Abstract
Verbal fluency tasks are generally thought to be mediated by frontal brain regions for letter fluency and temporal regions for category fluency. This idea, however, is primarily based on lesion studies and adapted versions of the fluency tasks in functional neuroimaging, without fundamental evidence from structural neuroimaging in healthy individuals. We investigated the cortical structural correlates of letter and category fluency, including overlapping and different regions, in 505 individuals who participated in a community-based study of healthy aging. The correlation between cortical thickness and verbal fluency in whole-brain analyses revealed distinct cortical signatures for letter fluency, primarily in frontal regions, and category fluency, in frontal and temporal-parietal regions. There was a dissociation in the left inferior frontal gyrus between letter and category fluency, with increased thickness in the posterior-dorsal versus anterior-ventral parts, respectively. These results distinguish the detailed anatomical correlates for verbal fluency within the coarse frontal-temporal distinction inferred from lesion studies and among the mixture of regions identified in functional neuroimaging. The evidence for the anatomical substrates of letter and category fluency, each recruiting slightly different language and cognitive processes, can serve both clinical applications as well as a deeper theoretical understanding of the organization of the cerebral cortex.
Keywords: frontal, inferior frontal gyrus, phonemic fluency, semantic fluency, temporal
Introduction
Verbal fluency tasks—naming as many items of a given criterion, typically letters or categories, under time constraints—are widely administered and popular tools in both research and clinical settings. Given the identical administration, yet distinct content, letter and category tasks provide intraindividual as well as group-based contrasts for wide-ranging clinical diagnostic applications and research purposes (e.g., Eng et al. 2018). For example, individuals with Alzheimer’s disease generally perform worse on category than letter fluency (e.g., Monsch et al. 1992), while children with the auditory linguistic/dysphonetic form of developmental dyslexia can be differentiated from normally developing children based on their poor letter fluency score (Cohen et al. 1999).
The contrast in performance between the 2 tasks is thought to result from the recruitment of partly overlapping as well as partly dissociable cognitive processes and brain regions. With regard to cognitive processes, both verbal fluency tasks involve executive functioning skills (i.e., updating and inhibition) and indices of verbal ability (i.e., vocabulary size and lexical access) (e.g., Sauzéon et al. 2011; Shao et al. 2014). Nonetheless, letter fluency is more strongly linked to executive function abilities while category fluency relies more strongly on verbal abilities (e.g., Martin et al. 1994; Luo et al. 2010; Shao et al. 2014). Similarly, a dissociation has been suggested with regard to the brain regions that mediate performance on these 2 tasks. The general pattern regarding the anatomical locus of fluency tasks is that letter fluency performance is more strongly mediated by left frontal brain regions, while category fluency performance is more strongly mediated by left temporal regions (e.g., Gourovitch et al. 2000; Brickman et al. 2005).
The data employed to identify the association with these anatomical regions are primarily based on patient populations. Selective impairment in letter versus category performance corresponding to lesions or atrophy in frontal and temporal regions have been shown in a variety of neurologically impaired individuals, such as those with poststroke aphasia, Alzheimer’s disease, primary progressive aphasia, vascular dementia, and amyotrophic lateral sclerosis (Hodges et al. 1992; Troyer et al. 1998; e.g., Baldo et al. 2006; Jones et al. 2006; Laws et al. 2010; Quinn et al. 2012). A meta-analysis by Henry and Crawford (2004) across 1 791 individuals with focal cortical lesions quantified the effects of cortical damage on verbal fluency performance. They showed that the largest deficit on letter fluency was associated with left-lateralized frontal lesions, while the largest deficit on category fluency was associated with left temporal lesions.
Although the lesion method has contributed tremendously to the understanding of brain-behavior relationships, there are some limitations, including the “modularity” or “localization” assumption (i.e., that a given cognitive function is mediated by only the region that is bound by the lesion) and the assumption that areas surrounding lesions are functioning normally (Rorden and Karnath 2004). Functional magnetic resonance imaging (fMRI) studies of verbal fluency tasks have increased the understanding of the functional neuroanatomy of verbal fluency to some extent (e.g., Gaillard et al. 2000; Pihlajamaki et al. 2000; Abrahams et al. 2003; Gaillard et al. 2003; Costafreda et al. 2006; Basho et al. 2007; Meinzer et al. 2009; Sheldon and Moscovitch 2012). However, results from fMRI experiments are variable and implicate a large number of regions inconsistently across studies. This variability may be due in part to paradigm-dictated adaptations of the task, in which word-generation is either cued or covert, as well as motion artefacts (for discussion, see Birn et al. 2004; Grogan et al. 2009; Birn et al. 2010; Di et al. 2012).
A previous volume-based analysis of structural brain differences among healthy adults in letter and category fluency exemplified the success of using structural imaging techniques (Grogan et al. 2009). The authors analyzed regions related to overall fluency performance and those that differed between letter and category fluency, by subtracting the behavioral scores of the fluency tasks and subsequently relating this difference score to cortical volume measures. They investigated regions-of-interest (ROIs) restricted to various coordinates based on the fMRI literature. One of their ROIs included the inferior frontal gyrus (IFG), as a large number of studies reported an anatomic dissociation for verbal fluency in the IFG, in which category fluency is represented in the more anterior-ventral and letter fluency in the more posterior-dorsal region (e.g., Bokde et al. 2001; Bookheimer 2002; Costafreda et al. 2006). Grogan and colleagues found a positive correlation with volume in the left inferior temporal lobe for category relative to letter fluency, but no additional relations between volume and fluency, potentially related to a relatively small sample size.
Recent advances in the analysis of structural MRI allow for the quantification of cortical thickness across the entire hemispheric cortical mantle, which provides a biologically meaningful assessment of the neuroanatomy underlying specific cognitive processes (Dickerson et al. 2008). Cortical volume is a function of the morphological properties of thickness and surface area, yet these measures are genetically and phenotypically independent (Winkler et al. 2010). Cortical thickness, over and above cortical volume, is one of the most important factors related to various cognitive functions, including executive abilities and vocabulary (e.g., Hartberg et al. 2010; Schmidt et al. 2016), especially when interrogated with vertex-specific general linear models (Righart et al. 2017).
To examine and derive the distinct neuroanatomical patterns that support the 2 verbal fluency tasks, the current study focused on vertex-wise analysis of cortical thickness as it relates to performance on verbal fluency in a large and diverse sample of healthy older adults. Based on previous lesion and imaging studies, we hypothesized that better letter fluency performance would be correlated with thicker cortical surface in the left frontal cortex, and better category fluency performance to be correlated with thicker cortex in left temporal-parietal regions. We moreover investigated whether there are regions in which cortical thickness uniquely correlates with only one of the fluency measures and regions that are common between both measures, and if so, if the strength of the correlation was similar or different between the 2 fluency measures. We hypothesized that there would be an anterior–posterior distinction between anatomical correlates of letter and category fluency, particularly in the IFG.
Materials and Methods
Participants, Materials, and Procedure
Participants were recruited from the Washington Heights-Inwood Columbia Aging Project (WHICAP), an ongoing community-based study of cognitive aging based in northern Manhattan. The project was designed to examine the epidemiology of cognitive aging and dementia with a focus on risk factors, biological markers, socio-demographic factors, and cognitive profiles of older adults living in the community (Stern et al. 1992; Tang et al. 2001). Participants were evaluated with a neuropsychological battery approximately every 18–24 months that included tests of memory, orientation, language, abstract reasoning, and visuospatial ability.
From a subgroup of 552 individuals who underwent 3 T MRI scanning, we examined a total of 505 (91%) individuals; inclusion criteria were for participants not to be diagnosed with dementia at the time of the MRI scan, have valid cortical thickness data, and have no missing fluency scores. Table 1 summarizes the characteristics of the participants.
Table 1.
Participant characteristics
| Variable | Measure |
|---|---|
| Age in years (mean+SD, range) | 74.1 ± 5.8, 62–96 |
| Education in yearsa (mean+SD, range) | 12.9 ± 4.4, 2–20 |
| Sex (n, %women) | 281, 55.6% |
| Language of administrationb (n, % English) | 376, 74.5% |
| Handednessc | |
| n, % right | 461, 91.3% |
| n, % left | 32, 6.3% |
| n, % ambidextrous | 7, 1.4% |
| n, % switched | 4, 0.8% |
| Race/ethnicityd | |
| n, % Black | 182, 36.0% |
| n, % Hispanic | 150, 29.7% |
| n, % other race | 14, 2.8% |
aMissing data for 2 participants.
bNeuropsychological testing occurred in a participant’s dominant language.
cMissing data for 1 participant; switched = switched from left to right.
dThe ethnic/racial breakdown was based on self-report using the format of the 2000 US Census.
As part of their neuropsychological evaluation, participants performed letter and category fluency tasks. Participants were asked to generate as many words as possible that begin with each of 3 letters (C, F, and L when tested in English; P, S, and V when tested in Spanish) and each of 3 semantic categories (animals, clothing, and food) in separate 60-s trials. The mean number of words generated over the 3-letter fluency trials and the mean number of words generated over the 3 category fluency trials were calculated; these 2 scores were used as the predictors in the models.
MRI Acquisition and Preprocessing
All participants underwent high-resolution structural brain MRI at Columbia University on a 3 T Philips Achieva scanner. Structural MRI sequences included a magnetization prepared rapid gradient echo (MPRAGE) to obtain whole-brain, 3-dimensional T1-weighted images (165 slices in transverse orientation; slice thickness = 1 mm; field of view = 256 mm × 200 mm × 165 mm; voxel size = 1.0 × 1.0 × 1.0 mm3; repetition time = 6.6 ms; echo time = 3 ms; flip angle = 8 degrees). The obtained T1-weighted images were used for vertex-wise cortical surface-based morphometry analyses in FreeSurfer (Version 6.0, http://surfer.nmr.mgh.harvard.edu/). Cortical thickness was derived with the FreeSurfer automated processing stream for cortical reconstruction.
Statistical Analysis
For behavioral analyses, we used descriptive statistics to report the distributional characteristics of performance on both the letter and category fluency tasks. We used a mixed design general linear model with letter and category fluency as repeated measures, age and years of education as continuous variables, and sex, language of administration, and handedness as fixed factors. We used Pearson (for continuous variables) and point biserial (for categorical variables) correlation coefficient analyses to calculate the correlations of the demographic variables with the 2 fluency measures and mean cortical thickness.
For imaging analyses, we used the Freesurfer QDEC analytic framework (version 1.5) to correlate regional cortical thickness with performance on letter and category fluency tasks. Analyses generated whole-brain vertex-wise parametric maps of correlation coefficients and associated P values. We corrected for multiple comparisons using Monte Carlo Z simulation at P < 0.005 with the sign positive to test whether thicker cortex was associated with better performance. A separate Monte Carlo Z simulation with the sign negative (indicating a correlation between better performance and thinner cortex) did not yield significant areas of association.
We first performed analyses using general linear models with cortical thickness as the dependent variable and letter or category fluency performance as the predictor variable in separate models for both hemispheres. We did not include demographic variables in the models as covariates, as all but age did not influence both the predictor and outcome variables (Table 2); age is likely in the causal pathway that links cortical thickness and verbal fluency and therefore conceptualized not as a confounder but rather a preceding causal factor in the relationship between cortical thickness and verbal fluency. Notably, a primary purpose of this study was to compare differences in patterns of brain regions associated with each fluency measure, and thus, the demographic variables are identical between these 2 models.
Table 2.
Correlation matrix of demographic variables with fluency performances and mean cortical thickness
| Letter fluency | Category fluency | Mean thickness | |
|---|---|---|---|
| Age | |||
| r | −0.072 | −0.356 | −0.305 |
| P | 0.107 | <0.001 | <0.001 |
| Sex | |||
| r | 0.022 | 0.097 | 0.191 |
| P | 0.622 | 0.030 | <0.001 |
| Education | |||
| r | 0.513 | 0.470 | 0.073 |
| P | <0.001 | <0.001 | 0.103 |
| Language of administration | |||
| r | −0.380 | −0.344 | −0.021 |
| P | <0.001 | <0.001 | 0.637 |
| Handedness | |||
| r | 0.018 | 0.027 | 0.021 |
| P | 0.679 | 0.541 | 0.642 |
Note: r = Pearson or point biserial correlation coefficient, P = P value.
Subsequently, to investigate the overlap and difference in regional variations in thickness between the 2 tasks, masks of significant clusters as identified by Monte Carlo simulation were created in MATLAB 2013b and overlaid on the same surface to demonstrate the spatial overlap in QDEC. Additionally, a Meng’s Z test (P < 0.005, one-sided; Meng et al. 1992) was used to test for a reliable difference in correlation coefficients from 2 dependent correlations. This procedure allowed us to identify regions that uniquely correlated with only one of the fluency measures and, in case of overlapping regions, to calculate if the strength of the correlation was similar or different between the 2 fluency measures. Meng’s Z test was restricted to the total surface encompassed by either mask of significant correlation.
Results
Behavioral analyses
Average performance on letter fluency across the 3 trials ranged from 1.30 to 28.70, with a mean of 11.63 items per individual (SD = 4.64). On category fluency, average performance across the 3 trials ranged from 3.33 to 33.00, with a mean of 16.87 items per individual (SD = 4.54).
Participants generated more words on category than letter fluency (F(1,498) = 105.516, P < 0.001). Increasing age (F(1,498) = 65.664, P < 0.001) and lower number of years of education (F(1,498) = 4.859, P = 0.028) were associated with worse performance. Overall fluency was higher in women than men (F(1,498) = 4.002, P = 0.046). Fluency performance was similar between those tested in English and Spanish (F(1,498) = <0.001, P = 0.997) and those who were right-handed and other than right-handed (F(1,498) = 0.001, P = 0.982).
Table 2 provides the correlation matrix of demographic variables with the 2 fluency performances and mean cortical thickness. Older age correlated with lower mean cortical thickness and lower category fluency performance, but not with letter fluency performance. Men had lower cortical thickness than women but sex did not correlate with fluency performance. More years of education and English compared with Spanish correlated with higher fluency performance but not with mean cortical thickness. Handedness did not correlate with either verbal fluency performance or mean cortical thickness.
Cortical Thickness and Fluency
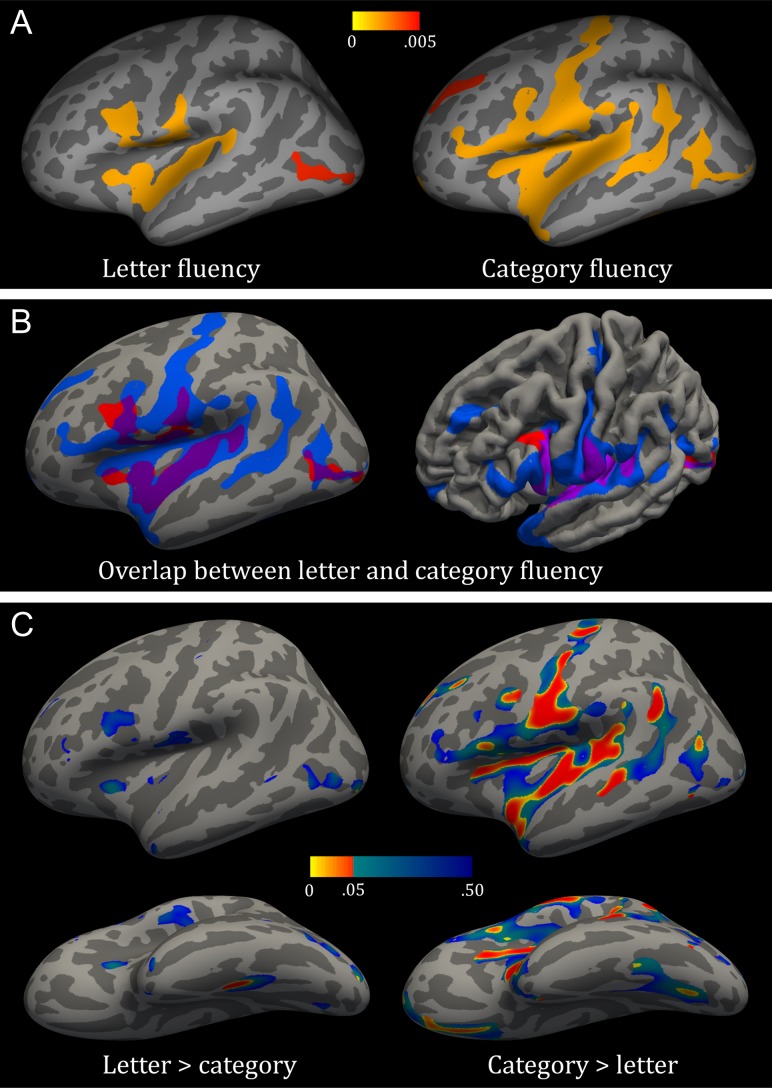
Statistical and visual details of significant clusters for letter and category fluency are presented in Table 3 and Figure 1A. Better letter fluency performance was associated with an increased cortical thickness in regions of the left frontal lobe, namely the pars opercularis in the IFG, the inferior part of the caudal middle frontal gyrus, the posterior-inferior part of the rostral middle frontal gyrus, and the inferior part of the precentral gyrus. Positive correlations in the left parietal lobe were seen in the inferior part of the postcentral gyrus and the precuneus. In the left temporal lobe, clusters of positive correlations involved the insula, superior temporal gyrus, transverse temporal (i.e., Heschl’s gyrus), and fusiform gyrus. Clusters of positive correlations in the left occipital lobe encompassed the lingual gyrus, transverse occipital sulcus, cuneus, and pericalcarine. The pattern in the right hemisphere was largely symmetrical to that in the left hemisphere; however, differences included less immersion of the pars opercularis and insula, and more of the postcentral gyrus and inferior parietal lobule.
Table 3.
Cortical thickness clusters associated with letter and category fluency represented by the coordinates of each cluster’s maximum
| Region at cluster maximum | Hemisphere | x | y | z | CWP | Size | NVtxs |
|---|---|---|---|---|---|---|---|
| Letter fluency | |||||||
| Superior temporal gyrus | L | −46.2 | −24.1 | 7.7 | 0.0001 | 2126.73 | 4949 |
| R | 47.7 | −9.9 | −14.9 | 0.0173 | 422.49 | 742 | |
| Rolandic operculum | L | −45.0 | −0.7 | 11.6 | 0.0001 | 1489.96 | 3513 |
| R | 54.6 | 1.0 | 5.4 | 0.0029 | 575.16 | 1117 | |
| R | 45.5 | −11.8 | 28.0 | 0.0001 | 1502.3 | 3559 | |
| Lingual gyrus | L | −26.0 | −63.6 | −1.8 | 0.0001 | 919.22 | 1525 |
| Superior occipital gyrus | L | −11.5 | −80.0 | 6.4 | 0.0001 | 731.55 | 1235 |
| Precuneus | R | 6.7 | −57.3 | 21.3 | 0.0001 | 1516.34 | 2670 |
| Middle occipital gyrus | L | −33.4 | −88.3 | −2.7 | 0.0034 | 535.17 | 878 |
| Supramarginal gyrus | R | 49.1 | −35.3 | 26.4 | 0.0001 | 3314.91 | 8094 |
| Angular gyrus | R | 42.0 | −67.1 | 15.7 | 0.0047 | 526.57 | 795 |
| R | 24.8 | −75.6 | 28.1 | 0.0345 | 363.83 | 515 | |
| Precentral gyrus | R | 11.0 | −26.6 | 58.1 | 0.0094 | 469.52 | 1203 |
| Fusiform gyrus | R | 31.7 | −47.3 | −9.1 | 0.0002 | 1334.84 | 2057 |
| Category fluency | |||||||
| Superior temporal gyrus | L | −46.5 | −18.7 | 1.7 | 0.0001 | 9018.45 | 20 866 |
| R | 49.7 | −20.7 | −4.7 | 0.0001 | 10 194.12 | 24 411 | |
| Angular gyrus | L | −48.9 | −51.8 | 29.0 | 0.0001 | 1167.49 | 2678 |
| Fusiform gyrus | L | −30.0 | −60.3 | −2.6 | 0.0001 | 1129.88 | 1825 |
| R | 31.3 | −46.8 | −9.4 | 0.0001 | 2018.18 | 3117 | |
| Gyrus rectus | L | −9.3 | 23.4 | −17.2 | 0.0001 | 877.91 | 1548 |
| Middle temporal gyrus | L | −43.2 | −67.6 | 12.5 | 0.0001 | 717.83 | 1269 |
| L | −52.9 | −11.1 | −18.1 | 0.0223 | 390.20 | 694 | |
| Middle frontal gyrus | L | −25.8 | 30.3 | 29.7 | 0.0035 | 531.04 | 948 |
| Mmiddle occipital gyrus | L | −31.8 | −71.8 | 22.3 | 0.0378 | 346.15 | 535 |
| R | 40.6 | −64.2 | 5.6 | 0.0120 | 448.93 | 751 | |
| Postcentral gyrus | L | −40.2 | −26.2 | 39.1 | 0.0410 | 337.97 | 924 |
| Precuneus | R | 4.6 | −56.6 | 19.70 | 0.0001 | 2006.28 | 3649 |
| Precentral gyrus | R | 6.4 | −20.9 | 51.3 | 0.0094 | 470.13 | 1011 |
| Pars opercularis | R | 50.8 | 13.2 | 4.1 | 0.0041 | 547.74 | 1043 |
| Pars orbitalis | R | 32.9 | 29.9 | −11.5 | 0.0065 | 498.26 | 848 |
| Cuneus | R | 7.0 | −85.4 | 18.5 | 0.0041 | 549.44 | 707 |
Note: Annotation of regions based on the Automated Anatomical Labeling (AAL) atlas. Coordinates are in Talairach space. CWP = cluster-wise P value; size = surface area (mm2) of cluster; NVtxs = number of vertices in cluster.
Figure 1.
The relation between cortical thickness and verbal fluency conditions. (A) Patterns of thickness related to letter fluency (left) and category fluency (right); color-scale bar represents P values. (B) Overlap in regions associated with letter (red) versus category fluency (blue) on an inflated surface (left) and tilted pial surface to highlight the dissociation in the inferior frontal gyrus from a light anterior-dorsal angle (right) (C) Statistically stronger correlation with cortical thickness for letter than category fluency (left) and category than letter fluency (right) in lateral (top) and ventral (bottom) views; color-scale bar represents P values.
For category fluency, clusters in the left frontal lobe indicating positive correlations between performance and cortical thickness included the pars triangularis and pars opercularis of the IFG, precentral gyrus, superior, rostral, and caudal middle frontal gyri, and the medial orbitofrontal gyrus. In the left parietal lobe, correlations encompassed the postcentral gyrus and the inferior parietal lobule including the supramarginal and angular gyri. Clusters of positive correlations in the left temporal pole included the insula, superior, middle, and inferior temporal gyri, superior temporal sulcus, transverse temporal, entorhinal cortex, and fusiform gyrus. Lastly, positive correlations in the left occipital lobe were seen in the lateral occipital cortex and lingual gyrus. Again, clusters in the right hemisphere were in large part symmetrical to those in the left hemisphere; however, less immersion was seen of the pars triangularis and inferior parietal lobule, and more of the precuneus.
The overlap and differences in regions associated with each fluency task in the left hemisphere are visualized in Figure 1B. Statistical comparison testing for each task’s independent effect on cortical thickness (Fig. 1C) showed that the positive correlation between thickness and category fluency was stronger (P < 0.05) than that with letter fluency in the ventral-anterior part of the pars opercularis, middle part of the precentral gyrus, dorsal part of the rostral middle frontal gyrus, medial orbitofrontal cortex, posterior-inferior part of the caudal middle frontal gyrus, middle and dorsal parts of the postcentral gyrus, insula, transverse temporal, superior temporal gyrus, supramarginal gyrus, angular gyrus, and lateral occipital gyrus. In contrast, the positive correlation between thickness and letter fluency was significantly stronger than that with category fluency in the middle part of the fusiform gyrus.
Discussion
This study determined the structural cortical brain correlates of letter and category fluency task-performance in older adults. Although several previous studies were designed with the assumption that letter fluency is a frontal process and category fluency a temporal process (e.g., Martin et al. 1994; Shao et al. 2014), little evidence from structural MRI data exists to support this dissociation. Our results are consistent with this assumption that different brain regions are involved in verbal fluency. They highlight that letter fluency is mediated by inferior frontal, and medial and insular temporal regions, while category fluency is mediated by a large network including frontal as well as unique regions in the posterior temporal and inferior parietal cortex.
The left IFG in particular is associated consistently with both letter and category fluency in both lesion and fMRI studies (Pihlajamaki et al. 2000; e.g., Abrahams et al. 2003; Gaillard et al. 2003; Henry and Crawford 2004; Basho et al. 2007; Meinzer et al. 2009; Sheldon and Moscovitch 2012). Previous literature proposes functional fractionation within the IFG when it comes to the exact coordinates of letter versus category fluency (e.g., Bokde et al. 2001). Letter fluency, calling on mainly phonological processes, has been proposed to have a posterior-dorsal locus within the IFG. Category fluency, calling on mainly semantic processes, has been proposed to have an anterior-ventral locus. A review by Bookheimer (2002) of 15 fMRI studies on the centers of activation regarding semantic and phonological aspects of word processing showed both the dorsal–ventral and anterior–posterior dissociation. A meta-analysis by Costafreda et al. (2006) compared peak activations in the IFG for each task across 17 studies and found that letter fluency activates the IFG more dorsally whereas category fluency activates the IFG more ventrally. In line with these previous reports, our results showed a clear dissociation in the IFG between letter and category fluency in both the anterior–posterior and ventral–dorsal directions. Thicker cortex in the anterior-ventral part of the IFG correlated with better performance on category fluency, while thicker cortex in the posterior-dorsal part correlated with better letter fluency. This finding is also in line with the broader picture of the localization of language in the brain, as the IFG has been reliably implicated in word production and word comprehension in both lesion and fMRI studies (e.g., Baldo et al. 2006; Costafreda et al. 2006).
Besides a relation between fluency and thickness in the left hemisphere, we also found clusters associated with each fluency variant in the right hemisphere, more or less mirrored to the fluency patterns for each task in the left hemisphere. As it is well-known that language is primarily left lateralized, these results are most likely explained by the generally symmetrical distribution and individual differences of cortical thickness in the 2 hemispheres (e.g., Lemaitre et al. 2012). Important differences to note between regions associated with fluency in the right versus left hemisphere, however, are the absence of an association with the anterior IFG for category fluency and the dorsal IFG for letter fluency, as well as an absence of the posterior temporal associations with category fluency in the right hemisphere. Thus, the regions specifically of interest for letter and category fluency based on the previous literature were only observed in the left hemisphere. This asymmetry provides additional evidence for the left-lateralized localization of letter fluency in frontal regions, and category fluency in temporal-posterior and inferior frontal regions, in agreement with the difference in fluency performance shown in individuals with left versus right focal frontal- and temporal-lobe lesions (Troyer et al. 1998).
Besides the difference in measurement (volume versus thickness), the analytic approach of the current study diverges from the structural MRI study on verbal fluency by Grogan et al. (2009) in several ways. Grogan and colleagues focused on the contrast between letter and category fluency, while we additionally evaluated the common anatomical regions to both fluency task. Our goal was to identify with whole-brain analyses the cortical maps for letter and category fluency separately, as well as visual and statistical overlap and unique associations of regions mediated by each fluency task. In our study, we had a substantially larger sample size, and by statistically comparing the tasks at a neuroanatomical level using the sensitive measure of cortical thickness we built on the initial steps taken by Grogan et al. to provide structural MRI evidence in healthy individuals of the localization of verbal fluency.
Our findings provide an important foundation for various applications, as the verbal fluency tasks are a widely applied test and one of the cornerstones of the prevailing neuropsychological battery. Among the reasons for this popularity are its low costs, simplicity in administration, thorough appraisal in previous literature, available norms, indicative power in cognitively normal and impaired populations across the lifespan, and hybrid character of testing both executive control and verbal ability. By elucidating cortical signature of each fluency task, we can further build our understanding of the meaning of verbal fluency scores in various clinical populations and the theoretical mechanisms of executive functions and lexical processing.
Funding
This work was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH, grant number UL1TR001873). Data collection and sharing for this project was supported by the Washington Heights-Inwood Columbia Aging Project (WHICAP), funded by the National Institute on Aging (NIA), National Institutes of Health (grant numbers P01 AG07232, R01 AG037212, RF1 AG054023, R01 AG034189, R01 AG054520, and R56 AG034189). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Notes
This manuscript has been reviewed by WHICAP investigators for scientific content and consistency of data interpretation with previous WHICAP Study publications. We acknowledge the WHICAP study participants and the WHICAP research and support staff for their contributions to this study. Conflict of Interest: None declared.
References
- Abrahams S, Goldstein LH, Simmons A, Brammer MJ, Williams SC, Giampietro VP, Andrew CM, Leigh PN. 2003. Functional magnetic resonance imaging of verbal fluency and confrontation naming using compressed image acquisition to permit overt responses. Hum Brain Mapp. 20:29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo JV, Schwartz S, Wilkins D, Dronkers NF. 2006. Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. J Int Neuropsychol Soc. 12:896–900. [DOI] [PubMed] [Google Scholar]
- Basho S, Palmer ED, Rubio MA, Wulfeck B, Müller R-A. 2007. Effects of generation mode in fMRI adaptations of semantic fluency: paced production and overt speech. Neuropsychologia. 45:1697–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Cox RW, Bandettini PA. 2004. Experimental designs and processing strategies for fMRI studies involving overt verbal responses. Neuroimage. 23:1046–1058. [DOI] [PubMed] [Google Scholar]
- Birn RM, Kenworthy L, Case L, Caravella R, Jones TB, Bandettini PA, Martin A. 2010. Neural systems supporting lexical search guided by letter and semantic category cues: a self-paced overt response fMRI study of verbal fluency. Neuroimage. 49:1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokde AL, Tagamets M-A, Friedman RB, Horwitz B. 2001. Functional interactions of the inferior frontal cortex during the processing of words and word-like stimuli. Neuron. 30:609–617. [DOI] [PubMed] [Google Scholar]
- Bookheimer S. 2002. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 25:151–188. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Paul RH, Cohen RA, Williams LM, MacGregor KL, Jefferson AL, Tate DF, Gunstad J, Gordon E. 2005. Category and letter verbal fluency across the adult lifespan: relationship to EEG theta power. Arch Clin Neuropsychol. 20:561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MJ, Morgan AM, Vaughn M, Riccio CA, Hall J. 1999. Verbal fluency in children: developmental issues and differential validity in distinguishing children with attention-deficit hyperactivity disorder and two subtypes of dyslexia. Arch Clin Neuropsychol. 14:433–443. [PubMed] [Google Scholar]
- Costafreda SG, Fu CH, Lee L, Everitt B, Brammer MJ, David AS. 2006. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: role of the left inferior frontal gyrus. Hum Brain Mapp. 27:799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X, Kannurpatti SS, Rypma B, Biswal BB. 2012. Calibrating BOLD fMRI activations with neurovascular and anatomical constraints. Cereb Cortex. 23:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Fenstermacher E, Salat DH, Wolk DA, Maguire RP, Desikan R, Pacheco J, Quinn BT, Van der Kouwe A, Greve DN, et al. 2008. Detection of cortical thickness correlates of cognitive performance: reliability across MRI scan sessions, scanners, and field strengths. Neuroimage. 39:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng N, Vonk JMJ, Salzberger M, Yoo N. 2018. A cross-linguistic comparison of category and letter fluency: Mandarin and English. Q J Exp Psychol (Hove). 1747021818765997. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Hertz–Pannier L, Mott SH, Barnett AS, LeBihan D, Theodore WH. 2000. Functional anatomy of cognitive development fMRI of verbal fluency in children and adults. Neurology. 54:180–180. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, Braniecki SH, McKinney CM, Hunter K, Xu B, et al. 2003. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Hum Brain Mapp. 18:176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourovitch ML, Kirkby BS, Goldberg TE, Weinberger DR, Gold JM, Esposito G, Van Horn JD, Berman KF. 2000. A comparison of rCBF patterns during letter and semantic fluency. Neuropsychology. 14:353. [DOI] [PubMed] [Google Scholar]
- Grogan A, Green DW, Ali N, Crinion JT, Price CJ. 2009. Structural correlates of semantic and phonemic fluency ability in first and second languages. Cereb Cortex. 19:2690–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartberg CB, Lawyer G, Nyman H, Jönsson EG, Haukvik UK, Saetre P, Bjerkan PS, Andreassen OA, Hall H, Agartz I. 2010. Investigating relationships between cortical thickness and cognitive performance in patients with schizophrenia and healthy adults. Psychiatry Res. 182:123–133. [DOI] [PubMed] [Google Scholar]
- Henry JD, Crawford JR. 2004. A meta-analytic review of verbal fluency performance following focal cortical lesions. Neuropsychology. 18:284–295. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. 1992. Semantic dementia: progressive fluent aphasia with temporal lobe atrophy. Brain. 115:1783–1806. [DOI] [PubMed] [Google Scholar]
- Jones S, Laukka EJ, Bäckman L. 2006. Differential verbal fluency deficits in the preclinical stages of Alzheimer’s disease and vascular dementia. Cortex. 42:347–355. [DOI] [PubMed] [Google Scholar]
- Laws KR, Duncan A, Gale TM. 2010. ‘Normal’ semantic–phonemic fluency discrepancy in Alzheimer’s disease? A meta-analytic study. Cortex. 46:595–601. [DOI] [PubMed] [Google Scholar]
- Lemaitre H, Goldman AL, Sambataro F, Verchinski BA, Meyer-Lindenberg A, Weinberger DR, Mattay VS. 2012. Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiol Aging. 33:617.e611–617.e619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Luk G, Bialystok E. 2010. Effect of language proficiency and executive control on verbal fluency performance in bilinguals. Cognition. 114:29–41. [DOI] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Lalonde F, Mack C. 1994. Word retrieval to letter and semantic cues: a double dissociation in normal subjects using interference tasks. Neuropsychologia. 32:1487–1494. [DOI] [PubMed] [Google Scholar]
- Meinzer M, Flaisch T, Wilser L, Eulitz C, Rockstroh B, Conway T, Gonzalez-Rothi L, Crosson B. 2009. Neural signatures of semantic and phonemic fluency in young and old adults. J Cogn Neurosci. 21:2007–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Rosenthal R, Rubin DB. 1992. Comparing correlated correlation coefficients. Psychol Bull. 111:172–175. [Google Scholar]
- Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. 1992. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch Neurol. 49:1253–1258. [DOI] [PubMed] [Google Scholar]
- Pihlajamaki M, Tanila H, Hanninen T, Kononen M, Laakso MP, Partanen K, Soininen H, Aronen HJ. 2000. Verbal fluency activates the left medial temporal lobe: a functional magnetic resonance imaging study. Ann Neurol. 47:470–476. [PubMed] [Google Scholar]
- Quinn C, Elman L, McCluskey L, Hoskins K, Karam C, Woo JH, Poptani H, Wang S, Chawla S, Kasner SE, et al. 2012. Frontal lobe abnormalities on MRS correlate with poor letter fluency in ALS. Neurology. 79:583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righart R, Schmidt P, Dahnke R, Biberacher V, Beer A, Buck D, Hemmer B, Kirschke J, Zimmer C, Gaser C, et al. 2017. Volume versus surface-based cortical thickness measurements: a comparative study with healthy controls and multiple sclerosis patients. PLoS One. 12:e0179590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Karnath H-O. 2004. Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nat Rev Neurosci. 5:812–819. [DOI] [PubMed] [Google Scholar]
- Sauzéon H, Raboutet C, Rodrigues J, Langevin S, Schelstraete M-A, Feyereisen P, Hupet M, N’Kaoua B. 2011. Verbal knowledge as a compensation determinant of adult age differences in verbal fluency tasks over time. J Adult Dev. 18:144–154. [Google Scholar]
- Schmidt EL, Burge W, Visscher KM, Ross LA. 2016. Cortical thickness in frontoparietal and cingulo-opercular networks predicts executive function performance in older adults. Neuropsychology. 30:322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Janse E, Visser K, Meyer AS. 2014. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Front Psychol. 5:772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon S, Moscovitch M. 2012. The nature and time‐course of medial temporal lobe contributions to semantic retrieval: an fMRI study on verbal fluency. Hippocampus. 22:1451–1466. [DOI] [PubMed] [Google Scholar]
- Stern Y, Andrews H, Pittman J, Sano M, Tatemichi T, Lantigua R, Mayeux R. 1992. Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Arch Neurol. 49:453–460. [DOI] [PubMed] [Google Scholar]
- Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, Merchant C, Lantigua R, Costa R, Stern Y, et al. 2001. Incidence of Alzheimer’s disease in African-Americans, Caribbean Hispanics and Caucasians in northern Manhattan. Neurology. 56:49–56. [DOI] [PubMed] [Google Scholar]
- Troyer AK, Moscovitch M, Winocur G, Alexander MP, Stuss D. 1998. Clustering and switching on verbal fluency: the effects of focal frontal- and temporal-lobe lesions. Neuropsychologia. 36:499–504. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Duggirala R, Glahn DC. 2010. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 53:1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]