are defined by local contrast (see Supplemental Figure 1A, B). The temporal threshold task (TTH) provides an estimate of the minimum amount of time that is required to reliably perceive a fixed amount of contrast. On each trial, the participants pressed one button to indicate that they perceived the presence of the stimulus and another to indicate absence. Note that a worker's performance picking tea leaves can be determined both by the ease or accuracy with which they can find appropriate leaves (contrast detection as assessed by the CTH) and by the speed with which they can find appropriate leaves (as assessed by the TTH). The attentional network task (ANT) (20) probes 3 functions of attention: low-level attentional capture, mid-level spatial selective attention, and high-level control in the context of nominally irrelevant information. On each trial, the participants pressed a button to indicate whether the arrow stimulus pointed to the left or right, while attempting to disregard flanking elements (congruent, incongruent, or neutral distractors) on either side of the stimulus. The composite face effect task (CFE) (21) assesses the extent to which semantic memory can influence visual selective attention. The CFE involves the presentation of facial stimuli (all Indian women, Figure 2) in which the top and bottom portions are drawn from either the same or different faces, and in which those 2 portions are either aligned or misaligned. After training, the participants pressed a button to identify whether one half of the face (top or bottom) was person A or person B. The cued recognition task (CRT) is a variation on the classic recognition memory paradigm (22), in which an individual is presented with a set of items to be remembered, is tested on those items and an equal number of previously unseen items, and is then asked (for each test item) whether the item was previously seen (an “old” item) or not (a “new” item). Test items were presented with 0, 1, or 2 quadrants of the image blacked out in order to vary workload. This task was included because of evidence suggesting that decrements in the amount of work that can be accomplished during recognition is correlated with the level of compromise to neural circuits that support recognition memory (23).
Statistical analyses
Statistical analyses were performed by using SAS 9.3 (SAS Institute, Inc.). All RTs were first summarized for each participant in each group, at each time point, as the median for correct responses only. All trials with RTs <200 ms (anticipatory responses) or >2000 ms (lapses of attention) were excluded. Observations >3 SDs from the participant mean for that variable were replaced with the mean. This is consistent with standard practice in the literature on RTs (24). Total replacements were <2% of all observations. Unless specified otherwise, α = 0.05 for all analyses.
Where appropriate, response accuracy was summarized by using the measures from univariate equal-variance Gaussian signal detection theory; sensitivity and bias were estimated by using models for 2-alternative forced-choice tasks. The critical measures for the ANT were calculated as in the study by Fan et al. (20). Finally, the capacity and proportional change in capacity measurements used for the CRT were obtained by fitting the Cox proportional hazards model to the RT data for individual participants as a function of the number of cues, which has limited and conservative bias with small sample sizes (23).
Each of the blood variables was analyzed by using a 2 (treatment group: control or DFS) × 2 (assessment time: baseline or endline) repeated-measures ANOVAs, with treatment as a between-participants factor and assessment time as a within-participant factor. Each of the dependent endline variables from the perceptual and cognitive tasks was subjected to 2 classes of analyses. The primary (per-protocol) analyses were of the same form as the blood analyses, while additionally controlling for age at baseline and ethnicity. Initial exploratory analyses showed that these 2 variables were significant (P < 0.05) covariates in many cases and at both time points. Consequently, they were used as covariates in all the analyses. Additionally, the categorical variables pertinent to nutritional and iron status were analyzed by using weighted-least-square models with repeated measures to estimate main effects for the treatment group and assessment time along with their interaction. Note that all the analyses involved separate dependent variables; consequently, there was no need for any adjustments for α-inflation.
The secondary analyses were 2 different regression analyses intended to assess the plausibility that the provision of DFS was responsible for the changes in the perceptual and cognitive measurements. These analyses also provided a source of converging evidence with respect to the effects of the intervention. The first analysis regressed change in the behavioral variables in each task onto change in the iron biomarkers on the premise that those whose iron status improved the most would show the greatest change in perception and cognition. The second analysis regressed change in the behavioral variables onto baseline concentrations of iron biomarkers on the premise that those whose baseline iron concentrations were most compromised should show the largest potential for improvements in perception and cognition. In both analyses, the potential predictors were considered both alone and in interaction with the treatment group. When the interaction was significant, models were fit separately to the data for each group. These regression analyses involved choosing predictors by using step-wise model selection, with standard criteria (P < 0.15) for including variables in selected models. The “best” model in each analysis needed to be significantly superior to the null model (as assessed by the chi-square test) and to account for ≥10% of the total variance, by using the fewest possible number of predictors, as assessed by the likelihood ratio test for nested models and the Akaike Information Criterion (25) for nonnested models.
Results
Primary analyses
Blood variables.
Baseline characteristics of the sample are presented in Tables 2 and 3. Table 2 summarizes the results of the ANOVAs on each of the continuous blood biomarkers for iron status. For hemoglobin, sFt, log10(sFt), and BdFe the treatment × assessment (time) interaction was significant, showing a different rate of change in the iron status biomarker between the DFS and control groups. Only sTfR failed to show this interaction. Note that in the larger sample from which these 2 groups were drawn (13), the interaction was significant for all 5 variables. Table 3 summarizes the analyses of the categorical variables indexing prevalence of nutritional and iron status at baseline and endline. Significant treatment × assessment interactions were observed for the prevalence of anemia and ID and BdFe concentrations <0. In each case, the reduction in prevalence was significantly higher for the participants in the DFS group relative to those in the control group (Table 3). Only the reduction in prevalence of ID was not significant in the larger sample (13), although it was in this subsample, likely because of our choosing the most iron-deficient subjects for this substudy. Based on Indian RDAs (21 mg/d), >70% of women met the recommended daily intake for iron (26). DFS contributed to ∼8.4 mg Fe/d, which is estimated to meet the daily estimated average requirement (8.1 mg/d) for >50% of women of reproductive age (27).
TABLE 2.
Results of the analyses of the baseline, endline, and overall change values for each of the continuous blood variables1
| Mean ± SEM | ANOVA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Endline | Δ | T | A | T × A | |||||||
| Variable | DFS | CN | DFS | CN | DFS | CN | F | MSE | F | MSE | F | MSE |
| Hemoglobin, g/L | 117.1 ± 1.6 | 118.3 ± 1.6 | 118.1 ± 1.7 | 116.0 ± 1.4 | 1.0 ± 1.3 | −2.3 ± 1.0 | 0.00 | 2.71 | 1.16 | 0.40 | 5.78* | 0.40 |
| sFt, μg/L | 23.6 ± 3.1 | 25.4 ± 4.1 | 36.1 ± 3.2 | 31.2 ± 4.0 | 12.5 ± 2.4 | 5.8 ± 2.5 | 0.08 | 1484.9 | 26.22*** | 186.95 | 3.95* | 186.95 |
| log10(sFt), log10(μg/L) | 1.20 ± 0.05 | 1.18 ± 0.05 | 1.46 ± 0.04 | 1.33 ± 0.05 | 0.26 ± 0.03 | 0.13 ± 0.03 | 1.27 | 0.26 | 78.31*** | 0.03 | 7.74** | 0.03 |
| sTfR, mg/L | 7.06 ± 0.47 | 6.91 ± 0.46 | 6.45 ± 0.27 | 6.74 ± 0.25 | −0.61 ± 0.05 | −0.17 ± 0.42 | 0.00 | 13.56 | 1.58 | 3.60 | 1.39 | 3.60 |
| BdFe, mg/kg | 1.90 ± 0.58 | 1.78 ± 0.62 | 4.13 ± 0.37 | 1.82 ± 0.47 | 2.23 ± 0.43 | 0.04 ± 0.33 | 0.89 | 29.32 | 34.17*** | 4.39 | 6.09* | 4.39 |
| Vitamin B-12, μg/L | 248.8 ± 13.2 | 245.4 ± 12.9 | 234.0 ± 12.4 | 237.7 ± 13.8 | −14.8 ± 5.3 | −6.3 ± 4.5 | 0.05 | 19979 | 3.19+ | 738 | 0.93 | 738 |
n = 126. +0.05 ≤ P < 0.10, *P < 0.05, **P < 0.01, ***P < 0.001. A, assessment time; BdFe, total-body iron; CN, control group; DFS, double-fortified salt group; MSE, mean square errors; sFt, serum ferritin; sTfR, soluble transferrin receptor; T, treatment; Δ, endline − baseline.
TABLE 3.
Prevalence of nutritional and inflammatory statuses at baseline and endline along with tests for change in prevalence1
| Baseline | Endline | Δ | F-scores | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | DFS | CN | DFS | CN | DFS | CN | T | A | T × A |
| Hemoglobin <120 g/L (anemia) | 53 | 45 | 48 | 66 | −5 | 21 | 0.25 | 3.98* | 10.01*** |
| sFt <12 μg/L (iron deficiency) | 34 | 37 | 7 | 28 | −27 | −9 | 3.33 | 54.81*** | 9.22** |
| sTfR >8.6 mg/L | 28 | 21 | 12 | 14 | −16 | −7 | 0.11 | 14.96*** | 1.95 |
| BdFe <0 mg/kg | 31 | 28 | 7 | 28 | −24 | 0 | 1.91 | 12.10*** | 12.10*** |
| Vitamin B-12 <203 μg/L | 44 | 42 | 56 | 55 | 12 | 13 | 0.03 | 18.32*** | 0.16 |
| Folate <5 mg/L | 91 | 83 | 69 | 72 | −22 | −11 | 0.15 | 17.09*** | 2.78+ |
| CRP >5 mg/L | 3 | 6 | 3 | 2 | 0 | −4 | 0.05 | 1.22 | 1.22 |
| AGP >1 g/L | 23 | 24 | 25 | 23 | 2 | −1 | 0.00 | 0.00 | 0.22 |
Values are percentages, n = 126. +P < 0.10, *P < 0.05, **P < 0.01, ***P < 0.001. A, assessment time; AGP, α-1 acid glycoprotein; BdFe, total-body iron; CN, control group; CRP, C-reactive protein; DFS, double-fortified salt group; sFt; serum ferritin; sTfR, soluble transferrin receptor; T, treatment; Δ, endline − baseline.
SRT.
Table 4 summarizes the primary analyses for all variables in each of the 6 tasks, and Supplemental Figure 2 presents the means for the SRT. Participants in the DFS group showed larger reductions in median RT from baseline to endline than did participants in the control group, while controlling for age at baseline and ethnicity (Table 4). The magnitude of the treatment × assessment interaction effect, as indexed by the estimated proportion of variance accounted for by the effect of the treatment group at baseline and endline, showed that although the treatment group accounted for <1% of the variance at baseline, it accounted for 18% of the variance at endline (ηT2).
TABLE 4.
Results of the per-protocol analyses on each of the dependent measures in each of the 6 behavioral tasks, accounting for age at baseline and ethnicity1
| T | A | T × A | ηT 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Task | Variable | df | F | MSE | F | MSE | F | MSE | Baseline | Endline |
| SRT | Median RT | 120 | 5.14* | 43,242 | 1.07 | 42,493 | 9.09** | 42,493 | 0.00 | 0.18* |
| CTH | HR | 122 | 4.78* | 0.02 | 0.89 | 0.02 | 4.23* | 0.0200 | 0.00 | 0.12* |
| FAR | 122 | 3.48+ | 0.09 | 0.04 | 0.06 | 10.28** | 0.0600 | 0.00 | 0.11* | |
| d′ | 122 | 19.83*** | 1.6569 | 1.80 | 0.6419 | 49.28*** | 0.6419 | 0.00 | 0.31* | |
| c | 122 | 6.67* | 0.4083 | 0.02 | 0.3786 | 5.86* | 0.3786 | 0.00 | 0.10* | |
| τ | 122 | 29.99*** | 501 | 1.22 | 378 | 43.25*** | 378 | 0.00 | 0.39* | |
| TTH | HR | 122 | 1.32 | 0.0179 | 10.39*** | 0.0071 | 16.68*** | 0.0071 | 0.01 | 0.09* |
| FAR | 122 | 41.15*** | 0.0101 | 23.95*** | 0.0064 | 64.10*** | 0.0064 | 0.00 | 0.50* | |
| d′ | 122 | 16.17*** | 1.2883 | 22.93*** | 0.4756 | 61.59*** | 0.4756 | 0.00 | 0.27* | |
| c | 122 | 1.81 | 0.0364 | 5.60* | 0.0260 | 21.57*** | 0.0260 | 0.03 | 0.10* | |
| τ | 122 | 44.89*** | 370 | 31.66*** | 313 | 78.38*** | 313 | 0.01 | 0.44* | |
| ANT | RT, 0 cues | 118 | 0.77 | 99,795 | 3.58+ | 23,447 | 2.54 | 23,447 | 0.02 | 0.00 |
| RT, 2 cues | 118 | 0.02 | 103,913 | 6.30* | 27,798 | 8.36** | 27,798 | 0.01 | 0.02* | |
| Alerting | 118 | 9.68** | 10,659 | 1.83 | 9084 | 6.23* | 9,084 | 0.00 | 0.09* | |
| RT, center cues | 118 | 0.66 | 99,307 | 2.27 | 23,503 | 2 0.49 | 23,503 | 0.01 | 0.00 | |
| RT, spatial cues | 118 | 1.14 | 102,536 | 10.49** | 27,811 | 27.06*** | 27,811 | 0.02 | 0.10* | |
| Orienting | 118 | 27.04*** | 13,208 | 10.87** | 8780 | 44.61*** | 8780 | 0.00 | 0.29* | |
| RT, consistent flankers | 118 | 0.54 | 117,418 | 4.59* | 33,977 | 2.76+ | 33,977 | 0.01 | 0.00 | |
| RT, inconsistent flankers | 118 | 0.01 | 113,332 | 5.02* | 41,171 | 6.60* | 41,171 | 0.01 | 0.06* | |
| Conflict | 118 | 3.27+ | 15,538 | 0.29 | 12,056 | 3.83* | 12,056 | 0.00 | 0.04* | |
| CFE | RT | 122 | 0.18 | 16,468 | 2.59 | 22,623 | 3.66* | 22,623 | 0.01 | 0.11* |
| HR | 122 | 0.42 | 0.0224 | 0.35 | 0.0174 | 8.29** | 0.0174 | 0.02 | 0.06* | |
| FAR | 122 | 0.73 | 0.0065 | 0.00 | 0.0059 | 3.01+ | 0.0059 | 0.00 | 0.03* | |
| d′ | 122 | 5.46* | 0.5681 | 0.36 | 0.3309 | 3.71* | 0.3309 | 0.01 | 0.05* | |
| c | 122 | 1.48 | 0.1329 | 0.97 | 0.1138 | 0.08 | 0.1138 | 0.01 | 0.01 | |
| CRT | RT, 4 cues | 118 | 0.68 | 109,433 | 0.02 | 55,461 | 6.70* | 55,461 | 0.00 | 0.06* |
| HR, 4 cues | 118 | 7.47** | 0.0254 | 4.84* | 0.0110 | 5.68* | 0.0110 | 0.01 | 0.16* | |
| FAR, 4 cues | 118 | 1.56 | 0.0166 | 0.78 | 0.0095 | 1.76 | 0.0095 | 0.00 | 0.02 | |
| d′, 4 cues | 118 | 4.55* | 0.7508 | 7.63** | 0.2841 | 34.98*** | 0.2841 | 0.02 | 0.15* | |
| c, 4 cues | 118 | 2.17 | 0.1062 | 0.37 | 0.0861 | 0.07 | 0.0861 | 0.01 | 0.01 | |
| PCC | 118 | 13.97*** | 2760 | 2.72 | 2128 | 8.76** | 2128 | 0.01 | 0.16* | |
Variations in the number of df are due to differences in the number of participants for whom complete data were available for that task. +P < 0.10,*P < 0.05, **P < 0.01, ***P < 0.001. A, assessment time; ANT, attentional network task; c, bias; CFE, composite face effect task; CRT, cued recognition task; CTH, contrast threshold task; d′, sensitivity; FAR, false-alarm rate; HR, hit rate; MSE, mean SE; PCC, proportional change in capacity; RT, response time; SRT, simple reaction time task; T, treatment; TTH, temporal threshold task; ηT2, proportion of variance accounted for by the effect of treatment group; τ, threshold.
CTH and TTH.
The results for the 2 threshold measures were, with one exception, identical (Table 4). For the CTH, RTs were faster, hit rates (HRs) were higher, false-alarm rates were lower, sensitivity to the presence of the stimulus was greater, and thresholds (minimum amount of contrast needed for participants to reliably detect the contrast) were lower for the participants in the DFS group than in the control group at endline. The significant interaction for the bias measurement resulted from the fact that the HR increased so much more for the DFS participants than it did for the control participants (Supplemental Figure 3A, C). The results for the TTH data were the same except that the change in bias in the DFS group, instead of going from unbiased (no preference for reporting the presence of contrast) level to slightly liberal (preference for reporting the presence contrast, CTH), went from slightly conservative (preference for reporting the absence of contrast) to unbiased (TTH). It should be noted that the change for both tasks was in the same direction (an increased overall preference for reporting the presence of contrast) and of roughly the same magnitude (Supplemental Figure 4B, D). Overall, for these 2 tasks, treatment group accounted for 0.05% of the variance at baseline and for 21% of the variance at endline for the CTH and 1% of the variance at baseline and 28% of the variance at endline for the TTH.
ANT.
Alerting, orienting, and conflict scores each showed significant treatment × assessment time interactions (Table 4). Relative to participants in the control group, participants in the DFS group showed improvements in low (alerting), mid-level (orienting) and high-level (conflict resolution) functions of attention (Supplemental Figure 4). These changes were due to significant effects in only one component of each of the difference scores. Specifically, significant treatment × assessment time interactions were obtained only for trials with 2 rather than 0 cues, trials with spatial rather than central cues, and trials with inconsistent rather than consistent cues. That is, participants in the DFS group showed greater benefits when 2 cues were present and when cues indicated the location of the test stimulus and showed greater ability to filter out inconsistent aspects of the display relative to participants in the control group. For the variables with significant treatment × assessment interactions, the treatment effect accounted on average for 1% of the variance at baseline and 10% of the variance at endline.
CFE task.
Significant treatment × assessment time interactions were obtained for 3 of the 5 CFE scores: RT, HRs, and sensitivity (Table 4,Supplemental Figure 5). In each case, the increase from baseline to endline for the DFS group was much greater than the change for the control group. Each of the CFE scores were interaction contrasts for the interaction of memory (familiarity) and attention (alignment) and constructed so that values >0 indicate a cost because of the increased demands of efficient memory retrieval on selective attention. Consequently, these results indicate that the costs of improved memory retrieval on attention were observable in participants' speed, accuracy, and sensitivity to the identity of the image. For these 3 variables, the effect of the treatment group accounted for 1% of the variance at baseline and 6% of the variance at endline.
CRT.
Of the 6 variables in this task, 4 showed significant treatment × assessment time interactions: RTs for 4-cue trials, HRs for 4-cue trials, sensitivity for 4-cue trials, and proportional change in capacity across all levels of cuing (Table 4,Supplemental Figure 6). At the fixed level of workload (4 cues), participants in the DFS group reduced their RTs and increased their HRs and level of sensitivity across the trial more than those in the control group. With respect to the ability to adapt to increasing workload (increases in the number of cues), participants in the DFS group benefitted more from the increase in workload across time (an increase in the percentage of change in capacity) than did participants in the control group, who showed a small but significant cost of increasing workload. For these variables, the treatment effect accounted for 4% of the variance at baseline and 13% of the variance at endline.
Secondary analyses
The first of the analyses to assess the plausibility of the results of the primary analysis is an examination of the relation between the change in behavior and the change in the blood iron measurements (Table 5). At least one change variable in each of the tasks was successfully fit by a model meeting the criteria described in Methods. A change in hemoglobin predicted a change in performance for 3 variables in 2 tasks (SRT and CRT). A change in sFt predicted a change in performance in 8 variables in 4 tasks (CTH, TTH, ANT, and CFE), whereas a change in BdFe predicted a change in 4 variables in 3 tasks (CTH, TTH, and ANT). Change in sTfR did not predict a change in any variables, suggesting that sFt was likely the primary source of the effect attributed to BdFe. These models accounted for, on average, 8% of the variance for the control group and 16% of the variance for the DFS group.
TABLE 5.
Secondary analysis 1: change in behavioral measurements as a function of change in blood measurements of iron status1
| Predictor Δ | CN | DFS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Task | Dependent variable Δ | Hemoglobin | sFt | BdFe | Intercept | β | R2 | Intercept | β | R2 |
| SRT | Median RT | √ | −69 | −105 | 0.11 | −325 | −163 | 0.12 | ||
| CTH | HR | |||||||||
| FAR | ||||||||||
| d′ | √ | −0.12 | 0.01 | 0.04 | 0.87 | 0.23 | 0.13 | |||
| c | ||||||||||
| τ | √ | 8.44 | −2.19 | 0.05 | −24.20 | −1.83 | 0.17 | |||
| TTH | HR | √ | 0.019 | 0.002 | 0.14 | 0.074 | 0.003 | 0.18 | ||
| FAR | √ | −0.160 | −0.003 | 0.18 | −0.206 | −0.005 | 0.28 | |||
| d′ | √ | 0.671 | 0.040 | 0.02 | 1.796 | 0.122 | 0.12 | |||
| c | ||||||||||
| τ | √ | −95.22 | 2.880 | 0.00 | −70.686 | −3.718 | 0.14 | |||
| ANT | RT, 0 cues | |||||||||
| RT, 2 cues | √ | −32.73 | −2.12 | 0.14 | −109.56 | −4.27 | 0.13 | |||
| Alerting | ||||||||||
| RT, center cues | ||||||||||
| RT, | √ | 183.55 | 1.70 | 0.18 | −48.68 | −8.21 | 0.22 | |||
| Spatial cues | ||||||||||
| Orienting | √ | −24.07 | 1.22 | 0.21 | 63.21 | 2.69 | 0.19 | |||
| RT, consistent flankers | ||||||||||
| RT, inconsistent flankers | √ | 8.21 | 6.05 | 0.00 | −135.38 | −15.45 | 0.14 | |||
| Conflict | ||||||||||
| CFE | RT | √ | 128.55 | 2.68 | 0.04 | 12.21 | 4.86 | 0.16 | ||
| HR | ||||||||||
| FAR | ||||||||||
| d′ | √ | −2.01 | −0.01 | 0.00 | −1.02 | 0.02 | 0.16 | |||
| c | ||||||||||
| CRT | RT, 4 cues | √ | −8.19 | −35.08 | 0.05 | −84.96 | −73.11 | 0.10 | ||
| HR, 4 cues | ||||||||||
| FAR, 4 cues | ||||||||||
| d′, 4 cues | ||||||||||
| c, 4 cues | ||||||||||
| PCC | √ | −46.34 | 20.67 | 0.02 | −129.86 | 28.15 | 0.11 | |||
Check-marks indicate the variable that was selected as a predictor. Values for the intercept and variable estimates are reported only for those cases in which the variables were significantly different from 0 (P < 0.05). ANT, attentional network task; BdFe, total-body iron; c, bias; CFE, composite face effect task; CN, control; CRT, cued recognition task; CTH, contrast threshold task; d′, sensitivity; DFS, double-fortified salt; FAR, false-alarm rate; HR, hit rate; PCC, proportional change in capacity; RT, response time; sFt; serum ferritin; SRT, simple reaction time task; TTH, temporal threshold task; Δ, endline − baseline; τ, threshold.
The second set of analyses to test for plausibility examined the relation between changes in behavior and baseline concentrations of blood iron markers, guided by the idea that those with the lowest baseline levels of iron status should show the most improvement. Table 6 summarizes the results of these analyses. Consistent with the first set of plausibility analyses, we identified a model for ≥1 iron status variable in each task. Baseline hemoglobin predicted a change for 5 variables in 3 tasks (SRT, CTH, and CRT), baseline sFt predicted a change for 12 variables in 4 tasks (CTH, TTH, ANT, and CFE), and baseline BdFe predicted a change for 1 variable in 1 task (TTH). Identified models accounted for 14% of the variance for the control group and 25% of the variance for the DFS group.
TABLE 6.
Secondary analysis 2: change in behavioral measurements as a function of baseline concentrations of blood measurements of iron status1
| Baseline predictor | CN | DFS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Task | Dependent variable Δ | Hemoglobin | sFt | BdFe | Intercept | β | R2 | Intercept | β | R2 |
| SRT | Median RT | √ | −702 | 55 | 0.07 | −941 | 52 | 0.12 | ||
| CTH | HR | √ | 0.14 | −0.0004 | 0.36 | 0.24 | −0.0050 | 0.45 | ||
| FAR | ||||||||||
| d′ | √ | −0.28 | 0.02 | 0.00 | 1.54 | −0.03 | 0.24 | |||
| c | ||||||||||
| τ | √ | −11 | 1.42 | 0.01 | −92 | 5.43 | 0.12 | |||
| TTH | HR | √ | 0.068 | 0.001 | 0.01 | 0.158 | −0.002 | 0.19 | ||
| FAR | √ | −0.191 | 0.001 | 0.02 | −0.311 | 0.003 | 0.27 | |||
| d′ | √ | 2.138 | −0.116 | 0.03 | 4.502 | −0.205 | 0.15 | |||
| c | √ | −0.024 | 0.000 | 0.00 | −0.069 | −0.004 | 0.14 | |||
| τ | √ | −91.736 | −0.928 | 0.00 | −84.678 | 3.268 | 0.16 | |||
| ANT | RT, 0 cues | |||||||||
| RT, 2 cues | √ | −221.09 | 4.34 | 0.59 | −309.31 | 6.16 | 0.48 | |||
| Alerting | ||||||||||
| RT, center cues | ||||||||||
| RT, spatial cues | √ | −23.55 | 6.72 | 0.55 | −344.89 | 8.21 | 0.48 | |||
| Orienting | √ | 57.03 | −2.03 | 0.55 | 146.14 | −3.24 | 0.38 | |||
| RT, consistent flankers | ||||||||||
| RT, inconsistent flankers | √ | 6.37 | 0.26 | 0.00 | −257.03 | 3.80 | 0.19 | |||
| Conflict | √ | −13.48 | 0.66 | 0.14 | −70.59 | 2.15 | 0.14 | |||
| CFE | RT | √ | 260.21 | −4.33 | 0.09 | 135.32 | −3.93 | 0.34 | ||
| HR | ||||||||||
| FAR | ||||||||||
| d′ | √ | −2.35 | 0.01 | 0.00 | −0.97 | 0.05 | 0.19 | |||
| c | ||||||||||
| CRT | RT, 4 cues | √ | −230.05 | 21.19 | 0.01 | −781.13 | 62.27 | 0.20 | ||
| HR, 4 cues | ||||||||||
| FAR, 4 cues | ||||||||||
| d′, 4 cues | ||||||||||
| c, 4 cues | ||||||||||
| PCC | √ | 182.17 | −19.75 | 0.09 | 465.99 | −51.43 | 0.14 | |||
Check-marks indicate the blood measurements selected as the best predictor; note that soluble transferrin receptor was never selected as a predictor. Values for the intercept and variable estimate are reported only for those cases in which the variables were significantly different from 0 (P < 0.05). ANT, attentional network task; BdFe, total-body iron; c, bias; CFE, composite face effect task; CN, control; CRT, cued recognition task; CTH, contrast threshold task; d′, sensitivity; DFS, double-fortified salt; FAR, false-alarm rate; HR, hit rate; PCC, proportional change in capacity; RT, response time; sFt; serum ferritin; SRT, simple reaction time task; TTH, temporal threshold task; Δ, endline − baseline; τ, threshold.
Assessing relative impact
To assess the relative impact of DFS on specific aspects of perceptual and cognitive functioning, the variables were ranked (highest to lowest) in terms of the proportion of variance accounted for in each of the primary and 2 secondary analyses. In cases in which a model was not selected for a variable in either of the 2 secondary analyses, the R2 value for that variable was set to 0 for that analysis, and the rank was set to 99. The ranks were arbitrarily weighted to reflect our estimate of the relative importance of the evidence from each analysis, with the ranks from the primary (intention-to-treat) analysis weighted by 0.6 and the ranks from the 2 secondary analyses weighted by 0.3 and 0.1. The weighted ranks were then summed across the 3 analyses, and the ordering of the tasks according to the summed ranks is presented in Table 7. We identified 3 clusters of variables in these rankings. The first cluster included 9 variables for which no acceptable model could be identified in any of the secondary analyses. The second cluster included 11 variables for which only one of the 2 secondary analyses allowed for an acceptable model to be identified. The third cluster included 11 variables for which both secondary analyses allowed for an acceptable model to be identified. We should emphasize that, although the weights used for the combinations were arbitrary, the elements of the each of the clusters were quite stable for weightings that prioritized the primary over the secondary analyses, and that prioritized the first of the 2 secondary analyses.
TABLE 7.
Sum of the weighted ranks of the dependent variables in each of the tasks according to the proportion of variance accounted for in the primary (intention-to-treat) and two secondary analyses1
| Task | Dependent variable | Primary | Secondary analysis 1 | Secondary analysis 2 | Weighted sum |
|---|---|---|---|---|---|
| TTH | FAR | 1 | 1 | 6 | 1.5 |
| TTH | τ | 2 | 8 | 12 | 4.8 |
| CTH | τ | 3 | 5 | 17 | 5.0 |
| CTH | d' | 4 | 10 | 7 | 6.1 |
| TTH | d' | 6 | 12 | 13 | 8.5 |
| SRT | RT | 7 | 13 | 18 | 9.9 |
| ANT | RT spatial cues | 16 | 2 | 1 | 10.3 |
| CRT | PCC | 9 | 14 | 15 | 11.1 |
| TTH | HR | 17 | 4 | 9 | 12.3 |
| ANT | Conflict | 23 | 9 | 14 | 17.9 |
| ANT | RT 2 cues | 25 | 11 | 2 | 18.5 |
| CRT | HR 4 cues | 8 | 15 | — | 19.2 |
| CFE | HR | 19 | 6 | — | 23.1 |
| ANT | RT consistent flankers | 29 | 3 | — | 28.2 |
| CFE | c | 28 | 7 | — | 28.8 |
| ANT | Orienting | 5 | — | 4 | 33.1 |
| CTH | HR | 11 | — | 3 | 36.6 |
| CFE | RT | 13 | — | 5 | 38.0 |
| TTH | c | 14 | — | 16 | 39.7 |
| CRT | RT 4 cues | 20 | — | 8 | 42.5 |
| ANT | RT inconsistent flankers | 21 | — | 10 | 43.3 |
| CFE | d' | 22 | — | 11 | 44.0 |
| CRT | d' 4 cues | 10 | — | — | 45.6 |
| CTH | FAR | 12 | — | — | 46.8 |
| CTH | c | 15 | — | — | 48.6 |
| ANT | Alerting | 18 | — | — | 50.4 |
| CFE | FAR | 24 | — | — | 54.0 |
| CRT | FAR 4 cues | 26 | — | — | 55.2 |
| CRT | c 4 cues | 27 | — | — | 55.8 |
| ANT | RT 0 cues | 30 | — | — | 57.6 |
| ANT | RT central cues | 31 | — | — | 58.2 |
| Weightings | 0.6 | 0.3 | 0.1 |
Numbers at the bottom of the columns indicate the weighting for that analysis' ranks. A dash (—) indicates cases in which a model could not be identified for that variable. The variables were ranked within each analysis from highest (1) to lowest (31) in terms of proportion of total variance accounted for. ANT, attentional network task; c, bias; CFE, composite face effect task; CRT, cued recognition task; CTH, contrast threshold task; d′, sensitivity; FAR, false-alarm rate; HR, hit rate; PCC, proportional change in capacity; RT, response time; SRT, simple reaction time task; TTH, temporal threshold task; τ, threshold.
Discussion
The results described here were obtained in the context of a larger randomized controlled trial examining the efficacy of DFS in changing the blood iron status of female tea pickers in rural India (13). Provision of the DFS significantly reduced the prevalence of anemia, ID, iron depletion, and negative BdFe concentrations. Having established those effects in this subsample, we then assessed the extent to which changes in the biomarkers were associated with improvements in perception, attention, and memory. It should be noted that the participants in this subsample were more likely to be iron deficient than those in the larger sample and that there were only minor differences between this subsample and the larger sample in terms of improvements in the iron biomarkers and reductions in prevalence [see the study by Haas et al. (13) for additional details].
Of the 31 variables assessed in the 6 tasks, 24 showed significant treatment group × assessment time interactions in the primary analyses (Supplemental Table 1). Of those 24 variables, 16 had ≥1 significant secondary result, and 13 had 2 significant secondary results. In addition, every task had ≥1 variable that had both a significant treatment group × assessment time interaction in the primary analyses and 2 significant results in the secondary analyses. Together, these results suggest that this set of measurements of perception, attention, and memory was sensitive to the effects of iron status.
With respect to the ordering of the variables according to relative impact (Table 7), there are 4 points of interest. First, except for the percentage of change in capacity in the CRT, DFS had the most distinct effects on perceptual or attentional variables, including those from both visual threshold tasks, the ANT, and the most basic of the tasks, the SRT. Second, the variables associated with memory performance (coming from the CFE and CRT) showed significant relations revealed in only one of the 2 secondary analyses. Third, there was a set of variables for which the intervention produced essentially no patterned effects. All of this suggests that the effects of improved iron status were not uniform across all possible measures of perceptual and cognitive functioning but instead were most pronounced for perceptual awareness and spatial attention. Finally, for the variables in the top cluster, most of the effects revealed in the first set of secondary analyses were related to changes in either sFt or BdFe, with only the remaining 6 related to change in hemoglobin.
This last point suggests that the primary changes in brain function due to iron repletion may be those associated with neural signal quality by way of effects on neurotransmitters rather than with oxygen transport. We note, however, that our sample included individuals who were at best only mildly anemic, and their anemia may have been due to factors other than ID. Consequently, we do not have a group in which hemoglobin concentrations changed enough to have had much of an impact on oxygen transport. A second caution is that the model selection method biases selection to the simplest possible model, meaning that models composed of one predictor were preferred over more complex models. It is almost certain that improvements in iron status should confer benefits to brain function by way of multiple mechanisms; clearly, this question will require additional dependent measurements and increased methodological and quantitative sophistication. Finally, it should be noted that both the DFS and the control salt contained iodine. Consequently, it is possible that some of the improvements in the behavioral variables may be attributable to the iodine. However, any group differences over time would most likely be due to only the DFS.
Improvements in iron status may produce the perceptual and attentional changes observed in this study by affecting the brain systems that support learning in the domains of basic visual perception and spatial attention. Learning in both domains is supported by the procedural subsystem of human memory. This procedural subsystem includes the striatum, caudate, and globus pallidus and supports reinforcement learning by way of dopamine-related reward signaling (28). Critically, both perceptual learning and the learning associated with the allocation of spatial attention are dependent on reinforcement learning (28). In addition, these areas of the basal ganglia contain larger iron deposits than other brain areas, because iron is not distributed uniformly in the brain (29). Finally, these brain systems are also those in which ID has pronounced effects on dopamine regulation (30).
This possibility suggests that, when selecting assessments to evaluate the effects of variations in iron status on behavior, one must choose tasks that are sensitive to changes in the brain systems that are affected by changes in iron status. It further suggests that efforts to document effects of ID in perceptual and cognitive functioning with the use of measurements that are too general have limited motivation in terms of brain function or that are nonanalytic with respect to the combination of perceptual, attentional, and mnemonic function will probably be uninformative, unrelated to iron measures (or their change), or both. These possibilities were suggested almost 30 y ago (31, 32) and ≥1 review (33) supports both possibilities in the current literature.
There are, to our knowledge, only 2 published studies with adults whose measurements have been selected in such a principled manner (4, 5). The first study (4) overlaps with the present study in the sets of tasks used to assess cognitive functioning. The results were reported as composite z scores, so no task-specific comparisons are possible. However, results for change as a function of iron repletion in the domain of attention (which included measurements of simple RT and visual discrimination threshold) were found to be statistically significant, whereas the results for the domain of memory (which included a test of recognition memory) were only marginally significant. This is consistent with the ordering of effects over time obtained in the present study [acknowledging the numerous well-known dangers of interpreting relative magnitudes of P values (34)]. Both the second study (5) and the present study used the ANT. Although their results were only marginally significant, Scott and Murray-Kolb (5) did obtain associations between iron status and the orienting score in the ANT, consistent with the present study. Consequently, it is reasonable to conclude that the present results seem consistent with accumulating evidence on the potential specificity of ID on perceptual and cognitive functioning. Furthermore, progress in understanding that potential specificity is critically dependent on the neurobiologically motivated selection of functionally specific measures of perceptual and cognitive functioning.
One general weakness of the present study is the lack of measurements of either brain function (electroencephalography) or brain iron concentrations (as measured, e.g., by MRI). The hypothesized causal chain in the present effort is that a change in systemic and/or peripheral iron status will lead to changes in brain iron status, which will be expressed in changes in behavioral measurements of perceptual and cognitive performance. We suggest that, although the efforts reported here are positive initial steps, significant progress in understanding the mechanisms underlying behavioral change will be much more likely when behavioral measurements are augmented with measurements of brain dynamics (such as electroencephalography) and region-specific measurements of brain iron (including MRI), steps that have yet to be taken.
One additional issue with respect to the present study has to do with the size of the changes measured in the biomarkers and the behavioral variables. We would suggest that this might be something of a “red herring” for ≥2 reasons. First, although the levels of change in the behavioral variables were substantial, they were not in excess of what has been obtained with healthy adults under conditions of extended practice (see, e.g., references 18, 19, 35, 36). Second, the tasks used in the present study were, as noted above, selected specifically to reflect processes involved in the participants' job performance and to tap the use of cortical systems and circuits that have some dependency on iron status. This is not the case for the majority of extant studies of the functional consequences of ID. An understanding of the proportionality of the relation between changes in biomarkers and changes in behavioral measurements will require careful consideration of the specificity and sensitivity of the behavioral measurements.
In conclusion, we have reported evidence suggesting that repletion of iron status in women of reproductive age by using DFS is accompanied by measurable improvements in perceptual, attentional, and mnemonic functioning [see also the study by Vinodkumar et al. (37)]. Our analysis suggests that the most pronounced effects were at the levels of perception and attention, although effects at the level of memory function were also obtained, albeit not as pervasively. The results suggest the potential for DFS to improve brain health and function in this specific population and add to the accumulating evidence that repletion, generally, can efficiently and substantially benefit those with ID.
Supplementary Material
Acknowledgments
We thank Stephanie Rhoten for her helpful comments on an earlier version of the manuscript. We also thank Annie Wesley and Anand Lakshman from the Micronutrient Initiative and the staff of the Child in Need Institute (CINI) in Siliguri for logistical support. The authors' responsibilities were as follows—MJW: designed the study, selected and programmed the experimental tasks, developed the stimuli, trained the RAs, supervised data collection, analyzed the data, drafted the manuscript, and had primary responsibility for the final content; LEM-K: contributed to the study design, the selection of the tasks, the review of the analyses, and the writing of the manuscript; JEHN: contributed to endline data collection, the analyses of the data, and the writing of the manuscript; SV: coordinated data collection in the field and contributed comments; GAR: provided input to the design of the study and contributed comments; AW: contributed to the design and logistics of the study; JDH: was responsible for the design of the larger study of which the present effort was a part, the review of the analyses, and the writing of the manuscript; and all authors: read and approved the final version of the manuscript.
Abbreviations
- AGP
α-1 acid glycoprotein
- ANT
attentional network task
- BdFe
total-body iron
- CFE
composite face effect task
- CRP
C-reactive protein
- CRT
cued recognition task
- CTH
contrast threshold task
- DFS
double-fortified salt
- HR
hit rate
- ID
iron deficiency
- IDA
iron deficiency anemia
- RT
reaction time
- sFt
serum ferritin
- SRT
simple reaction time task
- sTfR
soluble transferrin receptor
- TTH
temporal threshold task
Footnotes
Supported by Nutrition International (formerly Micronutrient Initiative) and the Mathile Institute.
References
- 1. Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SRF, Branca F, Peña-Rosas JP, Bhutta ZA, Ezzati M; Nutrition Impact Model Study Group (Anaemia) . Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995-2011: a systematic analysis of population-representative data. Lancet 2013;1:e16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haas JD. The effects of iron deficiency on physical performance. Mineral requirements for military personnel levels needed for cognitive and physical performance during garrison training. Washington (DC): Food and Nutrition Board, National Academies Press; 2006. p. 451–61. [Google Scholar]
- 3. Haas JD, Brownlie T. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr 2001;131:676S–88S. [DOI] [PubMed] [Google Scholar]
- 4. Murray-Kolb LE, Beard JL. Iron treatment normalizes cognitive functioning in young women. Am J Clin Nutr 2007;85:778–87. [DOI] [PubMed] [Google Scholar]
- 5. Scott SP, Murray-Kolb LE. Iron status is associated with performance on executive functioning tasks in nonanemic young women. J Nutr 2016;146:30–7. [DOI] [PubMed] [Google Scholar]
- 6. Scott SP, De Souza MJ, Koehler K, Murray-Kolb LE. Combined iron deficiency and low aerobic fitness doubly burden academic performance among women attending university. J Nutr 2017;147:104–9. [DOI] [PubMed] [Google Scholar]
- 7. Otero GA, Pliego-Rivero FB, Contreras G, Ricardo J, Fernández T. Iron supplementation brings up a lacking P300 in iron deficient children. Clin Neurophysiol 2004;115:2259–66. [DOI] [PubMed] [Google Scholar]
- 8. Otero GA, Aguirre DM, Porcayo R, Fernandez T. Psychological and electroencephalographic study in school children with iron deficiency. Int J Neurosci 1999;99:113–21. [DOI] [PubMed] [Google Scholar]
- 9. Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet 2007;370:511–20. [DOI] [PubMed] [Google Scholar]
- 10. Andersson M, Thankachan P, Muthayya S, Goud RB, Kurpad AV, Hurrell RF, Zimmermann MB. Dual fortification of salt with iodine and iron: a randomized, double-blind, controlled trial of micronized ferric pyrophosphate and encapsulated ferrous fumarate in southern India. Am J Clin Nutr 2008;88:1378–87. [DOI] [PubMed] [Google Scholar]
- 11. Rajagopalan S, Vinodkumar M. Effects of salt fortified with iron and iodine on the haemoglobin levels and productivity of tea pickers. Food Nutr Bull 2000;21:323–9. [Google Scholar]
- 12. Asibey-Berko E, Zlotkin SH, Yeung GS, Nti-Nimako W, Ahunu B, Kyei-Faried S, Johnston JL, Tondeur MC, Mannar V. Dual fortification of salt with iron and iodine in women and children in rural Ghana. East Afr Med J 2007;84:473–80. [DOI] [PubMed] [Google Scholar]
- 13. Haas JD, Rahn M, Venkatramanan S, Marquis GS, Wenger MJ, Murray-Kolb LE, Wesley AS, Reinhart GA. Double-fortified salt is efficacious in improving indicators of iron deficiency in female Indian tea pickers. J Nutr 2014;144:957–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baxter J, Zlotkin S. Compendium of evidence on double fortified salt: technical report. Micronutrient initiative. Ottawa (Canada): Micronutrient Initiative; 2015. [Google Scholar]
- 15. Thurnham DI, McCabe LD, Haldar S, Wieringa FT, Northrop-Clewes CA, McCabe GP. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. Am J Clin Nutr 2010;92:546–55. [DOI] [PubMed] [Google Scholar]
- 16. Fechner GT. Elements of psychophysics (translated by H. E. Adler, 1966). Leipzig (Germany): Breitkopf and Hartel (Holt, Rinehart, and Winston); 1860. [Google Scholar]
- 17. Forster KI, Forster JC. DMDX: a windows display program with millisecond accuracy. Behav Res Methods Instrum Comput 2003;35:116–24. [DOI] [PubMed] [Google Scholar]
- 18. Wenger MJ, Rasche C. Perceptual learning in contrast detection: presence and costs of shifts in response criteria. Psychon Bull Rev 2006;13:656–61. [DOI] [PubMed] [Google Scholar]
- 19. Wenger MJ, Copeland AM, Bittner JL, Thomas RD. Evidence for criterion shifts in visual perceptual learning: data and implications. Percept Psychophys 2008;70:1248–73. [DOI] [PubMed] [Google Scholar]
- 20. Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci 2002;14:340–7. [DOI] [PubMed] [Google Scholar]
- 21. Hole GJ. Configurational factors in the perception of unfamiliar faces. Perception 1994;23:65–74. [DOI] [PubMed] [Google Scholar]
- 22. Ebbinghaus H. Über das Gedächtnis: Untersuchungen zur experimentellen Psychologie. [Memory: a contribution to experimental psychology.] Leipzig (Germany): Duncker and Humblot; 1885(in German). [Google Scholar]
- 23. Wenger MK, Negash S, Petersen RC, Petersen L. Modeling and estimating recall processing capacity: sensitivity and diagnostic utility in application to mild cognitive impairment. J Math Psychol 2010;54:73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luce RD. Reaction times: their role in inferring elementary mental organization: New York: Oxford University Press; 1986. [Google Scholar]
- 25. Bozdogan H. Model selection and Akaike's information criterion (AIC): the general theory and its analytical extensions. Psychometrika 1987;52:345–70. [Google Scholar]
- 26. Venkatramanan S, Marquis G, Neufeld L, Wenger MJ, Reinhart G, Haas JD. Double fortified salt intervention improved iron intake but not energy and other nutrient intakes in women tea plantation workers from West Bengal, India. Food Nutr Bull 2017Jan 1(Epub ahead of print; DOI: 10.1177/0379572117718121). [DOI] [PubMed] [Google Scholar]
- 27. Institute of Medicine Iron. In: Dietary reference intakes. Washington (DC): National Academy Press; 2005. p. 344. [Google Scholar]
- 28. Ashby FG, Ennis JM, Spiering BJ. A neurobiological theory of automaticity in perceptual categorization. Psychol Rev 2007;114:632–56. [DOI] [PubMed] [Google Scholar]
- 29. Beard JL, Connor JR. Iron status and neural functioning. Annu Rev Nutr 2003;23:41–58. [DOI] [PubMed] [Google Scholar]
- 30. Connor JR, Menzies SL, St Martin SM, Mufson EJ. Cellular distribution of transferrin, ferritin, and iron in normal and aged human brains. J Neurosci Res 1990;27:595–611. [DOI] [PubMed] [Google Scholar]
- 31. Haas JD, Fairchild MW. Summary and conclusions of the International Conference on Iron Deficiency and Behavioral Development. Am J Clin Nutr (Supplement) 1989;50:703–5. [Google Scholar]
- 32. Haas JD. Comments on “using developmental theory to identify children at risk.” Am J Clin Nutr (Supplement) 1989;50:595–6. [Google Scholar]
- 33. Falkingham M, Abdelhamid A, Curtis P, Fairweather-Tait S, Dye L, Hooper L. The effects of oral iron supplementation on cognition in older children and adults: a systematic review and meta-analysis. Nutr J 2010;9:4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berger JO, Sellke T. Testing a point null hypothesis: the irreconcilability of P values and evidence. J Am Stat Assoc 1987;82:112–22. [Google Scholar]
- 35. Wenger MJ, Payne DG. On the acquisition of mnemonic skill: application of skilled memory theory. J Exp Psychol Appl 1995;3:194–215. [Google Scholar]
- 36. Wenger MJ. On the whats and hows of retrieval in the acquisition of a simple skill. J Exp Psychol: Learn. 1999;25:1137–60. [DOI] [PubMed] [Google Scholar]
- 37. Vinodkumar M, Erhardt JG, Rajagopalan V. Impact of a multiple-micronutrient fortified salt on the nutritional status and memory of schoolchildren. Int J Vitam Nutr Res 2009;79:348–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.