Abstract
Commercial deficiency of practical system to label multiple targets in experimental mouse tissues significantly hinders the feasibility to study the potential association between/among multiple targets using tissue-based immunofluorescence (IF) staining. We have developed a new protocol to do dual - labeling immunofluorescences on mouse tissues by combining direct and indirect immunofluorescence, making it possible to use commercial antibodies from the same specious (rabbit) to detect multiple targets in formalin-fixed paraffin-embedded (FFPE) archival mouse tissues simultaneously. This method applies indirect immunofluorescence to assess the first antigen in mouse tissues by using a rabbit anti-mouse polyclonal antibody and goat anti-rabbit antibody. After that, normal rabbit serum was employed to blocking the free binding sites of the previous antibodies. Direct immunofluorescence was used to assess the second antigen by a commercial kit-labeled rabbit anti-human (mouse) antibody at different emission wavelength. At last, cell nuclei were co-stained by DAPI. The outcomes demonstrated that this protocol obtain promising signals of both antigens and the nuclei. Moreover, this method also works on infection disease models in which samples are often over fixed due to biosafety rules.
Keywords: Immunofluorescent staining, antibody, strategy
Introduction
Simultaneous detection of multiple tissue antigens is one of the most frequently used immunofluorescence techniques and plays an important role in studying colocalization of antigens. Both direct and indirect staining method can be used while indirect method is more preference due to relatively stranger signal and low cost. In order to avoid cross binding of secondary antibody with multiple primary antibodies, primary antibodies from different host species are recommended. However, in many cases, valid primary antibodies raised in different species are unavailable. In order to use primary antibodies raised in the same host species and avoid the risk of their cross-reaction with secondary antibodies, modifying of the primary antibodies can be applied so that they interact only with the secondary antibody of choice (1, 2).
Former reported methods for using primary antibodies raised in the same host species requires modifying of the primary antibodies by particular reagents (2, 3) Commercial labeling kits are available in market nowadays. However, those kits we chosen didn’t work effectively in our practice, which urge us to develop a more feasible protocol.
We developed this new method while detecting the expression level of fibrin in the mouse tissues to collect information during a study focusing on fibrin deposition in endothelium. The advantage of this new strategy was allowing two commercially available primary antibodies both raised from rabbit to be used simultaneously in one FFPE archival mouse tissue. Only one commercial labeling kit and normal rabbit serum are additionally needed comparing with the traditional indirect immunofluorescence staining.
Furthermore, tissues from some special disease models especially infection diseases are often over fixed by formalin due to bio-safety issues which makes it even more difficult to achieve antigen retrieval. Since the main research of our lab are focus on the infection diseases, we also applied this new method on Ebola Virus (EBOV) infected samples and achieved a good result.
Material
Wild-type and EPAC1-null mice
Rabbit polyclonal antibody against fibrin(ogen) (DAKO)
Rabbit polyclonal antibody against von Willebrand Factor (vWF) (Thermo Fisher Scientific)
Mouse monoclonal antibody against Fibrin(ogen) (Santa Cruz Biotechnology)
Mouse monoclonal antibody against vWF (Invitrogen)
AlexaFluor 594-conjugated goat anti-rabbit (Invitrogen)
AlexaFluor 488-conjugated goat anti-rabbit IgG (Invitrogen)
AlexaFluor 594-conjugated goat anti-mouse (Invitrogen)
AlexaFluor 488-conjugated goat anti-mouse IgG (Invitrogen)
DyLight 594 Microscale Antibody Labeling Kit (Thermo Fisher Scientific)
DyLight 488 Microscale Antibody Labeling Kit (Thermo Fisher Scientific)
Normal rabbit serum (DAKO)
Ultra V Block (Thermo Fisher Scientific)
ProLong Gold Antifade Mountant with DAPI (Invitrogen)
Proteinase K Solution (Invitrogen)
PH 6.0 citrate buffer solution
Olympus BX51 epifluorescence microscope
Method
Tissues collected from wild-type and EPAC1–null mice after extensive perfusion in vivo were fixed in 4% neutral buffered formaldehyde, embedded in paraffin, sectioned at 5 μm thickness.
Sections are heated at 70°C for two hours to make the tissues to strongly adhere to the slides.
Sections deparaffined and rehydrate by going through xylene and gradient ethanol.
Antigen retrieval: incubate in 10% PH 6.0 citrate buffer at 98°C for 30 minutes followed by proteinase K treatment for 3min.
Blocking: incubate with Ultra V Block for 10 min and normal rabbit serum for 30 minutes at 21°C.
- First antigen staining (indirect)
- Primary antibody incubation: rabbit anti-vWF polyclonal antibody (1:100) incubate over night at 4°C.
- Wash once by 0.02% T-PBS and distilled water 3 times each for 5 minutes.
- Second antibody incubation: AlexaFluor 488-conjugated goat anti-rabbit IgG (1:1000) incubate with light block for 30 minutes at 21°C.
- Wash once by distilled water.
Free binding sites blocking by incubation with normal rabbit serum for 30 minutes at 21°C with light block.
- Second antigen staining (direct)
- Label the anti-fibrin(ogen) rabbit polyclonal antibody by DyLight 594 Microscale Antibody Labeling Kit following the guideline of the kit.
- Incubate the sections with the labeled antibody (1:100 for brain; 1:500 for lung and kidney) overnight at 4°C with light block.
- Wash once by distilled water for 5 minutes.
Nuclei were counter-stained with DAPI.
Fluorescent images were analyzed using an Olympus BX51 epifluorescence microscope.
Result
We failed to acquire good results by applying classic indirect method using one rabbit and on mouse antibody. Only rabbit antibodies showed good signals in the mouse brain and long tissues for both Fibrin(ogen) (Figure 1) and vWF (Figure 2) antigens.
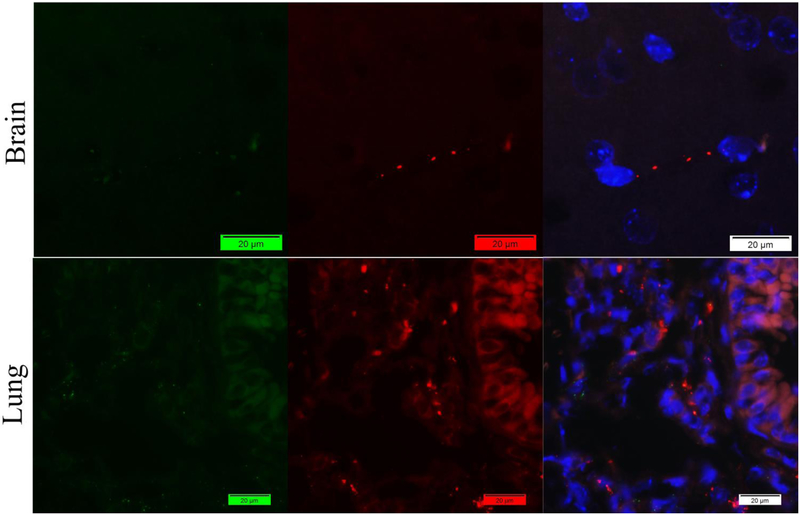
Figure 1.
Staining of mouse brain and lung tissues with mouse anti-vWF antibody (Green) and rabbit anti-fibrin(ogen) antibody (Red) by indirect method. Nuclei were counter-stained with DAPI (Blue). Only promising signals of Fibrin(ogen) were captured with little vWF in the lung.
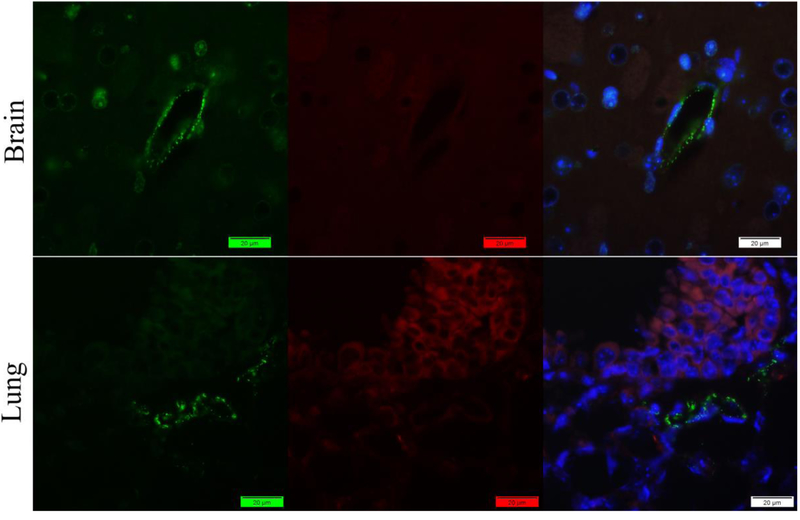
Figure 2.
Staining of mouse brain and lung tissues with rabbit anti-vWF antibody (Green) and Mouse anti-fibrin(ogen) antibody (Red) by indirect method. Nuclei were counter-stained with DAPI (Blue). Only promising signals of vWF were captured with little Fibrin(ogen) in the lung.
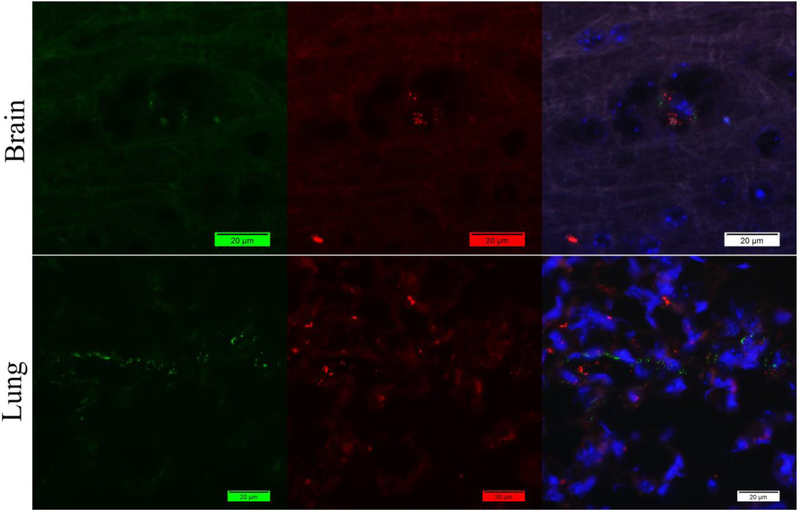
Then we tried to solve this problem by using antibody labeling kits to label both of the rabbit antibodies in order to use them simultaneously by applying direct method. However, the result is also disappointing. The antigens showed no colocalization which is not matched with our previous data (Figure 3).
Figure 3.
Staining of mouse brain and lung tissues with labeled rabbit anti-vWF antibody (Green) and labeled rabbit anti-fibrin(ogen) antibody (Red) by direct method. Nuclei were counter-stained with DAPI (Blue). Both vWF and fibrin(ogen) antigens are labeled but not colocalized.
After that we try to apply this hybrid method but without step of free binding sites blocking that has been described in method. Good signals of both colors were captured. But unfortunately, these two colors show extremely similar distribution to each other which indicates that cross-reaction occurred during the staining process (Figure 4).
Figure 4.
Staining of mouse brain and lung tissues with rabbit anti-vWF antibody (Green) and rabbit anti-fibrin(ogen) antibody (Red) by our new method but without normal rabbit serum. Promising vWf signals in brain and lung. However, red color shows similar distribution with the green ones which indicated the labeled rabbit anti-fibrin(gen) antibody have bounded with the free binding sits of previous antibodies.
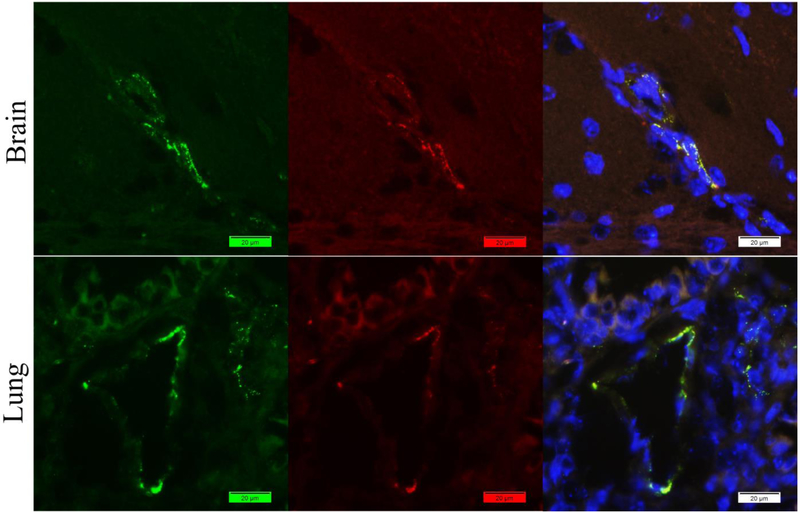
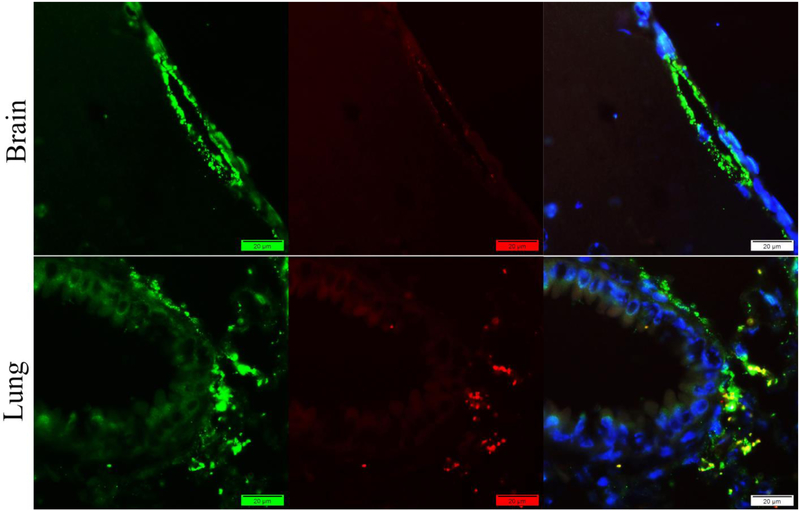
By applying our new method, both vWF and Fibrin(ogen) signals were captured from mouse brain and long tissues (Figure 5). The distribution of the signals meets the expression of the antigens in the tissues. Colocalization of these two antigens can be told after the merge of the two colors.
Figure 5.
Staining of mouse brain and lung tissues with rabbit anti-vWF antibody (Green) and rabbit anti-fibrin(ogen) antibody (Red) by our new method. Nuclei were counter-stained with DAPI (Blue). Promising signals of both vWF and Fibrin(ogen) were captured. Colocalization can be told after the merge of the two colors.
In order to further approve the superiority of this new method, we also applied it in another study of EBOV on mouse aortic ring tissues. The result is achieving. Significantly lower level of background and edge effect were achieved by employing rabbit anti-vwf antibody and rabbit anti-EBOV antibody with our new method compared with the traditional indirect method which employing rabbit anti-vwf antibody and mouse anti-EBOV antibody (6).
Discussion
Mouse is the most commonly used animal model in scientific researches while the most common primary antibodies in market were produced from mouse and rabbit. It is well known that staining of mouse tissue using mouse antibody often lead to high levels of background that is notoriously difficult to eliminate. Moreover, valid primary antibodies raised in different species are unavailable in many cases. In our study, we failed to acquire good signal by using mouse anti-vwf antibody (Figure 1) and mouse anti-fibrin(gen) antibody (Figure 2). This new method can perfectly avoid these problems by employing two rabbit antibodies.
Strict biosafety rules must be obeyed in processing infected animal models. That makes us often come across over fixed samples in infection disease researches. Our successful practice on the EBOV infected samples indicate this new method also works well on these kinds of samples.
Commercial labeling kits were available in market. However, they didn’t work well in our practice. Knowledge from technical support of the vender showed that the labeled antibody might affect each-other’s binding process to the antigen by modifying the primary antibody during the labeling process when more than one labeled antibodies were applied simultaneously. Although another kind of labeling kits are said to be available from that company which should avoid this affect, we prefer to develop more feasible protocol because the expensive price of more labeling kits as well as 100mg primary antibody needed for each labeling process. Our new method only need one labeled primary antibody which avoid the affect between two labeled antibodies, and on the other hand, save 100mg primary antibody and one more kit for labeling another antibody.
Normal rabbit serum is necessary to be applied between two staining process in order to block the free binding site of the previous antibodies. Otherwise, the labeled antibody will bind to the previous second antibody and present as completely colocalized with the first antigen (Figure 4).
To our experience, it is necessary to apply the indirect method preceding in this new method. We have no idea how strong the binding between antigen and labeled antibody is. If labeled antibody is applied first, it may be washed away during the followed indirect staining process. Due to the unavoidable washing steps during the staining, we also recommend labeled antibody to be applied on the antigen that relatively express lower in the tissue in order to achieve relatively high-level signal. Due to the extra cost of the antibodies and labeling kits, we did not repeat these experiments to approve our points above. This should be one of the limits of this study. Another limit is we have not applied this method to cell model yet, which needs further practice in the future.
Conclusion
As far as we know, this is the first strategy making it possible to use two commercial antibodies both from rabbit to detect multiple targets in FFPE archival mouse tissues simultaneously. All the antibodies, labeling kits and reagents used in this study are commercially available. This new method requires relatively less cost of funds and time while generate achieving results.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Kalyuzhny Alexander E. (ed.), Signal Transduction Immunohistochemistry: Methods and Protocols, Methods in Molecular Biology, vol. 717, DOI 10.1007/978-1-61779-024-9_13 [DOI] [Google Scholar]
- 2.Lim JCT, et al. An automated staining protocol for seven-colour immunofluorescence of human tissue sections for diagnostic and prognostic use. Pathology (2018). [DOI] [PubMed] [Google Scholar]
- 3.Frisch J, et al. Novel multicolor immunofluorescence technique using primary antibodies raised in the same host species. Methods in molecular biology 717, 233–244 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Bzorek M, Stamp IM, Petersen BL & Frederiksen L Use of commercially available rabbit monoclonal antibodies for immunofluorescence double staining. Applied immunohistochemistry & molecular morphology: AIMM 16, 387–392 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Donaldson JG Immunofluorescence staining. Current protocols in cell biology Chapter 4, Unit 4 3 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drelich Aleksandra, et al. Exchange Protein Directly Activated by cAMP Modulates Ebola Virus Uptake into Vascular Endothelial Cells. Viruses. 2018. October 16;10(10). pii: E563. doi: 10.3390/v10100563. [DOI] [PMC free article] [PubMed] [Google Scholar]