Abstract
Background:
Common variable immunodeficiency (CVID) and IgG deficiency are two of the more prevalent primary humoral immune defects. Whereas the former is defined by consensus with criteria for quantitative and qualitative antibody defects, the latter is used to describe patients with reduced IgG, who commonly have recurrent sinopulmonary infections but do not fulfill CVID criteria. However, these patients are often given this diagnosis.
Objective:
We compared immunological findings and clinical manifestations of two large cohorts of patients with CVID or IgG deficient to better delineate differences between those syndromes.
Methods:
We extracted clinical and laboratory data from electronic medical records of patients at our institution who had received International Classification of Disease codes for either CVID, or IgG deficiency. We gathered immunoglobulin levels, lymphocyte subpopulation counts, and serological vaccine responses. In some patients, we performed flow cytometry to determine percentages of memory and switched-memory B cells. We compiled and statistically compared clinical data related to infectious manifestations, bronchiectasis, autoimmune diseases, infiltrative inflammatory processes and lymphoid malignancies.
Results:
In contrast to IgG deficient patients, we found that CVID patients had lower IgG levels, greater unresponsiveness to most vaccines, lower percentages of memory and isotype switched-memory B cells, and lower CD4 T cell counts. Clinically, CVID patients presented similar rates of sinusitis and pneumonias, but a significantly higher prevalence of bronchiectasis and especially non-infectious complications.
Conclusion:
CVID and IgG deficiency do not share the same disease spectrum, the former being associated with immunodysregulative manifestations and markers of a more severe immune defect. These data may allow clinicians to distinguish these conditions and the management differences that these patients pose.
Keywords: Primary immunodeficiency, common variable immunodeficiency, IgG deficiency, cohort study, B cell phenotyping
INTRODUCTION
Common variable immunodeficiency (CVID) is a collection of hypogammaglobulinemia syndromes which form the most prevalent symptomatic primary immunodeficiency disease (PID).1 This defect is defined by markedly reduced serum IgG in combination with reduced IgA and/or IgM levels, deficient to absent antibody responses to infections or immunization, and absence of other defined primary or secondary immune defects.2,3 In addition to recurrent sinopulmonary infections, 30-50% of subjects have or will develop noninfectious manifestations: autoimmunity (cytopenias, vitiligo, arthritis, etc.), granulomas, interstitial lung diseases, enteropathy, lymphoid hyperplasia or lymphoid malignancies.4-6 While the definition of CVID has variously evolved7,8, symptomatic hypogammaglobulinemic subjects do not always fulfill all CVID criteria, having normal IgA and IgM and/or normal vaccine responses, and fall more logically in the category of IgG deficiency, which has a separate International Classification of Diseases code (ICD-10; D80.3). However, both CVID and IgG deficient subjects commonly come to medical attention due to recurrent sinopulmonary infections and as a general consensus9, subjects with demonstrable antibody deficiency are treated with IgG replacement. In many cases however, they are still given the ICD diagnosis codes for “CVID”, leading to both concern and added surveillance by other caregivers for the development of noninfectious complications and the potential morbidly associated with this diagnosis.6 By studying large cohorts of subjects with CVID or IgG deficiency, our goal was to compare the immunological markers and clinical manifestations of both groups of subjects to delineate key differences.
METHODS
Medical records:
Using an institutional review board approved protocol with signed consent, we extracted data from medical and laboratory records of subjects referred to our service for reduced immunoglobulin levels for a four-year period (January 2011 to February 2015) in the Epic electronic medical record system (Epic Systems Corporation). To aid in record retrieval, the ICD codes for both IgG deficiency (ICD-9 279.03; and more recently, D80.3 in ICD-10) and CVID (ICD-9 279.06; or D83.0; D83.1; D83.2; D83.8; D83.9 in ICD-10) were used. Patients who received either code were excluded from analysis if chart review revealed another cause for IgG deficiency such as intestinal loss, chronic lymphoid leukemia, corticosteroids or rituximab treatment, etc. In both groups, patients were excluded if there were insufficient data in the medical record. In addition, patients who received both 279.03 and 279.06 codes were kept in only one category after the correct diagnosis was established by chart review.
Laboratory data and immunizations:
Using electronic medical records (EMRs), baseline serum IgG, IgA and IgM levels were collected. IgG titers to protein and conjugate antigens (Haemophilus influenzae type b, measles, mumps, rubella, tetanus, diphtheria, and varicella) were obtained and positivity was determined using the provided laboratory cut-offs. Antibody responses to the 23-valent polysaccharide pneumococcal vaccine (PPV23) were considered protective when the titer was ≥ 1.3 μg/mL.10 For each patient, we entered the sum (out of 14 titers) of protective titers to pneumococcal serotypes. Lymphocyte T (CD3, CD4, CD8) and B cell (CD19 or CD20) populations were obtained from clinical laboratories and compiled for analysis.
B cell subpopulations:
Four-color flow cytometry with LSRII cytometer and FacsDIVA software (BD Biosciences) was performed on patient peripheral blood mononucleated cells using anti-human monoclonal antibodies CD19 PC5 (Beckman Coulter), CD27 FITC (Dako), IgM APC (Jackson ImmunoResearch Inc), and IgD PE (BD Pharmingen). Analysis was performed with FlowJo software (FlowJo, LLC). Isotype-switched memory B (smB) cells (CD19+CD27+IgD−) are expressed in percentage of the total CD19+ cell population.11
Clinical Records:
From the clinical record we compiled information on the presence or absence of autoimmune manifestations (rheumatoid arthritis, systemic lupus erythematous, seronegative arthritis, vasculitis, autoimmune thyroiditis, type 1 diabetes, pernicious anemia, autoimmune cytopenias, vitiligo, and other autoimmune processes), infiltrative inflammatory conditions (interstitial lung disease, lymphoid hyperplasia, granulomas, nodular regenerative hyperplasia, splenomegaly, enteropathy), lymphoid malignancies, as well as pneumonias, recurrent sinusitis, and bronchiectasis. Data on the use of Ig replacement was also recorded.
Statistics:
Descriptive data are presented as mean ± standard deviation. We compared categorical and continuous variables between groups by the chi-square X2 or Fisher exact test and Student t test or the non-parametric Mann–Whitney U-test, respectively, when appropriate. Data were analyzed with Prism 7 (GraphPad Software, Inc). A p value of ≤0.05 was considered statistically significant.
RESULTS
Population characteristics:
The initial list of patients retrieved from Epic EMR system included 147 patients categorized with CVID (ICD-9 279.06 code). Twenty-nine patients were excluded because there was insufficient data, two patients had a different PID (one case of X-linked agammaglobulinemia and one of transient hypogammaglobulinemia of infancy), and two patients had secondary hypogammaglobulinemia (one diagnosed with chronic lymphoid leukemia and one with non-Hodgkin’s lymphoma). Among the 172 patients coded 279.03 (IgG or other immunoglobulin deficiency), 17 were more correctly diagnosed as CVID and moved into this cohort. Eleven patients had an alternative PID (10 with IgG subclass deficiency and another with DiGeorge syndrome), 8 were excluded for insufficient EMR data, 7 had a secondary immunodeficiency (three with chronic lymphoid leukemia, three with lymphoma, one with lymphopenia secondary to chronic use of corticosteroids), and 5 had no detectable PID (normal workup). Thus our final analysis included 128 CVID patients and 124 IgG deficient patients. Mean ages (in years) of patients in the CVID and IgG deficiency groups were similar (45.4 vs 48.1, respectively). Although there was a trend toward a higher percentage of females in the IgG deficiency group (62.9 vs. 52.0%), these differences were not significant (p = 0.08).
Comparison of immunologic parameters:
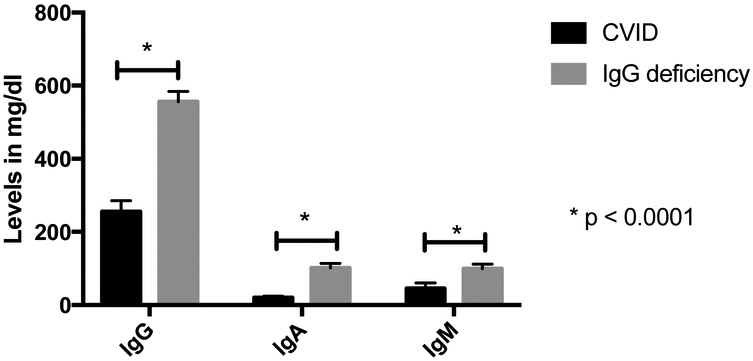
We first compared immunologic parameters between the 128 CVID and the 124 IgG deficient subjects. In CVID subjects, the serum IgG was significantly lower than in IgG deficient subjects, (means 255 mg/dl, range 226 to 285 vs 556 mg/dl, range 527 to 584; p < 0.0001). By definition2,7 IgA and IgM levels were of course significantly lower in subjects with CVID than those with IgG deficiency alone: IgA means 19.8 mg/dl (15.2 to 24.5) vs 102 mg/dl (90 to 113), p < 0.0001; and IgM 45.8 mg/dl (31.6 to 60.0) vs 99.5 mg/dl (87.1 to 112), p < 0.0001) (figure 1, table 1).
Figure 1.
Means of immunoglobulin levels in CVID and IgG deficient patients. IgG, IgA and IgM were all statistically lower in CVID patients.
TABLE I.
Comparison of immunologic parameters between CVID and IgG deficient patient cohorts
| CVID (total n = 128) |
IgG deficiency (total n = 124) |
p value | |
|---|---|---|---|
| Immunoglobulin level means (in mg/dl) | |||
| IgG (nl 767-1,590 mg/dL) | 255 (226 - 285) (n = 120) | 556 (527 - 584) (n =124) | <0.0001 |
| IgA (nl 61-356 mg/dL) | 19.8 (15.2 - 24.5) (n = 126) | 102 (90.0 - 113) (n = 124) | <0.0001 |
| IgM (nl 37-286 mg/dL) | 45.8 (31.6 - 60.0) (n = 126) | 99.5 (87.1 - 112) (n = 124) | <0.0001 |
| Percentage of patients with protective protein/conjugate serologic titers | |||
| Haemophilus influenzae B | 46.2 (30.3 - 62.1) (n = 39) | 66.3 (55.9 - 76.7) (n = 80) | 0.036 |
| Measles | 57.8 (43.2 - 72.4) (n = 45) | 88.5 (81.8 - 95.3) (n = 87) | <0.0001 |
| Mumps | 54.5 (39.6 - 69.4) (n = 44) | 80.9 (72.7 - 89.1) (n = 89) | 0.001 |
| Rubella | 84.4 (73.7 - 95.1) (n = 45) | 93.2 (87.9 - 98.5) (n = 88) | 0.11 |
| Tetanus | 63.3 (49.7 - 76.9) (n = 49) | 96.7 (93.1 - 100) (n = 88) | <0.0001 |
| Diphtheriaw | 71.1 (56.5 - 85.7) (n = 38) | 93.2 (87.3 - 99.0) (n = 73) | 0.001 |
| Varicella | 58.3 (42 - 74.6) (n = 36) | 93.4 (87.8 - 99.0) (n = 76) | <0.0001 |
| Sum of protective serologies to pneumococcal serotypes (means) | |||
| 3.08 (2.12 - 4.04) (n = 60) | 6.51 (5.78 -7.24) (n = 121) | <0.0001 | |
| T cell populations (absolute count means in cells/mm3) | |||
| CD3+ (nl 900- 2100) | 1209 (1070 - 1350) (n = 94) | 1344 (1150 - 1530) (n = 65) | 0.25 |
| CD3+CD4+ (nl 500-1500) | 680 (606 - 753) (n = 92) | 957 (812 - 1100) (n = 65) | 0.0004 |
| CD3+CD8+ (nl 150-1000) | 489 (403 - 574) (n = 92) | 362 (304 - 421) (n = 65) | 0.03 |
| B cells populations (means) | |||
| CD19+ (absolute, cells/mm3) nl = 74.4-441.1 | 205 (158 - 251) (n = 94) | 258 (202 - 313) (n = 67) | 0.15 |
| CD19+CD27+ (% of B cells) nl = 33.6-44.2 | 24.8 (20.9 - 28.8) (n = 111) | 41.0 (33.6 - 48.5) (n = 33) | 0.0002 |
| CD19+CD27+ (absolute cells/mm3) | 31.2 (22.4 - 40.0) (n = 85) | 97.5 (54.2 - 141) (n = 22) | <0.0001 |
| CD19+CD27+IgD- (% of B cells) nl = 20.8-24.3% | 2.85 (1.72 - 3.98) (n = 111) | 10.9 (8.28 - 13.4) (n = 33) | <0.0001 |
| CD19+CD27+IgD- (absolute, cells/mm3) | 3.65 (1.53 - 5.77) (n = 85) | 29.6 (16.5 - 42.7) (n = 22) | <0.0001 |
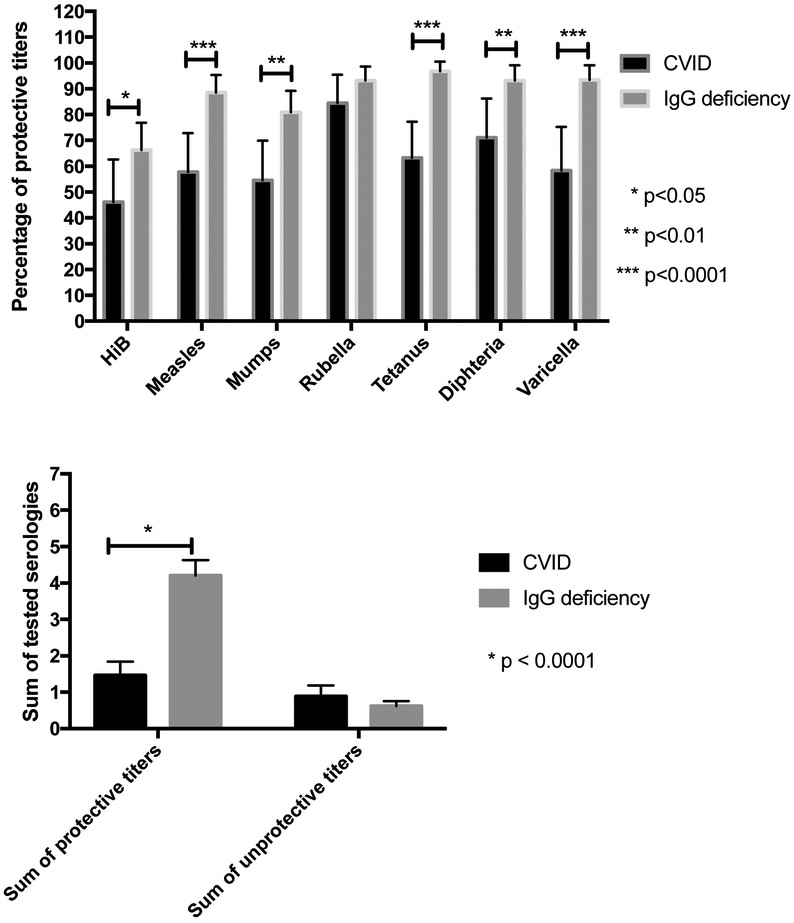
In terms of antibody comparisons, we examined IgG titers to the most commonly administered protein or conjugated vaccines (Haemophilus influenzae type b [Hib], measles, mumps, rubella, tetanus, diphtheria, and varicella). After immunization, CVID subjects had significantly fewer protective titers than IgG deficient subjects, for each of these serologic tests, except for rubella (Hib: 46.2% for CVID vs. 66.3% for IgG deficient patients, p = 0.04; measles: 57.8% vs. 88.5% p < 0.0001; mumps: 54.5% vs. 80.9%, p = 0.001; rubella: 84.4% vs. 93.2%, p = 0.11; tetanus: 63.3% vs. 96.7%, p < 0.0001; diphtheria: 71.1% vs. 93.2% p = 0.001; varicella: 58.3% vs. 93.4%, p < 0.0001) (figure 2A, table 1). We also aggregated protective titers for each patient and compared the means in each group (figure 2B). As expected, IgG deficient subjects had protective antibody to a significantly higher number of protein/conjugated antigens than CVID subjects (means 4.21 (range 3.78 to 4.63) vs. 1.46 (1.09 to 1.84) protective titers, p < 0.0001).
Figure 2.
Protein/conjugate vaccine serologies for CVID and IgG deficient patients. (A) Percentages of protective titers in each group of patients. CVID patients were significantly less protected against Haemophilus influenzae type B, measles, mumps, tetanus, diphtheria and varicella. (B) Sum of protective and unprotective titers in each group. CVID patients had lower numbers of protective titers (among those tested) than IgG deficient patients.
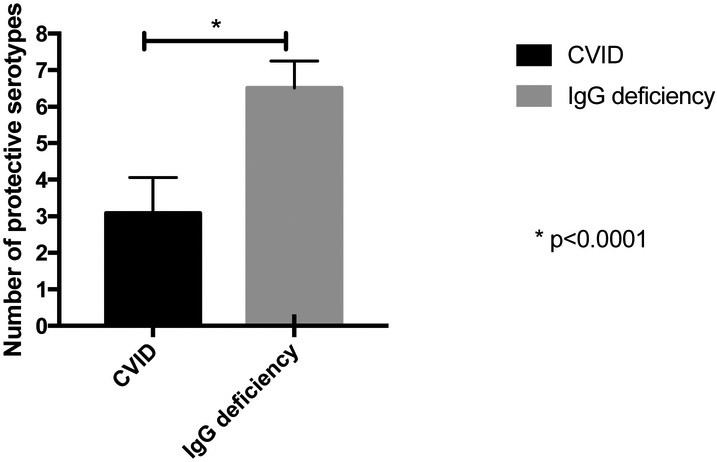
We also compared antibody responses for subjects who received the PPV23 and for whom responses were tested four weeks after vaccination. Using ≤ 1.3 μg/mL as a cut-off for each serotype, IgG deficient subjects had protective titers to twice as many serotypes as subjects diagnosed with CVID (means 6.51 (5.78 to 7.24) vs. 3.08 (2.12 to 4.04) serotypes, p < 0.0001) (figure 3, table 1).
Figure 3.
Pneumococcal titers post PPV23 vaccination. CVID patients had protective titers against fewer pneumococcal serotypes after PPV23 vaccination than IgG deficient patients.
B cell numbers and phenotypes:
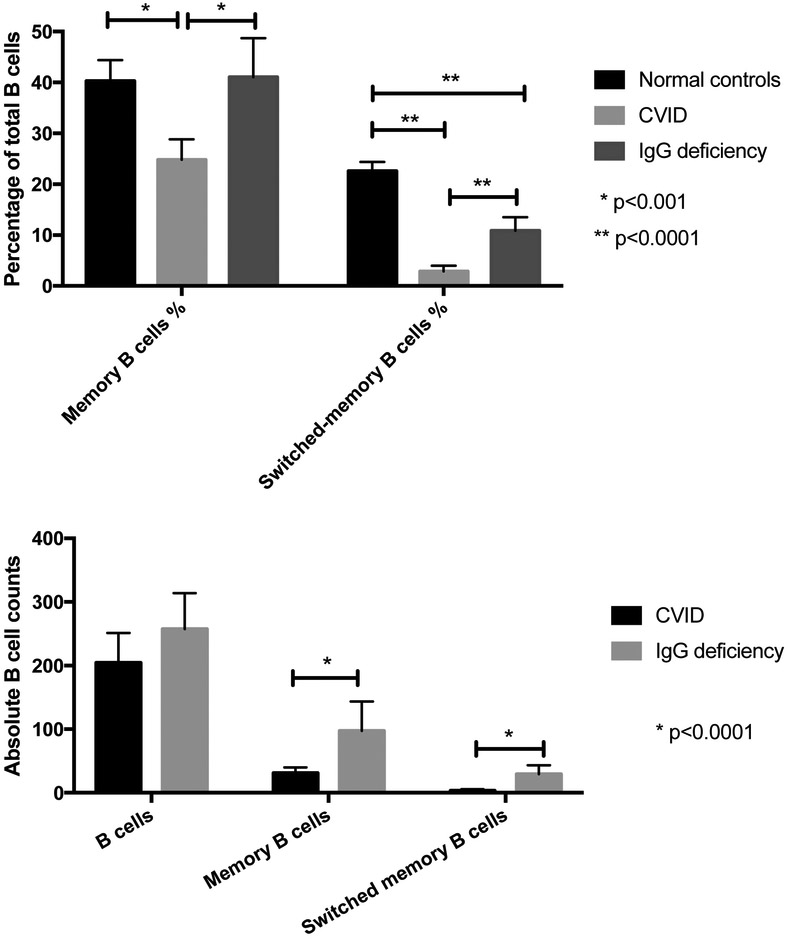
When comparing CVID and IgG deficient patients, there was no statistical difference between the two groups regarding the absolute peripheral blood B cell counts. However, 22.3% (21 out of 94) of the CVID subjects had B cell lymphopenia, in comparison to 11.9% (8 out 67) of the IgG deficient subjects. The memory B cell subpopulations of 111 CVID subjects and 33 IgG deficient subjects had been tested. As expected, CVID patients had both significantly lower percentages of both smB and memory B cells than 27 normal adult controls (2.85% (1.72 to 3.98) vs 22.6% (20.8 to 24.3), p < 0.0001; 24.8% (20.9 to 28.8) vs 40.3% (33.6 to 44.2), p = 0.0005). However, IgG deficient patients had similar percentages of memory B cells as compared to normal controls (means of 41.0% (33.6 to 48.5) vs 40.3% (33.6 to 44.2)), but significantly lower smB cells (10.9% (8.28 to 13.4) vs 22.6 % (20.8 to 24.3), p < 0.0001).
Comparing CVID to IgG deficient subjects, both smB cells and memory B cell percentages were both significantly lower in CVID subjects (2.85% vs. 10.9%), p < 0.0001; 24.8% vs 41.0%, p = 0.0002) (figure 4A). Comparing absolute cell counts (available for 85 CVID and 22 IgG deficient subjects), both smB and memory B cells were also significantly lower in CVID patients (3.65/μL vs 29.6/μL, p < 0.0001; 31.2/μL vs 97.5/μl, p < 0.0001) (figure 4B). Among CVID subjects, 80 (71%) had very low percentages of smB cells (≤2% of total B cells). (Table 1)
Figure 4.
B cell populations in CVID and IgG deficient patients. (A) Memory B cells and switched memory B cells expressed as percentages of total B cells. CVID patients had significantly lower percentages of memory and switched memory B cells compared to IgG deficient patients and normal controls. IgG deficient patients had similar percentages of memory B cell but lower switched-memory B cells compared to normal controls. (B) B cells populations and subpopulations (absolute counts). Similar deficiencies were demonstrated when comparing absolute cell counts.
As shown previously, very low numbers of smB cells were correlated with splenomegaly, autoimmunity, and granulomatous disease in CVID.11,12 Thus, as expected, all forms of non-infectious complications in CVID patients occurred in more subjects with very low smB cells than those with higher numbers (68.8% vs 42.0%). In addition, among the 33 subjects with IgG deficiency who were tested, only one (4.3%), a patient with a history of scleroderma, Raynaud’s disease and immune thrombocytopenic purpura, belonged to the low smB cell group.
T cell subpopulations:
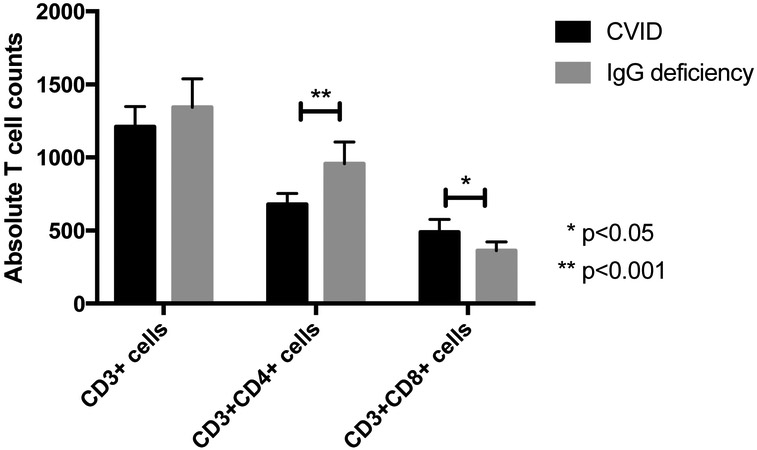
Functional T cell defects in CVID have been described in a number of studies13-19 as well as quantitative defects.20 Comparing T cell subpopulation absolute counts between 94 CVID and 65 IgG deficient subjects, there was no difference between total CD3+ cells, but CVID subjects had lower numbers of CD3+CD4+ T cells (680/μL vs 957/μL, p = 0.0004) and higher numbers of CD3+CD8+ T cells (489/μL vs 362/μL, p = 0.03) than IgG deficient patients (figure 5). While T cell subpopulation means were all within normal ranges, 31.5% (29 out of 92) CVID subjects had T helper lymphopenia in contrast with 7.7% (5 out of 65) of IgG deficient subjects.
Figure 5.
T cell counts. CVID patients had significantly fewer helper T cells and more CD8 T cells than IgG deficient patients.
Clinical Manifestations:
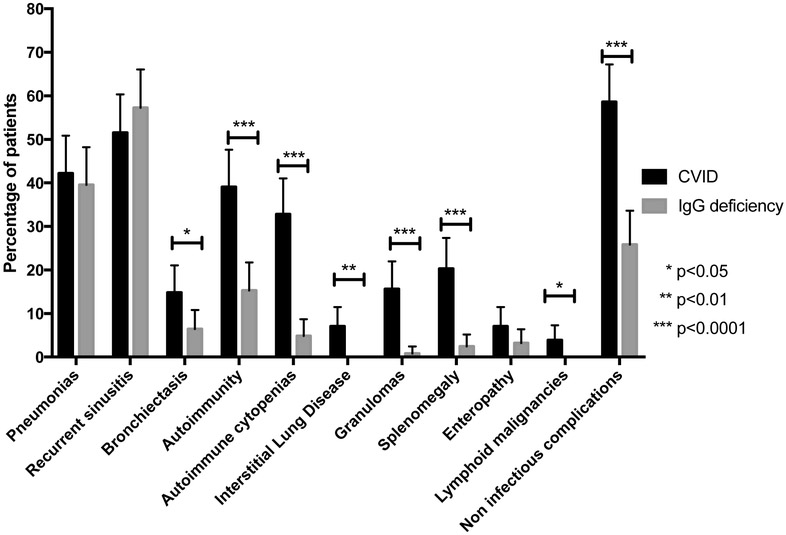
Both CVID and IgG deficiency confer susceptibility to respiratory tract infections, pneumonias and recurrent sinusitis and no differences in the prevalence of these illnesses were observed in these patient groups (table 2, figure 6). However, while IgG deficient subjects had numerous sinopulmonary infections (39.5% patients had pneumonias, and 57.3% had recurrent sinusitis, similar to CVID at 42.2% and 51.6% respectively), noninfectious complications, as suggested from the B cell phenotyping discussed above, were quite rare in the IgG deficient subjects. CVID subjects had strikingly more autoimmune complications (p < 0.0001), autoimmune cytopenias (p < 0.0001), interstitial lung disease (p = 0.003), granulomatous lesions on biopsy (p < 0.0001), splenomegaly (p < 0.0001), and lymphoid malignancies (p = 0.03) and non-infectious complications in general (p < 0.0001). Even though chronic respiratory infections were found in both groups, subjects with CVID still had more evidence of bronchiectasis (p = 0.03). Although there were also more biopsy-confirmed enteropathy cases among CVID patients (9 cases vs. 4 cases among IgG deficient subjects), these differences were not significantly different.
TABLE II.
Comparison of clinical manifestations between CVID and IgG deficient patients
| CVID (total n = 128) |
IgG deficiency (total n = 124) |
p value | |
|---|---|---|---|
| Infectious manifestations and complications (percentage) | |||
| Pneumonia | 42.2 (33.6 to 50.8) | 39.5 (30.9 to 48.2) | 0.67 |
| Recurrent sinusitis | 51.6 (42.9 to 60.3) | 57.3 (48.5 to 66) | 0.37 |
| Bronchiectasis | 14.9 (8.66 to 21) | 6.45 (2.11 to 10.8) | 0.03 |
| Non-infectious complications (percentage) | |||
| Autoimmunity (all causes) | 39.1 (30.6 to 47.6) | 15.3 (8.95 to 21.7) | <0.0001 |
| Autoimmune cytopenias | 32.8 (24.6 to 41) | 4.84 (1.05 to 8.63) | <0.0001 |
| Interstitial lung disease | 7.03 (2.58 to 11.5) | 0 | 0.003 |
| Granulomas | 15.6 (9.32 to 21.9) | 0.81 (−0.774 to 2.39) | <0.0001 |
| Splenomegaly | 20.3 (13.3 to 27.3) | 2.42 (−0.301 to 5.14) | <0.0001 |
| Enteropathy | 7.03 (2.58 to 11.5) | 3.23 (0.106 to 6.35) | 0.17 |
| Lymphoid malignancies | 3.91 (0.536 to 7.28) | 0 | 0.03 |
| Non infectious complications (any type of complication) | 58.6 (50 to 67.2) | 25.8 (18.1 to 33.5) | <0.0001 |
Figure 6.
Clinical characteristics. CVID patients had a significantly higher prevalence of bronchiectasis, autoimmunity (all types of manifestations), autoimmune cytopenias, interstitial lung disease, granulomas, splenomegaly, bronchiectasis, and non-infectious complications (of all causes).
IgG Replacement:
Similarly to CVID patients, subjects with IgG deficiency had a number of significant infections, but in contrast to CVID patients who were all treated with IgG replacement, only a certain percentage of IgG deficient patients were given this therapy. To better understand the immunological or clinical features that may have influenced the decision-making process, we compared the 34 IgG deficient patients who received IgG replacement therapy with the 90 IgG patients who had not. First, comparing baseline serum IgG levels, we found no difference between means of treated and untreated IgG deficient patients (569 vs 550 mg/dl) nor was there a difference in specific IgG subclass levels between the two groups. Examining antibody production, the rates of responses were also similar for both groups. A trend towards a lower response to the varicella vaccine among treated patients was observed, but this was not significant (81.8 % vs 95,4%, p = 0.09). However, aggregating vaccine responses showed that patients who were ultimately treated with IgG, had baseline protective titers against fewer antigens than patients who were not treated (2.7 (1.8 to 3.6) vs 4.7 (4.3 to 5.2) titers, p < 0.0001). In addition, responses to the PPV23 vaccine were significantly weaker in patients in whom IgG replacement was then initiated (5.4 (4.7 to 6.3) vs 6.9 (6.5 to 7.8) positive serotypes, p < 0.05). Second, amongst the IgG deficient patients, those who were given IgG replacement had had more episodes of pneumonia before treatment (58.8% (42.0 to 75.6) vs 32.2% (22.5 to 41.9), p = 0.007), were more likely to have bronchiectasis (14.7% (6.61 to 22.8) vs 3.33% (1.397 to 5.06), p = 0.02), and more historical episodes of sinusitis (70.6% vs 52.2%). When comparing individual noninfectious complications (autoimmune cytopenias, interstitial lung disease, granulomas, and enteropathy) no significant differences emerged between the two groups. However, when grouping all noninfectious manifestations together, patients in the IgG treatment group were more likely to have had at least one noninfectious manifestation before initiation of treatment (41.2% (24.4 to 58.0) vs 20.0% (17.0 to 23.0), p = 0.02).
DISCUSSION
We demonstrate the distinct clinical and immunological differences between subjects with consensus-defined CVID and others with IgG isotype deficiency. While both are B cell defects with variable losses of protective antibody, CVID subjects have additional losses of IgA and IgM, reflecting a more profound B cell defect.2 To further define CVID, Ameratunga et al.7 have proposed supportive laboratory criteria including low smB cells, increased CD21lo subsets, and gene mutations associated with CVID (such as TACI and BAFFR) while a current ESID working clinical definition8 includes reduced IgG and IgA and low smB cells as an alternative to antibody responses. Although these additional tests may further delineate CVID subcategories, most CVID subjects do not have known genetic defects, memory B cell panels have not been standardized, and clinicians commonly rely on immunoglobulin concentrations and vaccine responses to diagnose CVID according to published practice guidelines2,3. On the other hand, IgG deficiency has continued to have a more fluid definition and encompasses any subject with a lower than normal serum IgG level for age, with or without loss of documented antibody function. As subjects with low serum IgG are commonly labeled as having “CVID,” in this study we asked in what way does defined CVID and IgG deficiency overlap or differ? Previously addressing part of this question, Driessen et al.21 found that comparing 44 CVID and 21 IgG deficient subjects, both sets of patients had sinopulmonary infections, which we also found here. However, as for the cohorts examined here, the CVID group was more likely to have bronchiectasis, possibly associated with the lower baseline serum IgG levels in these subjects. In our cohort, CVID subjects also had significantly poorer vaccination responses to both protein and polysaccharide vaccines than IgG deficient subjects but also quite significant differences in clinical phenotypes. The CVID subjects had a much higher incidence of autoimmune cytopenias, interstitial lung disease, granulomas, splenomegaly, lymphoid malignancies and non-infectious complications in general than the IgG deficient group.
Low numbers of smB cells (CD19+CD27+IgD−) is not pathognomonic of CVID as it is associated with a number of other primary immune defects.22-26 However, very low numbers of smB cells have been correlated with higher incidence of splenomegaly, granulomas, and autoimmune diseases in CVID patients, as previously shown.11,12 As expected, this was found in this CVID group, reaffirming that smB cell reduction is associated with more severe CVID phenotypes that tend to manifest with features of immunodysregulation, in addition to susceptibility to infections. While fewer of our subjects with IgG deficiency had been examined for memory B cell populations, we found that smB cell percentage and absolute counts were significantly higher in IgG deficient subjects that in CVID subjects. More than 70% CVID subjects could be classified as smB cell deficient in cohort whereas only 4.3% (only 1 subject) of the IgG deficient patient group belonged to that category. When analyzing B cell subsets in their cohort of CVID and IgG deficient subjects, Driessen et al. found similar results: 57% of CVID subjects and 10% IgG deficient could be classified as smB cell deficient. These two sets of results suggest that B cell defects in IgG deficiency occur after germinal center maturation while a majority of CVID subjects exhibit an earlier and more severe defect.27 In practice, although normal isotype smB cell counts would be likely to exclude the diagnosis of CVID, impaired responses to vaccination have commonly been included as essential to this diagnosis, and are more commonly available. An interesting application of B cell subpopulation phenotyping could be in subjects already on IgG substitution and in whom the diagnosis of CVID is being revisited, either because of a milder phenotype or suspicion of a different PID. In these cases, smB cells may provide a useful marker to consider since neo-antigens are not readily available.
As demonstrated by Malphettes et al.20, a small percentage of patients with CVID present a more combined immunodeficiency with low CD4 T cells. In our cohort, CVID subjects had lower concentrations of helper T cells (with means still within the normal range) than IgG deficient subjects. We also found that almost a third of our CVID subjects had helper T cell lymphopenia; for these, non-infectious manifestations were more prevalent. As shown in other studies, for subjects with more profound T cell defects and/or opportunistic infections, a number of combined immune defects must be ruled out.28-33
Comparing clinical and immunological features of CVID and IgG deficient patients in a large cohort better differentiates these two clinical entities and allows us to conclude that these patient groups differ in essential ways that are likely to impact clinical management. CVID can be distinguished from IgG deficiency with more profound IgG deficiency, poorer vaccine responses, lower smB cells, and moderate T cell lymphopenia. More clinically important is that patients with CVID are significantly more prone to develop severe autoimmunity, interstitial lung disease, granulomatous infiltrations, and lymphoid hyperplasia, as well as lymphoid malignancies.
While IgG deficient subject may have perfectly normal vaccine responses and are not always treated with Ig replacement, greater losses of antibody, and/or clinical indicators such as chronic infections, or bronchiectasis in these patients, remain the most common factors that argue in favor of IgG replacement.34 IgG substitution may also have a positive impact on the frequency of sinusitis35 in addition to its already demonstrated benefit on pneumonia prevention in patients with primary antibody deficiency. However, regarding selection of the most cost-effective treatment, prospective studies about IgG deficient patients are needed to fully evaluate the success of therapies such as intermittent vaccination boosting, antibiotic prophylaxis vs. IgG replacement therapy.
What is already known about this topic?
Common variable immunodeficiency (CVID) is defined by a deficiency in IgG and one other isotype in addition to insufficient vaccine responses, whereas IgG deficiency does not meet all criteria for CVID. Both immune defects confer susceptibility to sinopulmonary infections.
What does this article add to our knowledge?
This large cohort study demonstrates that in contrast to IgG deficiency, CVID is an immunodeficiency that presents with lower IgG levels, greater unresponsiveness to most vaccines, lower percentages of memory and isotype switched-memory B cells, higher prevalence of non-infectious complications.
How does this article impact current management guidelines?
These data indicate that CVID and IgG deficiency do not share the same disease spectrum, the former being associated with immunodysregulative manifestations and markers of a more severe immune defect and may allow clinicians to better distinguish the two conditions.
Acknowledgments
Funding: This work was supported by the National Institutes of Health, AI-061093, AI-086037, AI-48693, the David S Gottesman Immunology Chair and an educational grant from Grifols.
ABBREVIATIONS USED
- CVID
Common variable immunodeficiency
- EMR
Electronic medical records
- ICD
International Classification of Diseases
- PID
Primary immunodeficiency disease
- PPV23
23-valent polysaccharide pneumococcal vaccine
- smB
isotype-switched memory B cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of potential conflict of interest: C. A. Filion received payment for lectures from CSL Behring. S. Taylor Black, P. J. Maglione, L. Radigan, and C. Cunningham-Rundles declare no relevant conflicts of interest.
REFERENCES
- 1.Chapel H, Cunningham-Rundles C. Update in understanding common variable immunodeficiency disorders (CVIDs) and the management of patients with these conditions. British Journal of Haematology 2009;145:709–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonilla FA, Barlan I, Chapel H, Costa-Carvalho BT, Cunningham-Rundles C, la Morena de MT, et al. International Consensus Document (ICON): Common Variable Immunodeficiency Disorders. J Allergy Clin Immunol Pract 2016;4:38–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Picard C, Bobby Gaspar H, Herz Al W, Bousfiha A, Casanova J-L, Chatila T, et al. International Union of Immunological Societies: 2017 Primary Immunodeficiency Diseases Committee Report on Inborn Errors of Immunity. J Clin Immunol 2018;38:96–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapel H, Lucas M, Lee M, Bjorkander J, Webster D, Grimbacher B, et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood 2008;112:277–86. [DOI] [PubMed] [Google Scholar]
- 5.Chapel H, Lucas M, Patel S, Lee M, Cunningham-Rundles C, Resnick E, et al. Confirmation and improvement of criteria for clinical phenotyping in common variable immunodeficiency disorders in replicate cohorts. J Allergy Clin Immunol 2012;130:1197–9. [DOI] [PubMed] [Google Scholar]
- 6.Resnick ES, Moshier EL, Godbold JH, Cunningham-Rundles C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood 2012;119:1650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ameratunga R, Woon S-T, Gillis D, Koopmans W, Steele R. New diagnostic criteria for common variable immune deficiency (CVID), which may assist with decisions to treat with intravenous or subcutaneous immunoglobulin. Clin Exp Immunol 2013;174:203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ESID Registry - Working Definitions for Clinical Diagnosis of PID. 2016;:1–25. Available from: https://esid.org/Working-Parties/Registry/Diagnosis-criteria
- 9.Perez EE, Orange JS, Bonilla F, Chinen J, Chinn IK, Dorsey M, et al. Update on the use of immunoglobulin in human disease: A review of evidence. J Allergy Clin Immunol 2017;139:S1–46. [DOI] [PubMed] [Google Scholar]
- 10.Orange JS, Ballow M, Stiehm ER, Ballas ZK, Chinen J, La Morena De M, et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: a working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol 2012;130:S1–24. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-Ramón S, Radigan L, Yu JE, Bard S, Cunningham-Rundles C. Memory B cells in common variable immunodeficiency: clinical associations and sex differences. Clin Immunol 2008;128:314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wehr C, Kivioja T, Schmitt C, Ferry B, Witte T, Eren E, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood 2008;111:77–85. [DOI] [PubMed] [Google Scholar]
- 13.Fischer MB, Hauber I, Eggenbauer H, Thon V, Vogel E, Schaffer E, et al. A defect in the early phase of T-cell receptor-mediated T-cell activation in patients with common variable immunodeficiency. Blood 1994;84:4234–41. [PubMed] [Google Scholar]
- 14.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol 1999;92:34–48. [DOI] [PubMed] [Google Scholar]
- 15.Di Renzo M, Serrano D, Zhou Z, George I, Becker K, Cunningham-Rundles C. Enhanced T cell apoptosis in common variable immunodeficiency: negative role of the fas/fasligand system and of the Bcl-2 family proteins and possible role of TNF-RS. Clin Exp Immunol 2001;125:117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horn J, Manguiat A, Berglund LJ, Knerr V, Tahami F, Grimbacher B, et al. Decrease in phenotypic regulatory T cells in subsets of patients with common variable immunodeficiency. Clin Exp Immunol 2009;156:446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serana F, Airò P, Chiarini M, Zanotti C, Scarsi M, Frassi M, et al. Thymic and bone marrow output in patients with common variable immunodeficiency. J Clin Immunol 2011;31:540–9. [DOI] [PubMed] [Google Scholar]
- 18.Ramesh M, Hamm D, Simchoni N, Cunningham-Rundles C. Clonal and constricted T cell repertoire in Common Variable Immune Deficiency. Clin Immunol 2017;178:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serrano D, Becker K, Cunningham-Rundles C, Mayer L. Characterization of the T cell receptor repertoire in patients with common variable immunodeficiency: oligoclonal expansion of CD8(+) T cells. Clin Immunol 2000;97:248–58. [DOI] [PubMed] [Google Scholar]
- 20.Malphettes M, Gerard L, Carmagnat M, Mouillot G, Vince N, Boutboul D, et al. Late-onset combined immune deficiency: a subset of common variable immunodeficiency with severe T cell defect. Clin Infect Dis 2009;49:1329–38. [DOI] [PubMed] [Google Scholar]
- 21.Driessen GJ, Dalm VASH, van Hagen PM, Grashoff HA, Hartwig NG, van Rossum AMC, et al. Common variable immunodeficiency and idiopathic primary hypogammaglobulinemia: two different conditions within the same disease spectrum. Haematologica 2013;98:1617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma CS, Pittaluga S, Avery DT, Hare NJ, Maric I, Klion AD, et al. Selective generation of functional somatically mutated IgM+CD27+, but not Ig isotype-switched, memory B cells in X-linked lymphoproliferative disease. J Clin Invest 2006;116:322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agematsu K, Nagumo H, Shinozaki K, Hokibara S, Yasui K, Terada K, et al. Absence of IgD-CD27(+) memory B cell population in X-linked hyper-IgM syndrome. J Clin Invest 1998;102:853–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warnatz K, Bossaller L, Salzer U, Skrabl-Baumgartner A, Schwinger W, van der Burg M, et al. Human ICOS deficiency abrogates the germinal center reaction and provides a monogenic model for common variable immunodeficiency. Blood 2006;107:3045–52. [DOI] [PubMed] [Google Scholar]
- 25.Bleesing JJ, Souto-Carneiro MM, Savage WJ, Brown MR, Martinez C, Yavuz S, et al. Patients with chronic granulomatous disease have a reduced peripheral blood memory B cell compartment. J Immunol 2006;176:7096–103. [DOI] [PubMed] [Google Scholar]
- 26.Speckmann C, Enders A, Woellner C, Thiel D, Rensing-Ehl A, Schlesier M, et al. Reduced memory B cells in patients with hyper IgE syndrome. Clin Immunol 2008;129:448–54. [DOI] [PubMed] [Google Scholar]
- 27.Roskin KM, Simchoni N, Liu Y, Lee J-Y, Seo K, Hoh RA, et al. IgH sequences in common variable immune deficiency reveal altered B cell development and selection. Sci Transl Med 2015;7:302ra135–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuetz C, Huck K, Gudowius S, Megahed M, Feyen O, Hubner B, et al. An immunodeficiency disease with RAG mutations and granulomas. N Engl J Med 2008;358:2030–8. [DOI] [PubMed] [Google Scholar]
- 29.Buchbinder D, Baker R, Lee YN, Ravell J, Zhang Y, McElwee J, et al. Identification of patients with RAG mutations previously diagnosed with common variable immunodeficiency disorders. J Clin Immunol 2015;35:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angulo I, Vadas O, Gargon F, Banham-Hall E, Plagnol V, Leahy TR, et al. Phosphoinositide 3-kinase δ gene mutation predisposes to respiratory infection and airway damage. Science 2013;342:866–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuehn HS, Ouyang W, Lo B, Deenick EK, Niemela JE, Avery DT, et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science 2014;345:1623–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schubert D, Bode C, Kenefeck R, Hou TZ, Wing JB, Kennedy A, et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med 2014;20:1410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milner JD, Vogel TP, Forbes L, Ma CA, Stray-Pedersen A, Niemela JE, et al. Early-onset lymphoproliferation and autoimmunity caused by germline STAT3 gain-of-function mutations. Blood 2015;125:591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agarwal S, Cunningham-Rundles C. Treatment of hypogammaglobulinemia in adults: a scoring system to guide decisions on immunoglobulin replacement. J Allergy Clin Immunol 2013;131:1699–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walsh JE, Gurrola JG, Graham SM, Mott SL, Ballas ZK. Immunoglobulin replacement therapy reduces chronic rhinosinusitis in patients with antibody deficiency. Int Forum Allergy Rhinol 2017;7:30–6. [DOI] [PubMed] [Google Scholar]