Abstract
Sexual differentiation of brain and behavior is largely a hormonally driven process occurring perinatally in rodents and prenatally in primates. Considered early life programming, this process occurs at a time when the brain is remarkably immature and often does not manifest until reproductive maturity, raising the question of how brief hormonal exposure early in life could have such an enduring effect. Epigenetic modifications that occur early and persist into adulthood is one feasible explanation. Sufficient evidence exists to confirm that there are indeed epigenetic changes to specific brain regions induced by steroid hormones in males to differentiate them from females, but whether they persist into adulthood is unclear. Regardless, there are strong correlations between early epigenetic changes and adult brain and behavior. Moreover, although generally referred to as a permanent process, there is evidence that adult sex-typic behaviors are malleable and even reversible in mammals under certain conditions and these may be a function of epigenetic maintenance of gene expression that impacts behavior.
Keywords: preoptic area, reproduction, steroid hormones, CpG’s, histones, sex differences
Any discussion of sex differences in brain and behavior is best begun with caveats. Most of what we know about the biological origins and mechanistic basis for sex differences in the brain comes from animal models, and most of those are laboratory rats and mice. These are good models in that they are mammals, but they are bad models for the inability to incorporate the enormous influences of culture, experience, societal expectations and gender bias that are an undeniable component of human brain development. They are also a bad model for all of the other ways in which reproductive physiology and behavior is manifest in the animal kingdom, such as hermaphroditic worms, parthenogenic lizards, sex changing fish and temperature-driven sex determination in some reptiles. That being said, the focus on rats and mice has given us substantial depth in our understanding of how and when sex differences occur in the brain, albeit at the expense of breadth.
In the majority of species and all mammals, sex is binomial, either male or female. It is also a direct by-product of sex chromosome complement. In mammals the Sry gene of the Y chromosome codes for a testis-determining factor that directs a gene expression cascade leading to differentiation of the gonadal anlage into testis. All subsequent male typical body characteristics are driven by the ensuing hormonal milieu which is initiated in the earliest stages of fetal development. The developing brain is likewise exposed to the hormonal profile established by the fetal testis and thereby programmed in a manner that supports both the reproductive physiology of the male and the requisite behaviors needed for reproductive success. By yoking the differentiation of the brain and the body to the gonads, nature has assured that brain reproductive phenotype matches body reproductive phenotype (see for review [1]).
Stated more factually, in males gamete production is ongoing, requiring continuous but pulsatile release of luteinizing hormone (LH) from the pituitary, which is directly controlled by the brain. Males also need to seek out, recognize and mate with females. These essential behaviors may additionaly require territory defense and/or direct male-to-male competition. Conversely, in females gameteogenesis is complete while still a fetus, and as an adult she must shed only a limited number of mature ova into the uterus on a cyclic basis. This requires a surge of LH from the pituitary and thereby distinct neuronal control from that seen in the male. Females must also recognize and mate with males, prepare an environment suitable for birthing, followed by active nursing and defensive protection of newborns. We tend to take all of this for granted in large part because it is so enormously invariant. Imagine a scenario in which a recently parturient female put her energy into territory defense and attacking other males instead of nurturing and protecting her offspring. Equally devastating would be a male attempting to nurse newborns and ward off all others in a misguided attempt at protection. But this never happens. We might see instances where females neglect or even mistreat their offspring, but it is not because they are behaving like males. And yes, male mammals will show some degree of nurturing towards newborns, but they do not show maternal-like aggression or attempts at lactation. Scientist are beginning to parse out the neural circuits regulating each component of the reproductive behavior sequela in each sex [2,3]. However, because the correlation between reproductive physiology and reproductive behavior is so strong we insufficiently question how this comes to be. One assumption is that adult behavior is driven by the adult hormonal profile which is a function of the mature adult gonad. The testis produces copious and continuous testosterone and the ovary produces cyclic estrogens and progestins, thus driving the different behavioral profiles. But is this assumption correct? A simple test is to remove the gonads of both males and females and then artificially impart on them the hormonal profile of the opposite sex. This has been done many times many decades ago using a variety of laboratory animals including rats, mice, gerbils, hamsters, guinea pigs and rhesus macaques. The answer is always the same, no, you cannot reverse the reproductive physiology or behavior of an adult animal by simply switching the hormonal profile [4]. There is an important exception discussed below, but for now we will continue with the notion that in the adult mammal reproductive behavior is not sex reversible.
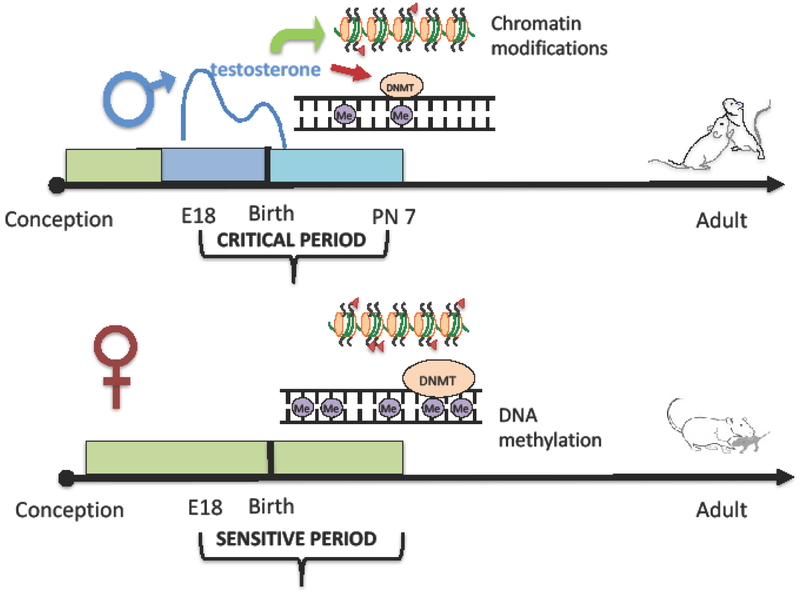
The lack of reversibility in adult reproductive physiology is a result of developmental sexual differentiation, an early life programming process in which the male brain phenotype is induced by the actions of testicular androgens and the female phenotype is induced in the absence of androgens or the aromatized byproduct, estrogens. Sexual differentiation occurs during a restricted critical period such that hormone exposure before or after that time has no lasting impact on the differentiation process (Figure 1). This is not unusual as there are many critical periods in brain development, but what is unusual is that the process occurs so early compared to the output. In rodents the critical period for sexual differentiation begins in the last 4 days of a 20-22 day gestation and ends within the first days of life. In primates, including humans, sexual differentiation begins in the 2nd trimester and is complete by birth (see for review [5]). Thus, the differentiation process occurs months to decades prior to the onset of reproductive competence, and at a time when the brain is still remarkably immature. So how can we reconcile the establishment of a neural circuitry regulating complex adult social behaviors and physiology at a time when most of the brain is not even fully developed much less properly connected? There are two not mutually exclusive hypotheses.
Figure 1: Epigenetics of Sexual Differentiation.
Sexual differentiation of the brain in the rodent begins prenatally during a critical period defined by the onset of androgen production by the fetal male testis. The critical period ends when removing androgens has no effect as the masculinization process has already occurred. In females there is a sensitive period, meaning the feminization process can be disrupted and directed toward masculinization if exposed to exogenous testosterone. Changes to both DNA methylation, via modification of DNMT activity, and the chromatin ensue in varying ways in distinct brain regions. The endurance of these changes into adulthood is not unambiguously clear but there are correlations between early epigenetic modifications and adult sex differences in brain and behavior. Although generally referred to as a permanent process, there is evidence that adult sex-typic behaviors are malleable and even reversible in mammals under certain conditions and these may be a function of epigenetic maintenance of gene expression that impacts behavior.
The first hypothesis is the historical view that invokes a hard wiring of the brain [6]. Neuroanatomical sex differences are a combination of the number of neurons, astrocytes and/or microglia within a particular nucleus or structure and the size of projections between regions. In several nuclei a sex difference in cell number is determined by differential cell death, with neurons dying in one sex either due to lack of trophic support from androgens or estrogens or as a direct result of steroid action [7]. Death being permanent, this would seem a sure way to permanently establish a sex difference. But surprisingly, the maintenance of the sex difference in cell number requires ongoing cell genesis into adulthood in at least some instances [8]. We also now understand that not all synapses are the same. Some can come and go, while others are remarkably stable, although the technical challenges of determining which are which is daunting. In the area of sex differences, few studies are longitudinal. But in one interesting case the authors analyzed dendritic morphology of neurons in a particular hypothalamic nucleus and found a sex difference in the very early neonatal period that then disappeared during the juvenile/adolescent period and re-emerged in precisely the same form in adults [9]. In studies from our laboratory, we find that a sex difference in synaptic density in a reproductively relevant brain region (the preoptic area) is evident at birth, but also evident in adolescent animals and similarly in adults [10] [11]. So, either the same synapses are persisting across the lifespan, which is possible, or there is a “memory” for how dense synapses should be along a particular stretch of dendrite in this brain region. The obvious, and perhaps only, source of such a memory is the epigenome.
Steroid hormones are perfectly poised to alter the epigenome due to their action via nuclear transcription factor receptors which affiliate with large multi-protein complexes that incorporate histone acetylating enzymes, thereby providing direct access to the DNA [12]. For this reason investigators have explored the potential for sex difference in chromatin as well as DNA methylation. There are two approaches to the question. One is to measure specific epigenetic modifications in the brains of males versus females. The other is to treat males and females with an epigenetic modifying drug and measure the impact on brain and behavior. Both approaches have proven fruitful but both have limitations.
A sufficient number of reports exist to convincingly demonstrate there are distinct epigenetic modifications in the brains of males and females [13-20] but there is insufficient information to reliably make declarative statements of fact, much less meaning, about said changes. Moreover, discussion of epigenetic origins of sex differences in the brain highlights how much we have ignored genetic origins. Every cell in the brain of mammals is either XX or XY. One of the most profound and robust epigenetic events in the life of a cell is the inactivation of one X chromosome in females. This has consequences for gene dosage (when inactivation is incomplete), parent of origin (males get their X only from their mothers) and heterochromatin (the extra X is a sink for epigenetic machinery). Evidence to-date reveals sex chromosome complement to be an important variable in adult physiology and behavior, but there has been no evidence for a role in the sexual differentiation of the brain (see for review [21]), although what that evidence would look like is unclear.
Returning to the question of epigenetics and establishment of brain sex differences, some interesting observations allow for speculation about generalities. This begins with observations regarding DNA. Methylation of cytosines proximal to guanines (CpG’s) is the canonical means of epigenetic silencing of gene expression. Frequently coalesced into islands located in gene regulatory regions, methylated CpG’s block access of the transcriptional apparatus both directly and in concert with recruitment of methylation-specific binding proteins and associated histone modifications. The promoters of genes considered central to sexual differentiation have been probed for CpG methylation and histone modifications and found to differ in males and females. These include Esr1 (estrogen receptor-alpha)[13,18,22], and Esr2 (estrogen receptor-beta)[23]. But the relationship between the amount of CpG methylation and measures of gene expression are often not correlated (see [23]). More surprisingly, in a case where the same promoter region was probed in animals at different developmental stages (i.e newborn, weanling and adult), the sex differences in the CpG methylation pattern shifted, disappearing from one CpG and appearing in others [23]. The changes were not random noise, being highly consistent across animals, but the functional significance and regulatory variables remain a mystery. A similar scenario was observed using a broader approach called Reduced Representation Bisulfite Sequencing (RRBS) in which all genomic regions enriched for CpG’s are probed. Here the authors explored the epigenome of newborn males and females and how that was impacted by testosterone treatment of females. Only a modest number of genes exhibited sex differences or hormonal modulation of CpG’s when newborns were compared, but a surprisingly large and different set of hormonally modified genes were identified in adult brains [24]. I have termed this an “epigenetic echo” in that some event initiated in the neonate returns in the adult but in a different form. What we are missing of course is a complete picture of all the potential epigenetic modifications in the neonate (chromatin, other portions of the genome), and any information about the intervening period from newborn to adulthood. This is one of the major challenges to understanding epigenetic modifications in the brain, our restriction to a snap shot in time, and, what hasn’t been discussed yet, our restriction to only one or a very few brain regions. Further complicating our understanding is the use of tissue homogenates which include all cell types so that we do not know if a particular epigenetic modification occurs in neurons, astrocytes, microglia, oligodendrocytes or some combination thereof. Technological advances will solve this particular problem very soon but may not be widely available to researchers for some time.
The most powerful approach for answering the question of whether sexual differentiation is epigenetic is the combination of identifying an epigenetic modification that differs in males versus females followed by pharmacological or genetic manipulation to establish a causal relationship. The aromatase enzyme converts androgens to estrogens and in rodents this is a requisite step for masculinization of reproductive physiology and behavior (see for review [25]). Histone acetylation of chromatin near promoters generally increases gene expression and is regulated by enzymes that either add or remove acetyl to lysine residues [26]. In a study of newborn rat pups, two forms of histone deacetylating enzymes were found associated with the Cyp19a promoter and this association was higher in neonatal males than females, consistent with higher aromatase expression [27]. Inhibition of the deacetylating enzymes in males during the perinatal critical period impaired the mating behavior of those animals in adulthood, strongly supporting the conclusion that during development, epigenetic modifications of the chromatin associated with the aromatase gene contributes to sex differences in expression and thereby estrogen production and masculinization [27]. But, females can easily be masculinized by treatment with exogenous testosterone, suggesting they have ample aromatase enzyme in the brain to convert androgens to estrogens. So, while the epigenetic changes to the aromatase promoter might be important, they cannot be construed as essential.
Returning to the methylome, we found the overall amount of CpG methylation to be higher in tissue homogenates from the POA of newborn females compared to males [19]. This was confirmed, with modifications, when the entire genome was assayed for CpG methylation. Importantly, the sex difference in methylation was reversed by treating females with a masculinizing dose of steroid during the critical period, thereby eliminating X-inactivation as the basis for higher methylation. Instead, the source of the higher methylation level on the DNA of females was traced to greater activity of the DNA methylation enzymes, the DNMT’s. The rates of enzyme activity were higher in females because activity was reduced in males by the higher levels of estrogens in their POA (mechanism unknown). If the level of CpG methylation in females was reduced pharmacologically by treatment with a DNMT inhibitor, those females exhibited a masculinized synaptic profile in the POA and displayed male-like mating behavior as adults when provided exogenous testosterone. Interestingly the same females showed no changes to their reproductive physiology, meaning the regulation of LH release from the pituitary remained feminized, nor did they lose the capacity to show nurturing behavior towards pups. Thus, the impact of the demethylation of the DNA was highly specific to masculinization of mating behavior. The same effect was seen in mice in which one specific form of the demethylating enzymes, DNMT3a, was genetically ablated in the POA as neonates. However, in this case the genetic deletion did not occur until outside the critical period due to the time delay created by injecting a Cre recombinase expressing virus at birth. This observation suggested that ongoing DNA methylation may be critical for closing the critical period and keeping it closed. This hypothesis was confirmed via pharmacological treatment to demethylate the DNA in older female rats, outside the critical period for sexual differentiation, and demonstration that their adult behavior was again masculinized. Females treated with a masculinizing dose of estradiol at the same time point, outside the critical period, were not masculinized indicating that the steroid-induced inhibition of DNMT activity was no longer in effect. This is the first, and potentially only, demonstration of a re-opening of the critical window for sexual differentiation and the strongest evidence to date that sexual differentiation of brain and behavior is maintained from early in development to adulthood vie epigenetic modifications. But there remain many unanswered questions, the most vexing of which is how such a highly specific effect is achieved by a seemingly broad pharmacological approach.
This brings us back to the question of the permanency of sexual differentiation and the important exception noted at the beginning of this discussion. How permanent the differentiation is depends on how you ask the question. As already discussed, if adult reproductively mature animals are treated with the hormonal milieu of the opposite sex, they do not take on the behavioral or physiological repertoire of that sex. However, if treated with high doses of long duration [28,29], what would be considered supra-physiological, or specific temporal patterns of hormone treatment [30], animals will begin to show opposite sex behaviors . Cells can’t come back from the dead, and rewiring circuits is a high bar to achieve, so what might explain this apparent semi-permanency of sexual differentiation? The key could lie in the ability of steroid hormones to modulate DNMT activity, as we demonstrated for the POA [19]. An emerging concept in neuroepigenetics is the need for continuous maintenance of epigenetic modifications, including methylation of CpG’s [31]. Inhibition of DNMT activity is documented to result in demethylation of the genome [32], but the process takes time as epigenetic modifications are meant to be enduring. It is possible that high dose long duration steroid exposure in the opposite sex changes DNMT activity in a way that over time allows for changes to CpG methylation in gene expression to be consistent with the sex normally exhibiting that hormonal profile. More specifically, it is possible that continuous high testosterone in adult females reduces DNMT activity in the POA over time, resulting in demethylation of the genes that normally repress male mating behavior. This hypothesis has not been tested, but intriguingly even the adult female ovary must continuously epigenetically repress the genetic program for testis development or it will begin to convert into a testis [33]. Moreover, an epigenetic mechanism of sexual differentiation would be adaptable to the many variants of the process in other species such as temperature dependency, socially-induced parthenogenesis and sex reversal or hermaphroditism. Reproduction is opportunistic, and it would seem that the best way to keep options open yet maintain reliability is to employ modifiable but durable epigenetic modifications.
Acknowledgements
This work was funded by RO1 MH52716 and R01DA039062 to MMM
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McCarthy MM, De Vries GJ, Forger NG: Sexual differentiation of the brain: A Fresh look at mode, mechanisms and meaning In Hormones, Brain and Behavior. . Edited by Pfaff DaJ M: Elsevier; 2017:3–32. vol 3.] [Google Scholar]
- 2.Yang T, Yang CF, Chizari MD, Maheswaranathan N, Burke KJ Jr., Borius M, Inoue S, Chiang MC, Bender KJ, Ganguli S, et al. : Social Control of Hypothalamus-Mediated Male Aggression. Neuron 2017, 95:955–970 e954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohl J, Babayan BM, Rubinstein ND, Autry AE, Marin-Rodriguez B, Kapoor V, Miyamishi K, Zweifel LS, Luo L, Uchida N, et al. : Functional circuit architecture underlying parental behaviour. Nature 2018, 556:326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker JB, Breedlove SM, Crews D, McCarthy MM: Behavioral Endocrinology edn 2nd. Cambridge, MA: MIT Press; 2002. [Google Scholar]
- 5.McCarthy MM, Herold K, Stockman SL: Fast, furious and enduring: Sensitive versus critical periods in sexual differentiation of the brain. Physiol Behav 2018, 187:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simerly RB: Wired for reproduction: Organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu. Rev. Neurosci. 2002, 25:507–536. [DOI] [PubMed] [Google Scholar]
- 7.Morris JA, Jordan CL, Breedlove SM: Sexual differentiation of the vertebrate nervous system. Nat. Neurosci. 2004, 7:1034–1039. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, Doncarlos LL, Sisk CL: Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pozzo-Miller LD, Aoki A: Stereological analysis of the hypothalamic ventromedial nucleus II. Hormone induced changes in the synaptogenic pattern. Developmental Brain Research 1991, 61:189–196. [DOI] [PubMed] [Google Scholar]
- 10.Amateau SK, McCarthy MM: Induction of PGE(2) by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci 2004, 7:643–650. [DOI] [PubMed] [Google Scholar]
- 11.Wright CL, Burks SR, McCarthy MM: Identification of prostaglandin E2 receptors mediating perinatal masculinization of adult sex behavior and neuroanatomical correlates. Dev Neurobiol. 2008, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spencer TE, Jenster G, Burcin MM, Allis CD, Zhou J, Mizzen CA, McKenna NJ, Onate SA, Tsai SY, Tsai MJ, et al. : Steroid receptor coactivator-1 is a histone acetyltransferase. Nature 1997, 389:194–198. [DOI] [PubMed] [Google Scholar]
- 13.Kurian JR, Olesen KM, Auger AP: Sex differences in epigenetic regulation of the estrogen receptor-alpha promoter within the developing preoptic area. Endocrinology 2010, 151:2297–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This manuscript was among the first to report a sex difference in CpG methylation in the promoter for the gene Esr1 – estrogen receptor alpha within the preoptic area of the brain, a region subject to sexual differentiation of male sex behavior.
- 14.Kolodkin MH, Auger AP: Sex difference in the expression of DNA methyltransferase 3a in the rat amygdala during development. J Neuroendocrinol 2011, 23:577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]; *The methylation of DNA is achieved via a class of enzymes called DNMTs. The authors examined a number of sexually dimorphic brain regions and found a sex difference in the amount of mRNA and protein for DNMT3a in the neonatal amygdala, being higher in females. They further demonstrated that treating females with a masculinizing dose of steroid reduced the amount of DNTM3a to that of males, suggesting a role for steroids in establishng epigenetic profiles.
- 15.Kigar SL, Chang L, Auger AP: Gadd45b is an epigenetic regulator of juvenile social behavior and alters local pro-inflammatory cytokine production in the rodent amygdala. Brain Behav Immun 2015, 46:60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]; *The Gadd45b enzyme is involved in the removal of methyl groups from CpG via the process of base-excision repair. Reducing expession of Gadd45b specifically in the amygdala of developing rats via siRNA impaired the later expression of juvenile social behaviors, many of which are expressed at different levels in males and females. This paper reveals that de-methylation may be as important a regulatory step as methylation.
- 16.Murray EK, Hien A, de Vries GJ, Forger NG: Epigenetic control of sexual differentiation of the bed nucleus of the stria terminalis. Endocrinology 2009, 150:4241–4247. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This was among the first reports of an impact of epigenetic modifying enzymes on sex differences in neuroanatomy during development. Treatment with the HDAC inhibitor, valproic acid, prevented the normal neuroprotective effect of steroids on cell survival in the BNST, thereby eliminating the sex difference by reducing the number of cells in male or masculnized females to that of control females.
- 17.Shen EY, Ahern TH, Cheung I, Straubhaar J, Dincer A, Houston I, de Vries GJ, Akbarian S, Forger NG: Epigenetics and sex differences in the brain: A genome-wide comparison of histone-3 lysine-4 trimethylation (H3K4me3) in male and female mice. Exp Neurol 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwarz JM, Nugent BM, McCarthy MM: Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span. Endocrinology 2010, 151:4871–4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, Russo SJ, Devine SE, McCarthy MM: Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci 2015, 18:690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This study was the first to marry epigenetic modifications and manipulations with a behavioral outcome. Focusing on the preoptic area and male sex behavior, the authors found females to have a higher level of overall methylation of CpG’s and when the was pharmacologically reduced early in development, masculinization of brain and behavior resulted but in the absence of steroid treatment.
- 20.Gagnidze K, Weil ZM, Pfaff DW: Histone modifications proposed to regulate sexual differentiation of brain and behavior. Bioessays 2010, 32:932–939. [DOI] [PubMed] [Google Scholar]
- 21.Arnold AP, Reue K, Eghbali M, Vilain E, Chen X, Ghahramani N, Itoh Y, Li J, Link JC, Ngun T, et al. : The importance of having two X chromosomes. Philos Trans R Soc Lond B Biol Sci 2016, 371:20150113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuda KI: Epigenetic changes in the estrogen receptor alpha gene promoter: implications in sociosexual behaviors. Front Neurosci 2014, 8:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nugent BM, Schwarz JM, McCarthy MM: Hormonally mediated epigenetic changes to steroid receptors in the developing brain: Implications for sexual differentiation. Horm Behav 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghahramani NM, Ngun TC, Chen PY, Tian Y, Krishnan S, Muir S, Rubbi L, Arnold AP, de Vries GJ, Forger NG, et al. : The effects of perinatal testosterone exposure on the DNA methylome of the mouse brain are late-emerging. Biol Sex Differ 2014, 5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Perhaps the only study to determine the impact on CpG methylation in the adult brain following neonatal hormonal treatment, the authors determined that the short-term impact in terms of number of genes in which the methylation pattern was modified was relatively few but that this increased by 20-fold in adulthood.
- 25.McCarthy MM: Estradiol and the developing brain. Physiol Rev 2008, 88:91–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strahl BD, Allis CD: The language of covalent histone modifications. Nature 2000, 403:41–45. [DOI] [PubMed] [Google Scholar]
- 27.Matsuda KI, Mori H, Nugent BM, Pfaff DW, McCarthy MM, Kawata M: Histone deacetylation during brain development is essential for permanent masculinization of sexual behavior. Endocrinology 2011, 152:2760–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sodersten P, de Jong FH, Vreeburg JT, Baum MJ: Lordosis behavior in intact male rats: absence of correlation with mounting behavior or testicular secretion of estradiol-17 beta and testosterone. Physiol Behav 1974, 13:803–808. [DOI] [PubMed] [Google Scholar]
- 29.Larsson K, Sodersten P, Beyer C, Morali G, Perez-Palacios G: Effects of estrone, estradiol and estriol combined with dihydrotestosterone on mounting and lordosis behavior in castrated male rats. Horm Behav 1976, 7:379–390. [DOI] [PubMed] [Google Scholar]
- 30.Olster DH, Blaustein JD: Progesterone facilitation of lordosis in male and female Sprague-Dawley rats following priming with estradiol pulses. Horm. Behav. 1989, 22:294–304. [DOI] [PubMed] [Google Scholar]
- 31.Sweatt JD: The emerging field of neuroepigenetics. Neuron 2013, 80:624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marquez VE, Kelley JA, Agbaria R, Ben-Kasus T, Cheng JC, Yoo CB, Jones PA: Zebularine: a unique molecule for an epigenetically based strategy in cancer chemotherapy. Ann N Y Acad Sci 2005, 1058:246–254. [DOI] [PubMed] [Google Scholar]
- 33.Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C, Holter NI, et al. : Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 2009, 139:1130–1142. [DOI] [PubMed] [Google Scholar]