Abstract
Craniosynostosis is the premature fusion of the calvarial sutures that is associated with a number of physical and intellectual disabilities spanning from pediatric to adult years. Over the past two decades, techniques in molecular genetics and more recently, advances in high-throughput DNA sequencing have been used to examine the underlying pathogenesis of this disease. To date, mutations in 57 genes have been identified as causing craniosynostosis and the number of newly discovered genes is growing rapidly as a result of the advances in genomic technologies. While contributions from both genetic and environmental factors in this disease are increasingly apparent, there remains a gap in knowledge that bridges the clinical characteristics and genetic markers of craniosynostosis with their signaling pathways and mechanotransduction processes. By linking genotype to phenotype, outlining the role of cell mechanics may further uncover the specific mechanotransduction pathways underlying craniosynostosis. Here, we present a brief overview of the recent findings in craniofacial genetics and cell mechanics, discussing how this information together with animal models is advancing our understanding of craniofacial development.
Keywords: Calvarial bone, Suture fusion, Molecular genetics, Development, Biomechanics
For Table of Contents Use Only
Introduction
The human calvaria consists of five major bones: the paired frontal and parietal bones and the occipital bone at birth. These bones develop through intramembranous ossification, where the radial growth of each bone from a central locus of osteogenesis, approximates with an unossified mesenchyme to form a suture. The unossified mesenchyme is presumed to serve two major functions: it allows for both temporary deformation of the skull during birth and expansion of the cranial vault during brain growth. In normal development, the metopic suture, located between the paired frontal bones fuses at three to nine months of age,1 while the other sutures fuse in the third decade of life.2 Prior to these events, the balance of sutural elasticity, calvarial osteogenesis, and brain growth maintains healthy calvarial development.
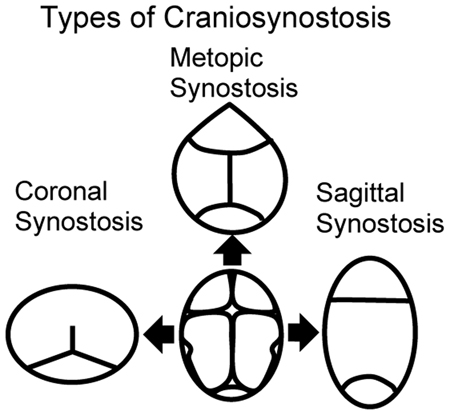
Excessive bone growth at the osteogenic fronts or untimely reduction in brain growth can result in premature suture fusion. The four common types of synostosis are: metopic, coronal, sagittal and lambdoid synostosis (Fig. 1). Craniosynostosis divides into syndromic and non-syndromic forms with syndromic forms defined as those with recognizable patterns of craniofacial and non-craniofacial malformations. A number of mutations are associated with syndromic craniosynostosis.3-6 Collectively, non-syndromic single-suture craniosynostosis (SSC) represents a common group of human malformations with a birth prevalence of 1 in 1,700-2,500 live births;7-8 whereas syndromic forms have a prevalence of approximately 1 in 25,000.9-11 Due both to its prevalence and the required medical and surgical management, craniosynostosis is one of the most clinically significant craniofacial disorders.
Figure 1.
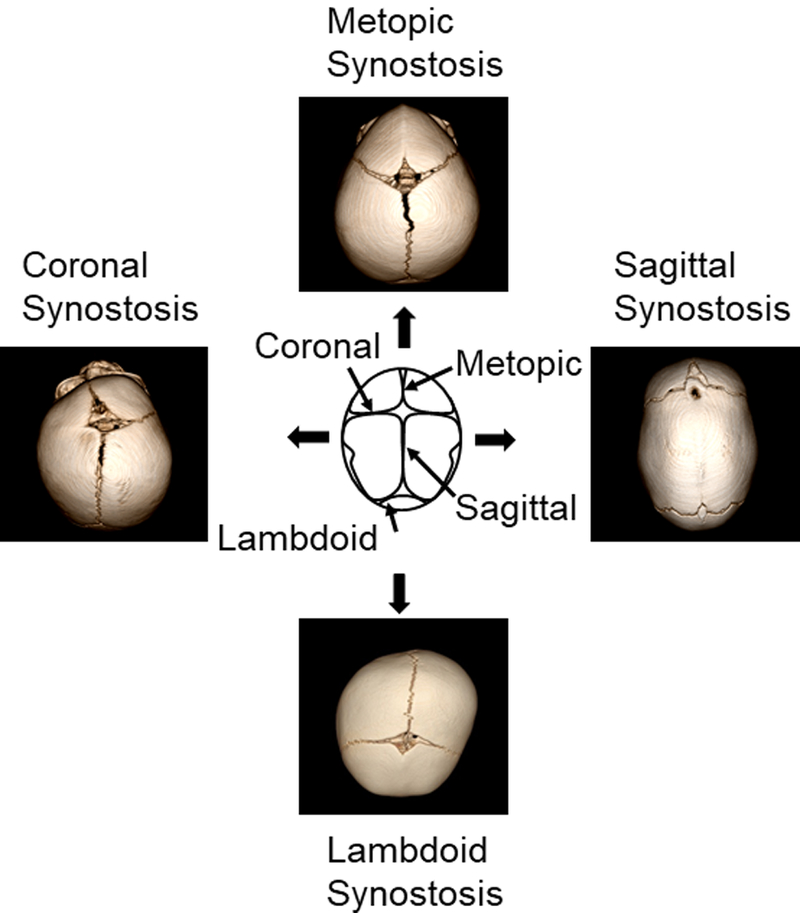
Types of craniosynostosis. Center: schematic representation of the top view of a normal cranium with all identified sutures (metopic, coronal, sagittal and lambdoid). To either side of the normal presentation of the skull, CT scans showing skull shapes with coronal (left) and sagittal (right) synostosis. Finally, metopic synostosis is shown at the top, while lambdoid synostosis is shown at the bottom.
Premature suture fusion results in abnormalities in skull shape, usually becoming apparent between the last trimester of pregnancy and the first few months of life. Early suture fusion reduces further growth of the adjoining bones, in a direction orthogonal to the suture. Consequently, the normal expansion of the brain promotes compensatory overgrowth at other sutures, leading to progressive distortion in the skull shape. These changes in head shape can be associated with increased intracranial pressure that when untreated, may result in permanent brain injury.12-13 In addition to these risks, craniosynostosis is also associated with alterations in craniofacial growth including mid-facial hypoplasia, abnormalities in dental alignment, orbital deformation, and other characteristics such as hearing loss or intellectual disability12-13. Generally, craniosynostosis is treated with cranioplasty in order to restore the normal shape of the head and relieve increased intracranial pressure. Due to its complexity, such procedures hold risk to significant morbidity.14-15 To date, craniosynostosis remains a significant medical and dental health issue where there are no pharmacological treatments, nor earlier interventions to prevent suture fusion.
More recently, it has become evident that abnormal suture fusion may be caused by an interaction of a number of factors. One of the least understood factors that may be involved in this process is the role of mechanical forces in expansion of the calvaria, brain growth and its effect in maintaining suture patency, which is the focus of this review. The second factor is the intrinsic property of the suture, which has been reviewed elsewhere.16 Finally, external forces acting on the calvaria, especially during fetal life, might also contribute to the onset of craniosynostosis, especially in non-syndromic cases of SSC.
Recent epidemiological evidence consistent with contributions from fetal head constraint showed positive associations of craniosynostosis with twin pregnancies, multiple pregnancies, and high birth weight.17 Previously, it was shown that compressive strain can increase osteogenesis at the suture.18 Furthermore, in vivo mouse models of head constraint have been shown to induce craniosynostosis.19 Recent work has demonstrated that the activity of an anabolic signaling factor as insulin growth factor 1 (IGF-1) affects human derived SSC osteoblast contractility and migration, providing valuable insight for phenotype-genotype correlation in SSC osteoblasts.20 It is evident therefore, that there exists a complex interplay between suture patency, genetics, signaling pathways, and mechanotransduction processes which may be related to the pathogenesis of craniosynostosis. The purpose of this review is to provide an overview of the underlying developmental biomechanics of suture formation, followed by a discussion of the recent molecular genetics of craniosynostosis, supporting a role of cell mechanics in this disease; and finally, a consideration of future ideas and directions.
Developmental biomechanics of suture formation
Calvarial bone formation and suture fusion
The human calvaria is formed through intramembranous ossification which occurs within a condensed region of mesenchymal stem cells. Its formation is in contrast to the formation of endochondral bone such as long bones and the skull base, which advance initially through a stage of chondrogenesis before proceeding to osteogenesis.21 The development of the human calvaria commences during the eighth week of gestation.9, 22-24 At the initial site of ossification (the ossification locus), mesenchymal osteoprogenitor cells differentiate into osteoblasts, secrete extracellular matrix (ECM) proteins, and initiate mineralization.9 Osteogenesis in the human calvaria requires the differentiation of mature osteoblasts from undifferentiated proliferating mesenchymal osteoprogenitor cells. Growth of the calvaria is radially outward from the locus of osteogenesis, eventually approximating the bones to form the suture (Fig. 2).23 The leading edges of these osteogenic fronts contain proliferative osteoprogenitor cells.25-26
Figure 2.
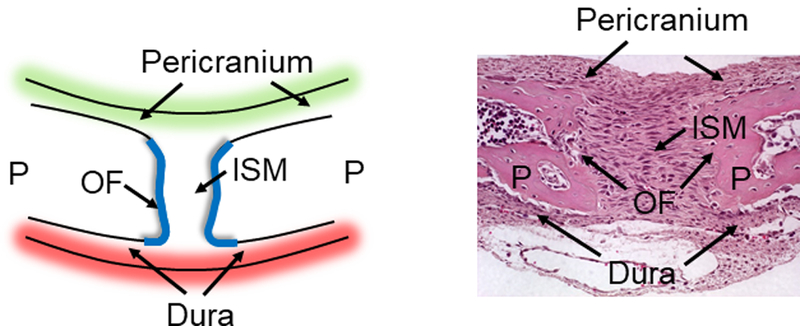
Schematic and histological presentation of the sagittal suture. (A) A schematic and (B) histological appearance of the sagittal suture showing the paired parietal bones (P) and the relative positions of the osteogenic fronts (OF), intrasutural mesenchyme (ISM), the pericranium and the dura mater. The leading edges of these osteogenic fronts contain proliferative osteoprogenitor cells and the sagittal suture is a composite structure that consists of the osteogenic fronts and the intrasutural mesenchyme.
Suture formation occurs through the progression of the two confronting osteogenic fronts. Therefore, a suture is a composite structure comprised of a region of unossified tissue between two calvaria bordered by osteogenic fronts and the overlying dura mater (the tough membrane that adheres to the inner surface of the cranial vault and separates it from the brain). Mature cranial sutures can withstand deformation in both tension and compression.27 Their primary function is to enable the growth of the skull in coordination with the rapid expansion of the calvaria during brain growth.28 Furthermore, the intracranial pressure of the brain growth produces tensile strains, which may either act directly on the suture or indirectly through mechanotransduction via the dura mater.18 In addition, sutures allow deformation of the skull during birth, absorb cyclic mechanical loading during mastication and locomotion, and act as shock absorbers against external forces.29
Although cranial sutures start off as simple lines of separation between developing bones, they become increasingly interdigitated with age.30 Mathematically, these meandering patterns have been previously described in terms of fractal geometry, with the fractal dimension increasing with age.30 Furthermore, there have been analytical attempts to account for this behavior by employing reaction-diffusion models which incorporate diffusible factors, positive and negative feedback loops, mechanical strain, and time-dependent processes.27, 30 Moreover, a recent study has suggested that the fractal nature of these meandering patterns may be due to the stochastic nature of craniosynostosis.31 Therefore, these theoretical findings demonstrate that suture growth is likely to incorporate the interplay of cellular signaling pathways that are responsive to mechanical strain.
The major calvarial sutures fuse at different times during normal development. In humans, the metopic suture (between the frontal bones) fuses at three to nine months of age1 while the others (coronal, sagittal and lambdoid) fuse in the third decade of life.2 Although some investigation into the molecular processes of suture fusion has been conducted in humans, much of our understanding is drawn from animal models. Immunohistochemical studies of the coronal sutures in rats (between the frontal and parietal bones) reveal high concentrations of alkaline phosphatase at the osteogenic fronts on fetal day 19 (F19) prior to apposition of their osteogenic fronts.32 At the time of apposition (F21), alkaline phosphatase activity decreases, demonstrating reduced bone formation, perhaps representing a mechanism serving to prevent synostosis. In contrast, in vitro studies of osteoblasts derived from prematurely fused human sutures demonstrate an increase in alkaline phosphatase production and osteocalcin expression, suggesting that osteogenic differentiation occurs in surplus of that present in normal sutures.33-34 These studies suggest that regulation of bone differentiation and matrix production plays an important role in suture patency.
Apoptosis (programmed cell death) has also been widely explored during suture fusion in rodents. Through histologic evaluation of fetal and newborn mice, apoptotic bodies have been observed at the osteogenic front during bone apposition.35-38 These findings suggest that the process of apoptosis may attenuate osteogenesis at the suture boundary, thereby preventing abnormal fusion. It appears therefore, that a harmonious balance of brain growth,2 inhibited mineralization of the intrasutural mesenchyme,39 growth of the calvarial bones at the osteogenic front,25-26 and programmed cell death36 maintains suture patency during skull growth. When the persistence of the unossified intrasutural mesenchyme of the calvaria is prematurely abolished or there exists an overgrowth of the osteogenic fronts, the neighboring calvaria begin to fuse, which then results into craniosynostosis. Craniosynostosis is therefore an etiologically heterogeneous condition with known genetic and presumed epigenetic causes.
Strain and suture patency
For over two decades, it has been suggested that in utero head constraint is associated with an increased incidence of premature calvarial suture fusion.2, 40-43 Previous studies have shown that early descent into the pelvis, primiparity and other forms of fetal constraint have been implicated as causing both metopic and sagittal synostosis.17, 40, 44-45 The proposed pathogenesis in these cases is that compression of the calvaria leads to reduced strain, at the osteogenic fronts and ultimately early suture fusion. These clinical examples are consistent with animal models of fetal constraint wherein cervical ligatures were used to prolong gestation resulting in craniosynostosis.46 In addition to in utero constraint, reduced brain growth resulting in severe microcephaly is well known to be associated with premature fusion of the calvaria.2, 43 Like in utero constraint, reduced brain growth has the effect of reducing quasi-static tensile strain across the calvarial sutures (Fig. 3). Similarly, treatment of hydrocephalus with ventriculoperitoneal shunting can lead to premature fusion of otherwise normal sutures.47 Shunting decompresses the enlarged brain resulting in a reduction of the tensile strain experienced by the suture microenvironment. Although the exact pathogenesis of synostosis in these examples remains unclear, they serve to illustrate a possible relationship between quasi-static tensile strain and homeostasis of the suture microenvironment. These observations suggest that inhibition of normal suture strain associated with brain growth can result in premature suture fusion. These clinical and experimental models are in apparent disagreement with well-established animal data, which suggest that even in the absence of normal suture strain, the dura mater has an intrinsic ability to maintain suture patency.48-50 Moreover, differential expression of transforming growth factors beta 1, beta 2, and beta 3 (TGF-β1,-β2, and -β3) and the type I TGF-β3 receptor in the suture microenvironment has been associated in the regulation of suture fusion through its control of proliferation and apoptosis.51-55 This apparent inconsistency emphasizes the importance of improving our understanding of the role of strain in suture patency and calvarial development.
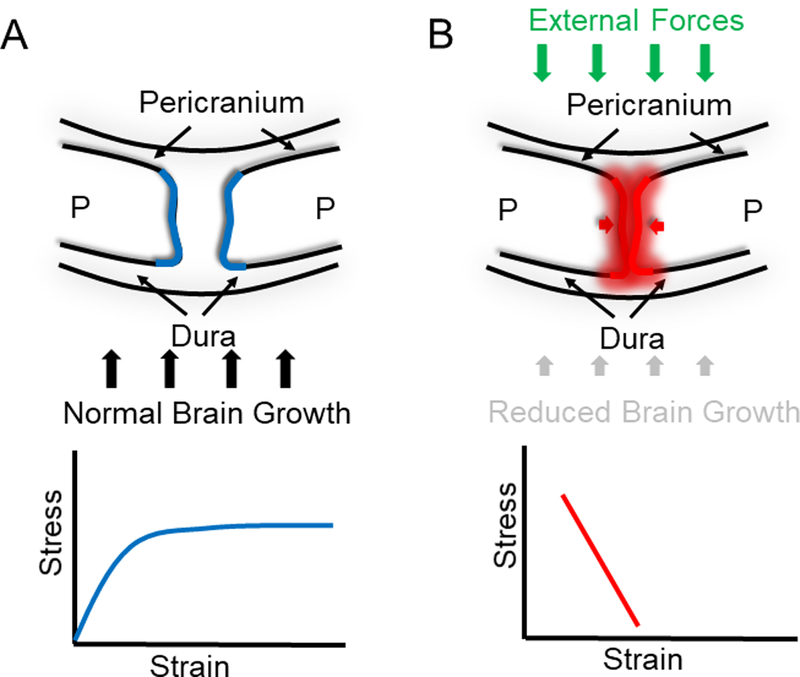
Figure 3.
Strains and suture patency. (A) Cross-sectional depiction of the sagittal suture depicting the paired parietal bones (P). The dura mater is the tough membrane that adheres to the inner surface of the cranial vault, which separates it from the brain. The pericranium is located apically. The growth of the cranial vault is regulated by a harmonious balance of proliferating and differentiating cells occurring within the suture (blue). This growth takes place in synchrony with an expanding brain (black arrows). Therefore, we can describe this behavior by plotting the effect of normal expanding brain and its effect on the suture as a stress-strain curve. (B) Conversely, in craniosynostosis, this balance is disturbed by external forces as in utero constraints during pregnancy (green arrows), poor brain expansion (vertical black arrows) and/or abnormal signal transduction within the suture (red shade). Generally, reduced brain growth has the effect of reducing quasi-static tensile strain across the calvarial suture as shown in the stress-strain graph.
Much of our knowledge of suture biology comes from studies of facial sutures, rather than cranial sutures. For example, both oscillatory and continuous strains on the facial sutures are known to stimulate suture growth.56 Tensile and compressive oscillatory strain of 1500 microstrain (με) have been demonstrated to increase suture growth, where enhanced expression of ECM and mass of both osteoblast and fibroblast cells were observed56-57 Fibroblast and osteoblast proliferation in response to mechanical strain is well recognized; however, there has been little work done in designing experimental models that mimic normal suture biology. As little as 500 με of oscillatory strain has been found to induce premaxillomaxillary suture osteogenesis.58 Therefore, the oscillatory strain experienced in facial sutures induces suture growth with both compressive and tensile strain having an anabolic effect on the suture microenvironment.56
Mechanical loading on sutures
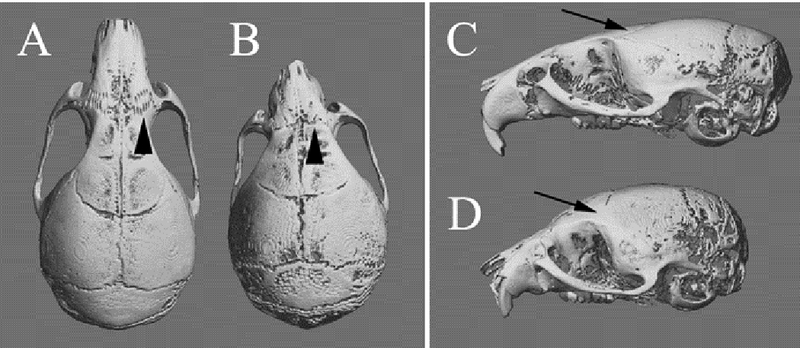
The earliest studies of cranial suture biology sought to relate the morphology of a suture to its mechanical microenvironment.59-61 More specifically, when sutures were transplanted into regions which did not experience mechanical loads, the new microenvironment was found to alter suture morphology.62 Furthermore, previous studies investigating the relationship between mechanical loadings as a result of mastication observed an upregulation of sutural bone growth.63 This study found that increased masticatory muscle mass and bite force would increase sagittal suture complexity in myostatin-knockout mice. Moreover, we have identified loss of nasofrontal suture complexity in the midface deficient FGFR2 mutant model of Apert syndrome, where loss of normal incisor occlusion occurs (unpublished data: Fig. 4).64 This suggests that the tissue surrounding the suture adapted to a particular mechanical loading regime achieved by differential bone growth at the suture63. In another study applying tensile testing on sagittal sutures from postnatal rats aged two to 60 days, increased sutural thickness and stiffness per length was also observed.65 Interestingly, these aforementioned properties were found to be age dependent, suggesting that during development, the rat sagittal suture changes significantly after exposure to in vivo quasi-static tensile strain due to intracranial pressure.65
Figure 4.
MicroCT images were obtained of the nasofrontal suture of both control and Apert mice carrying the Fgfr2(S252W/+) mutation. (A) The control mouse is showing the interdigitated suture (black arrow). (B) The Apert mouse does not have normal occlusion or maxillary and mandibular incisors; therefore, the nasofrontal suture is not strained and loses normal interdigitation (black arrow).
The craniofacial skeleton comprises intramembranous bones which exhibit growth following calvarial expansion66 and mastication.67 Change in masticatory forces has been shown to induce craniosynostosis, wherein osteopetrotic mice displayed premature fusion of the sagittal suture,68 while rats on much softer diets exhibited internasal synostosis.69 Expansion of the brain changes cranial growth, where absence of brain tensile forces observed in fetuses with microcephaly developed craniosynostosis.70-72 Conversely, the presence of external pathological forces can also have a major impact on skull development. For example, primiparity,17 multiple births,73 low pelvic station,41 and late-term pregnancies74 have all been associated with the development of SSC.
Modeling sutures and cranial growth
Novel developments in computer modeling are frequently employed when conducting detailed investigations into the effect of cranial vault loading. Analysis incorporating techniques in finite element analysis (FEA) and multibody dynamics analysis (MDA) have in the past, been used to examine such phenomena as musculoskeletal force generation, translations of bone plates and subsequent stress/strain distributions within the cranial vault. These techniques have previously been used to mechanically model biological systems, where understanding the force distribution of mastication in humans and other primates is under scrutiny.75-77 It is essential however, that when developing a suitable computational analogue, the specific material properties unique to the structure must be incorporated in the model to accurately predict the mechanical properties of the environment. This is more challenging in consideration of materials such as bone which are by their nature considered an anisotropic material where their properties vary not only between individuals but also throughout each specimen. Previously, one group performed a sensitivity study into the effects of using isotropic and anisotropic material properties in a Macaca fascicularis cranium.75 The group observed that while more detailed models were more accurate when compared to their experimental strain counterparts, investigations defined by solely isotropic material produced comparable results. Congruently, one other study also validated these conclusions,77 where they showed positive correlation to experimental data using isotropic material properties. These findings therefore indicate that investigations into complex three-dimensional structures applying isotropic materials yield highly successful results.78-80
FEA is generally used when addressing questions concerning the impact of patent sutures on skull stresses/strains. To produce accurate measurements of strains experimentally, strain gauges are fixed to the surfaces of bones.81-83 Generally, localized strains at these fixed locations are easily obtainable. However, to infer global strain measure over the entire cranial vault or the patent suture is a more challenging task. Therefore, FEA can be used to predict the stress/strain distributions for the entire structure.84-86 A previous study assessing local and global strains carried out on a lizard skull revealed. 87 The first was strain modification was found to be greater in global patent sutures when compared to fused sutures. The second being that strain found to decrease in some areas of the skull was seen to increase in others.87 In contrast, another study however suggested that patent sutures had little effect on skull strains in primates;88 but appeared more important in animals with more patent sutures or a greater suture to bone volume as reptiles.87, 89 These studies when combined with experimental data provide important information describing suture form and function.
In order to gain a wider insight in the impact of patent and fused sutures on load transfer within the cranial vault, more comprehensive analyses are needed. One way of doing this is to combine both MDA and FEA. These two techniques used in the reptile Sphenodon to predict separate biting loading regimen subsequently analyzed the structural performance of the skull under such regimens.90 Subjecting the skull to many different loading regimens is important because cranial vault deformation varies greatly depending on the loading position and magnitude.90 These findings demonstrated for the first time that patent sutures may in fact help in reducing the number of cranial areas with low-level strain throughout the reptile skull, leading to a more consistent method in predicting strain levels during mastication90 Such findings are of clinical relevance due to their implications in respect to the remodeling and growth of bone in both juvenile and adult skulls, ensuring the normal trajectory of bone development.
Molecular Genetics of Craniosynostosis
Our understanding of the genetic components of human craniosynostosis are modest at best. Presently, there are 57 genes known to be causally related to craniosynostosis, which have been reviewed in great detail elsewhere.16 Herein, this review will briefly describe the genetic pathophysiology linked to some of the more common forms of craniosynostosis. In humans, syndromic synostosis (hereditary) is caused by mutations in the genes for fibroblast growth factor receptors (FGFR) and twist-related protein 1 (TWIST1). The following syndromes - Apert, Crouzon, Pfeiffer, and Jackson Weiss - are all due to specific gain of function mutations of FGFR2 in either the second inter-loop domain (Apert) or third immunoglobulin-like domain (Crouzon, Pfeiffer, Jackson Weiss).91-96 This is similar to the gain of function mutations in the second inter-loop domains of FGFR1 and FGFR3, which result in Pfeiffer and “Muenke Type” craniosynostosis, respectively.97-100 Other less common mutations of FGFR2 and FGFR3 have been associated with syndromic craniosynostosis.101-102 A single point mutation in MSX2 is believed to increase transcriptional activity, resulting in “Boston-type” craniosynostosis.103 To date, the only other transcription factor found to be associated with craniosynostosis is the basic helix-loop-helix (bHLH) protein TWIST1. Several loss of function mutations in DNA binding and loop domains of TWIST1 have been found to be responsible for Saethre-Chotzen syndrome.104-109
Saethre-Chotzen syndrome
Saethre-Chotzen syndrome (SCS, acrocephalo-syndactyly type III) is one of the more common forms of syndromic craniosynostosis.109-110 Patients with SCS typically present premature fusion of one or more sutures of the calvaria, brachycephaly, facial asymmetry, a low frontal hairline, ptosis, maxillary hypoplasia, and small ears with a prominent superior crus.111 Although any sutures in the calvaria can undergo premature fusion in SCS, coronal sutures is the most common. Associated limb anomalies may include brachydactyly or cutaneous syndactyly of the second and third digits of the upper extremities. As SCS is an autosomal dominant trait, it is accepted that the SCS phenotype is caused by a functional haploinsufficiency of TWIST1.112 This is further supported by animal models such as the heterozygous TWIST1 mutant mouse (TWIST1tm1Bhr) that reveals premature coronal suture fusion mimicking that of the human SCS phenotype.105, 113-114
TWIST1+/−mutant and haploinsufficiency
High-throughput sequence analysis has identified many intragenic TWIST1 mutations in patients with SCS.109, 112 Nonsense mutations inhibiting translation of the DNA and the HLH domains have been identified from the 5’ end of the coding sequence to the end of the HLH motif. Though missense mutations cluster within the functional domains, specific mutational loci have yet to be identified. In recent studies, the functional effects of TWIST1 mutations have also been examined. In these studies, nonsense mutations were found to increase the synthesis of truncated proteins that rapidly degraded, leading to functional haploinsufficiency.115-116 Missense mutations involving helical domains were found in contrast, to result in a loss of heterodimer formation, which subsequently altered nuclear translocation.115-116 Moreover, in-frame insertion or missense mutations within the loop domain were found to alter dimer formation while these mutations in the basic domain altered DNA binding. Taken together, these findings suggest that both protein degradation and altered subcellular localization, may in part, account for the loss of functional TWIST1 protein (functional haploinsufficiency) in SCS patients.
TWIST1+/− mutant and cell specifications
TWIST1 and other bHLH transcription factors play an important role in specifying and maintaining cell identity.117-118 TWIST1 was initially characterized in Drosophila as being necessary during gastrulation in the establishment of the mesodermal germ layer and embryos with TWIST1 mutations failing to develop mesoderm.119 In Drosophila, TWIST1 expression persists at high levels in the mesoderm until its differentiation into the somatopleura and splanchnopleura when its expression diminishes.120 During mouse development, TWIST1 is expressed in the neural crest cells that populate the cephalic region and branchial arches, which differentiate into connective tissue, muscle, cartilage, and bone.121 Migratory populations of cephalic neural crest cells are the origin of the membranous bones of the skull and its intervening sutures, overlying dermis, and underlying dura mater,122-125 which infers a crucial role in early calvarial development. TWIST1 has also been shown previously to inhibit differentiation of multiple cell lineages, including muscle126-129 and bone.130-131 Taken together, these findings propose that TWIST1 may function to maintain cells in a less differentiated state during craniofacial development. In support of this hypothesis, recent studies suggests that TWIST1 is indeed necessary for normal osteocalcin expression in human osteoblasts,132 perhaps acting through a RUNX2 dependent pathway.133 While the precise function of osteocalcin still remains unclear, its secretion by osteoblasts during differentiation suggests a likely role in matrix mineralization. Furthermore, an additional investigation into the role of TWIST1 in osteoblast biology observed its binding to the promoter of periostin (OSF2) by which upregulating its expression.134 As a secreted ligand of α5β3 and α5β5 integrins, periostin is therefore believed to play a role in cellular adhesion. Together, these recent discoveries suggest that TWIST1 might serve to regulate both matrix mineralization and cellular adhesion. While information from disease-specific mutations and their genetic/biochemical characteristics provide a clear benefit to understanding craniosynostosis, it is when we view genetics in relation to cell mechanics (i.e. signaling pathways and mechanotransduction processes) that we gain a more detailed understanding of the external and internal factors influencing this disease.
Role of Cell Mechanics
Mechanical properties of sutures
Characterizing the mechanical properties (elastic modulus) of bone is an important step in the understanding of craniofacial development. By invoking tools in tensile testing or three-point bending, the elastic modulus of calvarial bones and sutures in normal skulls has been able to be quantified.135-138 More recently however, nanoindentation has been used as an alternative method in examining tissue samples less than 0.1 mm in size, making it an ideal method for measuring the properties of cranial bone, and even sutures, in rodents and other small animals.139 When using a Crouzon mouse model (Fgfr2C342Y/+), a difference in the elastic modulus of the frontal bones between wild type and Fgfr2C342Y/+mutant mice was observed at the early stages of post-natal development.139 In contrast however, this study also demonstrated that the elastic modulus of the parietal bones and their sutures were comparatively more similar between these two groups.139 It is therefore likely that such variations in the mechanical properties of the calvaria may result from different patterns of strain as a consequence of suture fusion.
Traction forces of sutures
In vivo, both mechanical forces and properties of the ECM influence cellular physiology. The translation of physical information into a cellular response is now believed to be a critical component in many biological pathways.140-142 Extracellular nanoscale forces have been shown to influence numerous signaling pathways both in vivo and in vitro.143-147 Such nanoscale forces can arise through stretch or compression of the microenvironment, fluid shear stress or localized forces occurring at focal adhesion sites, which have been shown to result in cytoskeletal remodeling, changes in cellular orientation and alignment, alterations in gene regulation, and the determination of cell fate.143-147 Understanding the role of mechanotransduction is therefore quintessential in expanding our understanding of how physical forces are generated and transmitted through living cells.
Mechanical forces imposed on osteoblasts are a well-known inductor of osteogenic markers. In particular, cyclic strain has been shown to induce the production of these osteogenic markers, including osteocalcin, osteopontin, alkaline phosphatase, and type I collagen.148 Osteoblasts differentiated from mesenchymal stems cells, have been shown to increase in response to mechanical factors like cell shape;149-150 substrate stiffness,151 and applied strain.152 Moreover, when a strain regimen was applied in vivo to the tibia of transgenic mice selectively overexpressing IGF-1, a five-fold increase in bone formation as compared to wild-type mice was observed.153 This suggests therefore that traction forces are an essential factor for the mechanotransduction of cell shape, substrate stiffness, and applied strain shape.140, 154-156
The causative factors leading to craniosynostosis is of great interest due to relatively high frequency of SSC when compared to other birth defects, and its far-reaching clinical burden. Previously, the family of TGF-β1, -β2, and -β3 were found to play an important role in suture morphogenesis by regulating and maintaining suture patency and calvarial bone growth.55 Furthermore, cyclical loading on murine calvaria was also found to induce suture fusion and show upregulation of alkaline phosphatase, a non-specific bone marker of osteoblastic activity.157 Recently, IGF-1 expression has been correlated to SSC osteoblast contractility and migration, where increased expression levels led to larger traction forces and reduced migrations speeds in diseased osteoblasts.20 Furthermore, in our previous study we identified a number of genes (FGFR3, TGFBR1, TGFB3, WNT3, WNT5B, WNT16, CTBP2, DTX4, DVL2 and ITGB1) whose expression was correlated with contractility and/or migration in SSC osteoblasts, all of which have been previously implicated in bone development.158-161 These findings suggest that there exists interplay between the IGF-1 pathway and the regulation of the aforementioned genes, which may act in an integrative manner leading to the development of SSC.
Migration of osteoblasts derived from sutures
Previous studies have implicated IGF-1 signaling in mediating focal adhesion formation and cell migration.162-163 Indeed recent transcriptomic studies reveal an upregulation in IGF-1 expression in calvarial osteoblasts derived from patients with SSC, which was accompanied by a further positive correlation to an increase of ECM-mediated focal adhesion proteins.164 This anabolic signaling factor appears to promote the association of the IGF-1 receptor to focal adhesion proteins, leading to increased cellular migration and invasion.162 In our previous study, we found that not only did IGF-1 expression correlate to cell contractility, but also to cell migration.20 Furthermore, a number of factors that have been found to influence skeletal development have been correlated to migration in osteoblasts derived from SSC patients.20 Previous work has identified RUNX2 as an osteogenic marker that induces osteoblast and chondrocyte differentiation by enhancing their migration through coupling with PI3K-Akt signaling.165 Furthermore, Akt signaling is activated by IGF-1 through the PI3K pathway and therefore, IGF-1 plays an important role in RUNX2-dependent osteoblastic differentiation in MC3T3-E1 cells. 165
Healthy patterned growth of the calvarium is dependent on a tightly regulated program of cell proliferation, differentiation, and migration. Investigating the contributions of these processes is crucial in understanding how the calvarial pattern is established in cranial growth and how developmental pathologies like craniosynostosis arise. Osteoblast migration has previously been demonstrated to be an important factor in the patterned growth of calvarial bones, where its impairment was found to lead to craniosynostosis in TWIST1 and EphA4 mutant mice.166 These findings were consistent with previous work,167 supporting the notion that cell migration is a significant morphogenetic force in the patterned growth of the skull vault. Therefore, it appears that the migration of osteoprogenitor cells from osteogenic front may contribute to the apical expansion of calvarial bones.
More precise techniques in identifying the progenitor cell populations which comprise the suture, as well as understanding the mechanotransduction processes that guide their migration and differentiation, will help further advance our understating of the mechanisms that underlie the patterned growth of the skull as well as the pathophysiology of craniosynostosis.
Future Ideas and Directions
One of the most exciting areas of craniofacial research is investigating the integrative role of mechanical forces, signal transduction, and gene regulation in the onset of craniosynostosis. By employing mutant mouse models, we can identify candidate genes affected as result of changes in mechanical strain mimicking that of an expanding brain. Furthermore, developing an in vitro model that allows us to study the transduction of mechanical signal into biochemical changes will advance our understanding of the role of strain induced by brain growth and other mechanical forces (mastication or pulsatile blood flow) in normal calvarial development and suture development. Given the importance of environmental factors in craniosynostosis, including frequent asymmetry in suture fusion, the contribution of genetic and epigenetic influences are all crucial areas of interest that should be explored further in hope to yield diagnostic treatments on a case-by-case basis.
While the precise mechanisms preceding craniosynostosis are complicated and unclear at present, current advances in the field suggest it is a bridge between suture biology and cell mechanics which may affect the normal onset of suture fusion. Further investigations which raise disease-specific cell mechanics to their genetic counterparts are therefore necessary in order to provide deeper insights into the mechanisms regulating the development of craniosynostosis and other developmental disorders.
Acknowledgments
Funding Sources
This work was supported by the National Institutes of Health Grants NIH/NIDCR R01DE018227 (MLC) and the Jean Renny Endowment for Craniofacial Research (MLC).
References
- 1.Vu HL; Panchal J; Parker EE; Levine NS; Francel P, The timing of physiologic closure of the metopic suture: a review of 159 patients using reconstructed 3D CT scans of the craniofacial region. J. Craniofac. Surg. 2001, 12 (6), 527–32. [DOI] [PubMed] [Google Scholar]
- 2.Cohen MM Jr., Sutural biology and the correlates of craniosynostosis. Am. J. Med. Genet. 1993, 47 (5), 581–616. [DOI] [PubMed] [Google Scholar]
- 3.Fitzpatrick DR, Filling in the gaps in cranial suture biology. Nat. Genet. 2013, 45 (3), 231–2. DOI: 10.1038/ng.2557. [DOI] [PubMed] [Google Scholar]
- 4.Sharma VP; Fenwick AL; Brockop MS; McGowan SJ; Goos JA; Hoogeboom AJ; Brady AF; Jeelani NO; Lynch SA; Mulliken JB; Murray DJ; Phipps JM; Sweeney E; Tomkins SE; Wilson LC; Bennett S; Cornall RJ; Broxholme J; Kanapin A; Johnson D; Wall SA; van der Spek PJ; Mathijssen IM; Maxson RE; Twigg SR; Wilkie AO, Mutations in TCF12, encoding a basic helix-loop-helix partner of TWIST1, are a frequent cause of coronal craniosynostosis. Nat. Genet. 2013, 45 (3), 304–7. DOI: 10.1038/ng.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Twigg SR; Vorgia E; McGowan SJ; Peraki I; Fenwick AL; Sharma VP; Allegra M; Zaragkoulias A; Sadighi Akha E; Knight SJ; Lord H; Lester T; Izatt L; Lampe AK; Mohammed SN; Stewart FJ; Verloes A; Wilson LC; Healy C; Sharpe PT; Hammond P; Hughes J; Taylor S; Johnson D; Wall SA; Mavrothalassitis G; Wilkie AO, Reduced dosage of ERF causes complex craniosynostosis in humans and mice and links ERK1/2 signaling to regulation of osteogenesis. Nat. Genet. 2013, 45 (3), 308–13. DOI: 10.1038/ng.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Twigg SR; Forecki J; Goos JA; Richardson IC; Hoogeboom AJ; van den Ouweland AM; Swagemakers SM; Lequin MH; Van Antwerp D; McGowan SJ; Westbury I; Miller KA; Wall SA; van der Spek PJ; Mathijssen IM; Pauws E; Merzdorf CS; Wilkie AO, Gain-of-Function Mutations in ZIC1 Are Associated with Coronal Craniosynostosis and Learning Disability. Am. J. Hum. Genet. 2015, 97 (3), 378–88. DOI: 10.1016/j.ajhg.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shuper A; Merlob P; Grunebaum M; Reisner SH, The incidence of isolated craniosynostosis in the newborn infant. Am. J. Dis. Child 1985, 139 (1), 85–6. [DOI] [PubMed] [Google Scholar]
- 8.French LR; Jackson IT; Melton LJ 3rd, A population-based study of craniosynostosis. J Clin. Epidemiol. 1990, 43 (1), 69–73. [DOI] [PubMed] [Google Scholar]
- 9.Cohen MM; MacLean RE, Craniosynostosis : diagnosis, evaluation, and management. 2nd ed; Oxford University Press: New York, 2000; p xx, 454. [Google Scholar]
- 10.Cohen MM Jr., Craniosynostosis and syndromes with craniosynostosis: incidence, genetics, penetrance, variability, and new syndrome updating. Birth Defects Orig. Artic. Ser. 1979, 15 (5B), 13–63. [PubMed] [Google Scholar]
- 11.Meyer JL, Apert’s syndrome: (acrocephalosyndactylism). J. Foot Surg. 1981, 20 (4), 210–13. [PubMed] [Google Scholar]
- 12.Thompson DN; Malcolm GP; Jones BM; Harkness WJ; Hayward RD, Intracranial pressure in single-suture craniosynostosis. Pediatr. Neurosurg. 1995, 22 (5), 235–40. [DOI] [PubMed] [Google Scholar]
- 13.Thompson DN; Harkness W; Jones B; Gonsalez S; Andar U; Hayward R, Subdural intracranial pressure monitoring in craniosynostosis: its role in surgical management. Childs Nerv. Syst. 1995, 11 (5), 269–75. [DOI] [PubMed] [Google Scholar]
- 14.Lee HQ; Hutson JM; Wray AC; Lo PA; Chong DK; Holmes AD; Greensmith AL, Analysis of morbidity and mortality in surgical management of craniosynostosis. J. Craniofac. Surg. 2012, 23 (5), 1256–61. DOI: 10.1097/SCS.0b013e31824e26d6. [DOI] [PubMed] [Google Scholar]
- 15.Han RH; Nguyen DC; Bruck BS; Skolnick GB; Yarbrough CK; Naidoo SD; Patel KB; Kane AA; Woo AS; Smyth MD, Characterization of complications associated with open and endoscopic craniosynostosis surgery at a single institution. J. Neurosurg. Pediatr. 2016, 17 (3), 361–70. DOI: 10.3171/2015.7.PEDS15187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Twigg SR; Wilkie AO, A Genetic-Pathophysiological Framework for Craniosynostosis. Am. J. Hum. Genet. 2015, 97 (3), 359–77. DOI: 10.1016/j.ajhg.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez-Lara PA; Carmichael SL; Graham JM Jr.; Lammer EJ; Shaw GM; Ma C; Rasmussen SA, Fetal constraint as a potential risk factor for craniosynostosis. Am. J. Med. Genet. A 2010, 152A (2), 394–400. DOI: 10.1002/ajmg.a.33246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herring SW, Mechanical influences on suture development and patency. Front. Oral Biol. 2008, 12, 41–56. DOI: 10.1159/0000115031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacob S; Wu C; Freeman TA; Koyama E; Kirschner RE, Expression of Indian Hedgehog, BMP-4 and Noggin in craniosynostosis induced by fetal constraint. Ann. Plast. Surg. 2007, 58 (2), 215–21. DOI: 10.1097/01.sap.0000232833.41739.a5. [DOI] [PubMed] [Google Scholar]
- 20.Al-Rekabi Z; Wheeler MM; Leonard A; Fura AM; Juhlin I; Frazar C; Smith JD; Park SS; Gustafson JA; Clarke CM; Cunningham ML; Sniadecki NJ, Activation of the IGF1 pathway mediates changes in cellular contractility and motility in single-suture craniosynostosis. J. Cell Sci. 2016, 129 (3), 483–91. DOI: 10.1242/jcs.175976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shapiro F, Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur. Cell. Mater. 2008, 15, 53–76. [DOI] [PubMed] [Google Scholar]
- 22.Sperber GH, Craniofacial development. B C Decker: London, 2001; p vi, 220. [Google Scholar]
- 23.Markens IS, Embryonic development of the coronal suture in man and rat. Acta. Anat. (Basel) 1975, 93 (2), 257–73. [DOI] [PubMed] [Google Scholar]
- 24.O’Rahilly R; Gardner E, The initial appearance of ossification in staged human embryos. Am. J. Anat. 1972, 134 (3), 291–301. [DOI] [PubMed] [Google Scholar]
- 25.Decker JD; Hall SH, Light and electron microscopy of the new born sagittal suture. Anat. Rec. 1985, 212 (1), 81–9. [DOI] [PubMed] [Google Scholar]
- 26.Johansen VA; Hall SH, Morphogenesis of the mouse coronal suture. Acta. Anat. (Basel) 1982, 114 (1), 58–67. [DOI] [PubMed] [Google Scholar]
- 27.Khonsari RH; Olivier J; Vigneaux P; Sanchez S; Tafforeau P; Ahlberg PE; Di Rocco F; Bresch D; Corre P; Ohazama A; Sharpe PT; Calvez V, A mathematical model for mechanotransduction at the early steps of suture formation. Proc. Biol. Sci. 2013, 280 (1759), 20122670 DOI: 10.1098/rspb.2012.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nieman BJ; Blank MC; Roman BB; Henkelman RM; Millen KJ, If the skull fits: magnetic resonance imaging and microcomputed tomography for combined analysis of brain and skull phenotypes in the mouse. Physiol. Genomics 2012, 44 (20), 992–1002. DOI: 10.1152/physiolgenomics.00093.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rice DP, Developmental anatomy of craniofacial sutures. Front. Oral Biol. 2008, 12, 1–21. DOI: 10.1159/0000115028. [DOI] [PubMed] [Google Scholar]
- 30.Miura T; Perlyn CA; Kinboshi M; Ogihara N; Kobayashi-Miura M; Morriss-Kay GM; Shiota K, Mechanism of skull suture maintenance and interdigitation. J. Anat. 2009, 215 (6), 642–55. DOI: 10.1111/j.1469-7580.2009.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshimura K; Kobayashi R; Ohmura T; Kajimoto Y; Miura T, A new mathematical model for pattern formation by cranial sutures. J. Theor. Biol. 2016, 408, 66–74. DOI: 10.1016/j.jtbi.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Markens IS; Oudhof HA, The presence of alkaline phosphatase in the coronal suture of rat. Acta. Anat. (Basel) 1978, 102 (3), 319–23. [DOI] [PubMed] [Google Scholar]
- 33.De Pollack C; Renier D; Hott M; Marie PJ, Increased bone formation and osteoblastic cell phenotype in premature cranial suture ossification (craniosynostosis). J. Bone Miner. Res. 1996, 11 (3), 401–7. [DOI] [PubMed] [Google Scholar]
- 34.Lomri A; Lemonnier J; Hott M; de Parseval N; Lajeunie E; Munnich A; Renier D; Marie PJ, Increased calvaria cell differentiation and bone matrix formation induced by fibroblast growth factor receptor 2 mutations in Apert syndrome. J. Clin. Invest. 1998, 101 (6), 1310–7. [PMC free article] [PubMed] [Google Scholar]
- 35.Ten Cate AR; Freeman E; Dickinson JB, Sutural development: structure and its response to rapid expansion. Am. J. Orthod. 1977, 71 (6), 622–36. [DOI] [PubMed] [Google Scholar]
- 36.Furtwangler JA; Hall SH; Koskinen-Moffett LK, Sutural morphogenesis in the mouse calvaria: the role of apoptosis. Acta. Anat. (Basel) 1985, 124 (1–2), 74–80. [DOI] [PubMed] [Google Scholar]
- 37.Rice DP; Kim HJ; Thesleff I, Apoptosis in murine calvarial bone and suture development. Eur. J. Oral Sci. 1999, 107 (4), 265–75. [DOI] [PubMed] [Google Scholar]
- 38.Bourez RL; Mathijssen IM; Vaandrager JM; Vermeij-Keers C, Apoptotic cell death during normal embryogenesis of the coronal suture: early detection of apoptosis in mice using annexin V. J. Craniofac. Surg. 1997, 8 (6), 441–5. [DOI] [PubMed] [Google Scholar]
- 39.Opperman LA, Cranial sutures as intramembranous bone growth sites. Dev. Dyn. 2000, 219 (4), 472–85. [DOI] [PubMed] [Google Scholar]
- 40.Graham JM Jr.; Smith DW, Metopic craniostenosis as a consequence of fetal head constraint: two interesting experiments of nature. Pediatrics 1980, 65 (5), 1000–2. [PubMed] [Google Scholar]
- 41.Graham JM Jr.; Badura RJ; Smith DW, Coronal craniostenosis: fetal head constraint as one possible cause. Pediatrics 1980, 65 (5), 995–9. [PubMed] [Google Scholar]
- 42.Higginbottom MC; Jones KL; James HE, Intrauterine constraint and craniosynostosis. Neurosurgery 1980, 6 (1), 39–44. [DOI] [PubMed] [Google Scholar]
- 43.Cohen MM Jr., Etiopathogenesis of craniosynostosis. Neurosurg. Clin. N. Am. 1991, 2 (3), 507–13. [PubMed] [Google Scholar]
- 44.Koskinen-Moffett LK; Moffett BC Jr.; Graham JM Jr., Cranial synostosis and intra-uterine compression: a developmental study of human sutures. Prog. Clin. Biol. Res. 1982, 101, 365–78. [PubMed] [Google Scholar]
- 45.Graham JM Jr.; deSaxe M; Smith DW, Sagittal craniostenosis: fetal head constraint as one possible cause. J. Pediatr. 1979, 95 (5 Pt 1), 747–50. [DOI] [PubMed] [Google Scholar]
- 46.Koskinen-Moffett L, Moffet BC, Sutures and intrauterine deformation In Scientific Foundations and Surgical Treatment of Craniosynostosis Ed. By Pershing JA, Edgerton MT and Jane JA, Williams and Wilkins: Baltimore MD, pp 96–106, 1989. [Google Scholar]
- 47.Albright AL; Tyler-Kabara E, Slit-ventricle syndrome secondary to shunt-induced suture ossification. Neurosurgery 2001, 48 (4), 764–9; discussion 769–70. [DOI] [PubMed] [Google Scholar]
- 48.Opperman LA; Passarelli RW; Morgan EP; Reintjes M; Ogle RC, Cranial sutures require tissue interactions with dura mater to resist osseous obliteration in vitro. J. Bone Miner. Res. 1995, 10 (12), 1978–87. [DOI] [PubMed] [Google Scholar]
- 49.Opperman LA; Chhabra A; Nolen AA; Bao Y; Ogle RC, Dura mater maintains rat cranial sutures in vitro by regulating suture cell proliferation and collagen production. J. Craniofac. Genet. Dev. Biol. 1998, 18 (3), 150–8. [PubMed] [Google Scholar]
- 50.Kim HJ; Rice DP; Kettunen PJ; Thesleff I, FGF-, BMP- and Shh-mediated signalling pathways in the regulation of cranial suture morphogenesis and calvarial bone development. Development 1998, 125 (7), 1241–51. [DOI] [PubMed] [Google Scholar]
- 51.Opperman LA; Adab K; Gakunga PT, Transforming growth factor-beta 2 and TGF-beta 3 regulate fetal rat cranial suture morphogenesis by regulating rates of cell proliferation and apoptosis. Dev. Dyn. 2000, 219 (2), 237–47. [DOI] [PubMed] [Google Scholar]
- 52.Opperman LA; Chhabra A; Cho RW; Ogle RC, Cranial suture obliteration is induced by removal of transforming growth factor (TGF)-beta 3 activity and prevented by removal of TGF-beta 2 activity from fetal rat calvaria in vitro. J. Craniofac. Genet. Dev. Biol. 1999, 19 (3), 164–73. [PubMed] [Google Scholar]
- 53.Opperman LA; Galanis V; Williams AR; Adab K, Transforming growth factor-beta3 (Tgf-beta3) down-regulates Tgf-beta3 receptor type I (Tbetar-I) during rescue of cranial sutures from osseous obliteration. Orthod. Craniofac. Res. 2002, 5 (1), 5–16. [DOI] [PubMed] [Google Scholar]
- 54.Opperman LA; Moursi AM; Sayne JR; Wintergerst AM, Transforming growth factor-beta 3(Tgf-beta3) in a collagen gel delays fusion of the rat posterior interfrontal suture in vivo. Anat. Rec. 2002, 267 (2), 120–30. [DOI] [PubMed] [Google Scholar]
- 55.Opperman LA; Nolen AA; Ogle RC, TGF-beta 1, TGF-beta 2, and TGF-beta 3 exhibit distinct patterns of expression during cranial suture formation and obliteration in vivo and in vitro. J. Bone Miner. Res. 1997, 12 (3), 301–10. DOI: 10.1359/jbmr.1997.12.3.301. [DOI] [PubMed] [Google Scholar]
- 56.Kopher RA; Mao JJ, Suture growth modulated by the oscillatory component of micromechanical strain. J. Bone Miner. Res. 2003, 18 (3), 521–8. [DOI] [PubMed] [Google Scholar]
- 57.Kopher RA; Nudera JA; Wang X; O’Grady K; Mao JJ, Expression of in vivo mechanical strain upon different wave forms of exogenous forces in rabbit craniofacial sutures. Ann. Biomed. Eng. 2003, 31 (9), 1125–31. [DOI] [PubMed] [Google Scholar]
- 58.Mao JJ; Wang X; Mooney MP; Kopher RA; Nudera JA, Strain induced osteogenesis of the craniofacial suture upon controlled delivery of low-frequency cyclic forces. Front. Biosci. 2003, 8, a10–7. [DOI] [PubMed] [Google Scholar]
- 59.Moss ML, Growth of the calvaria in the rat; the determination of osseous morphology. Am. J. Anat. 1954, 94 (3), 333–61. DOI: 10.1002/aja.1000940302. [DOI] [PubMed] [Google Scholar]
- 60.Moss ML, Experimental alteration of sutural area morphology. Anat. Rec. 1957, 127 (3), 569–89. [DOI] [PubMed] [Google Scholar]
- 61.Moss ML, Extrinsic determination of sutural area morphology in the rat calvaria. Acta. Anat. (Basel) 1961, 44, 263–72. [DOI] [PubMed] [Google Scholar]
- 62.Moss ML; Applebaum E, Differential growth analysis of vertebrate teeth. J. Dental Res. 1957, 36 (4), 644–51. [DOI] [PubMed] [Google Scholar]
- 63.Byron CD; Borke J; Yu J; Pashley D; Wingard CJ; Hamrick M, Effects of increased muscle mass on mouse sagittal suture morphology and mechanics. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2004, 279 (1), 676–84. DOI: 10.1002/ar.a.20055. [DOI] [PubMed] [Google Scholar]
- 64.Purushothaman R; Cox TC; Maga AM; Cunningham ML, Facial suture synostosis of newborn Fgfr1(P250R/+) and Fgfr2(S252W/+) mouse models of Pfeiffer and Apert syndromes. Birth Defects Res. A Clin. Mol. Teratol. 2011, 91 (7), 603–9. DOI: 10.1002/bdra.20811. [DOI] [PubMed] [Google Scholar]
- 65.Henderson JH; Chang LY; Song HM; Longaker MT; Carter DR, Age-dependent properties and quasi-static strain in the rat sagittal suture. J. Biomech. 2005, 38 (11), 2294–301. DOI: 10.1016/j.jbiomech.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 66.Huggare J; Ronning, Growth of the cranial vault: influence of intracranial and extracranial pressures. Acta Odontol. Scand. 1995, 53 (3), 192–5. [DOI] [PubMed] [Google Scholar]
- 67.Sun Z; Lee E; Herring SW, Cranial sutures and bones: growth and fusion in relation to masticatory strain. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2004, 276 (2), 150–61. DOI: 10.1002/ar.a.20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kawata T; Tokimasa C; Fujita T; Kawasoko S; Kaku M; Sugiyama H; Tanne K, Midpalatal suture of osteopetrotic (op/op) mice exhibits immature fusion. Exp. Anim. 1998, 47 (4), 277–81. [DOI] [PubMed] [Google Scholar]
- 69.Kiliaridis S, Masticatory muscle function and craniofacial morphology. An experimental study in the growing rat fed a soft diet. Sed. Dent. J. Suppl. 1986, 36, 1–55. [PubMed] [Google Scholar]
- 70.Davies BR; Duran M, Malformations of the cranium, vertebral column, and related central nervous system: morphologic heterogeneity may indicate biological diversity. Birth Defects Res. A Clin. Mol. Teratol. 2003, 67 (8), 563–71. DOI: 10.1002/bdra.10080. [DOI] [PubMed] [Google Scholar]
- 71.Chervenak FA; Jeanty P; Cantraine F; Chitkara U; Venus I; Berkowitz RL; Hobbins JC, The diagnosis of fetal microcephaly. Am. J. Obstet. Gynecol. 1984, 149 (5), 512–7. [DOI] [PubMed] [Google Scholar]
- 72.Moss ML; Young RW, A functional approach to craniology. Am. J. Phys. Anthropol. 1960, 18, 281–92. [DOI] [PubMed] [Google Scholar]
- 73.van Aalst JA; Schultz G; Eppley BL, Craniosynostosis anomalies in twins. J. Craniofac. Surg. 2005, 16 (4), 696–9. [DOI] [PubMed] [Google Scholar]
- 74.Hunenko O; Karmacharya J; Ong G; Kirschner RE, Toward an understanding of nonsyndromic craniosynostosis: altered patterns of TGF-beta receptor and FGF receptor expression induced by intrauterine head constraint. Ann. Plast. Surg. 2001, 46 (5), 546–53; discussion 553–4. [DOI] [PubMed] [Google Scholar]
- 75.Strait DS; Wang Q; Dechow PC; Ross CF; Richmond BG; Spencer MA; Patel BA, Modeling elastic properties in finite-element analysis: how much precision is needed to produce an accurate model? Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2005, 283 (2), 275–87. DOI: 10.1002/ar.a.20172. [DOI] [PubMed] [Google Scholar]
- 76.Ichim I; Swain M; Kieser JA, Mandibular biomechanics and development of the human chin. J. Dental Res. 2006, 85 (7), 638–42. [DOI] [PubMed] [Google Scholar]
- 77.Kupczik K; Dobson CA; Fagan MJ; Crompton RH; Oxnard CE; O’Higgins P, Assessing mechanical function of the zygomatic region in macaques: validation and sensitivity testing of finite element models. J. Anat. 2007, 210 (1), 41–53. DOI: 10.1111/j.1469-7580.2006.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rayfield EJ; Norman DB; Horner CC; Horner JR; Smith PM; Thomason JJ; Upchurch P, Cranial design and function in a large theropod dinosaur. Nature 2001, 409 (6823), 1033–7. DOI: 10.1038/35059070. [DOI] [PubMed] [Google Scholar]
- 79.Cattaneo PM; Dalstra M; Melsen B, The transfer of occlusal forces through the maxillary molars: a finite element study. Am. J. Orthod. Dentofacial Orthop. 2003, 123 (4), 367–73. DOI: 10.1067/mod.2003.73. [DOI] [PubMed] [Google Scholar]
- 80.Cruz M; Wassall T; Toledo EM; Barra LP; Lemonge AC, Three-dimensional finite element stress analysis of a cuneiform-geometry implant. Int. J. Oral Maxillofac. Implants 2003, 18 (5), 675–84. [PubMed] [Google Scholar]
- 81.Herring SW; Mucci RJ, In vivo strain in cranial sutures: the zygomatic arch. J. Morphol. 1991, 207 (3), 225–39. DOI: 10.1002/jmor.1052070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Popowics TE; Herring SW, Load transmission in the nasofrontal suture of the pig, Sus scrofa. J. Biomech. 2007, 40 (4), 837–44. DOI: 10.1016/j.jbiomech.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ross CF; Berthaume MA; Dechow PC; Iriarte-Diaz J; Porro LB; Richmond BG; Spencer M; Strait D, In vivo bone strain and finite-element modeling of the craniofacial haft in catarrhine primates. J. Anat. 2011, 218 (1), 112–41. DOI: 10.1111/j.1469-7580.2010.01322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Curtis N; Kupczik K; O’Higgins P; Moazen M; Fagan M, Predicting skull loading: applying multibody dynamics analysis to a macaque skull. Anat. Rec. (Hoboken) 2008, 291 (5), 491–501. DOI: 10.1002/ar.20689. [DOI] [PubMed] [Google Scholar]
- 85.Curtis N; Witzel U; Fitton L; O’Higgins P; Fagan M, The mechanical significance of the temporal fasciae in Macaca fascicularis: an investigation using finite element analysis. Anat. Rec. (Hoboken) 2011, 294 (7), 1178–90. DOI: 10.1002/ar.21415. [DOI] [PubMed] [Google Scholar]
- 86.Moazen M; Curtis N; O’Higgins P; Evans SE; Fagan MJ, Biomechanical assessment of evolutionary changes in the lepidosaurian skull. Proc. Natl. Acad. Sci. U. S. A. 2009, 106 (20), 8273–7. DOI: 10.1073/pnas.0813156106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moazen M; Curtis N; O’Higgins P; Jones ME; Evans SE; Fagan MJ, Assessment of the role of sutures in a lizard skull: a computer modelling study. Proc. Biol. Sci. 2009, 276 (1654), 39–46. DOI: 10.1098/rspb.2008.0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Q; Smith AL; Strait DS; Wright BW; Richmond BG; Grosse IR; Byron CD; Zapata U, The global impact of sutures assessed in a finite element model of a macaque cranium. Anat. Rec. (Hoboken) 2010, 293 (9), 1477–91. DOI: 10.1002/ar.21203. [DOI] [PubMed] [Google Scholar]
- 89.Metzger KA; Daniel WJ; Ross CF, Comparison of beam theory and finite-element analysis with in vivo bone strain data from the alligator cranium. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2005, 283 (2), 331–48. DOI: 10.1002/ar.a.20167. [DOI] [PubMed] [Google Scholar]
- 90.Curtis N; Jones ME; Evans SE; O’Higgins P; Fagan MJ, Cranial sutures work collectively to distribute strain throughout the reptile skull. J. R. Soc. Interface 2013, 10 (86), 20130442 DOI: 10.1098/rsif.2013.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reardon W; Winter RM; Rutland P; Pulleyn LJ; Jones BM; Malcolm S, Mutations in the fibroblast growth factor receptor 2 gene cause Crouzon syndrome. Nat. Genet. 1994, 8 (1), 98–103. [DOI] [PubMed] [Google Scholar]
- 92.Wilkie AO; Slaney SF; Oldridge M; Poole MD; Ashworth GJ; Hockley AD; Hayward RD; David DJ; Pulleyn LJ; Rutland P; et al. , Apert syndrome results from localized mutations of FGFR2 and is allelic with Crouzon syndrome. Nat. Genet. 1995, 9 (2), 165–72. [DOI] [PubMed] [Google Scholar]
- 93.Oldridge M; Lunt PW; Zackai EH; McDonald-McGinn DM; Muenke M; Moloney DM; Twigg SR; Heath JK; Howard TD; Hoganson G; Gagnon DM; Jabs EW; Wilkie AO, Genotype-phenotype correlation for nucleotide substitutions in the IgII-IgIII linker of FGFR2. Hum. Mol. Genet. 1997, 6 (1), 137–43. [DOI] [PubMed] [Google Scholar]
- 94.Hollway GE; Suthers GK; Haan EA; Thompson E; David DJ; Gecz J; Mulley JC, Mutation detection in FGFR2 craniosynostosis syndromes. Hum. Genet. 1997, 99 (2), 251–5. [DOI] [PubMed] [Google Scholar]
- 95.Yu K; Herr AB; Waksman G; Ornitz DM, Loss of fibroblast growth factor receptor 2 ligand-binding specificity in Apert syndrome. Proc. Natl. Acad. Sci. U. S. A. 2000, 97 (26), 14536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ibrahimi OA; Eliseenkova AV; Plotnikov AN; Yu K; Ornitz DM; Mohammadi M, Structural basis for fibroblast growth factor receptor 2 activation in Apert syndrome. Proc. Natl. Acad. Sci. U. S. A. 2001, 98 (13), 7182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reardon W; Wilkes D; Rutland P; Pulleyn LJ; Malcolm S; Dean JC; Evans RD; Jones BM; Hayward R; Hall CM; Nevin NC; Baraister M; Winter RM, Craniosynostosis associated with FGFR3 pro250arg mutation results in a range of clinical presentations including unisutural sporadic craniosynostosis. J. Med. Genet. 1997, 34 (8), 632–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Golla A; Lichmer P; von Gernet S; Winterpacht A; Fairley J; Murken J; Schuffenhauer S, Phenotypic expression of the fibroblast growth factor receptor 3 (FGFR3) mutation P250R in a large craniosynostosis family. J. Med. Genet. 1997, 34 (8), 683–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Muenke M; Gripp KW; McDonald-McGinn DM; Gaudenz K; Whitaker LA; Bartlett SP; Markowitz RI; Robin NH; Nwokoro N; Mulvihill JJ; Losken HW; Mulliken JB; Guttmacher AE; Wilroy RS; Clarke LA; Hollway G; Ades LC; Haan EA; Mulley JC; Cohen MM Jr.; Bellus GA; Francomano CA; Moloney DM; Wall SA; Wilkie AO; et al. , A unique point mutation in the fibroblast growth factor receptor 3 gene (FGFR3) defines a new craniosynostosis syndrome. Am. J. Hum. Genet. 1997, 60 (3), 555–64. [PMC free article] [PubMed] [Google Scholar]
- 100.Muenke M; Schell U; Hehr A; Robin NH; Losken HW; Schinzel A; Pulleyn LJ; Rutland P; Reardon W; Malcolm S; et al. , A common mutation in the fibroblast growth factor receptor 1 gene in Pfeiffer syndrome. Nat. Genet. 1994, 8 (3), 269–74. [DOI] [PubMed] [Google Scholar]
- 101.Oldridge M; Zackai EH; McDonald-McGinn DM; Iseki S; Morriss-Kay GM; Twigg SR; Johnson D; Wall SA; Jiang W; Theda C; Jabs EW; Wilkie AO, De novo alu-element insertions in FGFR2 identify a distinct pathological basis for Apert syndrome. Am. J. Hum. Genet. 1999, 64 (2), 446–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meyers GA; Orlow SJ; Munro IR; Przylepa KA; Jabs EW, Fibroblast growth factor receptor 3 (FGFR3) transmembrane mutation in Crouzon syndrome with acanthosis nigricans. Nat. Genet. 1995, 11 (4), 462–4. [DOI] [PubMed] [Google Scholar]
- 103.Ma L; Golden S; Wu L; Maxson R, The molecular basis of Boston-type craniosynostosis: the Pro148-->His mutation in the N-terminal arm of the MSX2 homeodomain stabilizes DNA binding without altering nucleotide sequence preferences. Hum. Mol. Genet. 1996, 5 (12), 1915–20. [DOI] [PubMed] [Google Scholar]
- 104.Howard TD; Paznekas WA; Green ED; Chiang LC; Ma N; Ortiz de Luna RI; Garcia Delgado C; Gonzalez-Ramos M; Kline AD; Jabs EW, Mutations in TWIST, a basic helix-loop-helix transcription factor, in Saethre-Chotzen syndrome. Nat. Genet. 1997, 15 (1), 36–41. [DOI] [PubMed] [Google Scholar]
- 105.el Ghouzzi V; Le Merrer M; Perrin-Schmitt F; Lajeunie E; Benit P; Renier D; Bourgeois P; Bolcato-Bellemin AL; Munnich A; Bonaventure J, Mutations of the TWIST gene in the Saethre-Chotzen syndrome. Nat. Genet. 1997, 15 (1), 42–6. [DOI] [PubMed] [Google Scholar]
- 106.Paznekas WA; Cunningham ML; Howard TD; Korf BR; Lipson MH; Grix AW; Feingold M; Goldberg R; Borochowitz Z; Aleck K; Mulliken J; Yin M; Jabs EW, Genetic heterogeneity of Saethre-Chotzen syndrome, due to TWIST and FGFR mutations. Am. J. Hum. Genet. 1998, 62 (6), 1370–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Seto ML; Lee SJ; Sze RW; Cunningham ML, Another TWIST on Baller-Gerold syndrome. Am. J. Med. Genet. 2001, 104 (4), 323–30. [DOI] [PubMed] [Google Scholar]
- 108.Lee S; Seto M; Sie K; Cunningham M, A child with Saethre-Chotzen syndrome, sensorineural hearing loss, and a TWIST mutation. Cleft Palate Craniofac. J. 2002, 39 (1), 110–4. [DOI] [PubMed] [Google Scholar]
- 109.Cunningham Ratisoontorn, (Updated 30 October, 2003). Saethre-Chotzen Syndrome In: GeneReviews at GeneTests: Medical Genetics Information Resource (database online). Copyright, University of Washington, Seattle: 1997–2003. Available at http://www.genetests.org.2003. [Google Scholar]
- 110.Cai J; Shoo BA; Sorauf T; Jabs EW, A novel mutation in the TWIST gene, implicated in Saethre-Chotzen syndrome, is found in the original case of Robinow-Sorauf syndrome. Clin. Genet. 2003, 64 (1), 79–82. [DOI] [PubMed] [Google Scholar]
- 111.Gallagher ER; Ratisoontorn C; Cunningham ML, Saethre-Chotzen Syndrome In GeneReviews(R), Pagon RA; Adam MP; Ardinger HH; Wallace SE; Amemiya A; Bean LJH; Bird TD; Fong CT; Mefford HC; Smith RJH; Stephens K, Eds. Seattle (WA), 1993. [PubMed] [Google Scholar]
- 112.Gripp KW; Zackai EH; Stolle CA, Mutations in the human TWIST gene. Hum. Mutat. 2000, 15 (2), 150–5. [DOI] [PubMed] [Google Scholar]
- 113.Bourgeois P; Bolcato-Bellemin AL; Danse JM; Bloch-Zupan A; Yoshiba K; Stoetzel C; Perrin-Schmitt F, The variable expressivity and incomplete penetrance of the twist-null heterozygous mouse phenotype resemble those of human Saethre-Chotzen syndrome. Hum. Mol. Genet. 1998, 7 (6), 945–57. [DOI] [PubMed] [Google Scholar]
- 114.Carver EA; Oram KF; Gridley T, Craniosynostosis in Twist heterozygous mice: a model for Saethre-Chotzen syndrome. Anat. Rec. 2002, 268 (2), 90–2. [DOI] [PubMed] [Google Scholar]
- 115.El Ghouzzi V; Legeai-Mallet L; Benoist-Lasselin C; Lajeunie E; Renier D; Munnich A; Bonaventure J, Mutations in the basic domain and the loop-helix II junction of TWIST abolish DNA binding in Saethre-Chotzen syndrome. FEBS Lett. 2001, 492 (1–2), 112–8. [DOI] [PubMed] [Google Scholar]
- 116.El Ghouzzi V; Legeai-Mallet L; Aresta S; Benoist C; Munnich A; de Gunzburg J; Bonaventure J, Saethre-Chotzen mutations cause TWIST protein degradation or impaired nuclear location. Hum. Mol. Genet. 2000, 9 (5), 813–9. [DOI] [PubMed] [Google Scholar]
- 117.Jan YN; Jan LY, HLH proteins, fly neurogenesis, and vertebrate myogenesis. Cell 1993, 75 (5), 827–30. [DOI] [PubMed] [Google Scholar]
- 118.Olson EN; Klein WH, bHLH factors in muscle development: dead lines and commitments, what to leave in and what to leave out. Genes Dev. 1994, 8 (1), 1–8. [DOI] [PubMed] [Google Scholar]
- 119.Thisse B; el Messal M; Perrin-Schmitt F, The twist gene: isolation of a Drosophila zygotic gene necessary for the establishment of dorsoventral pattern. Nucleic Acids Res. 1987, 15 (8), 3439–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thisse B; Stoetzel C; Gorostiza-Thisse C; Perrin-Schmitt F, Sequence of the twist gene and nuclear localization of its protein in endomesodermal cells of early Drosophila embryos. EMBO J. 1988, 7 (7), 2175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wolf C; Thisse C; Stoetzel C; Thisse B; Gerlinger P; Perrin-Schmitt F, The M-twist gene of Mus is expressed in subsets of mesodermal cells and is closely related to the Xenopus X-twi and the Drosophila twist genes. Dev. Biol. 1991, 143 (2), 363–73. [DOI] [PubMed] [Google Scholar]
- 122.Morriss-Kay GM, Derivation of the mammalian skull vault. J. Anat. 2001, 199 (Pt 1–2), 143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jiang X; Iseki S; Maxson RE; Sucov HM; Morriss-Kay GM, Tissue origins and interactions in the mammalian skull vault. Dev. Biol. 2002, 241 (1), 106–16. [DOI] [PubMed] [Google Scholar]
- 124.Couly GF; Coltey PM; Le Douarin NM, The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development 1993, 117 (2), 409–29. [DOI] [PubMed] [Google Scholar]
- 125.Couly GF; Coltey PM; Le Douarin NM, The developmental fate of the cephalic mesoderm in quail-chick chimeras. Development 1992, 114 (1), 1–15. [DOI] [PubMed] [Google Scholar]
- 126.Hebrok M; Fuchtbauer A; Fuchtbauer EM, Repression of muscle-specific gene activation by the murine Twist protein. Exp. Cell Res. 1997, 232 (2), 295–303. [DOI] [PubMed] [Google Scholar]
- 127.Rohwedel J; Horak V; Hebrok M; Fuchtbauer EM; Wobus AM, M-twist expression inhibits mouse embryonic stem cell-derived myogenic differentiation in vitro. Exp. Cell Res. 1995, 220 (1), 92–100. [DOI] [PubMed] [Google Scholar]
- 128.Hebrok M; Wertz K; Fuchtbauer EM, M-twist is an inhibitor of muscle differentiation. Dev. Biol. 1994, 165 (2), 537–44. [DOI] [PubMed] [Google Scholar]
- 129.Spicer DB; Rhee J; Cheung WL; Lassar AB, Inhibition of myogenic bHLH and MEF2 transcription factors by the bHLH protein Twist. Science 1996, 272 (5267), 1476–80. [DOI] [PubMed] [Google Scholar]
- 130.Murray SS; Glackin CA; Winters KA; Gazit D; Kahn AJ; Murray EJ, Expression of helix-loop-helix regulatory genes during differentiation of mouse osteoblastic cells. J. Bone Miner. Res. 1992, 7 (10), 1131–8. [DOI] [PubMed] [Google Scholar]
- 131.Lee MS; Lowe GN; Strong DD; Wergedal JE; Glackin CA, TWIST, a basic helix-loop-helix transcription factor, can regulate the human osteogenic lineage. J. Cell. Biochem. 1999, 75 (4), 566–77. [DOI] [PubMed] [Google Scholar]
- 132.Yousfi M; Lasmoles F; Lomri A; Delannoy P; Marie PJ, Increased bone formation and decreased osteocalcin expression induced by reduced Twist dosage in Saethre-Chotzen syndrome. J. Clin. Invest. 2001, 107 (9), 1153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yousfi M; Lasmoles F; Marie PJ, TWIST inactivation reduces CBFA1/RUNX2 expression and DNA binding to the osteocalcin promoter in osteoblasts. Biochem. Biophys. Res. Commun. 2002, 297 (3), 641–4. [DOI] [PubMed] [Google Scholar]
- 134.Oshima A; Tanabe H; Yan T; Lowe GN; Glackin CA; Kudo A, A novel mechanism for the regulation of osteoblast differentiation: transcription of periostin, a member of the fasciclin I family, is regulated by the bHLH transcription factor, twist. J. Cell. Biochem. 2002, 86 (4), 792–804. [DOI] [PubMed] [Google Scholar]
- 135.McElhaney JH; Fogle JL; Melvin JW; Haynes RR; Roberts VL; Alem NM, Mechanical properties on cranial bone. J. Biomech. 1970, 3 (5), 495–511. [DOI] [PubMed] [Google Scholar]
- 136.Jaslow CR, Mechanical properties of cranial sutures. J. Biomech. 1990, 23 (4), 313–21. [DOI] [PubMed] [Google Scholar]
- 137.McLaughlin E; Zhang Y; Pashley D; Borke J; Yu J, The load-displacement characteristics of neonatal rat cranial sutures. Cleft Palate Craniofac. J. 2000, 37 (6), 590–5. DOI: . [DOI] [PubMed] [Google Scholar]
- 138.Wang J; Zou D; Li Z; Huang P; Li D; Shao Y; Wang H; Chen Y, Mechanical properties of cranial bones and sutures in 1–2-year-old infants. Med. Sci. Monit. 2014, 20, 1808–13. DOI: 10.12659/MSM.892278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Moazen M; Peskett E; Babbs C; Pauws E; Fagan MJ, Mechanical properties of calvarial bones in a mouse model for craniosynostosis. PLOS One 2015, 10 (5), e0125757 DOI: 10.1371/journal.pone.0125757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Buxboim A; Ivanovska IL; Discher DE, Matrix elasticity, cytoskeletal forces and physics of the nucleus: how deeply do cells ‘feel’ outside and in? J. Cell Sci. 2010, 123 (Pt 3), 297–308. DOI: 10.1242/jcs.041186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jaalouk DE; Lammerding J, Mechanotransduction gone awry. Nat. Rev. Mol. Cell Biol. 2009, 10 (1), 63–73. DOI: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Janmey PA; Miller RT, Mechanisms of mechanical signaling in development and disease. J. Cell Sci. 2011, 124 (Pt 1), 9–18. DOI: 10.1242/jcs.071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sniadecki NJ; Anguelouch A; Yang MT; Lamb CM; Liu Z; Kirschner SB; Liu Y; Reich DH; Chen CS, Magnetic microposts as an approach to apply forces to living cells. Proc. Natl. Acad. Sci. U. S. A. 2007, 104 (37), 14553–14558. DOI: 10.1073/pnas.0611613104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Krishnan R; Park CY; Lin YC; Mead J; Jaspers RT; Trepat X; Lenormand G; Tambe D; Smolensky AV; Knoll AH; Butler JP; Fredberg JJ, Reinforcement versus fluidization in cytoskeletal mechanoresponsiveness. PLOS One 2009, 4 (5), e5486 DOI: 10.1371/journal.pone.0005486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chowdhury F; Na S; Li D; Poh YC; Tanaka TS; Wang F; Wang N, Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat. Mater. 2010, 9 (1), 82–8. DOI: 10.1038/nmat2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nagayama K; Adachi A; Matsumoto T, Heterogeneous response of traction force at focal adhesions of vascular smooth muscle cells subjected to macroscopic stretch on a micropillar substrate. J. Biomech. 2011, 44 (15), 2699–705. DOI: 10.1016/j.jbiomech.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 147.Yamamoto K; Sokabe T; Watabe T; Miyazono K; Yamashita JK; Obi S; Ohura N; Matsushita A; Kamiya A; Ando J, Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am. J. Physiol. Heart Circ. Physiol. 2005, 288 (4), H1915–24. DOI: 10.1152/ajpheart.00956.2004. [DOI] [PubMed] [Google Scholar]
- 148.Ignatius A; Blessing H; Liedert A; Schmidt C; Neidlinger-Wilke C; Kaspar D; Friemert B; Claes L, Tissue engineering of bone: effects of mechanical strain on osteoblastic cells in type I collagen matrices. Biomaterials 2005, 26 (3), 311–8. DOI: 10.1016/j.biomaterials.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 149.McBeath R; Pirone DM; Nelson CM; Bhadriraju K; Chen CS, Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 2004, 6 (4), 483–95. [DOI] [PubMed] [Google Scholar]
- 150.Kilian KA; Bugarija B; Lahn BT; Mrksich M, Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. U. S. A. 2010, 107 (11), 4872–7. DOI: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Engler AJ; Sen S; Sweeney HL; Discher DE, Matrix elasticity directs stem cell lineage specification. Cell 2006, 126 (4), 677–89. DOI: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 152.Rath B; Nam J; Knobloch TJ; Lannutti JJ; Agarwal S, Compressive forces induce osteogenic gene expression in calvarial osteoblasts. J. Biomech. 2008, 41 (5), 1095–103. DOI: 10.1016/j.jbiomech.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gross TS; Srinivasan S; Liu CC; Clemens TL; Bain SD, Noninvasive loading of the murine tibia: an in vivo model for the study of mechanotransduction. J. Bone Miner. Res. 2002, 17 (3), 493–501. DOI: 10.1359/jbmr.2002.17.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ingber DE, Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006, 20 (7), 811–27. DOI: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 155.Sniadecki NJ; Anguelouch A; Yang MT; Lamb CM; Liu Z; Kirschner SB; Liu Y; Reich DH; Chen CS, Magnetic microposts as an approach to apply forces to living cells. Proc. Natl. Acad. Sci. U. S. A. 2007, 104 (37), 14553–8. DOI: 10.1073/pnas.0611613104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Al-Rekabi Z; Pelling AE, Cross talk between matrix elasticity and mechanical force regulates myoblast traction dynamics. Phys. Biol. 2013, 10 (6), 066003 DOI: 10.1088/1478-3975/10/6/066003. [DOI] [PubMed] [Google Scholar]
- 157.Oppenheimer AJ; Rhee ST; Goldstein SA; Buchman SR, Force-induced craniosynostosis in the murine sagittal suture. Plast. Reconstr. Surg. 2009, 124 (6), 1840–8. DOI: 10.1097/PRS.0b013e3181bf806c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Coussens AK; Hughes IP; Wilkinson CR; Morris CP; Anderson PJ; Powell BC; van Daal A, Identification of genes differentially expressed by prematurely fused human sutures using a novel in vivo - in vitro approach. Differentiation 2008, 76 (5), 531–45. DOI: 10.1111/j.1432-0436.2007.00244.x. [DOI] [PubMed] [Google Scholar]
- 159.Yen HY; Ting MC; Maxson RE, Jagged1 functions downstream of Twist1 in the specification of the coronal suture and the formation of a boundary between osteogenic and non-osteogenic cells. Dev. Biol. 2010, 347 (2), 258–70. DOI: 10.1016/j.ydbio.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Brunner M; Millon-Fremillon A; Chevalier G; Nakchbandi IA; Mosher D; Block MR; Albiges-Rizo C; Bouvard D, Osteoblast mineralization requires beta1 integrin/ICAP-1-dependent fibronectin deposition. J. Cell Biol. 2011, 194 (2), 307–22. DOI: 10.1083/jcb.201007108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lories RJ; Corr M; Lane NE, To Wnt or not to Wnt: the bone and joint health dilemma. Nat. Rev. Rheumatol. 2013, 9 (6), 328–39. DOI: 10.1038/nrrheum.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Manes S; Llorente M; Lacalle RA; Gomez-Mouton C; Kremer L; Mira E; Martinez AC, The matrix metalloproteinase-9 regulates the insulin-like growth factor-triggered autocrine response in DU-145 carcinoma cells. J. Biol. Chem. 1999, 274 (11), 6935–45. [DOI] [PubMed] [Google Scholar]
- 163.Andersson S; D’Arcy P; Larsson O; Sehat B, Focal adhesion kinase (FAK) activates and stabilizes IGF-1 receptor. Biochem. Biophys. Res. Commun. 2009, 387 (1), 36–41. DOI: 10.1016/j.bbrc.2009.06.088. [DOI] [PubMed] [Google Scholar]
- 164.Stamper BD; Park SS; Beyer RP; Bammler TK; Farin FM; Mecham B; Cunningham ML, Differential expression of extracellular matrix-mediated pathways in single-suture craniosynostosis. PLOS One 2011, 6 (10), e26557 DOI: 10.1371/journal.pone.0026557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fujita T; Azuma Y; Fukuyama R; Hattori Y; Yoshida C; Koida M; Ogita K; Komori T, Runx2 induces osteoblast and chondrocyte differentiation and enhances their migration by coupling with PI3K-Akt signaling. J. Cell Biol. 2004, 166 (1), 85–95. DOI: 10.1083/jcb.200401138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ting MC; Wu NL; Roybal PG; Sun J; Liu L; Yen Y; Maxson RE Jr., EphA4 as an effector of Twist1 in the guidance of osteogenic precursor cells during calvarial bone growth and in craniosynostosis. Development 2009, 136 (5), 855–64. DOI: 10.1242/dev.028605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yoshida T; Vivatbutsiri P; Morriss-Kay G; Saga Y; Iseki S, Cell lineage in mammalian craniofacial mesenchyme. Mech. Dev. 2008, 125 (9–10), 797–808. DOI: 10.1016/j.mod.2008.06.007. [DOI] [PubMed] [Google Scholar]


