Abstract
Objectives
Vitamin D deficiency rickets remains a problem in Canada. Our primary objective was to determine the annual incidence of rickets and/or early vitamin D deficiency in Manitoba. Secondarily, we investigated if there was an increase in the annual incidence.
Methods
A retrospective chart review was undertaken to identify cases in our catchment area from 2003 to 2015. Data sources included endocrine and hospital charts and radiology reports. Early vitamin D deficiency was determined by review of all 25(OH)D tests from 2011 to 2015. Values less than 30 nmol/L with an elevated bone marker prompted a chart review in children under 7 years.
Results
We identified 46 cases of rickets and 68 with early vitamin D deficiency. For Manitoba, the annual incidence of rickets was 8.2 cases per 100,000 in infants, and 1.6 per 100,000 in children aged 1 to 7 years. Those with early vitamin D deficiency had annual incidences of 2.7 per 100,000 infants and 9.9 per 100,000 Manitoban children. No temporal trends were noted for either. For both disorders, most cases were from northern or rural locales; about 50% were of self-declared Indigenous or Inuit heritage, and the majority (>75%) of children were from families with high material deprivation using area-based socioeconomic measures.
Conclusion
Despite several decades of preventative efforts, the incidence of rickets was comparable to previous Canadian reports, particularly in infants. Greater education across the lifespan and engagement with communities and public health agencies will be needed to reduce the high incidence of this preventable disease.
Keywords: Incidence, Infants, Rickets, Socioeconomic status, Vitamin D
Vitamin D is essential for calcium homeostasis and bone and tooth health (1). Vitamin D deficiency can result in many abnormalities including rickets, the failure of mineralization in growing bone and cartilage. Infants and sometimes adolescents without defective mineralization of growth plates may present with other findings of rickets, such as hypocalcemic seizures. Additionally, infants may also present with dilated cardiomyopathy or proximal myopathy causing delayed gross motor milestones.
Prior to significant bony manifestations of rickets, biochemical signs of vitamin D deficiency are noted. These include elevations in alkaline phosphatase, parathyroid hormone (PTH), reduced 25-hydroxy vitamin D (25(OH)D), and possible reductions in serum calcium and /or phosphorus (stage 1 or ‘early’ vitamin D deficiency). Rachitic skeletal deformities are associated with stage 2 or 3 vitamin D deficiency (1).
Nutritional vitamin D deficiency is the most common cause of rickets (2). Risk factors include inadequate diet or reduced cutaneous synthesis of vitamin D due to northern latitude, nonwhite skin, or life style choices (e.g., sunscreen, limited outdoor time) (3). Maternal–fetal transfer of vitamin D is greatest in the third trimester, placing preterm and even term infants at risk of deficiency, particularly with poor gestational intake (4). Additionally, breast milk is not a rich source of vitamin D, and supplements should be provided to breast-fed infants (5). Formula fed infants may also be at risk if they do not consume a litre of formula per day (400 IU vitamin D/litre) and may require supplementation (6–8). Canada practices vitamin D fortification of commercial milk and margarine to reduce the incidence of rickets. Unfortunately, not all children consume vitamin D rich foods; socioeconomic status (SES) factors and location of residence may also contribute to inadequate intake or supplementation (9).
A 2-year survey (2004 to 2006) revealed an annual incidence rate of 2.9/100,000 of vitamin D deficiency rickets in Canadian children up to 7 years despite significant strategies to reduce risk (3). Recently in Quebec, we reported a tripling in the annual incidence of rickets over a decade to 7.9/100,000 (9). Clinical observation at the Children’s Hospital Winnipeg suggests that cases of rickets may be more frequent than generally believed.
Our primary objective was to determine the annual incidence of rickets and early vitamin deficiency in Manitoba, to examine temporal trends, and to characterize the clinical phenotype and risk factors for those with either clinical rickets or early vitamin D deficiency.
METHODS
A retrospective chart review was undertaken to determine cases of rickets in children <18 years in our catchment area (Manitoba, NW Ontario and W. Nunavut). Rickets cases were identified from endocrine and hospital charts with International Classification of Disease (ICD) codes and by reviewing radiology reports from January 1, 2003 until December 31, 2015.
Laboratory vitamin D data were obtained from the Health Sciences Centre (HSC) Clinical Chemistry laboratory, the single centre analyzing 25(OH)D for our entire catchment area from January 1, 2011 to December 31, 2015. Early vitamin D deficiency was defined by values of less than <30 nmol/L paired with an elevated PTH (>50 pmol/L) or alkaline phosphatase (greater than the upper reference interval for age), which prompted a chart and radiograph review in children < 7 years. Most PTH, serum calcium, phosphorus and alkaline phosphatase were analyzed using Roche Cobas e601 at HSC. For infants, 25(OH)D was performed by LC-MS/MS (ABI-Sciex, Mayo Clinic); for noninfants, DiaSorin RIA was used from 2003 to April 2012 and LC-MS/MS (Waters) thereafter at HSC. In all cases, coefficients of variation were ≤ 10%.
Data extracted from charts included age, sex, postal code, maternal vitamin D supplementation in pregnancy, maternal risk factors (e.g., presence of diabetes in pregnancy), feeding modality (breast or formula), supplement use, sun exposure, self-declared ethnicity (e.g., Indigenous), immigration status and if residing at a latitude above 55° north (10). We excluded children who were premature, had chronic disease or risks associated with non-nutritional vitamin D deficiency (e.g., hepatic, renal, gastrointestinal, congenital, or genetic causes).
Annual incidences were calculated based on the number of Manitoba children <1 year from census data (~15,000 children < 1 year, ~90,000 for those aged 1 to 6.999 years) (11). Medians and ranges are reported for continuous variables and percentage with 95% confidence intervals (CI) for categorical variables. These were compared using non-parametric testing given their non-normal distribution. Temporal trends in annual incidence rates were assessed by Poisson regression for count data. Data were analyzed using STATA 12 (College Station, TX, USA).
Postal codes were used to identify geographic location and area-based SES. Individual 6-digit postal codes were mapped to longitude, latitude, and census dissemination area (DA) using the PCCF+ (postal code conversion file, 2016) provided by Statistics Canada through the Data Liberation Initiative (12). Census DA’s are the smallest administrative unit published by Statistics Canada, each containing 400 to 700 individuals (12). The Raymond-Pampalon deprivation indices were assigned for each DA based on 2006 census data (13). Within each DA, the material deprivation quintile is a composite score based on neighbourhood household income, unemployment, and high school completion rates. Similarly, social deprivation quintiles are based on the proportion of single parent families; adults who are separated, divorced or widowed; and adults living alone. Due to privacy issues, only 104 postal codes were matched for deprivation indices, while all 114 cases were available for geomapping. Chi-squared tests were used to test the null hypothesis of homogeneity in the distribution of deprivation quintiles.
Approval was obtained from the University of Manitoba Health Ethics Research Board.
RESULTS
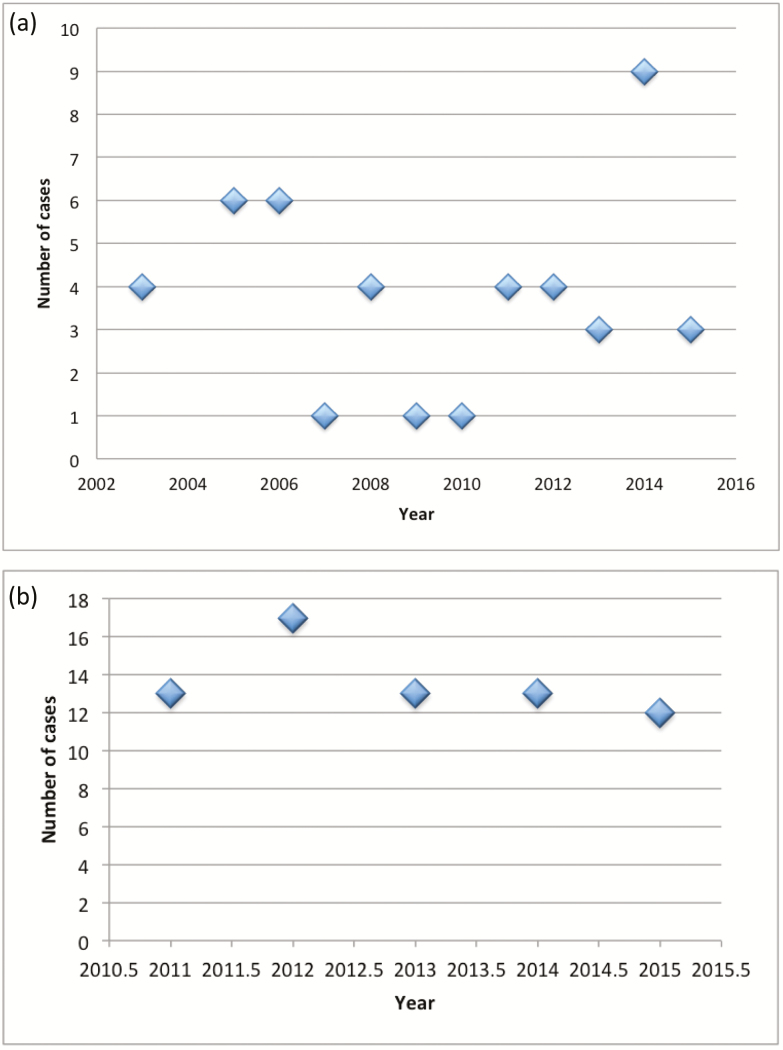
We identified 46 children with nutritional rickets from 2003 to 2015 (Figure 1a). Eighteen presented with hypocalcemic seizures (39%—18/46), 20 with bony changes (43%), 3 with failure to thrive, and 5 as part of a nutritional screen due to poor intake. The median was 4 cases annually in our catchment, giving Manitoba an annual incidence rate of rickets of 8.2 cases per 100,000 in infants and 1.6 cases per 100,000 in those aged 1 to < 7 years. Early vitamin D deficiency was detected 68 children <7 years between 2011 and 2015 (Figure 1b) with a median of 14 cases annually in the catchment area. This gives a Manitoba an annual incidence of 2.7 per 100,000 infants or 9.9 per 100,000 children aged 1 to <7 years. In both instances, there was no significant trend in incidence by Poisson regression (P=0.5, 0.3, respectively).
Figure 1.
(a) Incidence of rickets as identified through clinical data across our catchment area. (b) Incidence of early vitamin D deficiency as identified through laboratory data in our catchment area.
Comparing the rickets and vitamin D deficiency groups, those with rickets were overall younger (9.1 versus 47.7 months, P<0.001) (Table 1). Fewer in the deficiency group were detected in the vitamin D synthesizing season (33.8 versus 63.0%, P<0.01). Otherwise the two groups were similar, with low weight z-scores (median −0.9). Detailed biochemistry data were available only for those with rickets; serum phosphorus was typically normal (90% were above the lower reference interval for age and sex) while the median total calcium was low at 2.19 mmol/L (range 1.26 to 2.54). Few were taking vitamin D supplements, though the latter is based on a small sample size (n=37). Of those with more detailed data, 60% (35/58) had at least 1 risk factor for vitamin D deficiency (Table 1). All 114 children were subsequently treated with vitamin D supplements; those with hypocalcemia were also provided with calcium supplements.
Table 1.
Presenting features of cases
| Total (n=114) |
Rickets (n=46) |
Vitamin D deficiency (n=68) |
P-value | |
|---|---|---|---|---|
| Age (months) median range |
30.2 (0.1–193.8) |
9.1 (0.1–193.8) |
47.7 (0.1–95.9) |
<0.001 |
| Sex (M %) 95% CI |
55 (36.9–71.9) |
52.2 (36.9–71.2) |
60.2 (47.6–71.9) |
0.2 |
| % detected during April to September when cutaneous vitamin D synthesis is possible 95% CI |
45.6 (36.2–55.2) |
63.0 (47.5–76.7) |
33.8 (22.7–46.3) |
<0.01 |
| Weight Z-score median range |
−0.9 (−8.6–3.47) |
−0.9 (−8.6–3.47) |
−1.1 (−6.6–1.54) |
0.9 |
| Use of vitamin D supplement median 95% CI (n=37) |
22.5 (9.6–41.1) (n=31) |
16.7 (0.4–64.1) (n=6) |
0.8 | |
| Latitude median (degrees) range |
52.9 (48.65–66.5) |
50.2 (48.7–66.5) |
53.9 (42.98–62.8) |
0.09 |
| 25-hydroxyvitamin D concentration nmol/L (n=104) |
22.0 (6.2–48.0) |
19.5 (7.1–48) (n=36) |
24.0 (0–30.0) (n=68) |
0.1 |
| % with risk factors for rickets (nonwhite, immigrant, First Nations or Inuit status or latitude greater than 55°) mean 95% CI (n=58) |
78.2 (63.6–89.0) (n=36) |
32.3 (21.5–44.8) (n=22) |
95% CI-95% confidence intervals.
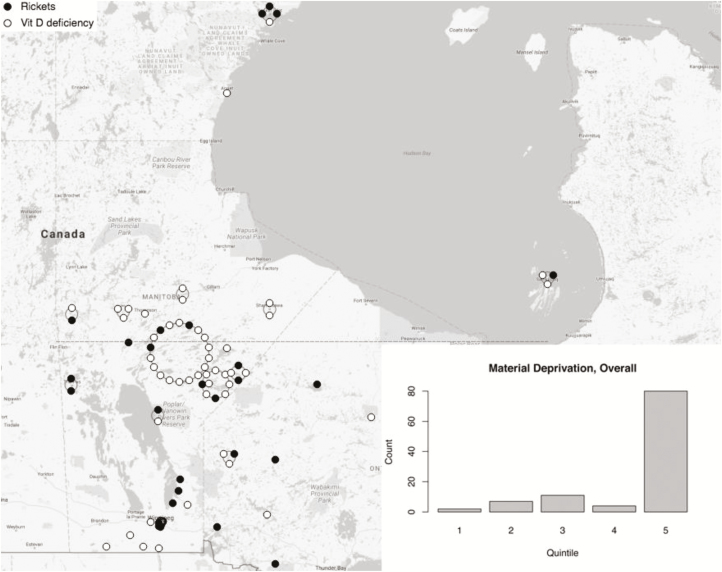
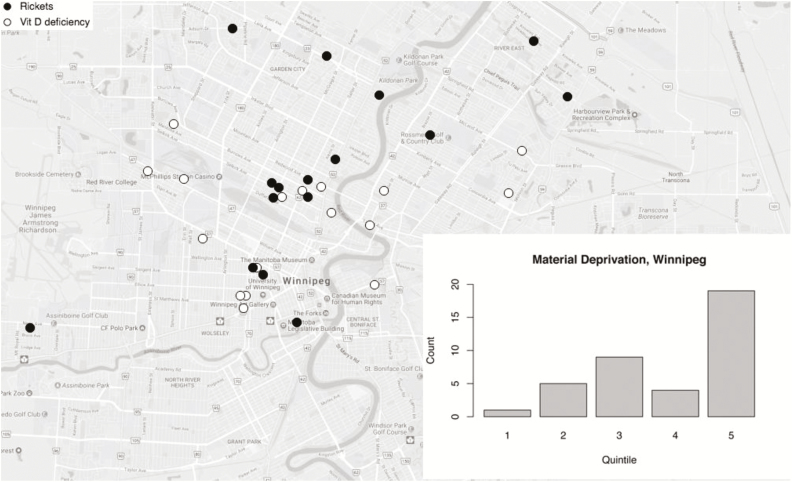
Geomapping revealed that two thirds of the cases (75/114) were located in rural or Northern regions (Figure 2a) with some from the Island Lake (18/75), a region particularly known for rickets in Manitoba. In this area three had rickets and 15 had early vitamin D deficiency. About 34% of those with rickets lived in Winnipeg (Figure 2b). When assessing SES status with area-based deprivation indices, greater than 75% in each group fell in the highest quintile of material deprivation (P<0.001) (Figure 2a and 2b). There was no inhomogeneity in the social deprivation across quintiles in both groups.
Figure 2.
(a) Geographic distribution of all cases in catchment area (Manitoba, NW Ontario and W. Nunavut). Each dot represents a case; dark circle = rickets, white circle = early vitamin D deficiency. The dotted line represents 55○ latitude. For clarity, overlapping location data are mapped to a large circle surrounding the central community i.e. jittered. The largest such cluster at 55○ latitude is Island Lake. The bar chart inset shows case counts by material deprivation quintile based on N=104. (b) Geographic distribution of cases in the city of Winnipeg, with material deprivation scores. Each dot represents a case; dark circle = rickets, white circle = early vitamin D deficiency. Inset bar chart shows counts per material deprivation quintile.
DISCUSSION
The annual incidence of rickets was 8.2 per 100,000 infants in Manitoba; this is comparable to the Canadian annual incidence of 9 cases per 100,000 infants using a survey targeting paediatricians, thereby missing cases managed by family doctors (3). We acknowledge that all studies, including ours, are still likely underestimating the incidence because case single centre assessments or physician surveys will not capture all cases. We noted no temporal trends; as in other reports, there was a predilection for rickets in sunny seasons, suggesting limited sun exposure and poor dietary intake (14,15).
Elsewhere in Canada, a recent Quebec assessment using linked administrative databases and ICD-10 codes found a tripling of the annual rickets incidence to 7.9 per 100,000 from 1998 to 2008 in term infants (9). When looking at other northern jurisdictions, such as Alaska, the annual incidence of rickets was similar to our infants at 11.2 per 100,000 children and stable over the past decade; this was about 5 times higher than southern states (16). Strikingly high rates have been found in immigrant children with darker skin pigmentation in other northern countries, (e.g., Denmark [17]). In that study, the annual incidence was found to be 60 per 100,000 children aged 0 to 14.9 years compared to 2.9 per 100,000 for all Danish children. Most other studies have reported higher risks in those with darker skin or immigrants (3,16,17).
Given the persistently high incidence of rickets and the availability of a unique laboratory dataset for our catchment area, we also examined the incidence of early vitamin D deficiency, a potential precursor to clinical rickets (3,15,17). Approximately 50% of these children were of First Nations or Inuit descent and two thirds were found at more northerly latitudes than Winnipeg (49.9°). They had a number of risk factors: darker skin pigmentation, which increases the amount of sun exposure required to synthesize vitamin D, and northern latitudes; having fewer months to synthesize vitamin D (18). Because these children did not have full radiographic screening, some of the children with early vitamin D deficiency might also have had rickets. Most reports list incidental cases as about 10% (3). However, an Australian surveillance program reported that 80% of their cases were actually incidental (lab derangements), although their approach was not as systematic as ours (15). Our access to lab data may be unique in the literature.
Importantly, we are not the first to report that Manitoba children have persistent rickets rates. Previous work identified the Island Lake region as particularly prone to rickets (4,19). For this reason, babies born there receive 100,000 IU of vitamin D orally at birth and their mothers receive two similar doses as prevention during pregnancy (personal communication, Head Nurse, Garden Hill Nursing Station). Given that 24% of our rural sample comes from the Island Lake area, this current perinatal public health intervention may not be sufficient; there were still 3 cases of rickets and 15 with deficiency. Apparently, this community and others in Manitoba have polymorphisms in their vitamin D receptors, which may impact 25(OH)D metabolism and function (20).
Striking disparities in material deprivation scores in both our cases of rickets and vitamin D deficiency likely underscores significant food insecurity. The fact that the material deprivation was strongly associated with cases even within the Winnipeg metropolitan area suggests that this is not simply an artifact of isolated northern communities, which do also include affluent neighbourhoods. Food insecurity typically reduces the family’s ability to obtain food rich in vitamin D and calcium (dairy) for mothers and young children (21,22). Exacerbating this situation is low uptake of maternal prenatal vitamin supplements (23). Additionally, low SES is also associated with increased rates of obesity; vitamin D is sequestered in fat, resulting in reduced availability of 25(OH)D (24). Unfortunately, as northern communities move away from traditional foods, rich sources of vitamin D and calcium may be lost (25). Moreover, as the dietary calcium intake is reduced, vitamin D catabolism is increased, resulting in even lower concentrations of 25(OH)D (26). Yet another risk factor for these families may be that as we increasingly encourage breastfeeding, vitamin D supplementation reinforcement may lag (9). This combination of environmental (latitude, darker skin, obesity, increased screen time with fewer outdoor activities), nutritional (departure from traditional foods), and genetic (vitamin D receptor polymorphisms) factors appear to have created a perfect storm with regards to the risk of rickets or early vitamin D deficiency in our catchment area.
Unfortunately, despite ongoing efforts to stem the tide of this easily preventable disease, our current efforts appear to be insufficient. Continued awareness in addition to new strategies is clearly needed. Options include fortification of additional foods, which is very effective and inexpensive (27), or Stoss therapy with large dose oral or intramuscular doses of vitamin D, which remains controversial in paediatrics (1). Ongoing campaigns to alert communities to risks of deficiency and the importance of supplements will be vital; promotion of easy to use preparations has improved uptake in Canada (28). There are lessons to be learned from Turkey and the city of Birmingham, that have implemented successful and inexpensive programs through ongoing education by all health care providers at every visit or patient contact (29,30). In Turkey, the prevalence of nutritional rickets was dramatically reduced through distributing free vitamin D supplements in an easy to use format, increased education of medical staff and clients, and universal promotion of vitamin D supplementation by health care providers (9,30).
Our research is not without limitations, including its retrospective nature and incomplete data; we were not able to assess the lab data prior to 2011. Additionally, we did not have complete clinical data available, and the uniqueness of our laboratory database means that our results may not be directly comparable to other studies lacking similar access to 25(OH)D concentrations. Lastly, we did not have complete diet information. As described above, adequate calcium impacts vitamin D metabolism (31). It is important to note that we cannot assume that the dietary intake of Indigenous or Inuit should be comparable to those in southern Canada (32). Important genetic adaptations have occurred to their typically low calcium environment (33). An important strength of our work was the assessment of vitamin D status in Indigenous communities using laboratory data. This provides a snapshot of high risk communities that are typically excluded from Statistics Canada surveys. Further study to better understand optimal calcium and vitamin D intakes in these divergent populations will be an additional important step prior to implementation.
CONCLUSION
Manitoba and our catchment area have a persistently high incidence of rickets, particularly in infants and despite current prevention strategies focused at high risk populations. The reasons for these levels of deficiency are likely multifactorial, including high material deprivation leading to food insecurity, darker skin (Indigenous and Inuit), and more northerly latitude. Education across the lifespan and enhanced engagement with public health partners and communities will be needed to reduce this high incidence, especially in infancy.
Acknowledgements
We are grateful for funding from the Children’s Hospital Research Institute of Manitoba.
Financial Disclosure: The authors have no financial relationship relevant to this article to disclose.
Funding Source: Internal funds.
Potential Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
REB: This study was reviewed and approved by the University of Manitoba Research Ethics Board.
References
- 1. Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M; Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society Vitamin D deficiency in children and its management: Review of current knowledge and recommendations. Pediatrics 2008;122(2):398–417. [DOI] [PubMed] [Google Scholar]
- 2. Wharton B, Bishop N. Rickets. Lancet 2003;362(9393):1389–400. [DOI] [PubMed] [Google Scholar]
- 3. Ward LM, Gaboury I, Ladhani M, Zlotkin S. Vitamin D-deficiency rickets among children in Canada. CMAJ 2007;177(2):161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gross ML, Tenenbein M, Sellers EA. Severe vitamin D deficiency in 6 Canadian first nation formula-fed infants. Int J Circumpolar Health 2013;72:20244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rodd C, Mushcab SA. Hypocalcemic seizures secondary to nutritional vitamin D deficiency in 3 infants fed soy formula. Clin Pediatr (Phila) 2005;44(5):455–7. [DOI] [PubMed] [Google Scholar]
- 6. Fatani T, Sharma AK, Weiler HA, Sheehy O, Bérard A, Rodd C. Differential low uptake of free vitamin d supplements in preterm infants: The Quebec experience. BMC Pediatr 2014;14:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Waiters B, Godel JC, Basu TK. Perinatal vitamin D and calcium status of northern Canadian mothers and their newborn infants. J Am Coll Nutr 1999;18(2):122–6. [DOI] [PubMed] [Google Scholar]
- 8. Godel JC, Hart AG. Northern infant syndrome: A deficiency state?Can Med Assoc J 1984;131(3):199–204. [PMC free article] [PubMed] [Google Scholar]
- 9. Millette M, Sharma A, Weiler H, Sheehy O, Bérard A, Rodd C. Programme to provide Quebec infants with free vitamin D supplements failed to encourage participation or adherence. Acta Paediatr 2014;103(10):e444–9. [DOI] [PubMed] [Google Scholar]
- 10. Munns CF, Shaw N, Kiely M, et al. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab 2016;101(2):394–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Statistics Canada, 2011 Census of Population Published 2011. 2016. <http://www12.statcan.gc.ca/census-recensement/2011/dp-pd/prof/index.cfm> (Accessed December 22, 2017).
- 12. Census Dictionary, Statistics Canada Published 2011. 2016. <http://www12.statcan.gc.ca/census-recensement/2011/ref/dict/geo021-eng.cfm> (Accessed December 22, 2017).
- 13. Pampalon R, Hamel D, Gamache P, Raymond G. A deprivation index for health planning in Canada. Chronic Dis Can 2009;29(4):178–91. [PubMed] [Google Scholar]
- 14. Wheeler BJ, Lawrence J, Chae M, et al. Intuitive eating is associated with glycaemic control in adolescents with type I diabetes mellitus. Appetite 2016;96:160–5. [DOI] [PubMed] [Google Scholar]
- 15. Munns CF, Simm PJ, Rodda CP, et al. ; APSU Vitamin D Study Group. Incidence of vitamin D deficiency rickets among Australian children: An Australian paediatric surveillance unit study. Med J Aust 2012;196(7):466–8. [DOI] [PubMed] [Google Scholar]
- 16. Singleton R, Lescher R, Gessner BD, et al. Rickets and vitamin D deficiency in Alaska native children. J Pediatr Endocrinol Metab 2015;28(7-8):815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beck-Nielsen SS, Brock-Jacobsen B, Gram J, Brixen K, Jensen TK. Incidence and prevalence of nutritional and hereditary rickets in southern Denmark. Eur J Endocrinol 2009;160(3):491–7. [DOI] [PubMed] [Google Scholar]
- 18. Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: Exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab 1988;67(2):373–8. [DOI] [PubMed] [Google Scholar]
- 19. Haworth JC, Dilling LA. Vitamin-D-deficient rickets in Manitoba, 1972-84. CMAJ 1986;134(3):237–41. [PMC free article] [PubMed] [Google Scholar]
- 20. Larcombe L, Mookherjee N, Slater J, et al. Vitamin D in a northern Canadian First Nation population: Dietary intake, serum concentrations and functional gene polymorphisms. Plos One 2012;7(11):e49872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Willows ND, Veugelers P, Raine K, Kuhle S. Prevalence and sociodemographic risk factors related to household food security in aboriginal peoples in Canada. Public Health Nutr 2009;12(8):1150–6. [DOI] [PubMed] [Google Scholar]
- 22. Power E. Food Security for First Nations and Inuit in Canada Background Paper. Health Canada: Prepared for the First Nations and Inuit Health Branch, 2007. [Google Scholar]
- 23. Heaman MI, Green CG, Newburn-Cook CV, Elliott LJ, Helewa ME. Social inequalities in use of prenatal care in Manitoba. J Obstet Gynaecol Can 2007;29(10):806–16. [DOI] [PubMed] [Google Scholar]
- 24. Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 2000;72(3):690–3. [DOI] [PubMed] [Google Scholar]
- 25. Sharma S, Gittelsohn J, Rosol R, Beck L. Addressing the public health burden caused by the nutrition transition through the healthy foods north nutrition and lifestyle intervention programme. J Hum Nutr Diet 2010;23 (Suppl 1):120–7. [DOI] [PubMed] [Google Scholar]
- 26. Ross AC. The 2011 report on dietary reference intakes for calcium and vitamin D. Public Health Nutr 2011;14(5):938–9. [DOI] [PubMed] [Google Scholar]
- 27. Pietrek J, Preece MA, Windo J, et al. Prevention of vitamin-D deficiency in Asians. Lancet 1976;1(7970):1145–8. [DOI] [PubMed] [Google Scholar]
- 28. Rodd C, Jean-Philippe S, Vanstone C, Weiler H. Comparison of 2 vitamin d supplementation modalities in newborns: Adherence and preference. Appl Physiol Nutr Metab 2011;36(3):414–8. [DOI] [PubMed] [Google Scholar]
- 29. Moy RJ, McGee E, Debelle GD, Mather I, Shaw NJ. Successful public health action to reduce the incidence of symptomatic vitamin D deficiency. Arch Dis Child 2012;97(11):952–4. [DOI] [PubMed] [Google Scholar]
- 30. Hatun Ş, Ozkan B, Bereket A. Vitamin D deficiency and prevention: Turkish experience. Acta Paediatr 2011;100(9):1195–9. [DOI] [PubMed] [Google Scholar]
- 31. Lips P. Vitamin D physiology. Prog Biophys Mol Biol 2006;92(1):4–8. [DOI] [PubMed] [Google Scholar]
- 32. Delormier TK,H. Dietary characteristics of eastern James Bay Cree women. Artic Institute of North America 1999;52(2):182–7. [Google Scholar]
- 33. Sellers EA, Sharma A, Rodd C. Adaptation of Inuit children to a low-calcium diet. CMAJ 2003;168(9):1141–3. [PMC free article] [PubMed] [Google Scholar]