Abstract
Background:
Three well-established intrinsic connectivity networks (ICNs) involved in cognitive-affective processing include the cognitive control network (CCN), default mode network (DMN), and salience and emotional network (SEN). Despite recent advances in understanding developmental changes in these ICNs, the majority of research has focused on single seeds or networks in isolation with limited age ranges. Additionally, although internalizing psychopathologies (IPs), such as anxiety and depression, are often characterized by maladaptive cognitive-affective processing styles, it is not clear how IP history may influence age-related changes in brain networks.
Method:
The current study aimed to characterize the normative development of the CCN, DMN, and SEN across a large age-span (7–29 year olds) of typically developing individuals (TD; n=97). We also explore how age may impact differences in network connectivity between TD individuals and patients with IPs.
Results:
Among TD individuals, DMN and CCN connectivity strengthened with age, whereas connectivity between the SEN and ventromedial prefrontal cortex weakened across development. When exploring group (IP versus TD) differences, the IP group was characterized by greater connectivity between the CCN and cerebellum and between the SEN and caudate from childhood to early adulthood, relative to TD individuals. Finally, patients with IPs, versus TD individuals, exhibited reduced connectivity between the SEN and medial frontal gyrus from adolescence to adulthood.
Conclusions:
The current findings shed light on differential age-related changes in brain network patterns among psychiatrically-free, TD individuals and those with a history of IPs, and may provide plausible targets for novel mechanism-based treatments that may differ based on developmental stage
Keywords: development, age, depression, anxiety, internalizing psychopathologies, intrinsic connectivity networks (ICN)
Introduction
Substantial evidence suggests that the brain is organized into many functional networks that flexibly interact to support various types of information processing (Menon, 2011; Seeley et al., 2007; Yeo et al., 2011). To capture a representation of these networks, researchers have examined functional connectivity patterns, measured via resting-state functional magnetic resonance imaging (rs-fMRI), which represent the temporal correlation of brain regions (Biswal et al., 2010) and capture spontaneous low frequency fluctuations in the blood oxygen level dependent signal (Fox & Raichle, 2007). In recent years, increased attention has been placed on examining age-related changes in these intrinsic connectivity networks (ICNs) to capture typical maturational shifts in brain development, and also to characterize the biological basis of psychiatric disorders in which normal developmental processes are disrupted (Kaiser & Pizzagalli, 2015).
Three ICNs that have been well established and serve distinct roles in cognitive and emotional processing include the default mode network (DMN), salience and emotional network (SEN), and cognitive control network (CCN) (Fox & Raichle, 2007; Menon, 2011; Seeley et al., 2007). Briefly, the DMN is hypothesized to support self-referential or introspective mental processes and includes regions that are typically active during rest and inactive during cognitively demanding tasks (e.g., Raichle et al., 2001). The SEN on the other hand, encompasses limbic and salience regions that are active during the detection and processing of external or internal emotional and novel stimuli (Seeley et al., 2007). Lastly, the CCN, also often referred to as the central executive network, is a frontoparietal system that is thought to be instrumental in problem-solving and executive functioning (Menon, 2011).
Given the importance of these three networks for key aspects of information processing implicated in internalizing disorders, increased attention has been placed in recent years to better understand age-related changes in the ICNs. Consistent with the notion of enhanced cognitive and mental processing with age, there is evidence that connectivity among regions in the DMN increases in strength from childhood to late adolescence (Fair et al., 2008; Power, Fair, Schlaggar, & Petersen, 2010; Rubia, 2013). Additional research has found evidence for increased connectivity among regions within the CCN among adults relative to children (Barber, Caffo, Pekar, & Mostofsky, 2013; Jolles, van Buchem, Crone, & Rombouts, 2010). These previous studies are consistent with functional magnetic resonance imaging (fMRI) studies highlighting increased activation in frontal and parietal regions from childhood to adulthood during cognitive control tasks, suggesting that efficient top-down control processes may not be fully developed until adulthood (i.e., Luna et al., 2001). Regarding SEN development, there is evidence for an increase in connectivity between the nucleus accumbens and subcortical regions among a sample of 8 to 25 year olds (van Duijvenvoorde, Achterberg, Braams, Peters, & Crone, 2016). However, connectivity between the nucleus accumbens and ventromedial prefrontal cortex (vmPFC), a region shown to support down regulation of key regions within the SEN (i.e., amygdala, striatum) (Etkin, Egner, & Kalisch, 2011), declined with age among these individuals. This is consistent with a separate study showing positive amygdala with the medial prefrontal cortex (mPFC) connectivity during childhood, versus the opposite pattern in adolescence and adulthood (Gee et al., 2013), perhaps implicating enhanced regulatory abilities with development.
Although these previous studies shed light on typical maturational processes of ICN patterns, the majority of past research has focused on single seeds or networks in isolation with limited age ranges. In contrast, a recent study did examine age-related changes in several resting-state networks from childhood to late-adolescence. Specifically, developmental changes in connectivity among six resting-state networks linked to cognitive and emotional processes were assessed among healthy youth ranging in age from 7–18 (Solé-Padullés et al., 2016). In this study, within CCN and DMN intrinsic connectivity increased from childhood to adolescence. Additionally, SEN was synchronized with more anterior regions with increasing age, such as the inferior and superior frontal cortices and the left insula. However, connectivity between the SEN and mPFC declined from childhood to adolescence, replicating previous studies (Gee et al., 2013; van Duijvenvoorde et al., 2016).
The current study sought to replicate and extend these previous findings in two important ways. First, we aimed to characterize the development of intrinsic connectivity within and between three networks (DMN, CCN, and SEN) among a larger age-span (i.e., 7–29 year olds) of psychiatrically-free, typically developing (TD) individuals by utilizing rs-fMRI. The inclusion of a wider age range when exploring age-related changes in network connectivity is essential given evidence that maturation of the corpus callosum occurs well into early adulthood (i.e., late 20s) (Keshavan et al., 2002; Pujol et al., 1993). Consistent with previous studies (Barber et al., 2013; Fair et al., 2008; Jolles et al., 2010; Power et al., 2010; Rubia, 2013; Solé-Padullés et al., 2016), we predicted that connectivity within the CCN and DMN would be positively associated with age among TD individuals. For the SEN, we predicted that there would be enhanced connectivity with subcortical regions (i.e., hippocampus, amygdala) with age (van Duijvenvoorde et al., 2016); however, consistent with previous studies (Solé-Padullés et al., 2016; van Duijvenvoorde et al., 2015) and the notion of enhanced regulatory abilities with age, we predicted that connectivity between the SEN and vmPFC would decline with age.
A second extension of the current study was to explore whether individuals with a history of internalizing psychopathologies (IPs) exhibit parallel patterns of network connectivity with development relative to the TD sample. Specifically, IPs, such as anxiety and depression, are highly comorbid across the lifespan (e.g., Kessler et al., 2005; Moffitt et al., 2007) and derive from common mechanisms. Indeed, there is substantial evidence demonstrating that depressive and anxiety disorders are characterized by deviant patterns of connectivity within DMN, SEN, and CCN regions relative to TD individuals in specific developmental and adult age spans (for reviews, see Hulvershorn, Cullen, & Anand, 2011; Kim et al., 2011; MacNamara, DiGangi, & Phan, 2016; Mulders, van Eijndhoven, Schene, Beckmann, & Tendolkar, 2015). However, few studies to date have examined network changes between individuals with IPs and psychiatrically-free individuals across the lifespan, particularly during childhood development when neural plasticity is most active. A better understanding of age-related changes in ICN patterns among individuals with IPs is essential to increase our understanding of these disorders across the lifespan and to develop plausible targets for early identification and novel mechanism-based treatments that may differ based on stage of development. Moreover, exploring the specificity of these network patterns early on in development (i.e., during childhood and adolescence) may provide insight into biological markers that can be targeted to prevent the development and persistence of anxiety and depression in youth.
Thus, this extension of the current study was to examine how developmental stage (i.e., age) may impact differences in network connectivity between TD individuals and patients with IPs. Consistent with previous adolescent and adult studies (for reviews, see Kaiser et al., 2015; MacNamara et al., 2015; Mulders et al., 2015), we predicted that relative to the TD group, individuals with a history of anxiety and/or depression would exhibit greater connectivity within the DMN from childhood to adulthood. Investigations of CCN and SEN connectivity across the lifespan have been more mixed among individuals with anxiety and depression (Kaiser et al., 2015; Mulders et al., 2015), with evidence for both decreased (Bluhm et al., 2009; Cullen et al., 2009; Sylvester et al., 2012; Veer et al., 2010) and increased (Davey, Harrison, Yücel, & Allen, 2012; Sheline, Price, Yan, & Mintun, 2010; Sylvester et al., 2012) connectivity within these networks among adolescent and adult populations. We have previously shown that within the CCN, adolescents (ages 12–17) with a history of depression exhibit increased connectivity (Peters, Burkhouse, Feldhaus, Langenecker, & Jacobs, 2016), whereas the opposite pattern is found among remitted depressed young adults ranging in age from 18 to 23 (Stange et al., 2017). Moreover, when examining cross-network connectivity patterns, remitted depressed adolescents exhibited increased connectivity between the SEN and DMN (Peters et al., 2016), while young adults in remission from MDD exhibited stable and reliable hyperconnectivities of the DMN and SEN with the CCN (Bessette et al., 2018; Jacobs et al., 2014). Thus, taken together, these previous studies suggest that developmental stage may impact the pattern of network connectivity among individuals with a history of depressive and anxiety disorders.
In the current study, we sought to extend these previous findings by including a larger sample of patients with internalizing disorders across a wider age span to explore how period of development may impact ICN patterns among individuals with IPs and no history of psychopathology. The inclusion of a larger age range is essential to characterize the biological basis of these disorders given that anxiety and depressive disorders are highly prevalent and cause significant disability across the lifespan, and knowing that cortex maturation occurs into early adulthood (Keshavan et al., 2002; Pujol et al., 1993). Given the mixed findings reported to date across a number of different age and developmental windows, we did not have a priori hypotheses regarding age-by-disorder interactions.
Method
Participants
The reported participant sample consisted of 233 children, adolescents, and adults recruited through the University of Michigan (UM) and University of Illinois at Chicago (UIC)1. Participants were enrolled in one of five research studies at UIC or UM. For each of these studies, participants were recruited using flyers and multiple postings on the internet. All participants completed an assessment battery of self-report and diagnostic measures, followed by an MRI scan. Youth diagnoses were obtained using the Schedule of Affective Disorders and Schizophrenia for School-Age Children (Kaufman et al., 1997). For adults, diagnoses were determined using either the Structured Clinical Interview for DSM-IV (SCID; First, Spitzer, & Gibbon, 2002) or the Diagnostic Interview for Genetic Studies (Nurnberger et al., 1994). To be included in the TD group, participants could not meet criteria for a current or past DSM-IV Axis-I psychological disorder. To be included in the IP patient group, participants had to meet criteria for a current or past DSM-IV depressive or anxiety disorder. Exclusionary criteria for the IP group included substance abuse or dependence within the past six months, history of bipolar or schizophrenia disorders, intellectual disability, and pervasive development disorders. The reported sample included 97 TD individuals and 136 patients with IPs between 7 and 29 years of age. Participant demographic and clinical characteristics are presented in Table 1. The current study was approved by the University of Michigan (UM) and the University of Illinois at Chicago (UIC) Institutional Review Boards and all adults and youth provided written informed consent and assent, respectively.
Table 1.
Demographics and clinical characteristics of the sample.
| TD (n = 97) |
IP (n = 136) |
Statistic (χ2 or t-value) |
|
|---|---|---|---|
| Demographics | |||
| Age (M, SD) | 19.26 (4.56) | 18.64 (4.47) | −1.03 |
| Sex (% female) | 58.8% | 65.4% | 0.38 |
| Race/Ethnicity | |||
| Caucasian | 58.7% | 59.6% | 0.18 |
| American Indian/Alaskan Native | 0% | 0.7% | 0.89 |
| African American | 8.2% | 11.0% | 0.64 |
| Asian | 18.6% | 5.1% | 10.99* |
| Other/Biracial | 11.3% | 21.3% | 4.04 |
| Hispanic | 10.4% | 19.8% | 3.83 |
| Diagnoses | |||
| Current Anxiety Disorder | 0.0% | 72.1% | - |
| Current MDD | 0.0% | 29.4% | - |
| Past MDD | 0.0% | 47.8% | - |
| Comorbid Anxiety/Depression History | 0.0% | 49.3% | - |
| Clinical Characteristics (M, SD) | |||
| Depressive Symptoms (Z Score) | −1.02 (0.41) | 0.45 (1.43) | 9.82** |
| Anxiety Symptoms (Z Score) | −0.51 (0.71) | 0.46 (1.06) | 7.87** |
| Study Characteristics | |||
| Study Site (% UIC) | 73.2% | 71.3% | 0.75 |
Note. TD = typically developing; IP = internalizing psychopathology; MDD = major depressive disorder;
p < .01;
p < .001.
Symptom Measures
Given that the current study consisted of data across 5 different studies, participants did not complete a uniformed measure of anxiety or depressive symptoms. To assess anxiety symptoms, participants either completed the Beck Anxiety Inventory (BAI; Beck & Steer, 1990) or the Multidimensional Anxiety Scale for Children (MASC; March et al., 1997). To assess depressive symptoms, participants either completed the Beck Depression Inventory (BDI; Beck, Steer, & Brown, 1996), Children’s Depression Inventory (CDI; Kovacs, 2004), or the Reynold’s Adolescent Depression Scale (RADS; Reynolds, 2010). As such, standardized anxiety and depressive scores were created for all participants by calculating age and where possible, sex- corrected Z scores utilizing published normative data from community samples for each of the measures (see Supplementary Table 1 for additional details).
rs-fMRI Acquisition
At UM an eyes-open resting state scan was acquired over eight minutes on a 3.0 T GE Signa scanner (Milwaukee, WI) using T2*-weighted single-shot reverse spiral sequence with the following parameters: 90-degree flip angle, field-of-view 20 cm, matrix size =64 × 64, slice thickness =4 mm, 30 ms echo time, 29 sequentially acquired slices. Eyes-open, resting scans at UIC were collected over eight minutes on a 3.0 T GE Discovery scanner (Milwaukee, WI) using parallel imaging with ASSET and T2* gradient-echo axial EPI with the following parameters: 90-degree flip angle, field-of-view 22 cm, matrix size =64 × 64, slice thickness = 3 mm, 22.2 ms echo time, 44 interleaved slices. At both sites, high-resolution anatomic T1 scans were obtained for spatial normalization; motion was minimized with foam pads, a visual tracking line (UIC only) and/or cross (UIC and UM) on the display, and by conveying the importance of staying still to participants, with TRs of 2000 ms and a total of 240 TRs.
rs-fMRI Preprocessing
Time series data were detrended and mean-centered. Physiologic correction was performed by regressing out white matter and cerebral spinal fluid signals (Behzadi et al., 2007). Motion parameters were regressed out (Jo et al., 2013). Based upon recent literature (Jo et al., 2013; Power et al., 2012), motion volumes were identified based on any TR to TR movement exceeding 1.5 degrees, or 3 consecutive TRS exceeding the same in any of direction pitch, roll, or yaw (Power et al., 2012). In addition, we examined normality plots of the average standard deviation of movement values in the pitch, roll, and yaw planes for outliers. Participants with movement values in any plane greater than .5 mm were excluded. IP and TD groups did not differ in regards to these movement values (i.e., pitch, roll, and yaw planes).
Slice timing was completed with SPM8 (version R4667) (http://www.fil.ion.ucl.ac.uk/spm/doc/) and motion detection algorithms were applied using FSL (version 5.0.0.2) (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). Co-registration of structural images to functional images was followed with spatial normalization of the coregistered T1-SPGR to the Montreal Neurological Institute (MNI) 152 brain template. The resulting normalization matrix was then applied to the slice-time-corrected time-series data. These normalized T2* time-series data were spatially smoothed with a 5 mm Gaussian kernel and spatially resampled to T2* images with isotropic voxels, 2 mm on each side.
To define the mask for the CCN, we selected key nodes based upon Yeo et al. (2011). We included bilateral dorsolateral prefrontal cortex nodes within the middle frontal gyrus (+/−45 29 32), inferior parietal lobule (+/−52 −50 49), and dorsal anterior cingulate (+/−5 22, 47) to use as seeds in the connectivity analysis (Yeo et al., 2011). For each of these three bilateral seeds, we ran cross-correlation time series analyses as described above (and similar to Stange et al., 2017). Similar procedures were used for three bilateral seeds for the SEN [amygdala (+/−23 −5 −19; Jacobs et al., 2014), ventral striatum inferior (+/−9 9 −8; diMartino et al., 2008), anterior insula (+/−33 12 −6; Deen et al., 2011)] and for the DMN [posterior cingulate (+/−5 −49 25), dorsal medial cingulate (+/−7 46 −2), temporoparietal junction (+/−49 −63 45)] (Yeo et al., 2011).
Analytical Plan
Using the three masks (CCN, DMN, and SEN) derived from the available download (Yeo et al., 2011), second-level models in SPM8 tested for voxels that were significantly related to age (as a continuous factor) within the masks among the TD group. In these analyses, we controlled for study site, sex, and movement parameters (i.e., average standard deviation of movement values in each of the pitch, roll, and yaw planes). The threshold of significance reported for the fMRI analyses was p < 0.0005 and k ≥ 150 [3dClustsim with whole brain corrected p-value of .001 per analysis; (December 16, 2015, updated release; 10,000 iterations; http://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html; Eklund, Nichols, & Knutsson, 2016)]. Using whole-brain correction thresholds, despite focused hypotheses that utilized only a subset of voxels (DMN, SEN, and CCN masks, for clarity in interpretation) represents a conservative approach (familywise WB error, p < .003). Data were extracted from the suprathreshold ROIs that were associated with age.
Next, we explored Group × Age interactions in SPM8. Specifically, an identical set of analyses to the TD model described above was conducted with the addition of the Group (IP, TD) × Age (continuous) interaction. Data were extracted from significant ROIs for the Age × Group interaction contrasts: positive contrast = IP group exhibits stronger connectivity with age, relative to the TD group; negative contrast = IP group exhibits weaker connectivity with age, relative to the TD group. To follow-up on significant interactions, we utilized the Johnson-Neyman technique in SPSS (Hayes & Rockwood, 2017), which outputs a value of the moderator (Age), at which the association between the independent variable (IP vs. TD) and outcome crosses the threshold for statistical significance (p < .01).
Results
Participant Characteristics.
As shown in Table 1, the TD and IP groups did not differ in regards to age, sex, ethnicity, and study site. However, a greater number of TD participants identified as Asian American, relative to the IP group. As expected, the IP group reported a greater number of anxiety and depressive symptoms, relative to the TD group.
Age Related Changes in Network Connectivity among TD individuals.
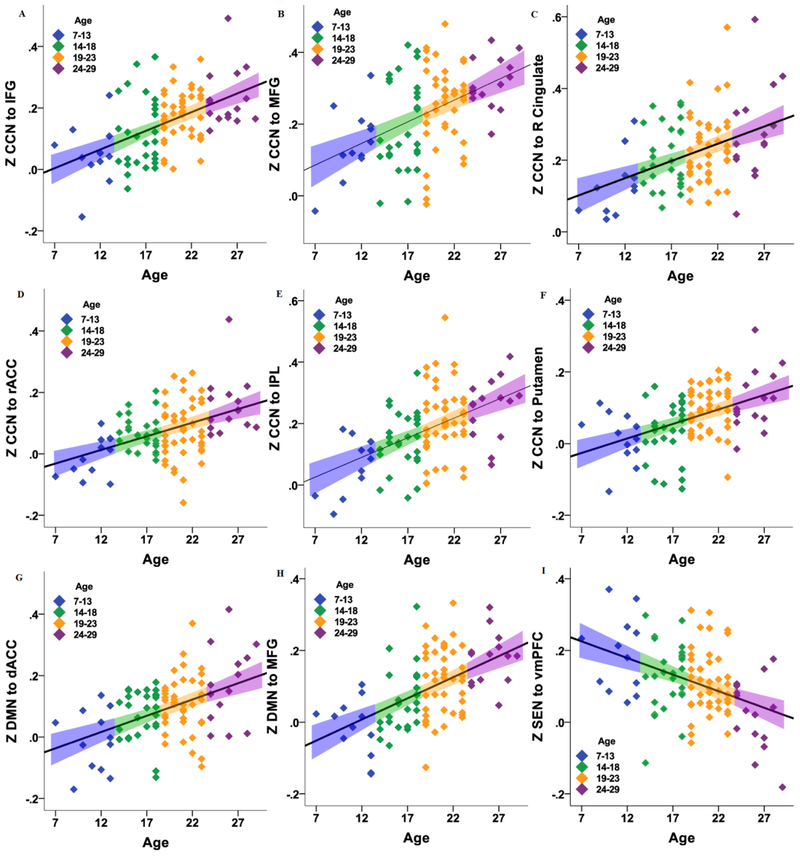
Table 2 displays the findings of the influence of age on connectivity within the three networks among TD participants. As shown in Figures 1 and 2, second-level analyses in SPM8 revealed a positive linear effect of age on connectivity between the CCN (average of connectivity between 3 bilateral seeds of CCN mask) and the following regions: inferior frontal gyrus (IFG), middle frontal gyrus (MFG), rostral anterior cingulate cortex (ACC), right ACC, inferior parietal lobe (IPL), and putamen. Age was also positively correlated with connectivity between the DMN (average of connectivity between 3 bilateral seeds of DMN mask) and dorsal anterior cingulate and middle prefrontal cortex. Finally, results revealed a negative correlation between age and SEN (average of connectivity between 3 bilateral seeds of SEN mask) connectivity with the vmPFC.
Table 2.
Location of clusters of connectivity that were significantly related to age among the typically developing (TD) participants, for each of the three networks.
| MNI Coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Lobe | Gyrus | BA | x | y | z | Z | k |
| Age Increasing | |||||||
| CCN Mask | |||||||
| Frontal | Inferior | 45 | 54 | 34 | 12 | 5.14 | 233 |
| Frontal | Middle | 9 | 38 | 10 | 36 | 4.37 | 222 |
| Limbic | Cingulate | 32 | 0 | 44 | 4 | 4.78 | 360 |
| Limbic | Cingulate | 32 | −2 | 24 | 34 | 4.23 | 165 |
| Parietal | Inferior | 40 | −40 | −52 | 32 | 4.05 | 209 |
| Subcortical | Putamen | -- | 18 | 14 | −4 | 4.87 | 273 |
| DMN Mask | |||||||
| Frontal | Cingulate | 32 | 6 | 20 | 38 | 4.82 | 456 |
| Frontal | Middle | 9 | 44 | 22 | 22 | 4.67 | 997 |
| Age Decreasing | |||||||
| SEN Mask | |||||||
| Frontal | Ventromedial | 9 | 2 | 54 | 4 | −4.78 | 462 |
Note. CCN = cognitive control network; DMN = default mode network; SEN = salience emotion network; k = number of voxels; MNI = Montreal Neurological Institute.
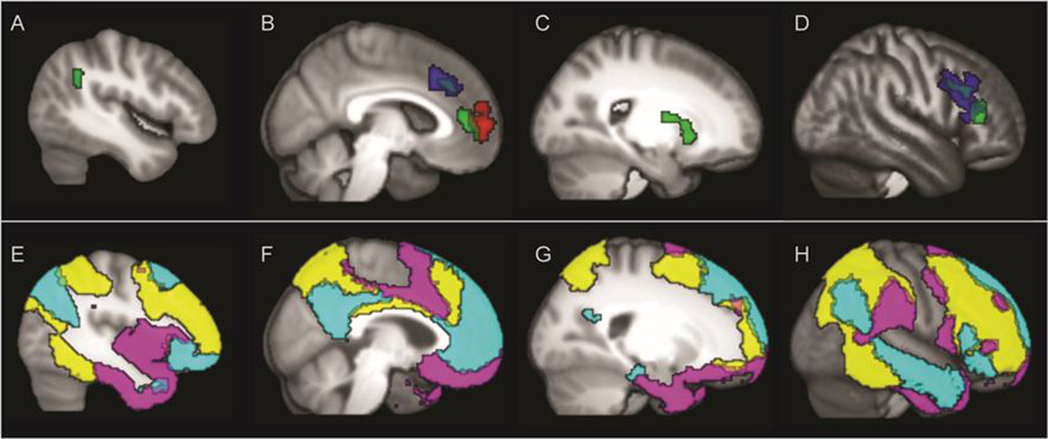
Figure 1.
Top Panel: The seed by cluster effects for the typically developing comparison subjects in panels A (left lateral), B (left medial), C (Right Putamen) and D (right inferior and middle frontal. Blue = increasing connectivity with default mode network (DMN) seeds with age. Red = decreasing connectivity with age to salience emotional network (SEN) seeds. Green = increasing connectivity with age in healthy controls for cognitive control network (CCN) seeds. Bottom Panels E through H illustrate the three primary network masks, DMN (cyan), SEN (violet), and CCN (yellow) in the same laterality of panels to match Panels A through D for easy comparison of within and across network connectivity comparisons.
Figure 2.
Scatter plots of the relation between age (continuous) and mean Z-corrected connectivity between a) cognitive control network (CCN) with inferior frontal gyrus (IFG); b) CCN with middle frontal gyrus (MFG); c) CCN with right cingulate; d) CCN with rostral anterior cingulate cortex (rACC); e) CCN with inferior parietal lobule (IPL); f) CCN with putamen; g) default mode network (DMN) with dorsal ACC (dACC); h) DMN with MFG; i) salience emotional network (SEN) with ventromedial prefrontal cortex (vmPFC). The colors represent the age ranges for illustrative purposes, and the linear error bar is the 95% confidence interval for the mean.
Group Differences in Network Connectivity.
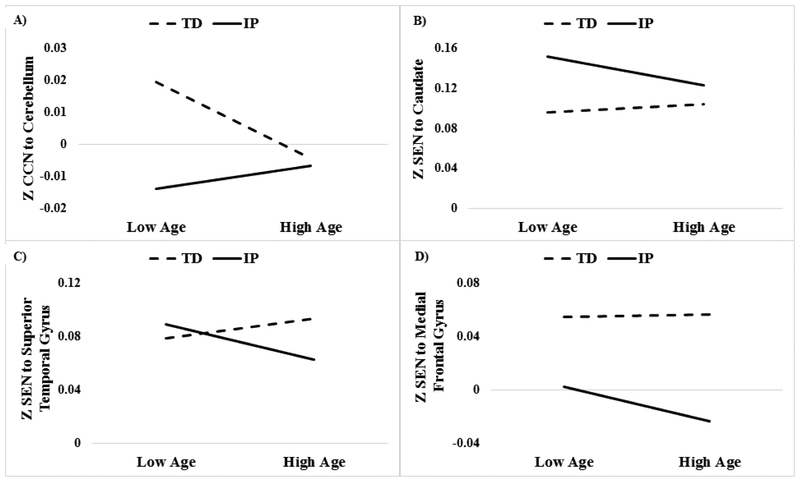
Group × Age interactions were examined in SPM8 (Table 3 and Figure 3). For the CCN model, results revealed significant Group × Age interactions with the Cerebellum. Follow-up analyses utilizing the Johnson-Neyman technique revealed that TD individuals, relative to patients, exhibited greater connectivity between the CCN and Cerebellum between the ages of 7 and 20. For the SEN model, significant Group × Age interactions were observed for the Caudate, Superior Temporal Gyrus, and the Medial Frontal Gyrus. Post-hoc analyses revealed that IP patients exhibited greater connectivity between the SEN and Caudate between ages 7 and 24, relative to the TD group. Conversely, the IP, relative to the TD, group exhibited reduced connectivity between the SEN and Medial Frontal Gyrus beginning at age 12. Additionally, patients exhibited reduced connectivity between the SEN with the Superior Temporal Gyrus later in development (i.e., ages 21–29), relative to TD individuals.
Table 3.
Location of significant clusters of connectivity for the Group (IP vs. TD) × Age interaction, for each of the three networks.
| MNI Coordinates | ||||||
|---|---|---|---|---|---|---|
| Cluster Location | BA | x | y | z | Z | k |
| CCN Mask - Positive | ||||||
| Cerebellum/Culmen/Midbrain/Pons | - | 12 | −36 | −24 | 3.91 | 273 |
| SEN Mask – Negative | ||||||
| Caudate | - | −10 | 18 | 6 | 4.41 | 153 |
| Superior Temporal Gyrus | 22 | −66 | −44 | 16 | 3.92 | 169 |
| Medial Frontal Gyrus | 6 | 8 | −30 | 60 | 3.91 | 641 |
Note: IP = internalizing psychopathology; TD = typically developing; Positive = regions in which the IP group exhibits stronger connectivity with age, relative to the TD group; Negative = regions in which the IP group exhibits weaker connectivity with age, relative to the TD group; CCN = cognitive control network; DMN = default mode network; SEN = salience emotion network; k = number of voxels; MNI = Montreal Neurological Institute.
Figure 3.
Scatter plot depicting group (TD versus IP) differences in network connectivity at high and low (±1 SD) age for illustrative purposes. A) = cognitive control network (CCN) to cerebellum: groups differ statistically from age 7–20; B) = Salience emotional network (SEN) to caudate: groups differ statistically from age 7–24; C) = SEN to superior temporal gyrus: groups differ statistically from age 21–29; and D) = SEN to medial frontal gyrus: groups differ from age 12–29.
Potential Confounds.
Findings were similar when excluding the 21 participants from the patient group taking a psychotropic medication at the time of the scan. We also conducted the above analyses utilizing data from UIC participants only (i.e., excluding UM participants) to rule out site differences. The findings described in detail above remained unchanged.
Discussion
The current study sought to examine age-related changes of three commonly studied ICNs (i.e., CCN, DMN, and SEN) across a broad age-span (i.e., 7–29 year olds) of psychiatrically-healthy individuals. We also explored regions of network connectivity that differed between psychiatrically-free, TD individuals and patients with IPs (i.e., anxiety and depression) across development. Several notable findings emerged from the current study. First, when focusing analyses on findings with psychiatrically-free individuals, we found age-related increases in CCN connectivity with several regions involved in higher level cognitive and mental processing (i.e., IFG, MFG, rostral ACC, right ACC, IPL, and putamen). Age was also positively correlated with connectivity between the DMN and several regions comprising the CCN (i.e., MFG, rostral ACC). These findings replicate previous studies (Barber et al., 2013; Fair et al., 2008; Jolles et al., 2010; Power et al., 2010; Rubia, 2013; Solé-Padullés et al., 2016) by demonstrating potential maturation of the DMN and CCN at rest throughout development among a larger and broader sample of individuals with no prior history of a psychological disorder.
When focusing on SEN development among healthy individuals, the current study found no evidence for increased connectivity with limbic regions with age when utilizing a salience emotional network. This contrasted with another recent study, which reported increased connectivity between the nucleus accumbens and other subcortical regions with age (van Duijvenvoorde et al., 2016). As we elected to only study regions within the network that had common patterns of decreasing connectivity, our results are not directly comparable to seed based analyses of the nucleus accumbens. Our finding revealing age-related decreases in connectivity between the SEN and vmPFC is consistent with previous studies suggesting that connectivity between limbic regions and the mPFC decreases with age during rest and face processing tasks (Gee et al., 2013; Solé-Padullés et al., 2016; van Duijvenvoorde et al., 2016). This might be reflective of age-specific increases in emotion regulatory capacity.
In a distinct set of analyses, we also explored how period of development may impact ICN patterns that differ between TD participants and patients with IPs. Although previous studies have examined differences in patterns of connectivity between TD individuals and patients with IPs, no studies to date have examined how these effects may differ across development utilizing a broad age-span of individuals ranging from childhood to early adulthood. Focusing first on the CCN, patients with IPs were characterized by increased connectivity between the CCN and cerebellum from childhood to early adulthood (ages 7–20), relative to the TD group. Studies have provided increasing evidence for the cerebellum’s involvement in cognitive function and emotional reactions, and subsequently, IP pathophysiology (Schmahmann, 2010; Stoodley, 2012). Notably, other studies have demonstrated that depressed adults exhibit decreased connectivity between the CCN and cerebellum (Liu et al.., 2012, Ma et al., 2013; Zeng et al., 2012), relative to TD individuals. Our findings contribute to the literature by highlighting that these group differences are most pronounced in young children, potentially suggesting a plausible brain-based target for early identification of IPs.
Next, when examining group differences in SEN connectivity across age, results revealed several interesting findings. First, patients, compared to TD individuals, exhibited increased connectivity between the SEN and caudate, a region of the striatum involved in the control of cognitive and emotional processes, between ages 7 and 24. Interestingly, studies to date provide evidence of cortical-striatal dysfunction in a variety of mood and anxiety disorders (for a review, see Marchand, 2010), and it has been posited that a cortical-striatal-thalamic circuit is involved in the pathophysiology of depression (Drevets, 2001). Results also revealed that patients, relative to TD individuals, exhibited reduced connectivity between the SEN and superior temporal gyrus later in development (i.e., ages 20–29). This finding is consistent with a separate study demonstrating that adults with depression exhibit a wide-spread reduction in intrinsic connectivity of the amygdala with several regions involved in emotion processing and regulation, including the superior temporal gyrus (Ramasubbu et al., 2014). Notably, this group difference was not observed among younger individuals; therefore, characteristics of the disease may be contributing to the observed connectivity pattern, as there is evidence for the role of number of episodes and length of illness on changes in neural structures (e.g., Frodl et al., 2003; MacQueen et al., 2003; Sheline, Sanghavi, Mintun, & Gado, 1999). The current study did not comprehensively assess disease characteristics for all participants; therefore, future studies are needed to assess specific factors contributing to these ICN patterns across development.
Replicating previous studies, we also found evidence for reduced connectivity between the SEN and medial frontal gyrus, which plays a pivotal role in the modulation and inhibition of excessive limbic activity (Etkin et al., 2011). Interestingly, the observed group difference began during the adolescent period (i.e., age 12), which is when rates of depression escalate, particularly among females (Hankin et al., 1998). This finding corresponds to previous research showing reduced connectivity between key regions within the SEN and mPFC among individuals with IPs (Gee et al., 2013; Kim et al., 2011; Pannekoek et al., 2014). Notably, there is increasing evidence in support of the theory that early adolescence and puberty-specific changes contribute to increased emotional and stress reactivity and responsiveness (Dahl & Gunnar, 2009, Spear, 2009), which have been linked to IPs across the lifespan (see Aldao, Nolen-Hoeksema, & Schweizer, 2010 for a meta-analysis). Although speculative, increased frontolimbic connectivity may be one potential mechanism implicated in the development of depression during the adolescent period. Future, longitudinal designs are needed to test this possibility.
Finally, contrary to our original hypothesis, we found limited evidence for group differences in connectivity within the DMN across developmental groups. This finding differs from some previous studies, which have provided support for DMN hyperconnectivity among adults with current depression (Mulders et al., 2015). However, studies examining DMN connectivity among anxious populations have been more mixed with evidence for increased (Liao et al., 2011), decreased (Sylvester et al., 2012), and intact (Pannekoek et al., 2013) DMN connectivity patterns. Future studies might specifically stratify recruitment of major depression, anxiety disorders, and comorbid groups to test additional interesting hypotheses, as well as including serial assessments to evaluate the effects of illness. Our findings do suggest intact DMN connectivity patterns during the resting state among individuals with highly overlapping anxiety and depressive disorders across a large age-span.
The current findings may provide plausible targets to identify risk for internalizing disorders. They could also be used as targets for novel mechanism-based treatments that may differ based on developmental stage. For instance, there is evidence that attention bias modification training and cognitive remediation can alter connectivity within the CCN among patients with IPs (Beevers, Clasen, Enock, & Schnyer, 2015; Lanius et al., 2015). Alternatively, neurofeedback training utilizing electroencephalography (EEG) has been shown to assist in the regulation of major brain networks such as the SEN (Lanius et al., 2015). Lastly, repetitive transcranial stimulation (Fischer et al., 2016), transcranial direct current stimulation, and novel pharmacotherapeutic treatments (Watts et al., 2013) developed specifically to target intrinsic network functioning may prove to be beneficial for patients with IPs.
There were several limitations to the current study that should be addressed. First, in order to fully understand the development of these three ICNs, a longitudinal investigation is needed to examine non-linear age effects, changes within individuals, and how different characteristics of IPs (i.e., episodes, length) influence ICN patterns over time. Second, given the role of pubertal status and sex hormones on connectivity patterns (Klapwijk et al., 2013), it will be important for future studies to examine their influence on patterns of network maturation. Next, because of the sample size and high documented comorbidity among individuals with IPs, we were unable to examine group differences based on specific diagnosis. Although the inclusion of a highly comorbid sample of individuals with IPs is highly generalizable to the community, it will be important for future larger studies to examine how these ICNs may differ for specific IPs across development. Fourth, despite the strength of rs-fMRI for identifying individual differences in ICNs, future studies involving task-based fMRI (e.g., those requiring cognitive control, emotion regulation, and internal thought) might provide additional information about age-related changes and differences in the functioning of these networks in other contexts (e.g., Spreng, 2012).
An additional limitation of the current study was the collection of data across five different studies, which had different goals ranging from trait assessments in the remitted state to pretreatment assessments in the active state. Despite being able to demonstrate that the current results did not differ across sites and scanners (UIC versus UM), we were unable to compare findings across the five studies due to inadequate statistical power and non-overlapping age ranges. Although study site was adjusted for in all analyses, independent replication is needed before strong conclusions can be generated. Additionally, given the use of data across several research studies, we were unable to have uniformed symptom or functioning measures that spanned across development, beyond the broad categorical definition provided. To explore symptom relations in the current study, standardized z-scores were created based on published normative data. However, an important direction for future studies will be to examine whether differences in ICN patterns that span across development relate to broader aspects of behavior and functioning.
Despite these limitations, the current study addresses a gap in the literature by being the first to examine how stage of development influences differences in network connectivity patterns between psychiatrically-free, TD individuals and those with a history of IPs. Future larger studies are needed to better understand illness/disease factors (e.g., number of episodes, chronicity, family history of IPs) contributing to the observed differences in ICN patterns among individuals with IPs across the different age groups. If replicated, these findings have the potential to inform novel brain-based therapeutics that may differ based on developmental stage.
Supplementary Material
Acknowledgements
Subsets of these samples have been reported in prior work, including from MH091811 awarded to Langenecker (Bessette et al., 2018; Bhaumik et al., 2017; DelDonno et al., 2017; Jacobs et al., 2014, 2016; Jenkins et al., 2017; Rao et al., 2016; Stange et al., 2017), MH086517 awarded to Monk and Langenecker (Hamm et al., 2014, Wu et al., 2015), UIC CCTS UL1TR000050, Mind and Life Foundation, and Klingenstein Third Generation Foundation Fellowship awarded to Jacobs (Jacobs et al., 2016; Peters et al., 2016,). Data is also present from MH101487 (awarded to Langenecker). Burkhouse is supported by K23-MH113793, Stange is supported by K23-MH112769, and Bessette is supported by T32-MH067631.
Footnotes
Twenty-six individuals (TD = 16, IP = 10) were excluded based on excessive movement (criteria described in method section) during the resting scan.
References
- Aldao A, Nolen-Hoeksema S, & Schweizer S (2010). Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical psychology Review, 30(2), 217–237. [DOI] [PubMed] [Google Scholar]
- Barber AD, Caffo BS, Pekar JJ, & Mostofsky SH (2013). Developmental changes in within-and between-network connectivity between late childhood and adulthood. Neuropsychologia, 51(1), 156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, & Steer RA (1990). Manual for the Beck anxiety inventory. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Beck depression inventory-II. San Antonio, 78(2), 490–8. [Google Scholar]
- Beevers CG, Clasen PC, Enock PM, & Schnyer DM (2015). Attention bias modification for major depressive disorder: Effects on attention bias, resting state connectivity, and symptom change. Journal of Abnormal Psychology, 124(3), 463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, & Liu TT (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage, 37(1), 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessette KL, Jenkins LM, Skerrett KA, Gowins JR, Deldonno SR, Zubieta JK, … & Langenecker SA (2018). Reliability, convergent validity and time invariance of default mode network deviations in early adult Major Depressive Disorder. Frontiers in Psychiatry, 9, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, … & Dogonowski AM (2010). Toward discovery science of human brain function. Proceedings of the National Academy of Sciences, 107(10), 4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm R, Williamson P, Lanius R, Théberge J, Densmore M, Bartha R, … & Osuch E (2009). Resting state default‐mode network connectivity in early depression using a seed region‐of‐interest analysis: Decreased connectivity with caudate nucleus. Psychiatry and Clinical Neurosciences, 63(6), 754–761. [DOI] [PubMed] [Google Scholar]
- Craighead WE, Smucker MR, Craighead LW, & Ilardi SS (1998). Factor analysis of the Children’s Depression Inventory in a community sample. Psychological Assessment, 10(2), 156–165. [Google Scholar]
- Crane NA, Jenkins LM, Bhaumik R, Dion C, Gowins JR, Mickey BJ, … & Langenecker SA (2017). Multidimensional prediction of treatment response to antidepressants with cognitive control and functional MRI. Brain, 140(2), 472–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KR, Gee DG, Klimes-Dougan B, Gabbay V, Hulvershorn L, Mueller BA, … & Lim KO (2009). A preliminary study of functional connectivity in comorbid adolescent depression. Neuroscience Letters, 460(3), 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE, & Gunnar MR (2009). Heightened stress responsiveness and emotional reactivity during pubertal maturation: implications for psychopathology. Development and Psychopathology, 21(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Dalley MB, Bolocofsky DN, Alcorn MB, & Baker C (1992). Depressive symptomatology, attributional style, dysfunctional attitude, and social competency in adolescents with and without learning disabilities. School Psychology Review, 21(3), 444–458. [Google Scholar]
- Davey CG, Harrison BJ, Yücel M, & Allen NB (2012). Regionally specific alterations in functional connectivity of the anterior cingulate cortex in major depressive disorder. Psychological Medicine, 42(10), 2071–2081. [DOI] [PubMed] [Google Scholar]
- Dozois DJ, Dobson KS, & Ahnberg JL (1998). A psychometric evaluation of the Beck Depression Inventory–II. Psychological Assessment, 10(2), 83–89. [Google Scholar]
- Drevets WC (2001). Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Current Opinion in Neurobiology, 11(2), 240–249. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, & Knutsson H (2016). Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences, 113(28), 7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, & Kalisch R (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in cognitive sciences, 15(2), 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, … & Schlaggar BL (2008). The maturing architecture of the brain’s default network. Proceedings of the National Academy of Sciences, 105(10), 4028–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, & Gibbon MW, (2002). Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition (SCID-I/P). New York: Biometrics Research. [Google Scholar]
- Fischer AS, Keller CJ, & Etkin A (2016). The clinical applicability of functional connectivity in depression: Pathways toward more targeted intervention. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 1(3), 262–270. [DOI] [PubMed] [Google Scholar]
- Fox MD, & Raichle ME (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature reviews. Neuroscience, 8(9), 700–711. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Born C, Jäger M, Groll C, … & Möller HJ (2003). Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biological Psychiatry, 53(4), 338–344. [DOI] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, … & Tottenham N (2013). A developmental shift from positive to negative connectivity in human amygdala–prefrontal circuitry. Journal of Neuroscience, 33(10), 4584–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis MM, Haaga DA, & Ford GT (1995). Normative values for the Beck Anxiety Inventory, Fear Questionnaire, Penn State Worry Questionnaire, and Social Phobia and Anxiety Inventory. Psychological Assessment, 7(4), 450. [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, & Angell KE (1998). Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology, 107(1), 128–140. [DOI] [PubMed] [Google Scholar]
- Hammar A, & Ardal G (2009). Cognitive functioning in major depression–a summary. Frontiers in Human Neuroscience, 3:26, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF, & Rockwood NJ (2017). Regression-based statistical mediation and moderation analysis in clinical research: Observations, recommendations, and implementation. Behaviour Research and Therapy, 98, 39–57. [DOI] [PubMed] [Google Scholar]
- Hulvershorn LA, Cullen K, & Anand A (2011). Toward dysfunctional connectivity: a review of neuroimaging findings in pediatric major depressive disorder. Brain Imaging and Behavior, 5(4), 307–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs RH, Jenkins LM, Gabriel LB, Barba A, Ryan KA, Weisenbach SL, … & Gotlib IH (2014). Increased coupling of intrinsic networks in remitted depressed youth predicts rumination and cognitive control. PloS one, 9(8), e104366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs RH, Watkins ER, Peters AT, Feldhaus CG, Barba A, Carbray J, & Langenecker SA (2016). Targeting ruminative thinking in adolescents at risk for depressive relapse: Rumination-focused cognitive behavior therapy in a pilot randomized controlled trial with resting state fMRI. PloS one, 11(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo HJ, Gotts SJ, Reynolds RC, Bandettini PA, Martin A, Cox RW, & Saad ZS (2013). Effective preprocessing procedures virtually eliminate distance-dependent motion artifacts in resting state FMRI. Journal of Applied Mathematics, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles DD, van Buchem MA, Crone EA, & Rombouts SA (2010). A comprehensive study of whole-brain functional connectivity in children and young adults. Cerebral Cortex, 21(2), 385–391. [DOI] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, & Pizzagalli DA (2015). Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry, 72(6), 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, & Pizzagalli DA (2015) Dysfunctional connectivity in the depressed adolescent brain. Biological Psychiatry, 78(9), 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao UMA, Flynn C, Moreci P, … & Ryan N (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry, 36(7), 980–988. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, DeBellis M, Dick E, Kotwal R, Rosenberg DR, … & Pettegrew JW (2002). Development of the corpus callosum in childhood, adolescence and early adulthood. Life Sciences, 70(16), 1909–1922. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 593–602. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, & Whalen PJ (2011). The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behavioural Brain Research, 223(2), 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapwijk ET, Goddings AL, Heyes SB, Bird G, Viner RM, & Blakemore SJ (2013). Increased functional connectivity with puberty in the mentalising network involved in social emotion processing. Hormones and Behavior, 64(2), 314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M (2004). Children’s depression inventory (CDI). Toronto: Multi-Health Systems Inc. [Google Scholar]
- Kujawa A, MacNamara A, Fitzgerald KD, Monk CS, & Phan KL (2015). Enhanced neural reactivity to threatening faces in anxious youth: evidence from event-related potentials. Journal of Abnormal Child Psychology, 43(8), 1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Frewen PA, Tursich M, Jetly R, & McKinnon MC (2015). Restoring large-scale brain networks in PTSD and related disorders: a proposal for neuroscientifically-informed treatment interventions. European Journal of Psychotraumatology, 6(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Xu Q, Mantini D, Ding J, Machado-de-Sousa JP, Hallak JE, … & Crippa JAS (2011). Altered gray matter morphometry and resting-state functional and structural connectivity in social anxiety disorder. Brain Research, 1388, 167–177. [DOI] [PubMed] [Google Scholar]
- Liu L, Zeng LL, Li Y, Ma Q, Li B, Shen H, & Hu D (2012). Altered cerebellar functional connectivity with intrinsic connectivity networks in adults with major depressive disorder. PloS one, 7(6), e39516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, … & Sweeney JA (2001). Maturation of widely distributed brain function subserves cognitive development. Neuroimage, 13(5), 786–793. [DOI] [PubMed] [Google Scholar]
- Ma Q, Zeng LL, Shen H, Liu L, & Hu D (2013). Altered cerebellar–cerebral resting-state functional connectivity reliably identifies major depressive disorder. Brain Research, 1495, 86–94. [DOI] [PubMed] [Google Scholar]
- March JS, Parker JD, Sullivan K, Stallings P, & Conners CK (1997). The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. Journal of the American academy of child & adolescent psychiatry, 36(4), 554–565. [DOI] [PubMed] [Google Scholar]
- Marchand WR (2010). Cortico-basal ganglia circuitry: a review of key research and implications for functional connectivity studies of mood and anxiety disorders. Brain Structure and Function, 215(2), 73–96. [DOI] [PubMed] [Google Scholar]
- MacNamara A, DiGangi J, & Phan KL (2016). Aberrant spontaneous and task-dependent functional connections in the anxious brain. Biological psychiatry: Cognitive Neuroscience and Neuroimaging, 1(3), 278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, … & Young LT (2003). Course of illness, hippocampal function, and hippocampal volume in major depression. Proceedings of the National Academy of Sciences, 100(3), 1387–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V (2011). Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences, 15(10), 483–506. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Harrington H, Caspi A, Kim-Cohen J, Goldberg D, Gregory AM, & Poulton R (2007). Depression and generalized anxiety disorder: cumulative and sequential comorbidity in a birth cohort followed prospectively to age 32 years. Archives of General Psychiatry, 64(6), 651–660. [DOI] [PubMed] [Google Scholar]
- Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, & Tendolkar I (2015). Resting-state functional connectivity in major depressive disorder: a review. Neuroscience & Biobehavioral Reviews, 56, 330–344. [DOI] [PubMed] [Google Scholar]
- Muris P, Merckelbach H, Ollendick T, King N, & Bogie N (2002). Three traditional and three new childhood anxiety questionnaires: Their reliability and validity in a normal adolescent sample. Behaviour research and therapy, 40(7), 753–772. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, … & Reich T (1994). Diagnostic interview for genetic studies: rationale, unique features, and training. Archives of General Psychiatry, 51(11), 849–859. [DOI] [PubMed] [Google Scholar]
- Pannekoek JN, Werff SJA, Meens PH, Bulk BG, Jolles DD, Veer IM, … & Vermeiren RR (2014). Aberrant resting‐state functional connectivity in limbic and salience networks in treatment‐naive clinically depressed adolescents. Journal of Child Psychology and Psychiatry, 55(12), 1317–1327. [DOI] [PubMed] [Google Scholar]
- Pannekoek JN, Veer IM, van Tol MJ, van der Werff SJ, Demenescu LR, Aleman A, … & van der Wee NJ (2013). Resting-state functional connectivity abnormalities in limbic and salience networks in social anxiety disorder without comorbidity. European Neuropsychopharmacology, 23(3), 186–195. [DOI] [PubMed] [Google Scholar]
- Peters AT, Burkhouse K, Feldhaus CC, Langenecker SA, & Jacobs RH (2016). Aberrant resting-state functional connectivity in limbic and cognitive control networks relates to depressive rumination and mindfulness: A pilot study among adolescents with a history of depression. Journal of Affective Disorders, 200, 178–181. [DOI] [PubMed] [Google Scholar]
- Perrin JS, Merz S, Bennett DM, Currie J, Steele DJ, Reid IC, & Schwarzbauer C (2012). Electroconvulsive therapy reduces frontal cortical connectivity in severe depressive disorder. Proceedings of the National Academy of Sciences, 109(14), 5464–5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Fair DA, Schlaggar BL, & Petersen SE (2010). The development of human functional brain networks. Neuron, 67(5), 735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Vendrell P, Junqué C, Martí‐Vilalta JL, & Capdevila A (1993). When does human brain development end? Evidence of corpus callosum growth up to adulthood. Annals of Neurology, 34(1), 71–75. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, & Shulman GL (2001). A default mode of brain function. Proceedings of the National Academy of Sciences, 98(2), 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasubbu R, Konduru N, Cortese F, Bray S, Gaxiola I, & Goodyear B (2014). Reduced intrinsic connectivity of amygdala in adults with major depressive disorder. Frontiers in Psychiatry, 5, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds WM (2010). Reynolds adolescent depression scale. John Wiley & Sons, Inc. [Google Scholar]
- Rubia K (2013). Functional brain imaging across development. European Child & Adolescent Psychiatry, 22(12), 719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD (2010). The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychology Review, 20(3), 236–260. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, … & Greicius MD (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, & Mintun MA (2010). Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences, 107(24), 11020–11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, & Gado MH (1999). Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. Journal of Neuroscience, 19(12), 5034–5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solé-Padullés C, Castro-Fornieles J, de la Serna E, Calvo R, Baeza I, Moya J, … & Sugranyes G (2016). Intrinsic connectivity networks from childhood to late adolescence: effects of age and sex. Developmental Cognitive Neuroscience, 17, 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2009). Heightened stress responsivity and emotional reactivity during pubertal maturation: Implications for psychopathology. Development and Psychopathology, 21(1), 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN (2012). The fallacy of a “task-negative” network. Frontiers in Psychology, 3, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange JP, Bessette KL, Jenkins LM, Peters AT, Feldhaus C, Crane NA, Ajilore O, Jacobs RH, Watkins ER, Langenecker SA. (2017). Attenuated intrinsic connectivity within cognitive control network among individuals with remitted depression: Temporal stability and association with negative cognitive styles. Human Brain Mapping. 38(6), 2939–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ (2012). The cerebellum and cognition: evidence from functional imaging studies. The Cerebellum, 11(2), 352–365. [DOI] [PubMed] [Google Scholar]
- Sylvester CM, Corbetta M, Raichle ME, Rodebaugh TL, Schlaggar BL, Sheline YI, … & Lenze EJ (2012). Functional network dysfunction in anxiety and anxiety disorders. Trends in Neurosciences, 35(9), 527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijvenvoorde ACK, Achterberg M, Braams BR, Peters S, & Crone EA (2016). Testing a dual-systems model of adolescent brain development using resting-state connectivity analyses. Neuroimage, 124, 409–420. [DOI] [PubMed] [Google Scholar]
- Veer IM, Beckmann CF, Van Tol MJ, Ferrarini L, Milles J, Veltman DJ, … & Rombouts SA (2010). Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Frontiers in Systems Neuroscience, 41, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts BV, Schnurr PP, Mayo L, Young-Xu Y, Weeks WB, & Friedman MJ (2013). Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. Journal of Clinical Psychiatry, 74(6), 541–550. [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, … & Fischl B (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LL, Shen H, Liu L, Wang L, Li B, Fang P, … & Hu D (2012). Identifying major depression using whole-brain functional connectivity: a multivariate pattern analysis. Brain, 135(5), 1498–1507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.