Abstract
Background
Prior to introducing social needs screening into our subspecialty clinics, we first wanted to understand the health effects of the major social challenges facing children with chronic diseases in British Columbia.
Methods
Using a strict prospective methodology, avoiding use of health databases and proxy end points, we studied the effects of five social health determinants (distance from care, family income, gender, ethnicity, caregiver education), on health outcomes in three groups of children with chronic diseases: cystic fibrosis (CF), type 1 diabetes (T1D), chronic kidney disease (CKD). Social determinant data were collected at a face-to-face interview during a clinic visit. These were correlated with diagnosis-specific health outcomes, measured at the same visit. Main outcomes were: forced expired volume in 1 second (FEV1) (CF group), HbA1c (T1D group), estimated glomerular filtration rate (CKD group).
Results
We studied 270 children: 85 CF, 89 T1D and 96 CKD. In all three groups, children from families with annual income less than $45,000 had significantly worse health than those from families above this cut-off. Lower caregiver education was related to worse health in the CKD and T1D groups. We found no adverse health effects associated with distance from subspecialty care, patient ethnicity or gender.
Conclusion
Even in a prosperous province, family poverty and lack of caregiver education still impose measurable adverse effects on the health of children with chronic diseases. We hope these results help support the integration of social needs screening into routine multidisciplinary outpatient clinics. Early detection of social problems and targeted interventions will hopefully help to equalize health outcomes between children from different social groups.
Keywords: Cystic fibrosis, Education, Health care access, Income, Kidney disease, Social determinants, Type 1 diabetes
“When it comes to health, your zip code is more important than your genetic code”
Dr Garth Graham (1).
From Hippocrates (2) to Engels (3), it has been known for centuries that poor social conditions are bad for health. Until recently, it was believed there was a threshold relationship between social disadvantage and health (4). The surprising finding from the influential Whitehall study (5) was that the relationship is actually a continuum across the whole social scale (6). Subsequent studies have confirmed that wherever there is a measurable social gradient, such as income, education level or employment grade, there is always an associated health gradient (7–9). The incremental benefits get smaller at higher social levels but the very rich are still healthier than the rich (10) and all are a great deal healthier than the poor.
The health effects of social deprivation are particularly important during childhood (11). Beginning in utero, the cumulative effects of adverse social factors lock a child into a trajectory of ill health that can last a lifetime (12). Social legislation aimed at equalizing health outcomes between rich and poor is now common practice in developed countries (13)—it is also a foundation of current WHO policy (14). A growing body of government reports (15), professional recommendations (16) and clinical research (17) emphasize that health workers can play an important part in this process by introducing social screening (social diagnosis) and targeted interventions (social prescribing), into routine clinic visits (18).
In our experience, social variables are particularly important modifiers of health in the growing numbers of children with chronic diseases (19). Prior to introducing social needs screening (backed by early referral to appropriate support services), into the clinics serving these children, we first wanted to understand which were the major social determinants that affected the health of children in our province with chronic health problems. Consequently, we examined the effects of distance from care, family income, gender, ethnicity and caregiver education, on health outcomes in three groups of children with chronic diseases (cystic fibrosis [CF], type 1 diabetes [T1D], chronic kidney disease [CKD]). A single-centre study allowed us to collect data directly from patients and their families which avoided the use of database reviews and proxy end points.
METHODS
Measuring SES
Socioeconomic status (SES) is a concept, not a measurable variable, so there is no accepted definition or single test to help quantify it (20). In adults, SES is usually inferred from measurements of one or more variables such as income or occupation. Unfortunately, there is no agreement on how to classify those who are not self-supporting or economically active, such as children (21). Further imprecision is added by the use of proxy measures of SES derived from health census databases or defined by family zip code (22). In order to capture the full effects of social variables upon child health (23), we studied a range of health determinants including two that were unique to the child (gender, ethnicity), plus three others that reflected the child’s social environment (family income, caregiver education, distance from subspecialty care). We did not use data bases or postal questionnaires. Responses to a structured questionnaire were collected at face to face interview by research assistants who were blinded to the child’s current measures of health.
Study setting, patient population and enrolment
Our study population included all children with a diagnosis of CF, T1D and nondialyzed CKD in British Columbia. All three patient groups are managed using a general model previously described by our Nephrology Division (24). Patients are reviewed at least three times a year by multidisciplinary subspecialty teams centralized to BC’s Children’s Hospital in Vancouver, BC. This is a 300 bed quaternary level facility serving the province’s roughly 1 million children from birth to 18 years.
After permission was provided by the research ethics committee of the University of British Columbia, eligible families were initially contacted by letter and later invited to join the study at their next clinic visit. Study consent was obtained from the parents and, where possible, study assent was also obtained from the children using age-appropriate forms. Patients were excluded from study if they were clinically unstable and, in the CF group, if they were unable to perform lung function tests.
Study design and measured end points
This was a prospective, cross-sectional observation study. After enrolment and consent were completed, we administered a questionnaire to the child’s primary caregiver in a private room or, in a few cases, by telephone. This was followed by a standard subspecialty clinic review that included tests specific for the child’s underlying chronic disease (detailed below). The questionnaire covered the following variables:
Social data: details of patient (age, gender, neonatal history, diagnosis, past significant medical issues), and primary caregiver (relation to child, partnership status, extended insurance, caregiver education, household income, home ownership, refugee status, immigrant status).
Ethnicity: the child was defined by the birth Mother’s reported ethnicity and classified into five groups—South Asian, First Nations, Euro-Canadian, East Asian, other.
Access to health care: defined by straight line distance, in kilometres, from the family home to BC’s Children’s Hospital.
Income: total family annual pretax income defined by four groups—<$25K/year, $25 to 45K/year, $45 to 65K/year, >$65K/year.
Education: primary caregiver’s highest level of education defined by five groups—did not graduate (DNG), high school, community college, university, post graduate.
To obtain accurate end points for comparison with social variables, the following disease-specific variables were measured and collected on the same day the questionnaire was administered:
The health of children in the CF group was assessed by lung function tests performed in an accredited paediatric lung function laboratory, following accepted guidelines (25). Principal end points were forced vital capacity and forced expired volume in 1 second (FEV1), both expressed as percentage of predicted values.
Children in the CKD group were assessed by their estimated glomerular filtration rate (eGFR), calculated from height and serum creatinine using the modified Schwartz formula (26). Their annualized GFR decline averaged over the preceding two years, was also calculated and expressed as mL/minute/1.73 m2/year (27).
In the type 1 diabetic group, glycemic control was assessed by standard hemoglobin A1c (HbA1c), expressed as a percentage of total hemoglobin (28). This reflects blood glucose control over the preceding 2 to 3 months.
Statistical analysis
Although the measured outcomes in the three study groups were all continuous variables (HbA1c, FEV1, GFR slope), none of the data sets passed the D’Agostino-Pearson omnibus test for normal distribution. Consequently, all data sets were managed using nonparametric methods. Values were expressed as median plus interquartile range. Two means were compared by the Mann–Whitney test. Three or more means were compared using the Kruskal–Wallace test, with Mann–Whitney post-hoc test. The correlation between health status and distance from subspecialty clinic was tested using linear regression (least squares fit). The degree of correlation was summarized by Pearson’s product-moment coefficient (r). Study sample size was limited by patient numbers in BC (roughly 100 with CKD and 90 with CF who could perform lung function). Assuming normally distributed data, a measurement standard deviation of 25%, 80% power and 5% chance of a type 1 error, the study required 40 subjects in each arm to detect a 15% difference between groups. Since all data sets were nonparametric, the sample size calculation can only be viewed as an estimate.
RESULTS
Main characteristics of study population
Over 12 months, we were able to enrol 96 of the 130 children in BC with nondialyzed CKD (74%) and 85 of the 96 children with CF (89%) who were able to perform lung function tests. We sampled roughly 5% of the 2,000 children with T1D living in BC. The final study total was 270 families (96 CKD, 85 CF and 89 T1D). The characteristics of the study children and their primary caregivers are listed in Table 1. Nineteen families refused to enter the study (13 CKD, 2 CF and 4 T1D). Those who enrolled did not show reluctance to answer sensitive questions concerning ethnicity or income.
Table 1.
Characteristics of study patients and their primary caregivers
| Chronic kidney disease | Cystic fibrosis | Type 1 diabetes | |
|---|---|---|---|
| Patient demographics | |||
| Number of patients | 96 | 85 | 89 |
| Age (months) | Median 125 (IQR 58–175). | Median 144 (IQR 106–184). | Median 156 (IQR 112–190). |
| Female | 31 (32%) | 39 (46%) | 36 (40%) |
| Preterm (<37 weeks) | 29 (30%) | 10 (12%) | 8 (9%) |
| Term (37–42 weeks) | 66 (69%) | 68 (80%) | 76 (85%) |
| Post-term (>42 weeks) | 1 (1%) | 3 (4%) | 5 (6%) |
| Birth weight (kg) | Median 3.26 (IQR 2.60–3.72) | Median 3.35 (IQR 2.97–3.66) | Median 3.50 (IQR 3.14–3.86) |
| Mechanical ventilation at birth | 17 (18%) | 5 (6%) | 3 (3%) |
| Has a family doctor | 86 (90%) | 76 (89%) | 86 (97%) |
| Has a paediatrician | 64 (67%) | 26 (31%) | 40 (45%) |
| Sibling with same illness | 0 (0%) | 19 (22.3%) | 2 (2.2%) |
| Primary caregiver demographics | |||
| Mother caregiver | 76 (79%) | 64 (75%) | 65 (73%) |
| Father caregiver | 15 (16%) | 19 (22%) | 22 (25%) |
| Other caregiver | 5 (5%) | 2 (2%) | 2 (2%) |
| Immigrant | 39 (41%) | 10 (12%) | 21 (24%) |
| Refugee | 2 (2%) | 0 (0%) | 1 (1%) |
| Extended health insurance | 68 (71%) | 64 (75%) | 72 (81%) |
| Ownership of: | |||
| Cellphone | 89 (93%) | 80 (94%) | 83 (93%) |
| Laptop | 77 (80%) | 72 (85%) | 80 (90%) |
| Car | 81 (84%) | 82 (96%) | 87 (98%) |
| House | 64 (67%) | 58 (68%) | 70 (78%) |
| Smoker | 8 (8%) | 5 (6%) | 10 (11%) |
| Has a major health condition | 28 (29%) | 26 (31%) | 26 (29%) |
| Married | 64 (67%) | 60 (71%) | 72 (81%) |
| Divorced | 10 (10%) | 9 (11%) | 8 (9%) |
| Single | 6 (6%) | 8 (9%) | 4 (5%) |
| Common-law | 16 (17%) | 7 (8%) | 4 (5%) |
| Unemployed/laid off | 17 (18%) | 13 (15%) | 10 (11%) |
| Disability Leave | 4 (4%) | 1 (1%) | 1 (1%) |
| Part time | 19 (20%) | 21 (25%) | 27 (30%) |
| Full time | 53 (55%) | 48 (57%) | 50 (56%) |
| Student | 3 (3%) | 2 (2%) | 1 (1%) |
The main characteristics of enrolled patients and their primary caregivers, listed by diagnostic group. Absolute numbers are followed by percentage of group total in brackets. Average values are expressed as median with IQR in brackets.
IQR Interquartile range.
Geographic distribution of study population
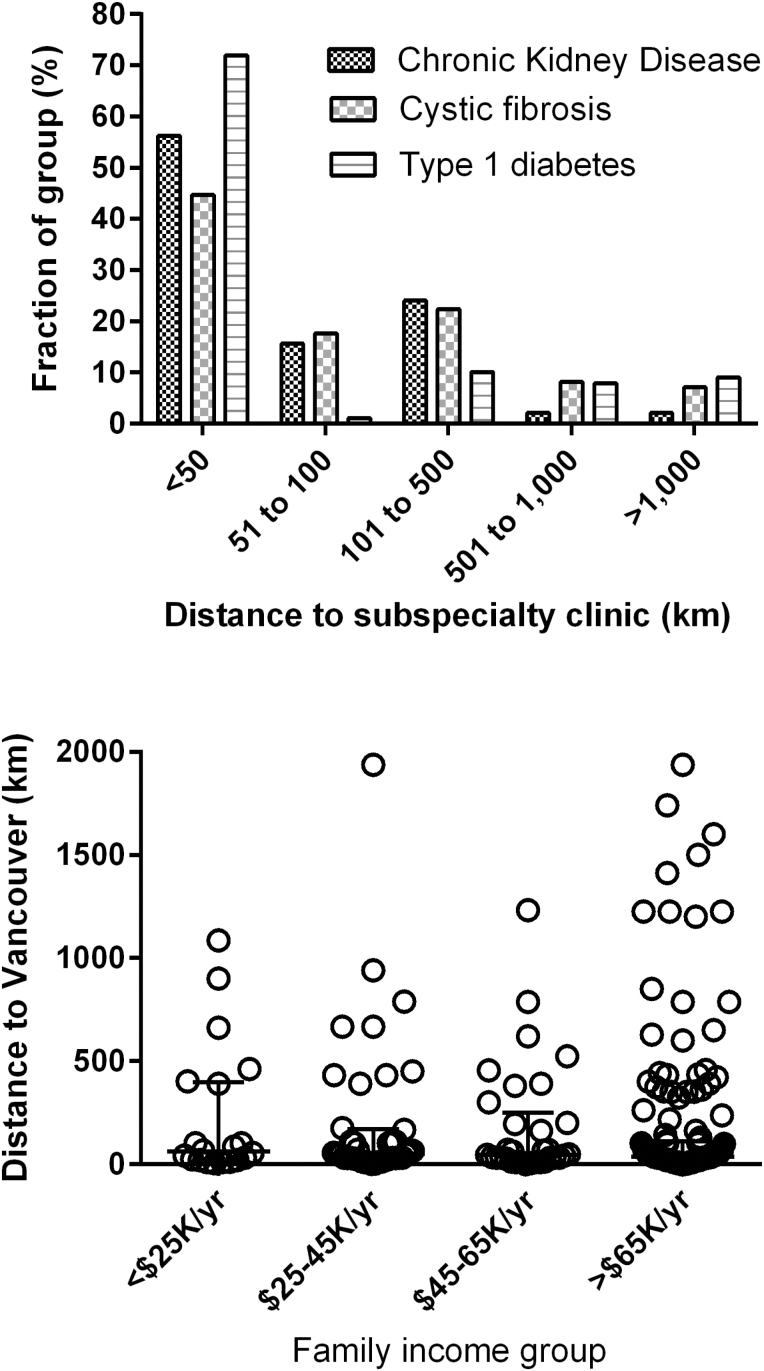
British Columbia is a large Province so travel distances are considerable—particularly for those travelling from Yukon Territory. The geographical distribution of the enrolled groups, reflected the normal population distribution in BC (Figure 1), where roughly 50% live within 50 km of Vancouver, 10% live in southern Vancouver Island (50 to 100 km) and 15% live in south-central BC (100 to 500 km) (29). More than 10% of the families made over a 1,000 km round trip for care, three or four times each year.
Figure 1.
Provincial distribution of family residence and family income groups. Top: Provincial distribution of the three patient groups, expressed as distance in kilometres from subspecialty clinics in Vancouver. Bottom: combined data from all three groups showing Provincial distribution of family income groups.
Health outcomes and distance to subspecialty care
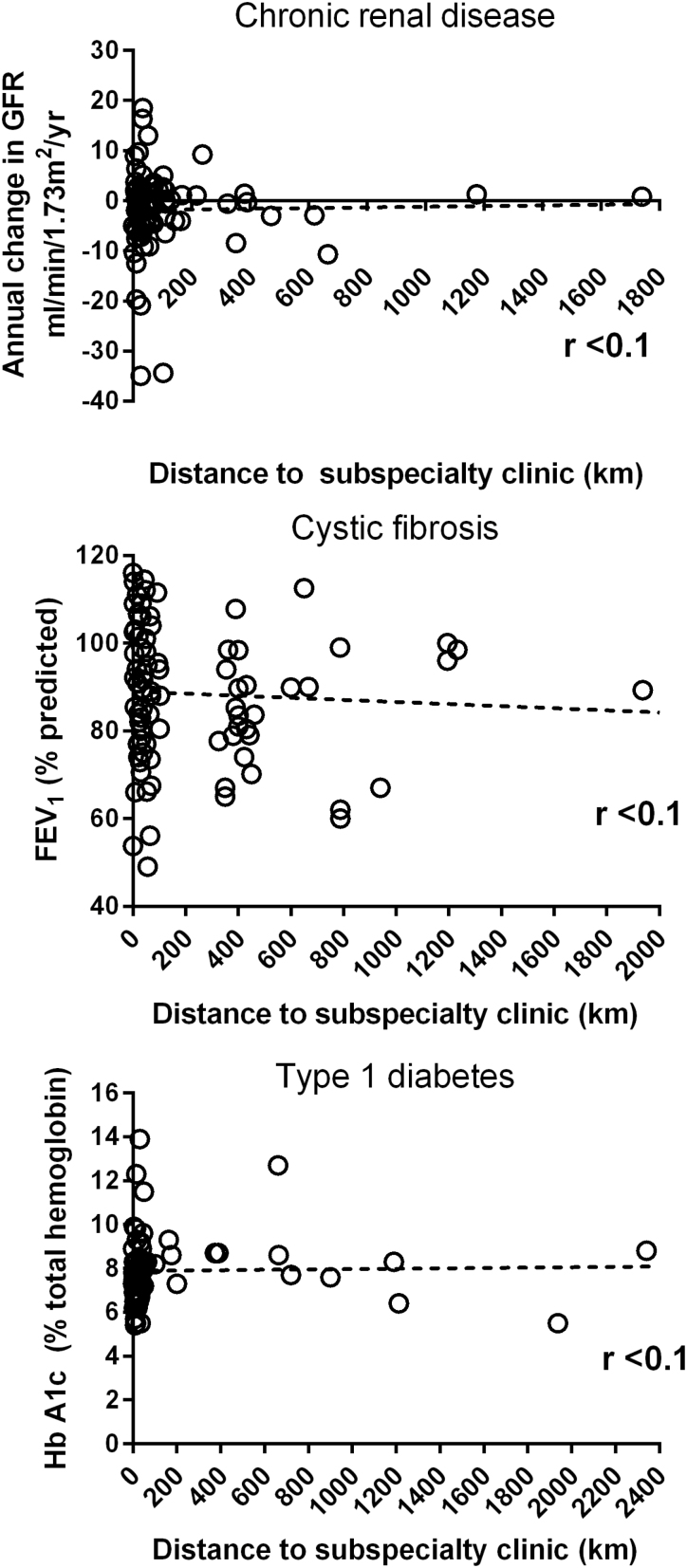
In contrast to findings from studies in other regions (30), we found no relationship between health outcomes in any of the three study groups and their distance from subspecialty care (Figure 2, Table 2). Similarly, while rural residence and poverty are closely related in some areas of the world (31), we found no evidence for a relationship between distance from Vancouver and family income (Figure 1, bottom; Table 2).
Figure 2.
Child health outcomes and distance from subspecialty care. The relationship between current health status and distance from subspecialty clinic plotted for three groups of children with chronic diseases. Top: chronic kidney disease, middle: cystic fibrosis, bottom: type 1 diabetes. The regression line is calculated by least squares fit and shown as a dotted line on each graph. The degree of correlation is summarized by Pearson’s product-moment coefficient (r).
Table 2.
Summary of results of statistical analyses
| Relationship tested | Statistical analysis | Chronic kidney disease GFR slope (mL/min/1.73 m2/year) |
Cystic fibrosis FEV1 (% predicted) |
Type 1 diabetes HbA1c (% total Hb) |
|---|---|---|---|---|
| Distance from clinic and child’s health. | Linear regression | No relationship, r<0.1 | No relationship, r<0.1 | No relationship, r<0.1 |
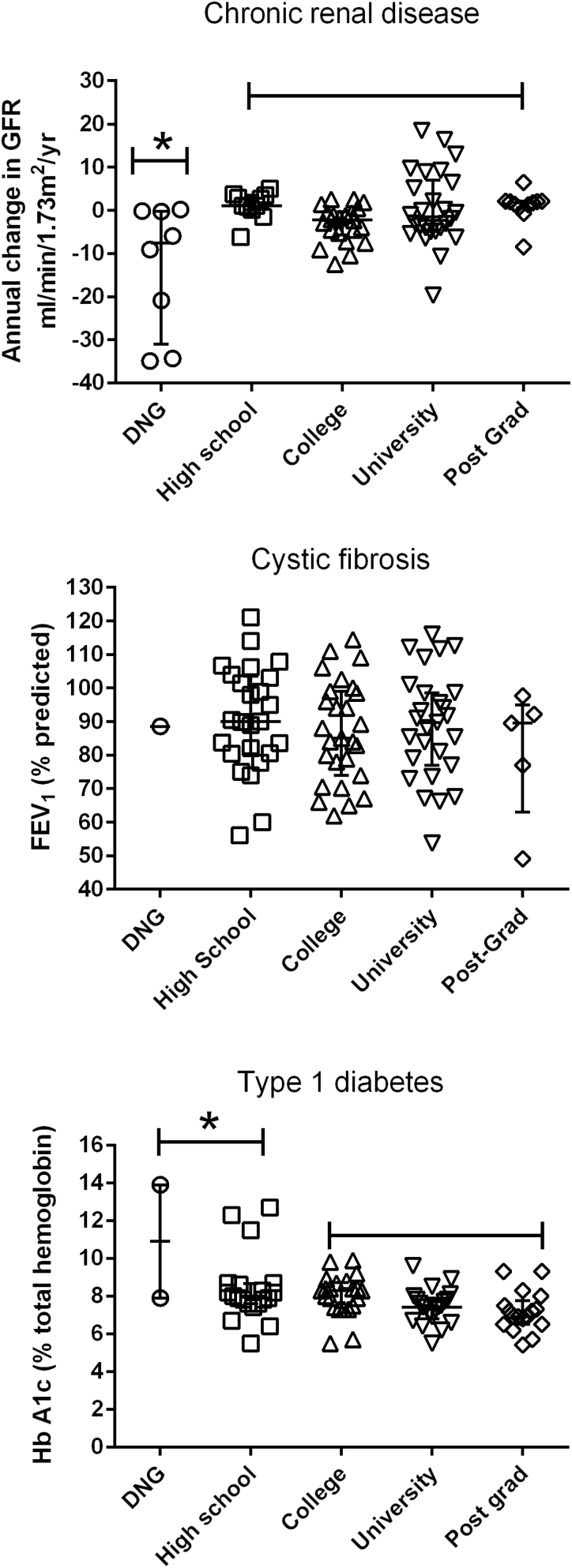
| Caregiver education and child’s health. | Kruskal–Wallace, post-hoc Mann–Whitney | DNG: –7.5(–31 to –0.2), ≥High school: -0.5(–4.1 to 2.1), Significant, P<0.01 | No significant group differences, P>0.05 | ≤High school: 8.0 (7.6–8.7), ≥College: 7.5 (6.9–8.4), Significant, P=0.04 |
| Patient ethnicity and child’s health | Kruskal–Wallace | No significant group differences. | Insufficient data spread | No significant group differences. |
| Patient gender and child’s health. | Mann–Whitney | Male: –0.4 (–3.9 to 2.4), Female: –2.1 (–7.8 to 1.3). Not significant, P=0.1 |
Male: 88.5 (74.8– 00.4), Female: 89.9 (80.4–98.5). Not significant, P=0.8 |
Male: 7.7(6.8–8.4), Female: 7.9 (7.3–8.6). Not significant, P=0.4 |
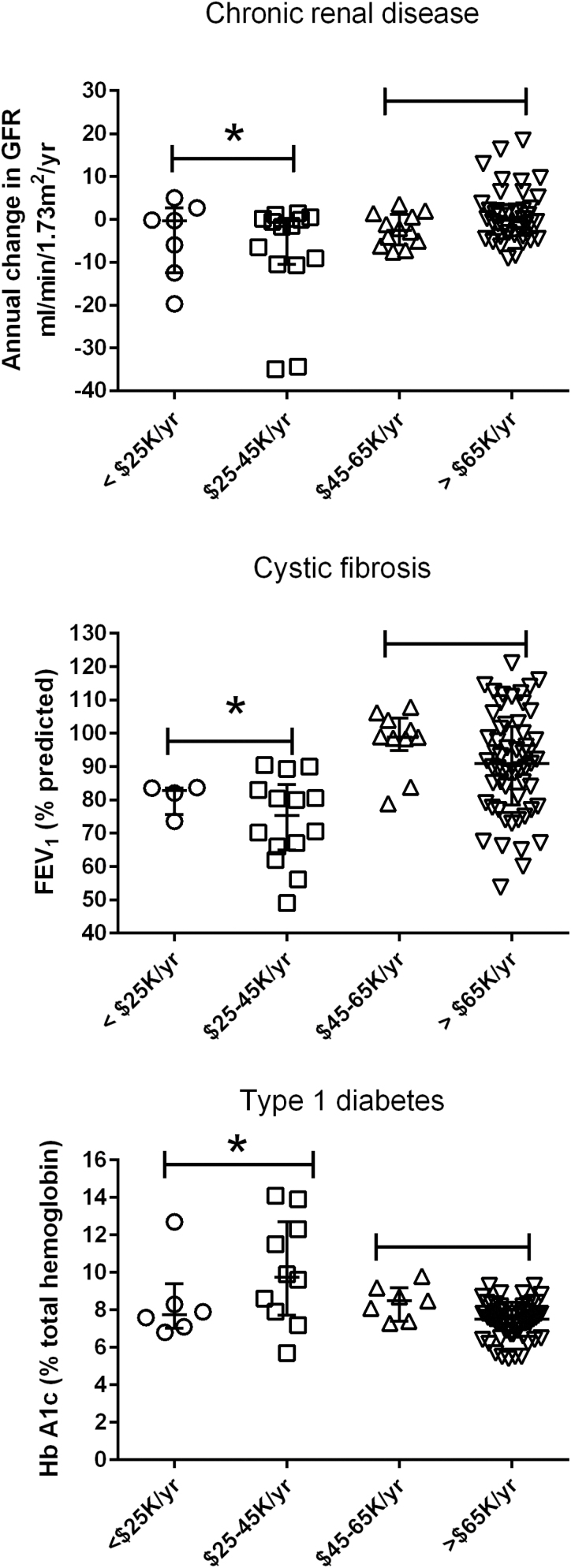
| Family income and child’s health. | Kruskal–Wallace, post-hoc Mann–Whitney | ≤$45K: –1.5 (–10.6 to 0.3) >$45K: –0.6 (–4 to 2.1). Significant P=0.03 |
≤$45K:80.2 (66.8–83.6) >$45K: 93.2 (81–102.6) Significant P<0.001 |
≤$45K: 8.5 (7.3–12.1) >$45K: 7.6 (6.9–8.3). Significant P<0.01 |
| Family income and distance from Vancouver. | Kruskal–Wallace, post-hoc Mann–Whitney | <$25K/year, 61.8 km (20.4–399) from Vancouver; $25–45K/year, 50 km (26.8–171); $45-65K/year, 45.7 km (20.5–250.3); >$65K/year, 37.5 km (19.4–110). No significant group differences, P=0.6 |
||
The relationship between five main social variables (distance from clinic, caregiver education, patient ethnicity, patient gender, family income) and the child’s current health in the three chronic disease groups (chronic kidney disease, cystic fibrosis, type 1 diabetes), plus the relationship between family income and distance from Vancouver. Values are expressed as median with interquartile range in brackets.
DNG Did not graduate; FEV 1 Forced expiratory volume in 1 s; GFR Glomerular filtration rate; HbA1c Hemoglobin A1c.
Health outcomes and family income
In keeping with the findings of many previous studies (32), family income appeared to be the primary determinant of health in our study population. In all three groups, children from families with total annual income below $45,000 had significantly worse health than those children living in families earning more than this cut-off (Figure 3, Table 2). The relationship was modest for the CKD group (P=0.03) but was much stronger for those children with T1D or CF (both P<0.01).
Figure 3.
Child health outcomes and family income. The relationship between current health status and annual family income plotted for three groups of children with chronic diseases. Top: chronic kidney disease, middle: cystic fibrosis, bottom: type 1 diabetes. Asterisks represent significant differences compared to others in the group (Kruskal–Wallace, Dunn’s post-hoc test, P<0.05).
Health outcomes and primary caregiver’s education
Most caregivers in our study had graduated from High School, so conclusions about a relationship between caregiver education and child health should be treated with caution. A significant relationship between less educated caregivers and poor child health appears to be present for the CKD group (Figure 4, Table 2). There was no difference between group means for the CF patients although there was only one caregiver who did not graduate. A modest difference in outcomes (P=0.04) was found in the T1D group by combining the DNG and High School groups but, again, the number of lower educated caregivers was small, so this finding should be treated with caution.
Figure 4.
Child health outcomes and primary caregiver’s education. The relationship between current health status and primary caregiver’s highest education level plotted for three groups of children with chronic diseases. Top: chronic kidney disease, middle: cystic fibrosis, bottom: type 1 diabetes. DNG: did not graduate. Asterisks represent significant differences compared to others in the group (Kruskal–Wallace, Dunn’s post-hoc test, P<0.05).
Health outcomes and patient gender and ethnicity
There were no differences between mean outcomes for the ethnic categories in the T1D and CKD study groups (Table 2). There was insufficient spread of ethnicities in the CF group to allow conclusions to be drawn. We found no evidence of decreased health outcomes amongst First Nations’ children, in any of the disease groups, but the sample sizes were small. Patient gender also had no measurable effect on outcome in any group (Table 2). This included the CF group where decreased survival amongst females is well described (33).
Health outcomes and other caregiver variables
We analyzed other caregiver variables and found no relationships between health outcomes in the child and the caregiver’s immigrant status, caregiver employment, caregiver’s health insurance status and family home ownership.
DISCUSSION
Using a strict methodology that relied on direct collection of five different social determinants compared with carefully quantified clinical end points, we found that children with chronic health conditions, living in families with annual incomes below $45,000, had significantly worse health outcomes, compared to those living in wealthier families (Figure 3). Caregiver education was the only other determinant predictive of poor outcomes but the relationship was modest compared to the effects of poverty (Figure 4). Explanations for the adverse health effects of poverty and low education levels, include limited access to health care, harmful living conditions, poor understanding of therapies and lower treatment compliance (8,34), but the exact causes are unknown.
It should be emphasized that screening for social problems in routine clinics, is not an end in itself. The detection of at-risk children must be backed up by substantive action (35). Once detected, families should be linked to appropriate resources and referred early to community services. Such a process is complicated so it is likely that the position of ‘patient navigator’ will become an essential part of the care team. Before planning such a clinical service, it is important to have a deeper understanding of the problem based on current data. While the link between poverty and ill health has already been described in single studies of children with T1D (36), CF (37) and CKD (38), our work provides more detailed information than earlier research. We hope it will be of practical value for those providing care for children with chronic health conditions—particularly in a Canadian setting.
When comparing different studies, it is important to remember that methodological variations in social determinant research can produce significant potential errors (21). Despite the multifactorial nature of SES, many studies only examine one or two dichotomous determinants (poor/nonpoor, rural/urban) and compare them to imprecise outcomes such as a parent-reported health score (37), when in reality, both social determinants and health outcomes are usually continuous across a wide range (20). These problems are exacerbated by the use of more easily obtained proxy markers in place of direct patient examination (postal code for social class or Medicaid status for poverty) and the use of databases of questionnaire responses stored over many years (22). We minimized these errors by measuring five different social determinants and, where possible, quantifying them over a range of values. We also measured end points that accurately expressed the child’s health on the same day the social questionnaire was administered.
Our methodology allows better insights into the relationships between social factors and health outcomes and provides reliable data for those planning to meet the health challenges associated with adverse social factors. For example, rather than simply concluding that poverty and low education levels are associated with poorer health outcomes, our data provide research support for the Canadian definition of poverty for a family of four (currently a pretax annual income below $44,320 [39]) and also provide information on the education levels needed to provide optimum care for a child with complex health needs.
For the purposes of planning an effective social screening program, we can also define which factors have the greatest adverse effects on the health of children with chronic disease. Clearly, family poverty and caregiver education are the most important determinants but we provide data on several others. In a large province with centralized care, it was reassuring to find that the health of rural patients was not affected by their distance from specialty care. We also found no adverse health effects associated with patient gender, ethnicity or immigrant status. However, the absence of a gradient associated with ethnicity, particularly First Nations heritage, should be interpreted with caution because both the CF and T1D groups are skewed toward European-Canadians. Before applying our results to clinical practice in other areas, it is important to remember that some social factors vary widely. While inequalities due to poverty and poor education are near universal findings, other variables, such as rural poverty, can vary between and even within countries (32). While we found no adverse health effects related to rural residence or gender in BC, both have been associated with poor health outcomes in studies from other regions (31,33).
The UK (13), Canada (15) and, more recently, the WHO (14), have led the way in incorporating health determinants research into long term population health planning. While many of those initiatives require actions at government level, physicians still have an important part to play in reducing social-based health inequities (15,16). Clinic visits provide a unique chance to address social problems (40)—chances that are unfortunately often missed (41,42). Early studies have already shown that clinic-based social screening can increase access to community services (17) and also improve caregiver assessment of child health (18). Our study was designed to help us prepare for social screening in our clinics serving children with chronic health problems. We believe that this will soon become a routine part of contemporary paediatric practice, so we hope our results are of value to others planning similar interventions.
Acknowledgements
We would like to acknowledge Lee Er and the BC Provincial Renal Agency for their support with renal outcome data collection and analysis. We also acknowledge the Judi Bowden Memorial Fund for Respiratory Research which provided funds to support the four research students (Ms Tourigny, Ms DeMello, Mr Garcia Espinosa and Mr Hamilton).
Conflicts of Interest: None of the eight authors has personal, financial or business conflicts of interest with any aspect of this research study.
Funding Source: The four research students (Ms Tourigny, Ms DeMello, Mr Garcia Espinosa and Mr Hamilton), were funded by the Judi Bowden Memorial Fund for Respiratory Research. Apart from providing money, the funding source had no role in any aspect of the conduct of the study or manuscript preparation.
Ethical Approval: The study was performed in accordance with current research standards of ethics and human rights. The study protocol was reviewed and given formal research approval by the University of British Columbia’s research ethics committee. Registration number: H15-01896.
Study Registration: The study is registered with ClinicalTrials.gov. Registration number: NCT02203084.
Author Contributions: Ms Tourigny, Ms DeMello, Mr Garcia Espinosa and Mr Hamilton were responsible for collecting and storing all patient data. Dr Yang (cystic fibrosis), Dr Amed (type 1 diabetes) and Dr Dionne (chronic renal disease) were responsible for supervising the conduct of the study in their respective clinics. All listed authors were then equally involved in data analysis and preparation of the final manuscript. The corresponding author had full access to all study data and had final responsibility for the decision to submit the finished manuscript for publication.
References
- 1. Graham GN. Why your ZIP code matters more than your genetic code: Promoting healthy outcomes from mother to child. Breastfeed Med 2016;11:396–7. [DOI] [PubMed] [Google Scholar]
- 2. Hippocrates. On Airs, Waters and Places. 400 BCE <http://classics.mit.edu/Hippocrates/airwatpl.1.1.html> (Accessed May 2017).
- 3. Engels F. The Condition of the Working Class in England 1884. <http://www.gutenberg.org/ebooks/17306> (Accessed May 2017).
- 4. Krieger N. Historical roots of social epidemiology: Socioeconomic gradients in health and contextual analysis. Int J Epidemiol 2001;30(4):899–900. [DOI] [PubMed] [Google Scholar]
- 5. Marmot MG, Rose G, Shipley M, Hamilton PJ. Employment grade and coronary heart disease in British civil servants. J Epidemiol Community Health 1978;32(4):244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: What the patterns tell us. Am J Public Health 2010;100(Suppl 1):S186–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilkinson RG, Pickett KE. Income inequality and socioeconomic gradients in mortality. Am J Public Health 2008;98(4):699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Conti G, Heckman J, Urzua S. The education-health gradient. Am Econ Rev 2010;100(2):234–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clougherty JE, Souza K, Cullen MR. Work and its role in shaping the social gradient in health. Ann N Y Acad Sci 2010;1186:102–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kosteniuk JG, Dickinson HD. Tracing the social gradient in the health of Canadians: Primary and secondary determinants. Soc Sci Med 2003;57(2):263–76. [DOI] [PubMed] [Google Scholar]
- 11. Adler NE, Stewart J. Health disparities across the lifespan: Meaning, methods, and mechanisms. Ann N Y Acad Sci 2010;1186:5–23. [DOI] [PubMed] [Google Scholar]
- 12. Singh-Manoux A, Ferrie JE, Chandola T, Marmot M. Socioeconomic trajectories across the life course and health outcomes in midlife: Evidence for the accumulation hypothesis?Int J Epidemiol 2004;33(5):1072–9. [DOI] [PubMed] [Google Scholar]
- 13. Marmot M, Allen J, Bell R, Bloomer E, Goldblatt P; Consortium for the European Review of Social Determinants of Health and the Health Divide WHO European review of social determinants of health and the health divide. Lancet 2012;380(9846):1011–29. [DOI] [PubMed] [Google Scholar]
- 14. WHO Commission on Social Determinants of Health. Closing the Gap in a Generation: Health Equity Through Action on the Social Determinants of Health 2008. <http://www.who.int/social_determinants/final_report/csdh_finalreport_2008.pdf> (Accessed May 2017).
- 15. Public Health Agency of Canada. Reducing Health Disparities – Roles of the Health Sector: Discussion Paper 2005. <http://publications.gc.ca/collections/collection_2008/phac-aspc/HP5-3-2005E.pdf> (Accessed May 2017).
- 16. Andermann A; CLEAR Collaboration Taking action on the social determinants of health in clinical practice: A framework for health professionals. CMAJ 2016;188(17–18):E474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garg A, Toy S, Tripodis Y, Silverstein M, Freeman E. Addressing social determinants of health at well child care visits: A cluster RCT. Pediatrics 2015;135(2):e296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gottlieb LM, Hessler D, Long D, et al. Effects of social needs screening and in-person service navigation on child health: A randomized clinical trial. JAMA Pediatr 2016;170(11):e162521. [DOI] [PubMed] [Google Scholar]
- 19. Cohen E, Kuo DZ, Agrawal R, et al. Children with medical complexity: An emerging population for clinical and research initiatives. Pediatrics 2011;127(3):529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic status in health research: One size does not fit all. JAMA 2005;294(22):2879–88. [DOI] [PubMed] [Google Scholar]
- 21. Shavers VL. Measurement of socioeconomic status in health disparities research. J Natl Med Assoc 2007;99(9):1013–23. [PMC free article] [PubMed] [Google Scholar]
- 22. Krieger N, Waterman P, Chen JT, Soobader MJ, Subramanian SV, Carson R. Zip code caveat: Bias due to spatiotemporal mismatches between zip codes and US census-defined geographic areas–the public health disparities geocoding project. Am J Public Health 2002;92(7):1100–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stevens GD. Gradients in the health status and developmental risks of young children: The combined influences of multiple social risk factors. Matern Child Health J 2006;10(2):187–99. [DOI] [PubMed] [Google Scholar]
- 24. Ajarmeh S, Er L, Brin G, Djurdjev O, Dionne JM. The effect of a multidisciplinary care clinic on the outcomes in pediatric chronic kidney disease. Pediatr Nephrol 2012;27(10):1921–7. [DOI] [PubMed] [Google Scholar]
- 25. Miller MR, Hankinson J, Brusasco V, et al. ; ATS/ERS Task Force Standardisation of spirometry. Eur Respir J 2005;26(2):319–38. [DOI] [PubMed] [Google Scholar]
- 26. Schwartz GJ, Muñoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol 2009;20(3):629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Levey AS, Inker LA, Matsushita K, et al. GFR decline as an end point for clinical trials in CKD: A scientific workshop sponsored by the national kidney foundation and the US food and drug administration. Am J Kidney Dis 2014;64(6):821–35. [DOI] [PubMed] [Google Scholar]
- 28. Rewers M, Pihoker C, Donaghue K, Hanas R, Swift P, Klingensmith GJ. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatr Diabetes 2009;10(Suppl 12):71–81. [DOI] [PubMed] [Google Scholar]
- 29. British Columbia Population Estimates. Government of British Columbia, BC Stats, 2015. <http://www.bcstats.gov.bc.ca/AboutUs/AboutBCStats/PublicSectorResearchEval.aspx> (Accessed May 2017).
- 30. Probst JC, Moore CG, Glover SH, Samuels ME. Person and place: The compounding effects of race/ethnicity and rurality on health. Am J Public Health 2004;94(10):1695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eberhardt MS, Pamuk ER. The importance of place of residence: Examining health in rural and nonrural areas. Am J Public Health 2004;94(10):1682–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gupta RP, de Wit ML, McKeown D. The impact of poverty on the current and future health status of children. Paediatr Child Health 2007;12(8):667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harness-Brumley CL, Elliott AC, Rosenbluth DB, Raghavan D, Jain R. Gender differences in outcomes of patients with cystic fibrosis. J Womens Health (Larchmt) 2014;23(12):1012–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Porterfield SL, McBride TD. The effect of poverty and caregiver education on perceived need and access to health services among children with special health care needs. Am J Public Health 2007;97(2):323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garg A, Boynton-Jarrett R, Dworkin PH. Avoiding the unintended consequences of screening for social determinants of health. JAMA 2016;316(8):813–4. [DOI] [PubMed] [Google Scholar]
- 36. Carter PJ, Cutfield WS, Hofman PL, et al. Ethnicity and social deprivation independently influence metabolic control in children with type 1 diabetes. Diabetologia 2008;51(10):1835–42. [DOI] [PubMed] [Google Scholar]
- 37. Quittner AL, Schechter MS, Rasouliyan L, Haselkorn T, Pasta DJ, Wagener JS. Impact of socioeconomic status, race, and ethnicity on quality of life in patients with cystic fibrosis in the United States. Chest 2010;137(3):642–50. [DOI] [PubMed] [Google Scholar]
- 38. Minnick ML, Boynton S, Ndirangu J, Furth S. Sex, race, and socioeconomic disparities in kidney disease in children. Semin Nephrol 2010;30(1):26–32. [DOI] [PubMed] [Google Scholar]
- 39. Statistics Canada. Low-income thresholds for households in Canada 2010. <https://www12.statcan.gc.ca/nhs-enm/2011/ref/dict/table-tableau/t-3-2-eng.cfm> (Accessed May 2017).
- 40. Berkowitz SA, Hulberg AC, Hong C, et al. Addressing basic resource needs to improve primary care quality: A community collaboration programme. BMJ Qual Saf 2016;25(3):164–72. [DOI] [PubMed] [Google Scholar]
- 41. Hassan A, Blood EA, Pikcilingis A, et al. Youths’ health-related social problems: Concerns often overlooked during the medical visit. J Adolesc Health 2013;53(2):265–71. [DOI] [PubMed] [Google Scholar]
- 42. Fleegler EW, Lieu TA, Wise PH, Muret-Wagstaff S. Families’ health-related social problems and missed referral opportunities. Pediatrics 2007;119(6):e1332–41. [DOI] [PubMed] [Google Scholar]