Abstract
Across cultures and throughout history, human beings have reported a variety of spiritual experiences and the concomitant perceived sense of union that transcends one’s ordinary sense of self. Nevertheless, little is known about the underlying neural mechanisms of spiritual experiences, particularly when examined across different traditions and practices. By adapting an individualized guided-imagery task, we investigated neural correlates of personally meaningful spiritual experiences as compared with stressful and neutral-relaxing experiences. We observed in the spiritual condition, as compared with the neutral-relaxing condition, reduced activity in the left inferior parietal lobule (IPL), a result that suggests the IPL may contribute importantly to perceptual processing and self-other representations during spiritual experiences. Compared with stress cues, responses to spiritual cues showed reduced activity in the medial thalamus and caudate, regions associated with sensory and emotional processing. Overall, the study introduces a novel method for investigating brain correlates of personally meaningful spiritual experiences and suggests neural mechanisms associated with broadly defined and personally experienced spirituality.
Keywords: functional magnetic resonance imaging, perception, spirituality, stress
Introduction
Across cultures and throughout recorded history, human beings have reported self-transcendent spiritual experiences (James 1902; Otto and Harvey 1926; Eliade 1959; Haidt 2006). Though varying widely in form, from ecstatic religious chanting to a felt oneness with the natural environment, these experiences share in common a perceived dissolution of the boundary between self and other and a sense of union with something larger than oneself (James 1902; Yaden et al. 2017). Nonetheless, little is known about how the experience of self-transcendence operates at the neural level. In this study, we set out to examine neural mechanisms that underlie personalized spiritual experiences.
A range of specific neural structures and systems have been linked to aspects of spirituality in religious and general contexts. In 16 healthy individuals, availability of the serotonin transporter in specific raphe nuclei has been linked to self-transcendence and spiritual acceptance (Kim et al. 2015). Among Carmelite nuns (n = 15) recollecting their most intense mystical experience in the order, multiple subcortical and cortical regions were implicated including the brainstem, striatum, and frontal and parietal cortices (Beauregard and Paquette 2006). Among Mormons (n = 19) and across tasks simulating religious practices, reward, salience and attentional systems were activated in regions including the ventral striatum (Ferguson et al. 2016). Among 104 individuals at varying risk for depression, a greater belief in the importance of religion and spirituality was associated with diminished default-mode connectivity (Svob et al. 2016). Among individuals with long-term substance-use, cortical thinning in the posterior cingulate cortex (PCC) was associated with measures of spirituality (Bouso et al. 2015). Aspects of spirituality have also been linked to lower brain volumes in regions including the PCC in a sample of 65 substance-abusing and non-abusing young adults (Simmons et al. 2012).
Although multiple brain regions may contribute to spirituality, the parietal cortex is arguably the most frequently implicated brain region linked to spirituality. For example, a self-reported sustained high importance of personal spirituality was associated with cortical thickness in parietal and precuneus regions (Miller et al. 2014). Spontaneous immersion of spiritual awareness, perception, and relationship to the transcendent was associated with regeneration around lesions in the parietal cortex (Urgesi et al. 2010). During religious recitation, religious individuals were found to engage fronto-parietal circuitry (Azari et al. 2001). Preliminary data have also suggested that the parietal cortex may also contribute to “Christmas spirit” amongst individuals with Christmas traditions (Hougaard et al. 2015). Using electroencephalography (EEG), posterior alpha has been associated with aspects of religiosity and spirituality (Travis 2001; Tenke et al. 2017), again implicating the parietal region. Some research has identified exceptional spiritual perceptions and mystical experiences in clergy (Beauregard and Paquette 2006), also showing associations with parietal cortical function. Neuroimaging studies of meditation and meditative prayer suggest an inverse relationship between parietal lobe activity and religious/spiritual beliefs (Herzog et al. 1990; Newberg and Iversen 2003; Newberg et al. 2003; Cahn and Polich 2006; Brefczynski-Lewis et al. 2007), with complementary findings observed in clinical populations (Johnstone and Glass 2008; Johnstone et al. 2012). In two reports from the same group, excitatory stimulation of the inferior parietal lobule (IPL) using intermittent theta burst stimulation was associated with decreases in implicit religiousness and spirituality (Crescentini et al. 2015), whereas a virtual lesion of the IPL using repetitive transcranial magnetic stimulation induced rapid increases in implicit religiousness and spirituality (Crescentini et al. 2014). Together, these findings provide strong support for the IPL in governing aspects of religiousness and spirituality.
In considering a theoretical foundation for why the parietal cortex has been repeatedly implicated in studies of spirituality, it is important to consider its function more broadly. The parietal cortex contributes to multiple processes including attention, impulse control, planned reasoning, sensory processing, and spatial reasoning, amongst other aspects of human functioning (Arnsten 2009; Teixeira et al. 2014; Barnby et al. 2015). Intriguingly given that spirituality involves a felt-sense of connection with someone or something outside of oneself, the parietal cortex, and perhaps particularly in the right hemisphere (Keenan and Gorman 2007; however, see Morin 2007), has been identified as contributing to a sense of self (Lou et al. 2017). For example, converging evidence using different methodologies suggests that the medial parietal cortex/precuneus, as part of a medial paralimbic network involving medial prefrontal/anterior cingulate regions, represents an important hub in a “neural signature” of self-awareness (Kjaer et al. 2002; Newen and Vogeley 2003; Northoff et al. 2006; Lou et al. 2017). Furthermore, this medial parietal region appears functionally connected with lateral regions of the parietal cortex associated with self-reference (Lou et al. 2004). Taken together with the findings above, data suggest that a felt-sense of connection outside of oneself that is a core component of spirituality may involve the IPL and related circuitry.
Although studies have linked specific brain measures to aspects of spirituality, none have sought to directly examine spiritual experiences, particularly when using a broader, modern definition of spirituality that may be independent of religiousness. A potential challenge in studying spiritual experiences involves the vast variety of spiritual experiences that may have varying degrees of relevance or meaning to different individuals. A potential solution to this situation involves the generation of personally-relevant, individually-tailored scripts that relate to spiritual experiences. In this study, we adapted a widely used guided-imagery task that we have used to study motivational and emotional processing relating to substance (alcohol, drug) cravings and stress responsivity to investigate directly the neural correlates of spiritual experiences (Potenza et al. 2012; Seo et al. 2016). We hypothesized that behavioral and neural responses to individualized spiritual cues would differ from those to stress and neutral-relaxing cues, with the spirituality condition being associated with higher self-reported spiritual feelings and relatively decreased parietal activation.
Material and Methods
Participants
Participant demographics are reported in Table 1. Participants were recruited from the New Haven community via advertisement. Twenty-seven English-speaking young adults (mean age: 22 ± 2.2 years, range 18–27 years; 12 female) completed the functional magnetic resonance imaging (fMRI) protocol. All participants completed a Structured Clinical Interview for Axis-I Psychiatric Disorders (First et al. 1995). Two participants met criteria for past major depressive disorder, three participants for past alcohol abuse, two participants for past cannabis abuse, one for past cannabis dependence, and one for past social phobia.
Table 1.
Participant demographic information
| n | 27 |
| Male/female | 15/12 |
| Age (SD) | 22.3 (2.21) |
| White/Black/Asian/Other | 18/5/3/1 |
| Non-Hispanic/Hispanic | 25/2 |
| Years of education | 15.15 (1.7) |
| Body mass index | 22.4 (2.2) |
| Self-report measures | |
| Relationship with G-da | 15.96 (2.5) |
| Eco-awareness | 27.21 (6.5) |
| Intrinsic spirituality | 5.33 (3.0) |
SD, standard deviation.
aSome of the authors do not write in full the name of G-d.
Eligibility criteria included that participants had no head injury or medical condition. Participants were excluded if they met any of the following criteria: pregnant, breast-feeding, color-blind, currently taking antidepressant medication or migraine medication, history of cardiovascular disease, BP > 145/90, bipolar disorder, psychosis, current substance-use disorder, history of seizure or neurologic disease, or uncontrolled diabetes. This protocol was approved by the Yale University School of Medicine Human Investigations Committee, and all participants provided written informed consent. All participants had negative urine screens for illicit substances.
Imagery Script Development Procedures
Approximately, 1 week prior to scanning, personalized guided-imagery scripts for spiritual, stress and neutral-relaxing conditions were developed with each participant. The personalized guided-imagery fMRI procedure is a standardized procedure for provoking of individual personal experiences and has previously been described and applied to elicit anxiety and food and drug craving in various populations (Potenza et al. 2012; Hommer et al. 2013; Jastreboff et al. 2013; Elsey et al. 2015). The Scene Development Questionnaire was used to develop the scripts as previously described (Sinha and Tuit 2012; Sinha 2013). Specific stimuli contexts as well as physiological, subjective, experiential and cognitive details relating to each situation were collected, and this information was then constructed into a script and recorded for presentation during fMRI, as per standardized procedures (Sinha and Tuit 2012).
For the spiritual script, participants were given the following instructions:
“We would like you to describe a situation in which you felt a strong connection with a higher power or a spiritual presence. Spiritual states are those that through a felt-sense connect you to something bigger than oneself, a oneness, or strong force which may be experienced as an energy, force, higher power, G-d, deity or transcendent figure or consciousness. Such states may be experienced in places of worship, at home, in your daily life, or outdoors in nature. Choose a personal lived situation that you directly experienced, whether others were present or not. Also, include in your description the bodily sensations you have experienced in these situations.
Some common experiences of transcendent connection include a two-way relationship with a higher power, a felt-sense of oneness in nature by the ocean or atop a mountain, being in a zone of intense physical activity (such as sports or yoga), sudden awareness, bodily felt connectivity or buoyancy, meditation or prayer. These may be extremely vivid or intense experiences, or these relatively accentuated experiences may filter into an ongoing felt transcendent connection or daily way of being connected to something more.
Sometimes it is difficult to think of a positive transcendent or spiritual experience “on the spot”. It may help to close your eyes and try to imagine yourself in the situation. While you are imagining the situation, try to generate the same sensations and feelings you would experience if you were actually in the situation. Describe the situation as though you are helping me see it as if I was there with you. (Please include such details as who was there; what you were doing; where you were; how things looked; what bodily sensations you experienced.)”
The spiritual imagery script was developed from a participant’s account of one of the most personally spiritual events experienced in the past year. Participants rated the spirituality on a 10-point Likert scale with “1 = not at all spiritual” and “10 = the most spiritual experience in the past year”. Script development occurred only for spiritual situations rated as an eight or above. Participants had diverse spiritual backgrounds; examples of spiritual situations included participating in a religious service at a house of worship, experiencing a connection with nature, and meditating. Participants also had diverse perspectives on the divine with individual script themes including unity/oneness, infinity/wonder and relationality, and communion. Food, sexual or drug-related scenarios were not included. Sample scripts are included in Supplemental section.
Stress and neutral-relaxing scripts were generated as described previously (Potenza et al. 2012; Hommer et al. 2013; Jastreboff et al. 2013; Elsey et al. 2015). The stress imagery script was developed from a participant’s account of one of the most personally stressful events occurring in the past 12 months. Participants rated the stressfulness on a 10-point Likert scale with “1 = not at all stressful” and “10 = the most stress they experience in the past year”. Script development occurred only for situations rated as an eight or above. Examples include situations such as illness/death of a loved one, unemployment-related stress, or strife with family. Trauma-related situations were not included. A neutral-relaxing script was developed from individual’s experience of neutral-relaxing situations, including reading in bed at night, watching television, or walking in nature.
Habituation and Imagery Training Session
Approximately, 1 h prior to the scanning session, participants were acclimatized to specific aspects of the study procedures and trained in guided-imagery and relaxation procedures as described previously (Potenza et al. 2012; Sinha and Tuit 2012; Hommer et al. 2013; Jastreboff et al. 2013; Elsey et al. 2015).
fMRI Procedure
The fMRI session consisted of participants being exposed to three imagery conditions: spiritual, stress and neutral-relaxing. Two scripts of each type were developed (i.e., two spiritual, two stress, two neutral-relaxing). Scripts were recorded using the same female voice.
Image Acquisition
MRI data were collected using a 3-T Siemens Trio MRI system equipped with a standard quadrature head coil, using T2*-sensitive gradient-recalled single-shot echo-planar pulse sequence. Anatomical images of the functional slice locations were acquired with spin-echo imaging in the axial plane parallel to the anterior cingulate-posterior cingulate (AC-PC) line with time repetition (TR) = 300 ms, time echo (TE) = 2.46 ms, bandwidth = 310 Hz/pixel, flip angle (FA) = 60°, field-of-view = 220 × 220 mm, matrix = 256 × 256, 32 slices with slice thickness = 4 mm and no gap. Functional images were collected with a single-shot gradient echo-planar imaging sequence. Thirty-two axial slices parallel to the AC-PC line covering the whole brain were acquired with TR = 2000 ms, TE = 25 ms, bandwidth = 2003 Hz/pixel, FA = 85°, field-of-view = 220 × 220 mm, matrix = 64 × 64, 32 slices with slice thickness = 4 mm and no gap. Then, a high-resolution 3D Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence (TR = 2530 ms; TE = 2.77 ms; bandwidth = 179 Hz/pixel; FA = 7°; slice thickness = 1 mm; field-of-view = 256 × 256 mm; matrix = 256 × 256) was used to obtain sagittal images for multi-subject registration.
Six fMRI trials (two per condition) were acquired using a block design. To control for nonspecific order effects, condition order was randomly assigned and counterbalanced across participants. Each script was presented only once for a subject, and scripts in the same condition were not presented consecutively. Each trial lasted 5 min, including a 1.5-min quiet baseline period followed by 2.5-min imagery (2 min of read imagery and 0.5 min of quiet imagery) and a 1-min quiet recovery. During baseline, participants were instructed to stay still in the scanner without engaging in any mental activity. Before and after each trial, anxiety, and spirituality ratings were elicited verbally for each using a 10-point Likert scale (0 = “not at all,” 10 = “extremely high”).
PreProcessing and First-Level Analyses
Functional MRI data were converted from Digital Imaging and Communication in Medicine (DICOM) format to analyze format using XMedCon. To achieve steady-state equilibrium between radiofrequency pulsing and relaxation, the first 10 images of each trial were discarded. Images were slice-time-corrected using a custom-designed MATLAB program. Motion correction was completed using SPM8 for three translational and three rotational directions, removing trials with linear motion greater than 1.5 mm and a rotation exceeding 2°. The recovery period (1 min) was excluded from the data analysis to prevent carryover effects from the imagery period.
Individual-level analysis was conducted using a general linear model (GLM) on each voxel in the entire brain volume with a task specific regressor (2.5-min imagery relative to a 1.5-min baseline) using Yale BioImageSuite (http://www.bioimagesuite.org/). To account for potential variability in baseline fMRI signal, drift correction was included in the GLM; drift regressors were used to remove the mean time course, linear trend, quadratic trend, and cubic trend for each run. Each trial was normalized against the immediate baseline period preceding the script and then two trials of the same type were averaged. Functional images were spatially smoothed with a 6-mm Gaussian kernel, resulting in normalized beta-maps in the acquired space (3.44 mm × 3.44 mm × 4 mm). To bring data into a common reference space, three registrations were calculated within BioImageSuite; a linear registration was computed from the individual functional image to the corresponding individual participant 2D anatomical image, a linear registration was computed from the 2D anatomical image to the individual participants’ 3D structural image and a nonlinear registration was computed between the individual 3D image and a standard reference 3D image, the Colin27 Brain. All three registrations were concatenated and applied as one registration to the normalized beta-maps in order to bring the data into a common reference space.
Second-Level Analyses
The Analysis of Functional Neuroimages (AFNI) software package (http://afni.nimh.nih.gov/afni) was used for whole-brain random-effects analysis to investigate main effects of condition (neutral-relaxing, stress, spirituality). Given significant results, these were further examined using BioImageSuite using two-tailed t-test maps to examine the source of the main effects of condition. Family-wise error (FWE) correction for multiple comparisons was conducted with 3dClustSim from AFNI using the autocorrelation function option and 10 000 iterations. Input smoothness for these simulations was estimated from the residuals of the t-tests. Results are shown at P < 0.05 corrected with an initial P threshold of P < 0.001.
Subjective Responses
Participants were questioned on their affective states immediately prior to and following listening to each of their individualized scripts. Participants were asked the following two questions: “On a scale of 0–10 what was the intensity of your spiritual connection?”; “On a scale of 0–10, how anxious do you feel?” Subjective ratings of vividness for each script were also obtained for each script, whereby an individual rated how well they were able to visualize their individual scenario during scanning (“On a scale of 0–10, how vivid was that story?”).
Results
Subjective Ratings
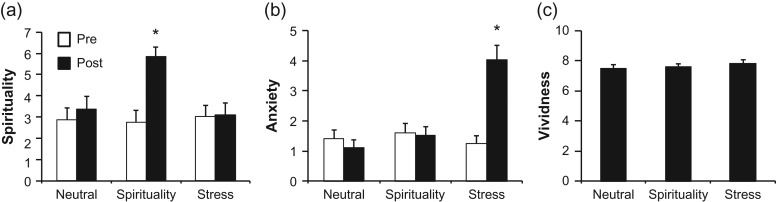
A repeated-measures ANOVA of the subjective measures of spiritual intensity showed a significant difference in spiritual connection following the spiritual scripts (F2,52 = 23.45, P < 0.05). Specifically, participants reported significantly more intense levels of spiritual connection following the spiritual scripts, relative to both the stress and the neutral-relaxing scripts (P < 0.05), with the last two not differing from one another (P > 0.05; Fig. 1a). Similarly, reports of anxiety differed between conditions (F2,52 = 28.98, P < 0.05); participants reported significantly higher levels of anxiety following the stress script (P < 0.05), relative to the spiritual or neutral-relaxing scripts, which did not differ from one another (P > 0.05; Fig. 1b). Reports of vividness between the three conditions did not differ (P > 0.05; Fig. 1c). Subjective reports are graphically depicted in Fig. 1.
Figure 1.
Subjective responses to the guided-imagery scripts. N = 27 participants were questioned prior to listening to their individualized script (white bars “Pre-script”) and following the script (black bars “Post-script”). (a) Subjective responses to the question “On a scale of 0–10 what was the intensity of your spiritual connection?” Participants reported significantly higher spiritual connection following the spiritual script (P < 0.05). (b) Subjective responses to the question: “On a scale of 0–10, how anxious do you feel?” Participants reported significantly higher anxiety following the stress script (P < 0.05). (c) Subjective responses to the question: “On a scale of 0–10, how vivid was that story?”.
fMRI Results
Task Main Effect
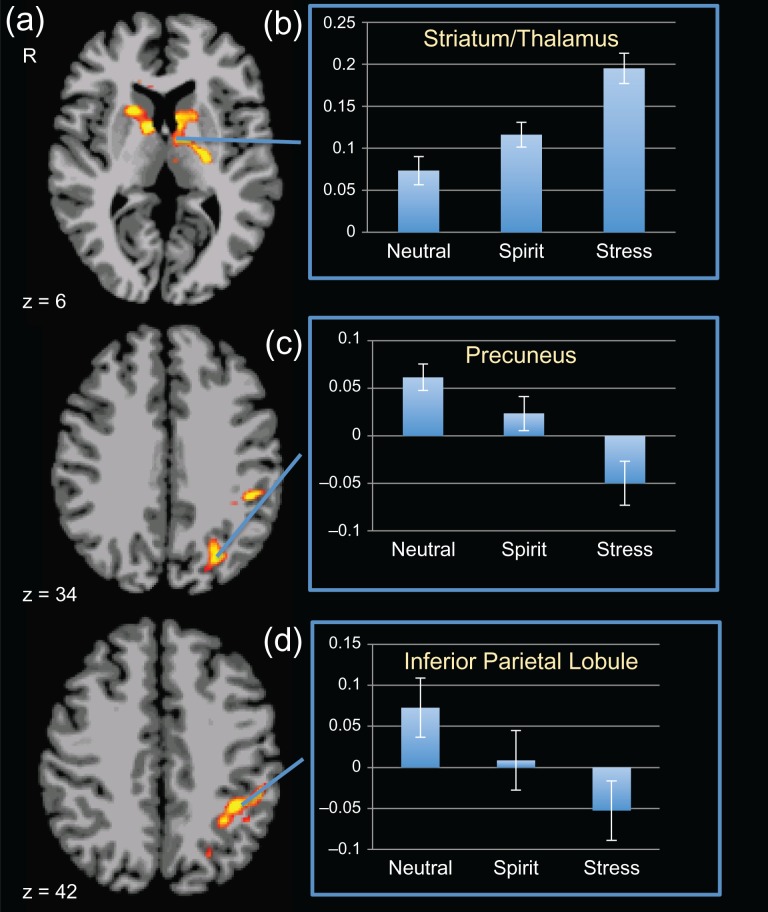
The main effect of task identified activation involving the medial thalamus extending to the caudate, the left IPL and the left precuneus (PFWE < 0.001; Table 2; Fig. 2a; Supplemental Fig. 1).
Table 2.
Main effect of cue condition (spiritual/stress/neutral-relaxing) on brain activations (PFWE < 0.001) in N = 27 participants
| Condition | Brain region | BA | Peak | Volume | k | F-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | (mm3) | Max | Mean | SD | ||||
| Task main effect | Medial thalamus/caudate | – | −6 | 0 | 0 | 7976 | 295 | 16.5 | 9.55 | 1.39 |
| Right claustrum/caudate | – | 24 | 24 | 12 | 1167 | 43 | 13.38 | 9.16 | 1.00 | |
| Left inferior parietal lobule | 7, 39, 40 | −33 | −51 | 54 | 3570 | 132 | 17.31 | 9.25 | 1.10 | |
| Left precuneus | 7, 39 | −27 | −75 | 36 | 829 | 31 | 14.30 | 9.30 | 1.09 | |
| Spiritual > Neutral | Left inferior parietal lobule | 40 | −51 | −36 | 39 | 772 | 29 | −4.48 | −3.53 | 0.21 |
| Stress > Neutral | Right temporal lobe | 20 | 30 | 3 | −39 | 677 | 25 | −4.44 | −3.59 | 0.21 |
| Right ventral striatum | 11 | 6 | 36 | −18 | 1320 | 49 | −5.23 | −3.63 | 0.30 | |
| Left ventral striatum | 11 | −12 | 18 | −9 | 727 | 27 | −4.51 | −3.63 | 0.29 | |
| Medial thalamus/caudate | – | −6 | 0 | 0 | 26 243 | 972 | 5.71 | 3.80 | 0.40 | |
| Bilateral precuneus/inferior parietal lobule | 7, 39, 40 | −33 | −51 | 54 | 20 313 | 752 | −5.84 | −3.71 | 0.33 | |
| Spiritual > Stress | Medial thalamus into striatum | – | −24 | −15 | 6 | 1289 | 48 | −4.63 | −3.55 | 0.24 |
| Thalamus | – | −12 | −6 | 6 | 448 | 17 | −4.23 | −3.52 | 0.19 | |
| Left white matter | – | −27 | 6 | 30 | 652 | 24 | −4.83 | −3.67 | 0.32 | |
Figure 2.
Main effect of condition and condition contrasts. Main effect of the guided-imagery scripts in the group (N = 27) during scanning (red box on left). (a) Maps depict axial views of z-levels 6 (striatum/thalamus), 34 (precuneus/superior occipital gyrus/parietal lobe), 42 (inferior parietal lobule) from figure top to bottom with significant differences across conditions. “Blue box on the right”: Between-condition differences on the guided-imagery scripts in the group (N = 27) during spiritual and stress scripts, relative to neutral-relaxing scripts and each other. Axial views show z-levels −18, −10, 6, 42 from left to right. Maps depict % blood oxygen level-dependent (BOLD) signal change in the (b) spiritual condition relative to the neutral-relaxing condition (“Spirit > Neutral”), (c) the stress condition relative to the neutral-relaxing condition (“Stress > Neutral”) and (d) the spiritual condition relative to the stress condition (“Spirit > Stress”). All maps are thresholded at an uncorrected level of P < 0.001 two-tailed and family-wise-error-corrected at P < 0.05. Red color indicates where participants show relatively greater activation, and blue color demonstrates areas where participants show relatively reduced activation. The right side of the brain is on the left.
Pairwise Contrasts Between Conditions
Spiritual versus neutral-relaxing condition
Pairwise contrasts between conditions were examined to understand the nature of the main effect findings. These showed that relative to the neutral-relaxing condition, the spiritual condition elicited less activity in the left IPL (Table 2; Fig. 2b; Supplemental Fig. S2).
Spiritual versus stress condition
Relative to the stress condition, the spiritual condition elicited decreased activity in a cluster in the medial thalamus and striatum (Table 2; Fig. 2c; Supplemental Fig. S3).
Stress versus neutral-relaxing condition
Relative to the neutral-relaxing condition, the stress condition elicited greater activity in a cluster in the bilateral thalamus/caudate. Relatively decreased activity was noted in clusters in the right temporal lobe, right and left ventral striatum extending into the orbitofrontal cortex and the bilateral precuneus extending into the IPL (Table 2; Fig. 2d; Supplemental Fig. S4).
Discussion
Spiritual experiences involve pronounced shifts in perception and buffer the effects of stress on mental health. Here, we provide mechanistic accounts of how these changes may occur at neural levels. Consistent with our hypotheses, spiritual cues relative to neutral-relaxing cues were associated with greater self-reported intensity of spiritual connection and reduced activation in the inferior parietal lobe (IPL), a region implicated in various perceptual processes. However, such a difference was not observed with respect to the stress cue. Additionally, we observed that, compared with the stress condition, the spirituality condition was associated with reduced activity in the medial thalamus and striatum, brain regions implicated in sensory and emotional processing. These results suggest neural foundations for spiritual experiences across different traditions and practices.
The present study showed reduced activity in the left IPL following spiritual cues, which is consistent with several previous investigations suggesting an inverse relationship between spiritual awareness and parietal activity. The posterior parietal cortex has been implicated in religiosity and spirituality across a wide variety of measures including importance of religion and spirituality (Miller et al. 2014), trait self-transcendence (Urgesi et al. 2010), implicit religiousness and spirituality (Crescentini et al. 2014, 2015), mindfulness meditation training (Lazar et al. 2000; Farb et al. 2007), and contemplative prayer (Newberg et al. 2003, 2015). Furthermore, activity in this region has been linked to spatiotemporal perceptual processes and, in particular, the representation of the human body in time and space (Assmus et al. 2003; Lou et al. 2004; Bolger et al. 2014). Since spiritual practices and experiences typically involve a perceived alteration in time and space (Newberg and Waldman 2009; Yaden et al. 2017), often an expanded sense of self in relation to the environment including subjective reports from the current study (see Supplementary Material), these findings lend support to our hypotheses and the relatively blunted activity of the IPL observed in the present study. The hemispheric lateralization of posterior parietal activation differs, however, across various studies of spirituality, which invites a more nuanced interpretation. Research has implicated the right IPL in the cognitive representation of one’s own body in space, while the left IPL has been linked to the visuo-spatial representation of others (Felician et al. 2003; Lou et al. 2004; Muhlau et al. 2005). Functionally connected to the ventral premotor cortex, the IPL contains both motor and mirror neurons which allow an observer to perceive individuals’ motor behaviors and intentions, and the left IPL in particular has been implicated in reading others’ intentions (Fogassi et al. 2005; D’Argembeau et al. 2008; Bonini et al. 2010). Moreover, research has linked the IPL to the attribution of agency, whereby left IPL activity may signal an attribution of agency outside of oneself (Farrer and Frith 2002). Taken together, the present finding suggests that spiritual experiences may involve a perceived encounter with a spacious “presence” or entity external to oneself. This interpretation is consistent with a strong feeling of connection or surrender to a deity or other revered figure, as often reported in religious and spiritual literature (James 1902; Wilber 2006).
The IPL has also been implicated in episodic memory retrieval as well as processing human faces (Leube et al. 2003; Mayes et al. 2004; Wagner et al. 2005), possibly suggesting that spiritual experiences interact with memory retrieval processes in a unique way. This possibility, however, is tempered by the fact that all three conditions involved re-experiencing highly salient memories, including those that may involve recollection of people (and thus their faces). As such, the experimental design argues against these possibilities. Nonetheless, future investigations involving larger samples may permit investigation of scripts with and without specific features (e.g., recollection of people or spiritual beings) to investigate such possibilities directly. Additionally, it is worth noting that the responses during the spiritual cue condition are congruent with the notion that systems-based changes may occur on neural levels in response to changes in perception (Freiwald et al. 2016; Mazzarella et al. 2013).
The spiritual condition in comparison with the stress condition revealed decreased activation in the medial thalamus and striatum, regions that also showed heightened activity following stress cues in comparison to the neutral-relaxing condition. These regions are involved in sensory and emotional processing and represent components of an identified cortico-striatal circuit related to stress response, whereby the thalamus relays information to striatal regions (Lanius et al. 2003; Sinha et al. 2004, 2016; Sommer 2003). Reduced activity in these regions suggests, therefore, that both spiritual and relaxing experiences may similarly differ from stressful experiences in activation of these subcortical regions.
In addition, at a methodological level, this study introduces a novel script-driven imagery approach to investigating neural correlates of spiritual experiences that may often occur spontaneously and are therefore difficult to study otherwise. The subjective responses demonstrate the ability of the task to generate personally meaningful spiritual experiences. As an alternative to studying particular volitional spiritual practices which typically come from a particular tradition, this method allows for the inclusion of a multiplicity of backgrounds.
While the results may have mental health implications given links between spirituality and better mental health (Miller 2012), it is important to note that our sample is derived from a healthy population. Future investigations of spiritual experiences in longitudinal studies or among clinical populations would produce further insights about such relationships. Limitations of the study include a relatively small sample of young adults. Although participants’ religious and spiritual backgrounds were relatively diverse, the study drew from a relatively small young adult sample. Further investigations should include larger samples of participants from multiple cultures, backgrounds, and age groups in order to understand better individual differences in the neural correlates of spirituality.
In conclusion, these results demonstrate neural mechanisms underlying spiritual experiences across diverse traditions and perspectives. Continuing to build our empirical understanding of how spiritual experiences are mediated by the brain and the future extension of similar studies to clinical populations could help facilitate the judicious integration of spirituality into treatment and prevention in areas of mental health conditions.
Supplementary Material
Notes
We are also grateful for the assistance that Chaim Kind, Candice Dwyer, and Trevor Friedman provided for this study. Conflict of Interest: None of the authors have any relevant financial disclosures. Dr M.N.P. has consulted for Ironwood, Lundbeck, Shire, INSYS, Rivermend Health, Opiant/Lakelight Therapeutics, and Jazz Pharmaceuticals; has received research support from the Pfizer, Mohegan Sun Casino and the National Center for Responsible Gaming; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse-control disorders or other health topics; has consulted for gambling and legal entities on issues related to impulse-control/addictive disorders; provides clinical care in a problem gambling services program; has performed grant reviews for the National Institutes of Health and other agencies; has edited journals and journal sections; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts.
Funding
This work was supported in part by the National Institutes of Health (R01DA039136); the Connecticut State Department of Mental Health and Addiction Services; the Connecticut Mental Health Center; the National Center on Addiction and Substance Abuse; a Center of Excellence in Gambling Research Award from the National Center for Responsible Gaming; and the Peter Boris Centre for Addictions Research.
References
- Arnsten AF. 2009. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology. CNS Drugs. 23:33–41. [DOI] [PubMed] [Google Scholar]
- Assmus A, Marshall JC, Ritzl A, Noth J, Zilles K, Fink GR. 2003. Left inferior parietal cortex integrates time and space during collision judgments. Neuroimage. 20(Suppl 1):S82–S88. [DOI] [PubMed] [Google Scholar]
- Azari NP, Nickel J, Wunderlich G, Niedeggen M, Hefter H, Tellmann L, Herzog H, Stoerig P, Birnbacher D, Seitz RJ. 2001. Neural correlates of religious experience. Eur J Neurosci. 13:1649–1652. [DOI] [PubMed] [Google Scholar]
- Barnby JM, Bailey NW, Chambers R, Fitzgerald PB. 2015. How similar are the changes in neural activity resulting from mindfulness practice in contrast to spiritual practice? Conscious Cogn. 36:219–232. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Paquette V. 2006. Neural correlates of a mystical experience in Carmelite nuns. Neurosci Lett. 405:186–190. [DOI] [PubMed] [Google Scholar]
- Bolger D, Coull JT, Schon D. 2014. Metrical rhythm implicitly orients attention in time as indexed by improved target detection and left inferior parietal activation. J Cogn Neurosci. 26:593–605. [DOI] [PubMed] [Google Scholar]
- Bonini L, Rozzi S, Serventi FU, Simone L, Ferrari PF, Fogassi L. 2010. Ventral premotor and inferior parietal cortices make distinct contribution to action organization and intention understanding. Cereb Cortex. 20:1372–1385. [DOI] [PubMed] [Google Scholar]
- Bouso JC, Palhano-Fontes F, Rodriguez-Fornells A, Ribeiro S, Sanches R, Crippa JA, Hallak JE, de Araujo DB, Riba J. 2015. Long-term use of psychedelic drugs is associated with differences in brain structure and personality in humans. Eur Neuropsychopharmacol. 25:483–492. [DOI] [PubMed] [Google Scholar]
- Brefczynski-Lewis JA, Lutz A, Schaefer HS, Levinson DB, Davidson RJ. 2007. Neural correlates of attentional expertise in long-term meditation practitioners. Proc Natl Acad Sci U S A. 104:11483–11488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn BR, Polich J. 2006. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychol Bull. 132:180–211. [DOI] [PubMed] [Google Scholar]
- Crescentini C, Aglioti SM, Fabbro F, Urgesi C. 2014. Virtual lesions of the inferior parietal cortex induce fast changes of implicit religiousness/spirituality. Cortex. 54:1–15. [DOI] [PubMed] [Google Scholar]
- Crescentini C, Di Bucchianico M, Fabbro F, Urgesi C. 2015. Excitatory stimulation of the right inferior parietal cortex lessens implicit religiousness/spirituality. Neuropsychologia. 70:71–79. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Feyers D, Majerus S, Collette F, Van der Linden M, Maquet P, Salmon E. 2008. Self-reflection across time: cortical midline structures differentiate between present and past selves. Soc Cogn Affect Neurosci. 3:244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliade M. 1959. The sacred and the profane; the nature of religion. New York: Harcourt. [Google Scholar]
- Elsey J, Coates A, Lacadie CM, McCrory EJ, Sinha R, Mayes LC, Potenza MN. 2015. Childhood trauma and neural responses to personalized stress, favorite-food and neutral-relaxing cues in adolescents. Neuropsychopharmacology. 40:1580–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z, Anderson AK. 2007. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Soc Cogn Affect Neurosci. 2:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer C, Frith CD. 2002. Experiencing oneself vs another person as being the cause of an action: the neural correlates of the experience of agency. Neuroimage. 15:596–603. [DOI] [PubMed] [Google Scholar]
- Felician O, Ceccaldi M, Didic M, Thinus-Blanc C, Poncet M. 2003. Pointing to body parts: a double dissociation study. Neuropsychologia. 41:1307–1316. [DOI] [PubMed] [Google Scholar]
- Ferguson MA, Nielsen JA, King JB, Dai L, Giangrasso DM, Holman R, Korenberg JR, Anderson JS. 2016. Reward, salience, and attentional networks are activated by religious experience in devout Mormons. Soc Neurosci. 13:104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. 1995. Structured clinical interview for DSM-IV, patient edition. Washington, DC: American Psychiatric Press, Inc. [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. 2005. Parietal lobe: from action organization to intention understanding. Science. 308:662–667. [DOI] [PubMed] [Google Scholar]
- Freiwald W, Duchaine B, Yovel G. 2016. Face processing systems: from neurons to real-world social perception. Annu Rev Neurosci. 39:325–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidt J. 2006. The happiness hypothesis: finding modern truth in ancient wisdom. New York: Basic Books. [Google Scholar]
- Herzog H, Lele VR, Kuwert T, Langen KJ, Rota Kops E, Feinendegen LE. 1990. Changed pattern of regional glucose metabolism during yoga meditative relaxation. Neuropsychobiology. 23:182–187. [DOI] [PubMed] [Google Scholar]
- Hommer RE, Seo D, Lacadie CM, Chaplin TM, Mayes LC, Sinha R, Potenza MN. 2013. Neural correlates of stress and favorite-food cue exposure in adolescents: a functional magnetic resonance imaging study. Hum Brain Mapp. 34:2561–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougaard A, Lindberg U, Arngrim N, Larsson HB, Olesen J, Amin FM, Ashina M, Haddock BT. 2015. Evidence of a Christmas spirit network in the brain: functional MRI study. BMJ. 351:h6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. 1902. The varieties of religious experience: a study in human nature. New York etc: Longmans, Green, and co. [Google Scholar]
- Jastreboff AM, Sinha R, Lacadie C, Small DM, Sherwin RS, Potenza MN. 2013. Neural correlates of stress- and food cue-induced food craving in obesity: association with insulin levels. Diabetes Care. 36:394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone B, Bodling A, Cohen D, Christ SE, Wegrzyn A. 2012. Right parietal lobe-related “selflessness” as the neuropsychological basis of spiritual transcendence. Int J Psychol Relig. 22:267–284. [Google Scholar]
- Johnstone B, Glass BA. 2008. Support for a neuropsychological model of spirituality in persons with traumatic brain injury. Zygon®. 43:861–874. [Google Scholar]
- Keenan JP, Gorman J. 2007. The causal role of the right hemisphere in self-awareness: it is the brain that is selective. Cortex. 43:1074–1082. [Google Scholar]
- Kim JH, Son YD, Kim JH, Choi EJ, Lee SY, Joo YH, Kim YB, Cho ZH. 2015. Self-transcendence trait and its relationship with in vivo serotonin transporter availability in brainstem raphe nuclei: an ultra-high resolution PET-MRI study. Brain Res. 1629:63–71. [DOI] [PubMed] [Google Scholar]
- Kjaer TW, Nowak M, Lou HC. 2002. Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. Neuroimage. 17:1080–1086. [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Hopper J, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS. 2003. Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biol Psychiatry. 53:204–210. [DOI] [PubMed] [Google Scholar]
- Lazar SW, Bush G, Gollub RL, Fricchione GL, Khalsa G, Benson H. 2000. Functional brain mapping of the relaxation response and meditation. Neuroreport. 11:1581–1585. [PubMed] [Google Scholar]
- Leube DT, Erb M, Grodd W, Bartels M, Kircher TT. 2003. Successful episodic memory retrieval of newly learned faces activates a left fronto-parietal network. Brain Res Cogn Brain Res. 18:97–101. [DOI] [PubMed] [Google Scholar]
- Lou HC, Changeux JP, Rosenstand A. 2017. Towards a cognitive neuroscience of self-awareness. Neurosci Biobehav Rev. 83:765–773. [DOI] [PubMed] [Google Scholar]
- Lou HC, Luber B, Crupain M, Keenan JP, Nowak M, Kjaer TW, Sackeim HA, Lisanby SH. 2004. Parietal cortex and representation of the mental Self. Proc Natl Acad Sci U S A. 101:6827–6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes AR, Montaldi D, Spencer TJ, Roberts N. 2004. Recalling spatial information as a component of recently and remotely acquired episodic or semantic memories: an fMRI study. Neuropsychology. 18:426–441. [DOI] [PubMed] [Google Scholar]
- Mazzarella E, Ramsey R, Conson M, Hamilton A. 2013. Brain systems for visual perspective taking and action perception. Soc Neurosci. 8:248–267. [DOI] [PubMed] [Google Scholar]
- Miller L. 2012. The Oxford handbook of psychology and spirituality. New York: Oxford University Press. [Google Scholar]
- Miller L, Bansal R, Wickramaratne P, Hao X, Tenke CE, Weissman MM, Peterson BS. 2014. Neuroanatomical correlates of religiosity and spirituality: a study in adults at high and low familial risk for depression. JAMA Psychiatry. 71:128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin A. 2007. Self-awareness and the left hemisphere: the dark side of selectively reviewing the literature. Cortex. 43:1068–1073. discussion 1074–1082. [DOI] [PubMed] [Google Scholar]
- Muhlau M, Hermsdorfer J, Goldenberg G, Wohlschlager AM, Castrop F, Stahl R, Rottinger M, Erhard P, Haslinger B, Ceballos-Baumann AO, et al. 2005. Left inferior parietal dominance in gesture imitation: an fMRI study. Neuropsychologia. 43:1086–1098. [DOI] [PubMed] [Google Scholar]
- Newberg AB, Iversen J. 2003. The neural basis of the complex mental task of meditation: neurotransmitter and neurochemical considerations. Med Hypotheses. 61:282–291. [DOI] [PubMed] [Google Scholar]
- Newberg AB, Pourdehnad M, Alavi A, d’Aquili EG. 2003. Cerebral blood flow during meditative prayer: preliminary findings and methodological issues. Percept Mot Skills. 97:625–630. [DOI] [PubMed] [Google Scholar]
- Newberg AB, Waldman MR. 2009. How God changes your brain: breakthrough findings from a leading neuroscientist. New York: Ballantine Books. [Google Scholar]
- Newberg AB, Wintering NA, Yaden DB, Waldman MR, Reddin J, Alavi A. 2015. A case series study of the neurophysiological effects of altered states of mind during intense Islamic prayer. J Physiol Paris. 109:214–220. [DOI] [PubMed] [Google Scholar]
- Newen A, Vogeley K. 2003. Self-representation: searching for a neural signature of self-consciousness. Conscious Cogn. 12:529–543. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. 2006. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 31:440–457. [DOI] [PubMed] [Google Scholar]
- Otto R, Harvey JW. 1926. The idea of the holy; an inquiry into the non-rational factor in the idea of the divine and its relation to the rational. London, New York, etc.: H. Milford, Oxford university press. [Google Scholar]
- Potenza MN, Hong KI, Lacadie CM, Fulbright RK, Tuit KL, Sinha R. 2012. Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am J Psychiatry. 169:406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Lacadie CM, Sinha R. 2016. Neural correlates and connectivity underlying stress-related impulse control difficulties in alcoholism. Alcohol Clin Exp Res. 40:1884–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons AN, Thayer RE, Spadoni AD, Matthews SC, Strigo IA, Tapert SF. 2012. The parametric, psychological, neuropsychological, and neuroanatomical properties of self and world evaluation. PLoS ONE. 7:e31509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. 2013. Modeling relapse situations in the human laboratory. Curr Top Behav Neurosci. 13:379–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Lacadie CM, Constable RT, Seo D. 2016. Dynamic neural activity during stress signals resilient coping. Proc Natl Acad Sci U S A. 113:8837–8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Wexler BE. 2004. Neural circuits underlying emotional distress in humans. Ann N Y Acad Sci. 1032:254–257. [DOI] [PubMed] [Google Scholar]
- Sinha R, Tuit K. 2012. Imagery script development procedures manual. Charleston, SC: CreateSpace. [Google Scholar]
- Sommer MA. 2003. The role of the thalamus in motor control. Curr Opin Neurobiol. 13:663–670. [DOI] [PubMed] [Google Scholar]
- Svob C, Wang Z, Weissman MM, Wickramaratne P, Posner J. 2016. Religious and spiritual importance moderate relation between default mode network connectivity and familial risk for depression. Neurosci Lett. 634:94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira S, Machado S, Velasques B, Sanfim A, Minc D, Peressutti C, Bittencourt J, Budde H, Cagy M, Anghinah R, et al. 2014. Integrative parietal cortex processes: neurological and psychiatric aspects. J Neurol Sci. 338:12–22. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J, Svob C, Miller L, Alvarenga JE, Abraham K, Warner V, Wickramaratne P, Weissman MM, Bruder GE. 2017. Association of posterior EEG alpha with prioritization of religion or spirituality: a replication and extension at 20-year follow-up. Biol Psychol. 124:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis F. 2001. Autonomic and EEG patterns distinguish transcending from other experiences during Transcendental Meditation practice. Int J Psychophysiol. 42:1–9. [DOI] [PubMed] [Google Scholar]
- Urgesi C, Aglioti SM, Skrap M, Fabbro F. 2010. The spiritual brain: selective cortical lesions modulate human self-transcendence. Neuron. 65:309–319. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. 2005. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 9:445–453. [DOI] [PubMed] [Google Scholar]
- Wilber K. 2006. Integral spirituality: a startling new role for religion in the modern and postmodern world. Boston: Integral Books. [Google Scholar]
- Yaden DB, Haidt J, Hood RW Jr, Vago DR, Newberg AB. 2017. The Varieties of Self-Transcendent Experience.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.