Abstract
Artificial ascites has been reported as an effective technique to reduce the risk of thermal injury in radiofrequency ablation of liver tumors by increasing the distance of collateral organs located next to the ablated sites. In this case report we share our experience with artificial ascites in an attempt to reduce the toxicity of collateral adjacent organs in the setting of re-irradiation for recurrent cervical cancer. A 52-year-old female who developed local recurrence after definitive radiation therapy was treated with interstitial re-irradiation by means of image-guided, (single-implant/multi fraction) high-dose-rate brachytherapy. Because the sigmoid colon was in close proximity to the recurrent tumor lesion, artificial ascites was generated before each treatment fraction by percutaneous injection of a defined amount of saline solution through the abdominal wall to create additional space between the two volumes. Artificial ascites showed a dosimetric improvement by reducing the sigmoid colon D0.1cc per fraction from 286 cGy before to 189 cGy after saline injection. No severe complication was associated with the injection procedure.
Introduction
Radiation therapy (RT) plays an important role in the management of uterine cervical cancer patients both as primary1–4 as well as postoperative adjuvant treatment.5, 6 However, when patients develop locally recurrent disease in pre-irradiated volumes, standard curative treatment consists of total pelvic exenteration (TPE)7, 8 because repeat dose-escalated external beam radiation therapy (EBRT) to the same localized site, although technically feasible, is not unreservedly implemented because of the high risk of severe side effects on account of previous RT which lower patient’s quality of life significantly.9 Notwithstanding, TPE per se constitutes a devastating surgical procedure which demands patients with both colostomy and cystostomy.10 Against that background, interstitial high-dose-rate (HDR) brachytherapy (BRT) has been tested as re-irradiation modality for salvage after local failure.9, 11 It generates biologically effective dose escalation to the treatment target whilet the versatility of intratarget dose modulation inherent to BRT can be controlled and directed to deliver higher doses to gross disease or to selectively reduce the dose to organs at risk (OARs).
From the standpoint of OARs protection, artificial ascites has been used in interventional radiology to avoid diaphragm or gastrointestinal tract damage when treating liver tumors with radiofrequency ablation (RFA).12–15 Factors impairing the therapeutic ratio of this thermal method are tumor size, with an accepted upper size limit of 3–4 cm for optimal treatment16 and the heat sink effect, stopping effective cytoreduction in perivascular lesions. In addition, there is a significant risk for thermal injury in the case of tumors abutting the diaphragm or close to the gastrointestinal tract, the bile duct, or the gallbladder. For those clinical scenarios, artificial ascites has been proven to be an effective method to increase safety space between risk structures and tumor lesions. To the best of our knowledge, there is no published experience describing the use of artificial ascites in association with RT. In the current report, we utilized it for the safe delivery of interstitial HDR BRT for the re-irradiation of recurrent cervical cancer by creating additional space between gastrointestinal tract volumes and the recurrence site.
Clinical presentation
A 52-year-old-female was treated with definitive concurrent chemoradiotherapy for FIGO stage IIB squamous cell uterine cervical cancer. In consequence of multiple thoracic and abdominal aortic dissections for Marfan-Syndrome, radical hysterectomy was not attempted in accordance with the patient´s wish. Chemoradiotherapy consisted of 2 cycles nedaplatin (100 mg m− 2) followed by 1 cycle of cisplatin (80 mg m− 2) [switch from nedaplatin to cisplatin because of drug induced skin rash] and 50.4 Gy conventionally fractionated whole pelvis EBRT (central shielding after 39.6 Gy) plus 24 Gy total physical dose HDR intracavitary BRT delivered in four fractions. The patient responded with complete clinical remission.
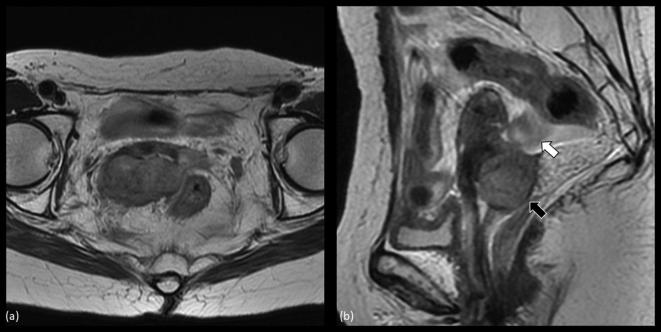
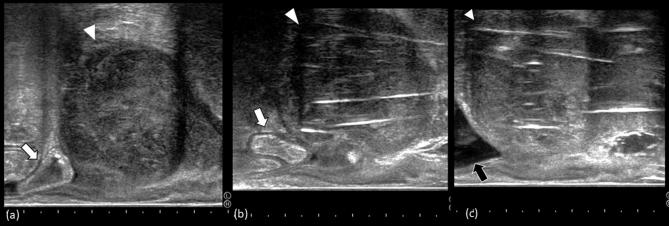
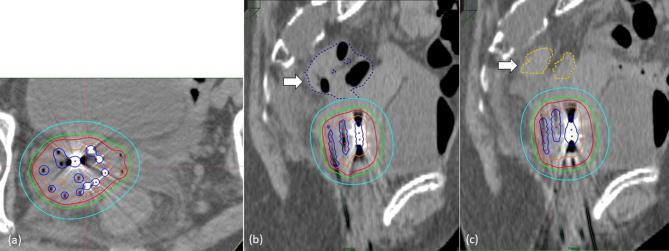
One year after completion of treatment, local recurrence was detected without regional or distant metastatic disease. As first non-invasive treatment approach, 12 cycles of polychemotherapy consisting of paclitaxel (135 mg m− 2) and cisplatin (50 mg m− 2) followed by 6 cycles of bevacizumab (15 mg kg−1) was administered resulting in partial response. Considering that recurrent disease was locally confined, TPE was planned but her cardiovascular comorbidities hindered this attempt. To this end, she was referred to our department for interstitial BRT as image-guided re-irradiation method. Figure 1 shows the recurrent tumor situated in the right parametrium and extending to the pelvic sidewall. On the sagittal view, the sigmoid colon can be demarcated next to the recurrence lesion (Figure 1b). The proximity of the sigmoid colon was also clearly visualized by trans-rectal ultrasonography (TRUS) (Figure 2a). Our technique of image-guided salvage interstitial BRT for cervical cancer has been described elsewhere.9, 11,16 In the current case, treatment consisted of sole HDR BRT with 48 Gy total physical dose being delivered in 8 fractions at 6 Gy, applied twice-daily with an interfractional interval of at least 6 h. No additional EBRT was prescribed. Figure 3a shows the isodose dose distribution of the implant. With regard to the procedure of ascites generation, a 20G needle (IntrocanⓇ Safety, B. Braun Medical Incorporated, Bethlehem, PA) was inserted percutaneously by ultrasound-guidance and 500 ml of saline solution was administered before every BRT fraction (Figure 4). Sagittal TRUS images before and after injection are displayed in Figure 2b–c. It is shown that the injection generated a displacement of the sigmoid colon from the recurrence region, recognizable as a shift of sigmoid volume outside the frame of the ultrasound (Figure 2c). No severe complication was observed during or after the injection procedure. From a dosimetric point of view, the displacement of the sigmoid resulted in a decrease of sigmoid D0.1cc from 286 to 189 cGy (Figure 3b–c) per fraction. For the purpose of the above dosimetric comparison, 3D treatment planning with anatomy-oriented dose optimization was performed before and after the first saline injection with the BRT catheters in situ based on repeated planning CT scans.
Figure 1.
MRI of recurrent cervical cancer before salvage brachytherapy. (a) shows an axial image of the tumor. The tumor extended beyond the right-sided parametrium to the pelvic wall. (b) depicts a sagittal image of the tumor (black arrow) visualizing that the sigmoid colon (white arrow) is located just next to the recurrent lesion.
Figure 2.
Trans-rectal ultrasonography sagittal view of the recurrent tumor. (a) shows the recurrent lesion before salvage brachytherapy. The white arrow head indicates the tumor with the white arrow marking the sigmoid colon located just next to the recurrence. b and c show the intrapelvic situs after interstitial catheter implantation. (b) depicts the situs before artificial ascites injection. It can be recognized that the sigmoid colon is situated next to the recurrent tumor. (c) is characterized by the shift of sigmoid volume outside the frame of the ultrasound (black arrow).
Figure 3.
Isodose distribution of the interstitial implant. (a) demonstrates an axial view of the tumor with the red and blue line representing the 100 and 200% isodose, respectively. (b, c) depict a sagittal view before and after artificial ascites injection. It is clear that the distance between sigmoid colon and high-dose volumes is increased after artificial ascites injection.
Figure 4.
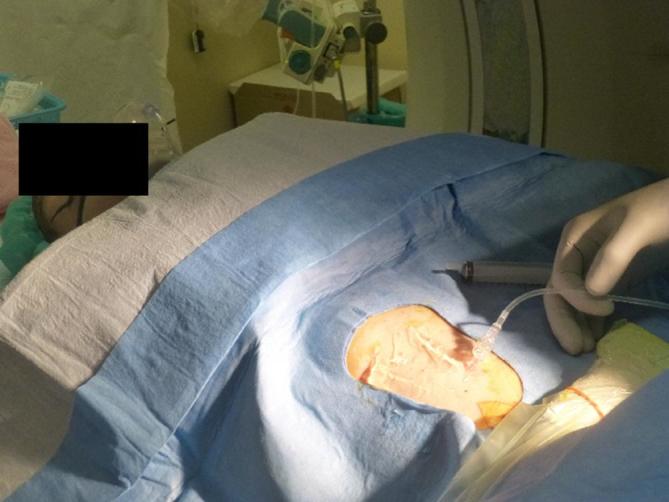
Artificial ascites injection procedure. A 20 G needle is inserted percutaneously under ultrasound guidance to avoid bowel injury and 500 ml saline solution are injected through a catheter.
Written informed consent was obtained from the patient for artificial ascites injection and BRT treatment and this case report was approved by the Institutional Review Board of the National Cancer Center Hospital (approval number 2017–331) according to the ethical standards laid down in the Declaration of Helsinki.
Discussion
Standard therapy for patients with non-metastatic recurrent cervical cancer who have past history of pelvic RT is TPE.7, 8 This approach poses a mutilating surgical procedure with a high incidence of complications and significant impairment of quality of life.10 In unresectable disease or in patients refusing surgery, radical re-irradiation with EBRT has been tried17, 18 but remains a choice of excessive morbidity which may outweigh the benefits of therapy. Chemotherapy alone, on the other hand, continues despite its excessive use in oncological reality to be of limited benefit with poor response and sobering outcome.19, 20
In this demanding clinical setting, some patients favor re-irradiation and interstitial BRT has demonstrated effectiveness in the management of locally recurrent cervical cancer developing within previously irradiated volumes.9, 11 There are, however, no well-defined recommendations for selecting patients for interventional radiooncological treatment and in most cases the decision is made individually. Notwithstanding this, the rationale for re-irradiation by means of HDR cannot be called into question considering that it offers radiobiological and technical advantages. As normal tissue toxicity after repeated full course conventional EBRT has shown to be significant, it seems reasonable to assume that further improvements in the therapeutic ratio can be generated by escalating the treatment dose while ameliorating conformity. Interstitial HDR BRT meets this objective optimally by exploiting the radiobiological advantage of larger fraction sizes while prospective 3D dosimetry provides anatomy-oriented dose optimization for highly conformal intensity modulated RT. At this point, the intrinsic characteristic of HDR to generate high intratarget dosing is of particular importance as it facilitates the application of ablative doses to central tumor volumes that are thought to experience increased radioresistance after previous irradiation.21, 22
Bearing in mind the predominantly palliative intention of re-irradiation, higher grade toxicity rates are of particular relevance the more so as a balance must be achieved between the probability of LC and the probability of toxic complications. Our group previously reported 2-year local control rates of 51.3% for patients who received image-guided interstitial BRT for recurrent cervical cancer at the cost of developing late severe complications greater than Grade 2 in 27.8% of patients.9 Therefore, further technical improvements are necessary to facilitate the safe delivery of cytotoxic doses. One approach could be the avoidance of hot spots in OARs by increasing the distance between target volume and OARs. In this report, we utilized artificial ascites for the safe delivery of interstitial HDR BRT of recurrent cervical cancer by creating additional space between gastrointestinal tract volumes and the recurrence site. It could be demonstrated that the procedure itself is safe and reproducible, confirming the experiences from its use in RFA of liver tumors.11–14 From a dosimetric point of view, the displacement of the sigmoid resulted in a decrease of sigmoid D0.1cc from 286y to 189 cGy per fraction.
To the best of our knowledge, this is the first report on the feasibility and safety of artificial ascites for OARs sparing in intrapelvic BRT. Concerning its long-term efficacy, further clinical research is needed to define which kind of patients will most benefit from this technique and whether this method significantly reduces late severe adverse effects in the re-irradiation settings.
Learning points
Artificial ascites has been reported as an effective technique to reduce the risk of thermal injury in radiofrequency ablation of liver tumors by increasing the distance of collateral organs located next to the ablated sites. In this case report it was suggested that a novel technique of artificial ascites in image-guided interstitial high-dose-rate brachytherapy could be generated safely in order to reduce the radiation exposure of organs at risk in the case of intrapelvic re-irradiation.
Footnotes
AcknowledgmentFunding: This study was partially supported by the Japan Agency for Medical Research and Development, AMED, the National Cancer Center Research and Development Fund (26-A-18 and 26-A-28), and JSPS KAKENHI Grant Number 15K19836.
Consent: Written informed consent for the case to be published (including images, case history and data) was obtained from the patient(s) for publication of this case report, including accompanying images.
Contributor Information
Naoya Murakami, Email: ore.murakami@gmail.com; namuraka@ncc.go.jp.
Satoshi Shima, Email: sshima@ncc.go.jp.
Kae Okuma, Email: kokuma@ncc.go.jp.
Kotaro Iijima, Email: koiijima@ncc.go.jp.
Nikolaos Tselis, Email: ntselis@hotmail.com.
Masakazu Uematsu, Email: muematsu@ncc.go.jp.
Yoshiaki Takagawa, Email: ytakagaw@ncc.go.jp.
Tairo Kashihara, Email: kashiharatairo@gmail.com.
Koji Masui, Email: mc0515kj@koto.kpu-m.ac.jp.
Ken Yoshida, Email: rad113@osaka-med.ac.jp.
Kana Takahashi, Email: kantakah@ncc.go.jp.
Koji Inaba, Email: koji_i_14@yahoo.co.jp.
Hiroshi Igaki, Email: higaki@ncc.go.jp.
Yuko Nakayama, Email: yukonak4@ncc.go.jp.
Jun Itami, Email: jitami@ncc.go.jp.
REFERENCES
- 1. Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol 1999; 17: 1339–48. doi: 10.1200/JCO.1999.17.5.1339 [DOI] [PubMed] [Google Scholar]
- 2. Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med 1999; 340: 1144–53. doi: 10.1056/NEJM199904153401502 [DOI] [PubMed] [Google Scholar]
- 3. Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med 1999; 340: 1137–43. doi: 10.1056/NEJM199904153401501 [DOI] [PubMed] [Google Scholar]
- 4. Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet 1997; 350: 535–40. doi: 10.1016/S0140-6736(97)02250-2 [DOI] [PubMed] [Google Scholar]
- 5. Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group Study. Gynecol Oncol 1999; 73: 177–83. doi: 10.1006/gyno.1999.5387 [DOI] [PubMed] [Google Scholar]
- 6. Peters WA, Liu PY, Barrett RJ, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol 2000; 18: 1606–13. doi: 10.1200/JCO.2000.18.8.1606 [DOI] [PubMed] [Google Scholar]
- 7. Nagase S, Inoue Y, Umesaki N, Aoki D, Ueda M, Sakamoto H, et al. Evidence-based guidelines for treatment of cervical cancer in Japan: Japan Society of Gynecologic Oncology (JSGO) 2007 edition. Int J Clin Oncol 2010; 15: 117–24. doi: 10.1007/s10147-010-0061-x [DOI] [PubMed] [Google Scholar]
- 8. Kasamatsu T, Onda T, Yamada T, Tsunematsu R. Clinical aspects and prognosis of pelvic recurrence of cervical carcinoma. Int J Gynaecol Obstet 2005; 89: 39–44. doi: 10.1016/j.ijgo.2004.12.020 [DOI] [PubMed] [Google Scholar]
- 9. Umezawa R, Murakami N, Nakamura S, Wakita A, Okamoto H, Tsuchida K, et al. Image-guided interstitial high-dose-rate brachytherapy for locally recurrent uterine cervical cancer: a single-institution study. Brachytherapy 2018; 17: 368–76. doi: 10.1016/j.brachy.2017.11.011 [DOI] [PubMed] [Google Scholar]
- 10. Brunschwig A. Complete excision of pelvic viscera for advanced carcinoma; a one-stage abdominoperineal operation with end colostomy and bilateral ureteral implantation into the colon above the colostomy. Cancer 1948; 1: 177–83. doi: [DOI] [PubMed] [Google Scholar]
- 11. Murakami N, Kato T, Miyamoto Y, Nakamura S, Wakita A, Okamoto H, et al. Salvage high-dose-rate interstitial brachytherapy for pelvic recurrent cervical carcinoma after hysterectomy. Anticancer Res 2016; 36: 2413–21. [PubMed] [Google Scholar]
- 12. Wang CC, Kao JH. Artificial ascites is feasible and effective for difficult-to-ablate hepatocellular carcinoma. Hepatol Int 2015; 9: 514–9. doi: 10.1007/s12072-015-9639-8 [DOI] [PubMed] [Google Scholar]
- 13. Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation with artificial ascites for hepatocellular carcinoma in the hepatic dome: initial experience. AJR Am J Roentgenol 2008; 190: 91–8. doi: 10.2214/AJR.07.2384 [DOI] [PubMed] [Google Scholar]
- 14. Lee EJ, Rhim H, Lim HK, Choi D, Lee WJ, Min KS. Effect of artificial ascites on thermal injury to the diaphragm and stomach in radiofrequency ablation of the liver: experimental study with a porcine model. AJR Am J Roentgenol 2008; 190: 1659–64. doi: 10.2214/AJR.07.2993 [DOI] [PubMed] [Google Scholar]
- 15. Kondo Y, Yoshida H, Shiina S, Tateishi R, Teratani T, Omata M. Artificial ascites technique for percutaneous radiofrequency ablation of liver cancer adjacent to the gastrointestinal tract. Br J Surg 2006; 93: 1277–82. doi: 10.1002/bjs.5374 [DOI] [PubMed] [Google Scholar]
- 16. Murakami N, Kobayashi K, Kato T, Nakamura S, Wakita A, Okamoto H, et al. The role of interstitial brachytherapy in the management of primary radiation therapy for uterine cervical cancer. J Contemp Brachytherapy 2016; 8: 391–8. doi: 10.5114/jcb.2016.62938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seo Y, Kim MS, Yoo HJ, Jang WI, Rhu SY, Choi SC, et al. Salvage stereotactic body radiotherapy for locally recurrent uterine cervix cancer at the pelvic sidewall: feasibility and complication. Asia Pac J Clin Oncol 2016; 12: e280–e288. doi: 10.1111/ajco.12185 [DOI] [PubMed] [Google Scholar]
- 18. Park HJ, Chang AR, Seo Y, Cho CK, Jang WI, Kim MS, et al. Stereotactic body radiotherapy for recurrent or oligometastatic uterine cervix cancer: a cooperative study of the Korean Radiation Oncology Group (KROG 14-11). Anticancer Res 2015; 35: 5103–10. [PubMed] [Google Scholar]
- 19. Monk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, Boardman CH, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol 2009; 27: 4649–55. doi: 10.1200/JCO.2009.21.8909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moore DH, Blessing JA, McQuellon RP, Thaler HT, Cella D, Benda J, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol 2004; 22: 3113–9. doi: 10.1200/JCO.2004.04.170 [DOI] [PubMed] [Google Scholar]
- 21. Spratt DE, Evans MJ, Davis BJ, Doran MG, Lee MX, Shah N, et al. Androgen receptor upregulation mediates radioresistance after ionizing radiation. Cancer Res 2015; 75: 4688–96. doi: 10.1158/0008-5472.CAN-15-0892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dahan P, Martinez Gala J, Delmas C, Monferran S, Malric L, Zentkowski D, et al. Ionizing radiations sustain glioblastoma cell dedifferentiation to a stem-like phenotype through survivin: possible involvement in radioresistance. Cell Death Dis 2014; 5 e1543. doi: 10.1038/cddis.2014.509 [DOI] [PMC free article] [PubMed] [Google Scholar]