Abstract
Extensive MRI evidence indicates early brain overgrowth in autism spectrum disorders (ASDs). Local gyrification may reflect the distribution and timing of aberrant cortical expansion in ASDs. We examined MRI data from (Study 1) 64 individuals with ASD and 64 typically developing (TD) controls (7–19 years), and from (Study 2) an independent sample from the Autism Brain Imaging Data Exchange (n = 31/group). Local Gyrification Index (lGI), cortical thickness (CT), and surface area (SA) were measured. In Study 1, differences in lGI (ASD > TD) were found in left parietal and temporal and right frontal and temporal regions. lGI decreased bilaterally with age, but more steeply in ASD in left precentral, right lateral occipital, and middle frontal clusters. CT differed between groups in right perisylvian cortex (TD > ASD), but no differences were found for SA. Partial correlations between lGI and CT were generally negative, but associations were weaker in ASD in several clusters. Study 2 results were consistent, though less extensive. Altered gyrification may reflect unique information about the trajectory of cortical development in ASDs. While early overgrowth tends to be undetectable in later childhood in ASDs, findings may indicate that a trace of this developmental abnormality could remain in a disorder-specific pattern of gyrification.
Keywords: cerebral cortex, cortical folding, morphology, neuroanatomy, neurodevelopment
Autism spectrum disorders (ASDs) are neurodevelopmental conditions characterized by sociocommunicative deficits and restricted and repetitive behaviors (American Psychiatric Association 2013). Prevalence has recently been estimated at 1 in 45 for children aged 3–17 years (Zablotsky et al. 2015). Despite numerous functional and anatomical brain findings on ASDs, no consensus on crucial brain substrates has been reached.
A large body of neuroimaging evidence supports a trajectory of early cerebral overgrowth in the first 2 years of life in ASDs followed by abnormally slow growth across later childhood and adolescence (Courchesne et al. 2001, 2003; Redcay and Courchesne 2005; Shen et al. 2013; Hazlett et al. 2017). Others have suggested this atypical growth trajectory may be specific to a subgroup of individuals with megalencephaly (Libero et al. 2016) or related to elevated extra-axial fluid (Shen et al. 2013, 2017), and a few studies found no significant difference (Williams et al. 1980; Haznedar et al. 2000). Cortical differences have been reported in volume, surface area (SA), and cortical thickness (CT) measures. This overgrowth may be more evident in frontal, temporal, and possibly parietal regions (Carper et al. 2002; Hazlett et al. 2011; Schumann et al. 2010), but its distribution across different parameters of cortical morphometry has yet to be fully established.
Atypical growth trajectories in ASDs reported for SA and CT mostly correspond to volumetric findings. Both cross-sectional and longitudinal studies have shown differences in CT in ASDs, with regional findings of increased thickness in childhood to young adulthood (Chung et al. 2005; Hardan et al. 2006; Hyde et al. 2010) and reduced thickness in later adulthood (Hadjikhani et al. 2005), along with differences in the rate of cortical thinning during adolescence and adulthood (Hardan et al. 2009; Scheel et al. 2011; Zielinski et al. 2014). SA is often greater in toddlers with ASDs compared with controls (Hazlett et al. 2011), but differences are less pronounced or absent in later childhood and adolescence (Wallace et al. 2013; Yang et al. 2016), paralleling the atypical trajectory of early volumetric growth in ASDs described above. For both SA and CT, the largest group differences have been reported in frontal and temporal lobes (Carper et al. 2002; Schumann et al. 2010).
Postmortem studies also indicate aberrant cortical development, with localized areas of altered neuronal packing density, patches of abnormal laminar organization, and cortical dysgenesis reported in both adult and child cases (Bailey et al. 1998; Hutsler et al. 2007). Similarly, abnormal minicolumnar organization has been reported in ASDs (Casanova et al. 2002; Buxhoeveden et al. 2006; McKavanagh et al. 2015). Disruptions in CT, SA, laminar organization, and minicolumn structure are likely related to neuronal proliferation and migration in prenatal development (Caviness et al. 1995; Orosco et al. 2014; Packer 2016), and may thus have implications for emerging cortical gyrification. Several models posit that gyrification is influenced by axonal tension (Van Essen 1997; Raj and Chen 2011), differential rates of expansion of cortical layers (Armstrong et al. 1995; Reillo et al. 2010; Ronan et al. 2014), or a combination of such intrinsic forces (Striedter et al. 2015). In ASDs, these forces may be impacted by abnormal neuronal proliferation and migration, leading to altered gyrification patterns. Hence, an additional goal for our study was to examine potential correlations between measures of gyrification and measures of SA and CT for possible insight into underlying mechanisms of observed anomalies. For instance, an area of altered local Gyrification Index (lGI) in ASD might also show a strong correlation with SA or CT in the same area, suggesting a potential driver of observed lGI effects.
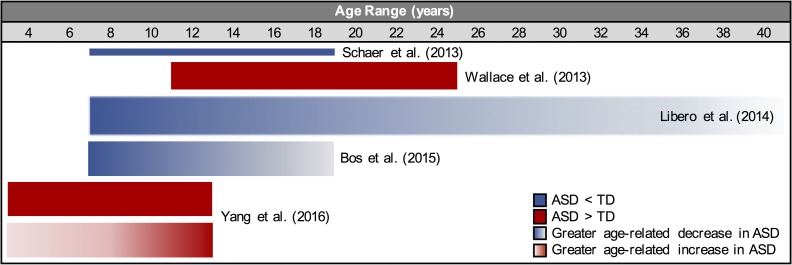
In typical development, cerebral gyrification may increase by about 16% and 7% in the first and second years of life, respectively (Li et al. 2014), followed by a gradual decline from late childhood into adulthood (Su et al. 2013; Klein et al. 2014). Previous studies have examined cortical morphology in ASDs, primarily in children and adolescents. Investigations of gyrification index at the whole brain (Williams et al. 2012) or lobar level (Hardan et al. 2004) have reported increased gyrification, particularly in frontal and parietal lobes. This is consistent with more localized measures of sulcal morphology which found increased sulcal depth, length, or area in regions including the insula, parietal operculum, and intraparietal sulcus (Nordahl et al. 2007; Shokouhi et al. 2012). In contrast, a study restricted to a sample of simplex cases of ASD found only small areas of decreased sulcal depth in (Dierker et al. 2013). A few studies have investigated gyrification in ASDs using the IGI measure, which has been suggested to be the most sensitive measure of cortical folding for detecting group differences when compared with local curvature and sulcal depth (Shimony et al. 2016). These studies have varied substantially in sample sizes, age ranges, and examination of age effects, as summarized in Figure 1. Overall, they indicate greater and atypically increasing gyrification in children with ASDs (Wallace et al. 2013; Yang et al. 2016), followed by increased decline across adolescence and adulthood, with findings mostly localized to frontal and parietal regions (Libero et al. 2014; Bos et al. 2015). Contradictory findings by Schaer et al. (2013) may be attributed to small sample size (only 11 participants with ASD) and failure to examine age-related changes. Additionally, some of these previous studies have been methodologically limited. Some had reduced spatial sensitivity either by using a lobar level average of lGI (Bos et al. 2015), or by using smoothed lGI data (Ecker et al. 2016), which may not be necessary in light of the inherent smoothness of the lGI measure, calculated for a 25 mm radius sphere surrounding each vertex (Schaer et al. 2008).
Figure 1.
Gyrification effects by age, as reported in previous ASD studies using the local Gyrification Index (lGI). Solid blue indicates lower reported lGI in ASD (frontal, parietal, and occipital regions). Solid red indicates reported greater gyrification in ASD (frontal, parietal, and temporal regions). Red gradient indicates reported greater age related increase in gyrification in ASD (frontal and parietal regions). Blue gradient indicates reported greater age related decline in gyrification (frontal and parietal regions). Bar width corresponds to relative sample size.
Given the small number and methodological limitations in the gyrification literature on ASDs, further investigation into age trajectories of gyrification is warranted. The goals of the present study were therefore to 1) examine age effects and group differences between children and adolescents with ASD and typically developing (TD) controls, using the IGI; and 2) examine the relationships between lGI, CT, and SA to better understand the mechanisms underlying gyrification differences in ASDs. These goals were addressed by examining 2 independent samples, a large in-house sample and a sample available from the Autism Brain Imaging Data Exchange (ABIDE; Di Martino et al. 2014) to test for replicability of findings.
Materials and Methods
Study 1: In-House Sample (SDSU)
Participants
A total of 206 individuals (108 ASD; 98 TD) were scanned for the present study. Participants with a history of neurological (e.g., epilepsy, tuberous sclerosis) or genetic (e.g., fragile X, Rett syndrome) conditions other than ASD were excluded. Inclusion in the TD group required the absence of personal or family history of autism, and of personal history of other neurological or psychiatric conditions. ASD diagnoses were confirmed by a clinical psychologist based on Diagnostic and Statistical Manual of Mental Disorders fifth edition criteria (American Psychiatric Association 2013), supported by the Autism Diagnostic Interview, Revised (ADI-R) (Lord et al. 1994) and the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) (Lord et al. 2012). IQ was assessed using the Wechsler Abbreviated Scale of Intelligence, second edition (WASI-II) (Weschler 1999). Social skills and executive function were further assessed with 2 parent reports, the Social Responsiveness Scale (SRS) (Constantino et al. 2003), which evaluates a child’s ability to engage in emotionally appropriate reciprocal social interactions, along with communicative deficits and restricted/stereotypic behaviors or interests, and the Behavior Rating Inventory of Executive Function (BRIEF) (Gioia et al. 2015), which assesses the everyday behavioral manifestations of executive control functions in children.
Imaging Data
High-resolution anatomical images were obtained using a 3 Tesla GE Discovery MR750 scanner with an 8-channel head coil and a T1-weighted inversion recovery fast spoiled gradient echo sequence (repetition time = 8.108 ms, echo time = 3.172 ms, flip angle = 8°, 172 slices, 1 mm3 resolution). Participants with abnormal neuroanatomical findings were excluded (3 ASD; 2 TD). Abnormal neuroanatomical findings included cysts (2 ASD), abnormally enlarged ventricles (1 ASD), and cerebellar hypoplasia (2 TD), as evaluated by a senior neuroanatomist (R.A.C.). Following visual assessment of MRI data and FreeSurfer output, 41 ASD and 32 TD participants were excluded due to insufficient quality of raw scans or surface reconstruction (e.g., due to ringing or ghosting artifacts). There were no statistically significant differences between the individuals excluded versus those included on measures on age, IQ, or autism severity. Data from 64 ASD and 64 TD participants between the ages of 7 and 19 years were analyzed, with groups matched on age, nonverbal IQ, gender, and handedness (Table 1).
Table 1.
Participant demographics.
| ASD (SDSU n = 64, NYU n = 31) | TD (SDSU n = 64, NYU n = 31) | ||||
|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | P-value | |
| SDSU | |||||
| Age (years) | 13.32 ± 2.65 | 7.85–18.31 | 13.53 ± 2.95 | 6.90–18.92 | 0.67 |
| TBV (cm3) | 1312.48 ± 120.86 | 1032.73–1654.40 | 1278.28 ± 118.11 | 1050.16–1683.22 | 0.11 |
| SRS total | 83.07 ± 10.53 | 56–112 | 42.69 ± 5.82 | 35–65 | <0.001 |
| WASI | |||||
| Verbal | 103.59 ± 17.60 | 56–147 | 105.94 ± 10.47 | 74–133 | 0.36 |
| Nonverbal | 108.20 ± 17.60 | 53–140 | 106.10 ± 14.10 | 62–137 | 0.46 |
| Full scale | 106.67 ± 16.88 | 66–141 | 106.45 ± 14.07 | 77–130 | 0.93 |
| BRIEF | |||||
| BRI | 70.93 ± 12.79 | 39–90 | 44.78 ± 8.79 | 37–81 | <0.001 |
| MET | 66.80 ± 9.02 | 44–82 | 46.81 ± 9.47 | 36–74 | <0.001 |
| ADOS-2 | |||||
| SA | 10.33 ± 3.53 | 5–18 | |||
| RRB | 3.13 ± 1.70 | 1–8 | |||
| Total | 13.38 ± 4.14 | 6–23 | |||
| Severity | 7.43 ± 1.87 | 3–10 | |||
| ADI | |||||
| Soc | 17.98 ± 5.40 | 6–28 | |||
| Com | 13.14 ± 5.40 | 2–24 | |||
| Rep | 5.97 ± 1.96 | 2–11 | |||
| Female | n = 12 | n = 9 | |||
| Left handed | n = 7 | n = 7 | |||
| NYU | |||||
| Age (years) | 11.44 ± 2.84 | 7.15–18.58 | 11.78 ± 2.57 | 7.26–18.31 | 0.62 |
| TBV (cm3) | 1243.65 ± 113.07 | 1020.60–1622.70 | 1273.46 ± 98.54 | 1044.91–1487.62 | 0.27 |
| SRS total | 90.03 ± 25.76 | 36–150 | 22.16 ± 13.56 | 5–56 | <0.001 |
| WASI | |||||
| Verbal | 99.39 ± 8.77 | 84–118 | 110.84 ± 12.90 | 80–128 | <0.001 |
| Nonverbal | 106.90 ± 14.10 | 72–129 | 107.97 ± 11.97 | 83–128 | 0.75 |
| Full Scale | 103.23 ± 10.05 | 76–124 | 110.45 ± 11.85 | 81–128 | 0.01 |
ASD, autism spectrum disorder; TD, typically developing; SRS, social responsiveness scale; WASI, Wechsler abbreviated scales of intelligence; BRIEF, behavior rating inventory of executive function; BRI, behavioral regulation index; MET, metacognition index; ADOS, autism diagnostic observation schedule-second edition; SA, social affect; RRB, restricted and repetitive behavior; ADI, autism diagnostic interview-revised; Soc, social interaction subscale; Com, communication subscale; Rep, restricted and repetitive behaviors subscale.
Study 2: ABIDE Sample (NYU)
Participants
Data were available from the ABIDE (Di Martino et al. 2014; Di Martino et al. 2017) for 137 male participants aged 7–18 years (60 ASD, 77 TD) from the New York University contributing site. Further details about the sample are available on the ABIDE website (http://fcon_1000.projects.nitrc.org/indi/abide/). NYU was selected for its large data set and similar age range to the SDSU sample.
Imaging Data
High resolution T1-weighted MRI sequences were obtained on a Siemens Allegra MR 2004a scanner (repetition time = 2530 ms, echo time = 3.25 ms, flip angle = 7°, 171 slices, 1.3 × 1.0 × 1.3 mm3 resolution). Following visual assessment of MRI data and FreeSurfer output, 24 ASD and 37 TD participants were excluded due to insufficient quality of raw scans or surface reconstruction errors. There were no statistically significant differences between the individuals excluded versus those included on measures on age, IQ, or autism severity. Additionally, 5 ASD and 9 TD participants at the extremes on measures of age and nonverbal IQ were excluded to match groups and for comparability to the SDSU sample. Data from 31 ASD and 31 TD participants were included in analyses (Table 1).
Image Processing: Cortical Reconstruction and Quality Assessment
FreeSurfer version 5.3.0 was employed to perform semiautomated cortical reconstruction on data from both study samples (Dale et al. 1999; Fischl et al. 1999). All FreeSurfer output was examined on a slice-by-slice basis to identify any inaccuracies in surface placement. Inaccuracies were corrected with white matter control points as needed, and then reassessed for accuracy. Scans that still showed inaccuracies were excluded. Scans with major artifacts, such as ghosting or ringing, or inaccuracies deemed unlikely to be ameliorated by manual edits (based on past experience) were excluded. SA and CT were automatically measured at each FreeSurfer surface vertex and lGI was measured using an added flag to the FreeSurfer reconstruction processing stream (Schaer et al. 2008). lGI is a 3D surface-based method for calculating the ratio of cortical surface area within the sulcal folds (pial surface) relative to the amount of cortex on the outer visible cortex (cortical hull). This calculation was made within a sphere of 25 mm radius around the pial surface vertex. This automated reconstruction feature has been validated as a reliable measure of gyrification against manual measurement (Schaer et al. 2012).
Statistical Analyses
Statistical analyses were performed for each vertex using a 2-step general linear model (GLM) approach (Yang et al. 2016), with separate models for CT, SA, and lGI as outcomes. All analyses were conducted both with and without total brain volume (TBV) as covariate to control for individual differences in brain size. TBV was calculated as the sum of supratentorial volume and cerebellar volume in FreeSurfer. A smoothing kernel of 15-mm full width half maximum was applied for CT and SA analyses, while no smoothing was implemented for lGI.
Corrections for vertex-wise multiple comparisons were conducted using FreeSurfer’s Monte Carlo null-z simulations based on precomputed simulation data available in the software, with a cluster forming threshold of P < 0.01 and a cluster-wise significance threshold of P < 0.05 (Hagler et al. 2006).
Main Effects of Group and Age
We tested for the main effects of group and age, utilizing the DOSS (different offset, same slope) matrix design:
Group by Age Interactions
We tested for group by age interactions utilizing the DODS (different offset, different slope) matrix design as implemented in FreeSurfer:
Secondary GLM analyses, testing the same outcome models, were conducted for lGI using Permutation Analysis of Linear Models (PALM) software to allow for permutation testing of the results (Winkler et al. 2014). Overall, 1000 permutations were run using tail approximation and threshold free cluster enhancement (TFCE). The significance threshold was set at P < 0.05, with family wise error (FWE) correction for multiple comparisons.
Correlations Between Morphometric Features
Clusters identified in the Monte Carlo simulations were used as regions of interest, for which partial correlations were calculated between lGI and CT and between lGI and SA, separately for each group while controlling for age. Correlation coefficients were Fisher-z transformed and entered into a z-test (Fisher 1938) in order to elucidate between-group differences in the relationships between morphometric features.
Correlations With Behavioral Measures
Partial correlations controlling for age were run to examine the relationships between mean lGI in each cluster (above) and t-scores from the SRS and BRIEF.
Results
Study 1: In-House Sample (SDSU)
local Gyrification Index
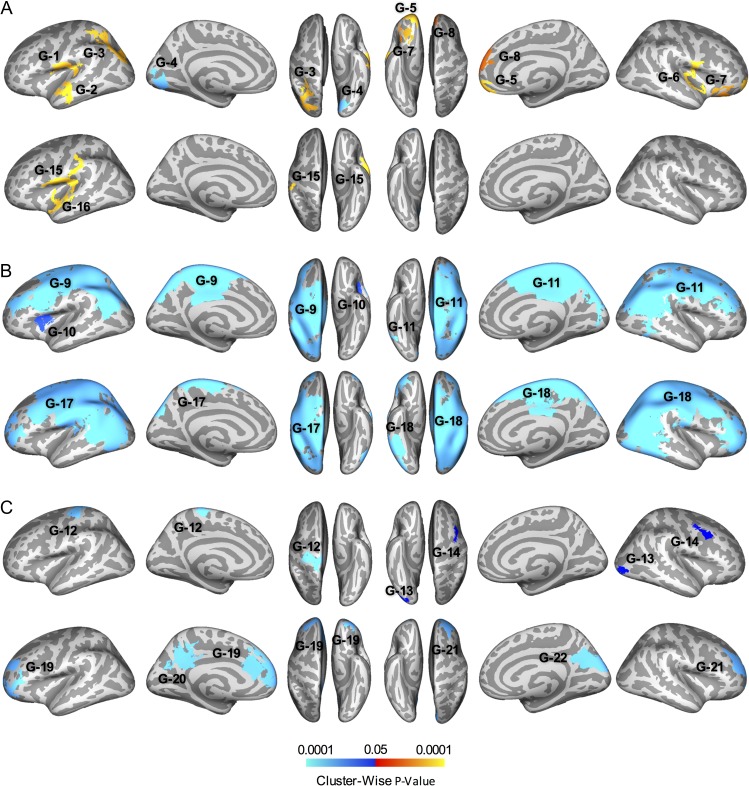
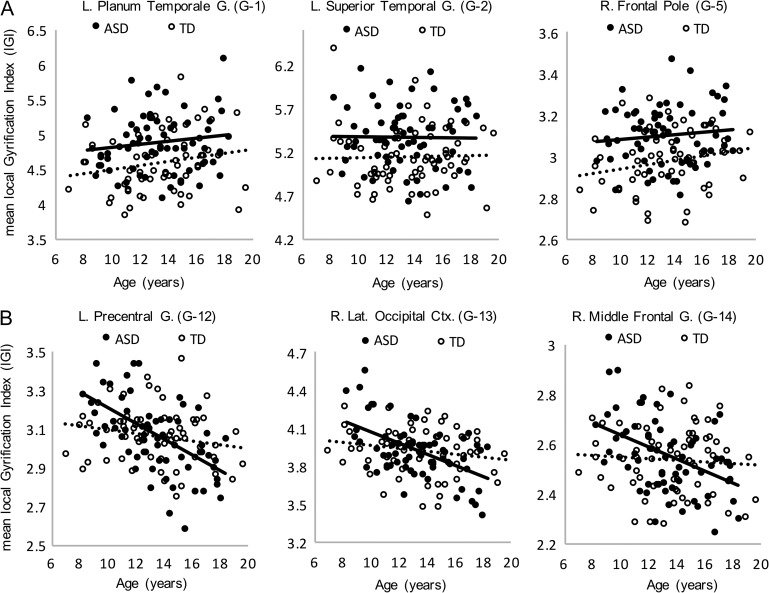
The lGI model revealed several clusters of greater lGI in the ASD group, controlling for age, with medium to large effect sizes (Fig. 2A, Fig. 3A, Supplementary Table S1). Peaks were localized in the precentral gyrus (G-1), superior temporal gyrus (G-2), and superior parietal lobule (G-3) on the left hemisphere, and frontal pole (G-5), perisylvian/precentral gyrus (G-6), lateral orbitofrontal cortex (G-7), and superior frontal gyrus (G-8) on the right. In contrast, lGI was lower in the ASD group in a single cluster with peak in left lingual gyrus (G-4). lGI also showed a negative main effect of age across groups in large clusters encompassing much of the dorsal and medial surfaces of the left and right frontal, parietal, and occipital lobes (G-9, G-10, and G-11; Fig. 2B). Group by age interaction effects for lGI were detected in 3 clusters (Figs 2C and 3B). The negative slope of the relationship between lGI and age was greater in the ASD than in the TD group in the central sulcus (G-12), right lateral occipital cortex (G-13), and right caudal middle frontal gyrus (G-14).
Figure 2.
Effects of group and age on local Gyrification Index (lGI). Top row of each panel shows SDSU sample; bottom row shows NYU sample. (A) Main effects of group, warm colors indicate ASD > TD. (B) Main effects of age in combined groups, cool colors indicate negative slope with age. (C) Interactions between group and age, cool colors indicate more negative slope in ASD than TD. Color reflects average significance across each cluster. Cluster forming threshold of P < 0.01, cluster-wise significance threshold of P < 0.05.
Figure 3.
(A) Main effects of group on lGI. Plots illustrate greater average gyrification index in the ASD group in 3 representative clusters (linear fits show age effects on lGI by group; Cluster G-1: ASD R2 = 0.018, TD R2 = 0.041; G-2: ASD R2 = 0.001, TD R2 = 0.001; G-5: ASD R2 = 0.011, TD R2 = 0.038). (B) Group by age interaction effects on local Gyrification Index (lGI). The negative effect of age is greater in the ASD group in each of 3 clusters exhibiting group by age interaction effects (SDSU Sample; Cluster G-12: ASD R2 = 0.278, TD R2 = 0.033; G-13: ASD R2 = 0.288, TD R2 = 0.032; G-14: ASD R2 = 0.201, TD R2 = 0.005). Cluster labels correspond to those in Figure 2.
Since little data on lGI in this age range is available in the published literature, a secondary analysis was performed using permutation testing. With this alternative approach, only the main effects of age were identified (clusters G-10, G-11). The group effect in cluster G-1 (precentral gyrus/planum temporale) approached the P = 0.05 threshold, with a minimum vertex value of P = 0.06.
Cortical Thickness
CT was found to be lower in the ASD group in a single cluster with a peak value in the insula (T-1; Supplementary Fig. S1A, Supplementary Table S2). Additionally, a main effect of age was found, with the cortex becoming thinner with age in large clusters across almost the entire left and right hemispheres across groups (T2 and T3, Supplementary Fig. S1C). No group by age interaction effects were found for CT.
Surface Area
There were no main effects of group, nor any group by age interaction effects. However, a positive main effect of age was found in several clusters (Supplementary Fig. S1D, Supplementary Table S2). In the left hemisphere, these clusters had peak values in precentral gyrus (A-1), lateral orbitofrontal cortex (A-2), and lateral occipital cortex (A-3). In the right hemisphere, peaks occurred in superior frontal gyrus (A-4), superior parietal lobule (A-5), fusiform gyrus (A-6), and lateral orbitofrontal cortex (A-7).
Correlations Between Morphometric Features
There was a strong positive relationship between lGI and SA across both groups in most clusters examined (Table 2). In contrast, the correlations between lGI and CT tended to be negative in the TD group. Notably, a reduction in strength or even a reversal of this relationship was shown in the ASD group in some clusters. Z-tests revealed between-group differences in the strength of the relationship between lGI and CT in 3 clusters. In cluster G-6, located in the Perisylvian region, the negative correlation observed in the TD group was reversed in the ASD group, which exhibited a modest positive correlation between lGI and CT (z=−3.06, P = 0.001). The negative correlation between lGI and CT observed in the TD group was reduced in magnitude in the ASD group in cluster G-13 in the lateral occipital cortex (z = −1.97, P = 0.04) and cluster G-3 spanning from the superior parietal lobule to anterior occipital lobe (z = −1.86, P = 0.03).
Table 2.
Partial correlations with local Gyrification index (controlling for age)
| Surface area | Cortical thickness | |||||||
|---|---|---|---|---|---|---|---|---|
| Cluster | Location | TD | ASD | P-value† | TD | ASD | P-value† | |
| Main effect of group | G-1 | Precentral gyrus/planum temporale | 0.256* | 0.326** | 0.337 | −0.403** | −0.297* | 0.251 |
| G-2 | Superior–middle temporal gyrus | 0.146 | 0.024 | 0.248 | −0.021 | 0.122 | 0.215 | |
| G-3 | Superior parietal lobule/anterior occipital lobe | 0.505** | 0.58** | 0.278 | −0.364** | −0.044 | 0.031 | |
| G-4 | Lingual gyrus–calcarine sulcus | 0.324** | 0.412** | 0.287 | −0.292* | −0.316* | 0.440 | |
| G-5 | Frontal pole | 0.133 | 0.355** | 0.095 | −0.128 | 0.084 | 0.119 | |
| G-6 | Perisylvian, insular, precentral cortex | 0.517** | 0.485** | 0.405 | −0.414** | 0.113 | 0.001 | |
| G-7 | Lateral orbitofrontal cortex | 0.185 | −0.031 | 0.115 | −0.363** | −0.260* | 0.264 | |
| G-8 | Superior frontal gyrus | 0.107 | 0.119 | 0.472 | −0.424** | −0.340** | 0.295 | |
| G-15 | Circular insular sulcus/postcentral sulcus | 0.577** | 0.350 | 0.274 | −0.057 | −0.057 | 1.000 | |
| G-16 | Insular cortex/transverse temporal sulcus | 0.157 | 0.253 | 0.707 | −0.446* | −0.099 | 0.155 | |
| Interaction | G-12 | Precentral gyrus/central sulcus | 0.337** | 0.256* | 0.312 | −0.164 | −0.135 | 0.436 |
| G-13 | Lateral occipital cortex | 0.209 | 0.357** | 0.187 | −0.375** | −0.037 | 0.024 | |
| G-14 | Caudal middle frontal gyrus/precentral sulcus | 0.054 | −0.163 | 0.348 | −0.138 | −0.205 | 0.352 | |
| G-19 | Frontal pole/anterior cingulate gyrus | 0.462* | 0.399* | 0.776 | −0.375* | −0.239 | 0.573 | |
| G-20 | Precuneus cortex | 0.553** | 0.259 | 0.181 | −0.371* | −0.001 | 0.145 | |
| G-21 | Superior frontal gyrus–sulcus | 0.383* | 0.287 | 0.685 | −0.234 | 0.105 | 0.198 | |
| G-22 | Cuneus cortex | 0.440* | 0.439 | 0.996 | 0.124 | −0.137 | 0.961 | |
Correlation analyses were restricted to clusters exhibiting interaction effects or between group differences.
Bold indicates significant difference between groups, uncorrected.
Italics indicate results from NYU sample.
*P < 0.05, **P < 0.01. †P-value corresponds to z-test of between group difference in correlation.
Behavioral Correlations
Correlations between lGI and behavioral measures are summarized in Supplementary Table S3. For the BRIEF, negative correlations in 4 of the 11 clusters examined were found within frontal and parietal lobes (G-3, G-5, G-7, G-8), reflecting an association between lower lGI and greater impairment on the Metacognition Index (MET) in the ASD group. In contrast, positive correlations were observed in the TD group between lGI and MET scores in occipital cluster G-13, and between lGI and the Behavioral Regulation Index (BRI) in parieto-occipital cluster G-3. BRIEF MET scores serve as a measure of ability to initiate, plan, organize, and sustain working memory, while BRI scores reflect the ability to shift cognitive set and modulate emotions and behavior using appropriate inhibitory control (Gioia et al. 2015). No correlations were found with SRS scores in either group. Behavioral correlation analyses did not survive FDR correction for multiple comparisons.
Study 2: ABIDE Sample (NYU)
local Gyrification Index
We found 2 clusters of greater gyrification in the ASD group in the left hemisphere, with peak values in the insula (G-15) and superior temporal gyrus (G-16) and medium effect sizes in both (Fig. 2A, Supplementary Table S1). Negative effects of age on lGI across groups were observed in the left hemisphere extending from the superior frontal and parietal lobes to the lateral occipital lobe (G-17) and in the right hemisphere encompassing much of the frontal, parietal, and occipital cortices (G-18; Fig. 2B). Interaction effects were observed in several clusters, with a greater negative effect of age on lGI in the ASD group in left and right rostral middle frontal gyri (G-19 and G-21), left precuneus (G-20), and right cuneus (G-22; Fig. 2C). When permutation testing was used as an alternative approach, only the main effects of age were identified (clusters G17 and G-18).
Cortical Thickness
No group by age interactions nor group differences were found for CT. A negative effect of age was observed across both groups in large clusters encompassing the majority of the cortex across both hemispheres (T-4, T-5, T-6; Supplementary Fig. S1E, Supplementary Table S1).
Surface Area
No group by age interactions nor group differences were found for SA, but in a single cluster in left superior frontal and paracentral gyri, a positive effect of age on SA was observed (A-8; Supplementary Fig. S1B, Supplementary Table S2).
Correlations Between Morphometric Features
There was a strong positive relationship between lGI and SA in many clusters (Table 2), similar to findings for the in-house sample. Also in line with these findings, the correlations between lGI and CT tended to be negative, but a reduction in strength or even reversal of this relationship was shown in the ASD group in some clusters. However, z-tests failed to reveal any between-group differences in the relationship between lGI and CT, nor between lGI and SA in the NYU sample.
Behavioral Correlations
No relationships between gyrification and SRS were found. BRIEF scores were not available from the ABIDE database.
Results Without TBV Covariate, Female, and Left-Handed Participants
The models for lGI, CT, and SA were also run without the TBV covariate. Results from these analyses were generally consistent with those from the models that included TBV, although several clusters did not survive correction for multiple comparisons, including clusters in the SDSU sample (G-4, G-5, G-7, G-8, G-10, G-13, G-14, T1, and A7) and the NYU sample (G16; Supplementary Tables S4 and S5). Additionally, the large cluster G-9 split into 2 smaller clusters, and a new cluster (A-9) met threshold for a positive effect of age in the model for SA in the NYU sample, extending from the right superior frontal to paracentral gyrus. Notably, however, main effects on lGI in the Perisylvian area (e.g., G-1, G-6, G-15), which had shown the greatest consistency between samples and between hemispheres, were still present.
The samples of female and left-handed participants were each too small to examine independently in the SDSU analysis. However, because results remained unchanged when excluding them from the sample, they were retained in the main analysis to conserve statistical power. The ABIDE sample did not include females or left-handed participants.
Discussion
The present study is among the first to compare multiple features of cortical morphology between individuals with ASDs and TD peers, and has to our knowledge the largest sample size on record for an analysis of gyrification in ASD. Results indicate that whereas lGI tends to be greater in children with ASD in some cortical regions, it also decreases more steeply with age than in typical development in spatially discrete regions. Further, we find that the group differences in lGI may be more closely tied to differences in CT than SA.
Gyrification
Across both groups, lGI showed negative effects of age, concordant with several previous studies using the same measure (Hogstrom et al. 2012; Mutlu et al. 2013; Klein et al. 2014) or related sulcal-depth based, 2D slice-based, and postmortem approaches (Armstrong et al. 1995; Zilles et al. 1988).
Our results also revealed several differences of medium to large effect size between ASD and TD groups independent of age. The ASD group showed greater gyrification in 7 out of 8 clusters, which were located mainly in Perisylvian regions in both the NYU and SDSU samples, with some additional effects in parietal and frontal areas in the SDSU sample only. This effect is likely driven by differences in the shape of the Sylvian fissure and, given the way lGI is calculated and the chronology of early cortical expansion, it may reflect broadly atypical growth trajectories of neighboring frontal, parietal, and temporal regions or interior aspects of the operculae. Consistent with our findings in the SDSU and NYU samples, increased sulcal depth, length, or surface area for the insula and parietal operculum were primary findings in 2 other studies of children and adolescents with ASD (Nordahl et al. 2007; Shokouhi et al. 2012), though a study limited to simplex ASD cases did not find the same effect (Dierker et al. 2013). Increased depth of the intraparietal sulcus was also found in the right hemisphere (Shokouhi et al. 2012) and bilaterally for a group with Asperger syndrome but not a group with ASD (Nordahl et al. 2007). The direction of this group difference is largely concordant with several previous studies of lGI in ASD with overlapping age ranges (Ecker et al. 2016; Wallace et al. 2013; Yang et al. 2016), although effects have varied spatially between studies, possibly due to small sample sizes and cohort effects. While the exact regions exhibiting such effects did not completely overlap between the 2 samples in the present study, there was some convergence onto lGI increases in earlier developing sulci.
The trajectory of lGI maturation also differed between groups in our analyses, with a greater negative effect of age for individuals with ASDs in several clusters in both the NYU and SDSU samples, albeit in different regions. The interaction effect is also consistent with the results of a previous study of the same age range limited to lobar gyrification measures, which showed a greater negative effect of age in ASD for frontal and parietal cortices (Bos et al. 2015). In contrast, Yang et al. (2016) found atypical positive effects of age in frontal and parietal regions in children with ASDs, which were, however, younger (4–12 years of age) than our sample. Taken together, these findings might indicate nonlinear age trajectories in certain regions in ASDs.
The lateral (or Sylvian) fissure is the first primary sulcus to develop, beginning to emerge as early as gestational week (GW) 16 and clearly separating the frontal and temporal lobes by GW 26 (Chi et al. 1977; Garel et al. 2001). It is followed closely by other primary sulci, including the cingulate, calcarine, and parieto-occipital sulci. The circular, superior frontal, intraparietal, precentral, and orbital sulci appear between gestational weeks 25 and 28 (Armstrong et al. 1995). Many of the clusters exhibiting differences between ASD and TD participants in our analyses fall in these earlier developing regions of gyrification, suggesting that lGI findings may reflect very early growth anomalies in ASDs. Although most older children and adults display TBV in the normal range, it is possible that some anomalous patterns of regional gyrification might still manifest remnants of early overgrowth. Moreover, lGI variability within the ASD cohort may provide some insight and potentially allow us to differentiate children with and without a history of overgrowth.
Cortical Thickness and Surface Area
Atypically reduced CT observed in the insula in the SDSU sample compares to previous findings of concordant effects in samples of similar age ranges, albeit in varying loci (Wallace et al. 2010; Ecker et al. 2014; Richter et al. 2015). There have also been reports of increased CT in ASD in early childhood with differences diminishing by adulthood (Libero et al. 2014; Khundrakpam et al. 2017), indicating that the direction of group effects may largely depend on age. Although our study was unable to detect a specific group by age interaction in this age range, we observed widespread thinning across both groups. This is expected from the literature (Gogtay et al. 2004; Sowell et al. 2004; O’donnell et al. 2005), although the precise shape of this decline, whether linear or curvilinear, has been debated. Some studies have also indicated regional differences (Ducharme et al. 2016; Wierenga et al. 2014).
We found positive age effects on SA across both groups. While the direction of this effect was consistent between the NYU and SDSU samples, the localization differed, adding to mixed findings from previous studies. A general trend in the literature shows increasing SA through later childhood, reaching a maximum in adolescence (Amlien et al. 2014; Schnack et al. 2015), followed by relative stability in young adulthood, or in some cases a decline (Vijayakumar et al. 2016; Tamnes et al. 2017). The timing and trajectory of SA growth also differs regionally in the cortex (Wierenga et al. 2014; Ducharme et al. 2016). Although we observed main effects of age, we detected no group difference or group by age interaction for SA. Previous studies have been mixed with reduced (Ecker et al. 2014; Libero et al. 2014; Mensen et al. 2017), increased (Hazlett et al. 2011; Ohta et al. 2015), or comparable SA (Raznahan et al. 2011; Wallace et al. 2010, 2013; Yang et al. 2016). These inconsistencies are not easily accounted for by sample age and may reflect high levels of interindividual variability. Only studies with much larger samples than currently available may have sufficient power to detect consistent group differences or group by age interactions. Our study provides no evidence for a difference in age related changes in these features of cortical morphology in this age range. This may suggest similar trajectories of CT and SA in children and adolescents with ASDs and their TD peers. Lack of findings for CT and SA accompanied by effects for lGI in the same samples suggest that spatial and maturational patterns of gyrification may provide unique information about the altered developmental trajectory of cortical morphology in ASD.
Relationships Between Features of Cortical Morphology
Correlations between lGI and CT and lGI and SA were examined to probe potential explanations for observed differences between groups, since the developmental processes underlying CT and SA are known to be mechanistically discreet (White et al. 2010). In many regions exhibiting main effects of group, lGI was positively correlated with SA, but negatively with CT, similar to previous reports (Hogstrom et al. 2012; Klein et al. 2014). Similar relationships are found in cross-species studies showing that gyrification increases with SA in a nonlinear fashion across different mammalian species (Hofman 1985), but is negatively associated with CT when SA is relatively constant across species (Pillay and Manger 2007). While previous research has established that CT and SA are driven by different growth processes under separate genetic influences and follow independent growth trajectories (Panizzon et al. 2009), these relationships with lGI could reflect mechanical processes contributing to specific gyrification patterns observed in the TD cortex (Armstrong et al. 1995; Ronan et al. 2014; Van Essen 1997).
Cortical thickness and thinning are thought to be affected by a number of factors at different points in development, including neuroproliferation (Rakic and Swaab 1988; Caviness et al. 1995), dendritogenesis (Huttenlocher and Dabholkar 1997; Shaw et al. 2008), and myelination (Gogtay and Thompson 2010; Deoni et al. 2015). Postmortem studies have provided evidence of differences in CT in ASDs that would be consistent with a possible role in the lGI differences we observed here (Bailey et al. 1998; Hutsler et al. 2007). Differences in the lGI–CT relationship observed between the ASD and TD groups might indicate that disrupted gyrification in ASD is more closely related to biological factors that drive the development of CT than to those that determine SA.
Correlations with Behavior
While our correlational analyses did not indicate links between gyrification and symptomatology as measured by the SRS, the few correlations of medium effect size were detected between lGI and BRIEF scores, with lower gyrification being associated with reduced executive function. While the mostly frontal localization of these effects is in line with the role of prefrontal cortex in executive function (Alvarez and Emory 2006), this direction of this relationship was unexpected given our findings of regionally greater gyrification in the ASD group, along with previous findings of executive dysfunction in ASDs (Craig et al. 2016). However, these modest behavioral correlations need to be viewed with great caution, as they did not survive correction for multiple comparisons.
Limitations and Future Directions
Given that the statistical distribution of the lGI measure across the cortex remains largely unexplored, permutation testing was conducted in addition to Monte Carlo simulations in order to correct for multiple comparisons. While the main effects of group and group by age interaction effects did not remain significant after permutation testing, the survival of the main effects of age lends some support to the overall results. Relatively subtle differences at the group level may be in part attributed to expected etiological heterogeneity in ASDs (Geschwind and State 2015). Medium to large effect sizes in the clusters showing main effects of group nonetheless suggest meaningful differences in lGI between groups. Our further analyses of the relationships between features of cortical morphology also demonstrate that there are noteworthy group differences within those clusters, regardless of the significance level of the main effect of group.
The cross-sectional nature of the data prevented us from directly testing age trajectories of different cortical morphology features, and therefore the age-related findings should be interpreted with caution. Additionally, the inclusion of mostly high-functioning individuals, while necessary to obtain high quality neuroimaging data, limits the generalizability of findings to only a portion of the full spectrum of ASDs. Although exploratory in nature, it is also important to acknowledge some of the statistical shortcomings of the correlation analyses. Correlations between different morphological features were conducted on a cluster-wise basis, which may have more limited sensitivity than vertex-wise analyses due to the averaging of values across clusters. Finally, both the behavioral and morphometric correlations are prone to potential false positive results due to the high number of comparisons. These types of analyses might benefit from a multivariate approach in the future.
Conclusions
Previous studies of CT and SA in ASDs have shown mixed findings, often lacking evidence for a reliable group difference in these morphological measures beyond early childhood. In contrast, the present study suggests a greater degree of cortical folding in ASDs in later childhood and adolescence. This increased gyrification may reflect a history of early cortical overgrowth in ASDs, displaying a morphological trace of early developmental abnormalities. Gyrification anomalies in ASDs may be related to factors influencing CT, in addition to SA. These findings have implications for understanding the potential cytoarchitectural abnormalities that may underlie differences observed in cortical macrostructure in ASDs.
Supplementary Material
Notes
Our sincere thanks to our participants and their families for sharing their time with us. We also thank the ABIDE initiative for providing access to data used in Study 2. Additional thanks to Samantha Marshall for her assistance in quality assessment of the data. Conflict of interest: None declared.
Funding
National Institutes of Health (R01-MH081023 and K01-MH097972).
References
- Alvarez JA, Emory E. 2006. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 16:17–42. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association 2013. Diagnostic and statistical manual of mental disorders. 5th ed Washington, DC: Author. [Google Scholar]
- Amlien IK, Fjell AM, Tamnes CK, Grydeland H, Krogsrud SK, Chaplin TA, Rosa MG, Walhovd KB. 2014. Organizing principles of human cortical development—thickness and area from 4 to 30 years: insights from comparative primate neuroanatomy. Cereb Cortex. 26:257–267. [DOI] [PubMed] [Google Scholar]
- Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K. 1995. The ontogeny of human gyrification. Cereb Cortex. 5:56–63. [DOI] [PubMed] [Google Scholar]
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Rutter M, Lantos P. 1998. A clinicopathological study of autism. Brain. 121:889–905. [DOI] [PubMed] [Google Scholar]
- Bos DJ, Merchán-Naranjo J, Martínez K, Pina-Camacho L, Balsa I, Boada L, Schnack H, Oranje B, Desco M, Arango C, et al. . 2015. Reduced gyrification is related to reduced interhemispheric connectivity in autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 54:668–676. [DOI] [PubMed] [Google Scholar]
- Buxhoeveden D, Semendeferi K, Buckwalter J, Schenker N, Switzer R, Courchesne E. 2006. Reduced minicolumns in the frontal cortex of patients with autism. Neuropathol Appl Neurobiol. 32:483–491. [DOI] [PubMed] [Google Scholar]
- Carper RA, Moses P, Tigue ZD, Courchesne E. 2002. Cerebral lobes in autism: early hyperplasia and abnormal age effects. Neuroimage. 16:1038–1051. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. 2002. Minicolumnar pathology in autism. Neurology. 58:428–432. [DOI] [PubMed] [Google Scholar]
- Caviness V, Takahashi T, Nowakowski R. 1995. Numbers, time and neocortical neuronogenesis: a general developmental and evolutionary model. Trends Neurosci. 18:379–383. [DOI] [PubMed] [Google Scholar]
- Chi JG, Dooling EC, Gilles FH. 1977. Gyral development of the human brain. Ann Neurol. 1:86–93. [DOI] [PubMed] [Google Scholar]
- Chung MK, Robbins SM, Dalton KM, Davidson RJ, Alexander AL, Evans AC. 2005. Cortical thickness analysis in autism with heat kernel smoothing. Neuroimage. 25:1256–1265. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, Metzger LM, Shoushtari CS, Splinter R, Reich W. 2003. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 33:427–433. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Carper R, Akshoomoff N. 2003. Evidence of brain overgrowth in the first year of life in autism. J Am Med Assoc. 290:337–344. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns C, Davis H, Ziccardi R, Carper R, Tigue Z, Chisum H, Moses P, Pierce K, Lord C, et al. . 2001. Unusual brain growth patterns in early life in patients with autistic disorder an MRI study. Neurology. 57:245–254. [DOI] [PubMed] [Google Scholar]
- Craig F, Margari F, Legrottaglie AR, Palumbi R, de Giambattista C, Margari L. 2016. A review of executive function deficits in autism spectrum disorder and attention-deficit/hyperactivity disorder. Neuropsychiatr Dis Treat. 12:1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. 1999. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 9:179–194. [DOI] [PubMed] [Google Scholar]
- Deoni SC, Dean DC, Remer J, Dirks H, O’Muircheartaigh J. 2015. Cortical maturation and myelination in healthy toddlers and young children. Neuroimage. 115:147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, O’Connor D, Chen B, Alaerts K, Anderson JS, Assaf M, Balsters JH, Baxter L, Beggiato A, Bernaerts S, et al. . 2017. Enhancing studies of the connectome in autism using the autism brain imaging data exchange II. Sci Data. 4:170010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Yan C-G, Li Q, Denio E, Castellanos FX, Alaerts K, Anderson JS, Assaf M, Bookheimer SY, Dapretto M, et al. . 2014. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol Psychiatry. 19:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierker DL, Feczko E, Pruett JR Jr, Petersen SE, Schlaggar BL, Constantino JN, Harwell JW, Coalson TS, Van Essen DC. 2013. Analysis of cortical shape in children with simplex autism. Cereb Cortex. 25:1042–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme S, Albaugh MD, Nguyen T-V, Hudziak JJ, Mateos-Pérez J, Labbe A, Evans AC, Karama S, Group BDC . 2016. Trajectories of cortical thickness maturation in normal brain development—the importance of quality control procedures. Neuroimage. 125:267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker C, Andrews D, Dell’Acqua F, Daly E, Murphy C, Catani M, de Schotten MT, Baron-Cohen S, Lai M, Lombardo M, et al. . 2016. Relationship between cortical gyrification, white matter connectivity, and autism spectrum disorder. Cereb Cortex. 26:3297–3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker C, Shahidiani A, Feng Y, Daly E, Murphy C, D’Almeida V, Deoni S, Williams S, Gillan N, Gudbrandsen M. 2014. The effect of age, diagnosis, and their interaction on vertex-based measures of cortical thickness and surface area in autism spectrum disorder. J Neural Transm. 121:1157–1170. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. 1999. Cortical surface-based analysis: II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 9:195–207. [DOI] [PubMed] [Google Scholar]
- Fisher RA. 1938. The statistical utilization of multiple measurements. Ann Hum Genet. 8:376–386. [Google Scholar]
- Garel C, Chantrel E, Brisse H, Elmaleh M, Luton D, Oury J-F, Sebag G, Hassan M. 2001. Fetal cerebral cortex: normal gestational landmarks identified using prenatal MR imaging. AJNR Am J Neuroradiol. 22:184–189. [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, State MW. 2015. Gene hunting in autism spectrum disorder: on the path to precision medicine. Lancet Neurol. 14:1109–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. 2015. Behavior rating inventory of executive function. 2nd ed. Torrance, CA: Western Psychological Services. [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, et al. . 2004. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Thompson PM. 2010. Mapping gray matter development: implications for typical development and vulnerability to psychopathology. Brain Cogn. 72:6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. 2005. Anatomical differences in the mirror neuron system and social cognition network in autism. Cereb Cortex. 16:1276–1282. [DOI] [PubMed] [Google Scholar]
- Hagler DJ, Saygin AP, Sereno MI. 2006. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 33:1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardan AY, Jou RJ, Keshavan MS, Varma R, Minshew NJ. 2004. Increased frontal cortical folding in autism: a preliminary MRI study. Psychiatry Res. 131:263–268. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Libove RA, Keshavan MS, Melhem NM, Minshew NJ. 2009. A preliminary longitudinal magnetic resonance imaging study of brain volume and cortical thickness in autism. Biol Psychiatry. 66:320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardan AY, Muddasani S, Vemulapalli M, Keshavan MS, Minshew NJ. 2006. An MRI study of increased cortical thickness in autism. Am J Psychiatry. 163:1290–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett HC, Gu H, Munsell BC, Kim SH, Styner M, Wolff JJ, Elison JT, Swanson MR, Zhu H, Botteron KN. 2017. Early brain development in infants at high risk for autism spectrum disorder. Nature. 542:348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett HC, Poe MD, Gerig G, Styner M, Chappell C, Smith RG, Vachet C, Piven J. 2011. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch Gen Psychiatry. 68:467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haznedar MM, Buchsbaum MS, Wei T-C, Hof PR, Cartwright C, Bienstock CA, Hollander E. 2000. Limbic circuitry in patients with autism spectrum disorders studied with positron emission tomography and magnetic resonance imaging. Am J Psychiatry. 157:1994–2001. [DOI] [PubMed] [Google Scholar]
- Hofman MA. 1985. Size and shape of the cerebral cortex in mammals (Part 1 of 2). Brain Behav Evol. 27:28–40. [DOI] [PubMed] [Google Scholar]
- Hogstrom LJ, Westlye LT, Walhovd KB, Fjell AM. 2012. The structure of the cerebral cortex across adult life: age-related patterns of surface area, thickness, and gyrification. Cereb Cortex. 23:2521–2530. [DOI] [PubMed] [Google Scholar]
- Hutsler JJ, Love T, Zhang H. 2007. Histological and magnetic resonance imaging assessment of cortical layering and thickness in autism spectrum disorders. Biol Psychiatry. 61:449–457. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. 1997. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 387:167–178. [DOI] [PubMed] [Google Scholar]
- Hyde KL, Samson F, Evans AC, Mottron L. 2010. Neuroanatomical differences in brain areas implicated in perceptual and other core features of autism revealed by cortical thickness analysis and voxel‐based morphometry. Hum Brain Mapp. 31:556–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khundrakpam BS, Lewis JD, Kostopoulos P, Carbonell F, Evans AC. 2017. Cortical thickness abnormalities in autism spectrum disorders through late childhood, adolescence, and adulthood: a large-scale MRI study. Cereb Cortex. 27:1721–1731. [DOI] [PubMed] [Google Scholar]
- Klein D, Rotarska-Jagiela A, Genc E, Sritharan S, Mohr H, Roux F, Han CE, Kaiser M, Singer W, Uhlhaas PJ. 2014. Adolescent brain maturation and cortical folding: evidence for reductions in gyrification. PLoS One. 9:e84914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Wang L, Shi F, Lyall AE, Lin W, Gilmore JH, Shen D. 2014. Mapping longitudinal development of local cortical gyrification in infants from birth to 2 years of age. J Neurosci. 34:4228–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libero LE, DeRamus TP, Deshpande HD, Kana RK. 2014. Surface-based morphometry of the cortical architecture of autism spectrum disorders: volume, thickness, area, and gyrification. Neuropsychologia. 62:1–10. [DOI] [PubMed] [Google Scholar]
- Libero LE, Nordahl CW, Li DD, Ferrer E, Rogers SJ, Amaral DG. 2016. Persistence of megalencephaly in a subgroup of young boys with autism spectrum disorder. Autism Res. 9:1169–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, DiLavore PC, Gotham K. 2012. Autism diagnostic observation schedule. CA: Western Psychological Services Torrance. [Google Scholar]
- Lord C, Rutter M, Couteur A. 1994. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 24:659–685. [DOI] [PubMed] [Google Scholar]
- McKavanagh R, Buckley E, Chance SA. 2015. Wider minicolumns in autism: a neural basis for altered processing? Brain. 138:2034–2045. [DOI] [PubMed] [Google Scholar]
- Mensen VT, Wierenga LM, van Dijk S, Rijks Y, Oranje B, Mandl RC, Durston S. 2017. Development of cortical thickness and surface area in autism spectrum disorder. NeuroImage Clin. 13:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu AK, Schneider M, Debbané M, Badoud D, Eliez S, Schaer M. 2013. Sex differences in thickness, and folding developments throughout the cortex. Neuroimage. 82:200–207. [DOI] [PubMed] [Google Scholar]
- Nordahl CW, Dierker D, Mostafavi I, Schumann CM, Rivera SM, Amaral DG, Van Essen DC. 2007. Cortical folding abnormalities in autism revealed by surface-based morphometry. J Neurosci. 27:11725–11735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Nordahl CW, Iosif AM, Lee A, Rogers S, Amaral DG. 2015. Increased surface area, but not cortical thickness, in a subset of young boys with autism spectrum disorder. Autism Res. 9:232–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orosco LA, Ross AP, Cates SL, Scott SE, Wu D, Sohn J, Pleasure D, Pleasure SJ, Adamopoulos IE, Zarbalis KS. 2014. Loss of Wdfy3 in mice alters cerebral cortical neurogenesis reflecting aspects of the autism pathology. Nat Commun. 5:4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’donnell S, Noseworthy MD, Levine B, Dennis M. 2005. Cortical thickness of the frontopolar area in typically developing children and adolescents. Neuroimage. 24:948–954. [DOI] [PubMed] [Google Scholar]
- Packer A. 2016. Neocortical neurogenesis and the etiology of autism spectrum disorder. Neurosci Biobehav Rev. 64:185–195. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, et al. . 2009. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 19:2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay P, Manger PR. 2007. Order‐specific quantitative patterns of cortical gyrification. Eur J Neurosci. 25:2705–2712. [DOI] [PubMed] [Google Scholar]
- Raj A, Chen YH. 2011. The wiring economy principle: connectivity determines anatomy in the human brain. PLoS ONE. 6:e14832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Swaab D. 1988. Defects of neuronal migration and the pathogenesis of cortical malformations. Prog Brain Res. 73:15–37. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, Clasen L, Gogtay N, Giedd JN. 2011. How does your cortex grow? J Neurosci. 31:7174–7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, Courchesne E. 2005. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biol Psychiatry. 58:1–9. [DOI] [PubMed] [Google Scholar]
- Reillo I, de Juan Romero C, García-Cabezas MÁ, Borrell V. 2010. A role for intermediate radial glia in the tangential expansion of the mammalian cerebral cortex. Cereb Cortex. 21:1674–1694. [DOI] [PubMed] [Google Scholar]
- Richter J, Henze R, Vomstein K, Stieltjes B, Parzer P, Haffner J, Brandeis D, Poustka L. 2015. Reduced cortical thickness and its association with social reactivity in children with autism spectrum disorder. Psychiatry Res. 234:15–24. [DOI] [PubMed] [Google Scholar]
- Ronan L, Voets N, Rua C, Alexander-Bloch A, Hough M, Mackay C, Crow TJ, James A, Giedd JN, Fletcher PC. 2014. Differential tangential expansion as a mechanism for cortical gyrification. Cereb Cortex. 24:2219–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaer M, Cuadra MB, Schmansky N, Fischl B, Thiran J-P, Eliez S. 2012. How to measure cortical folding from MR images: a step-by-step tutorial to compute local gyrification index. J Vis Exp. (59):e3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaer M, Cuadra MB, Tamarit L, Lazeyras F, Eliez S, Thiran J-P. 2008. A surface-based approach to quantify local cortical gyrification. IEEE Trans Med Imaging. 27:161–170. [DOI] [PubMed] [Google Scholar]
- Schaer M, Ottet M-C, Scariati E, Dukes D, Franchini M, Eliez S, Glaser B. 2013. Decreased frontal gyrification correlates with altered connectivity in children with autism. Front Hum Neurosci. 7:750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel C, Rotarska-Jagiela A, Schilbach L, Lehnhardt FG, Krug B, Vogeley K, Tepest R. 2011. Imaging derived cortical thickness reduction in high-functioning autism: key regions and temporal slope. Neuroimage. 58:391–400. [DOI] [PubMed] [Google Scholar]
- Schnack HG, Van Haren NE, Brouwer RM, Evans A, Durston S, Boomsma DI, Kahn RS, Pol HEH. 2015. Changes in thickness and surface area of the human cortex and their relationship with intelligence. Cereb Cortex. 25:1608–1617. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Bloss CS, Barnes CC, Wideman GM, Carper RA, Akshoomoff N, Pierce K, Hagler D, Schork N, Lord C. 2010. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J Neurosci. 30:4419–4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, et al. . 2008. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 28:3586–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MD, Kim SH, McKinstry RC, Gu H, Hazlett HC, Nordahl CW, Emerson RW, Shaw D, Elison JT, Swanson MR. 2017. Increased extra-axial cerebrospinal fluid in high-risk infants who later develop autism. Biol Psychiatry. 82:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MD, Nordahl CW, Young GS, Wootton-Gorges SL, Lee A, Liston SE, Harrington KR, Ozonoff S, Amaral DG. 2013. Early brain enlargement and elevated extra-axial fluid in infants who develop autism spectrum disorder. Brain. 136:2825–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimony JS, Smyser CD, Wideman G, Alexopoulos D, Hill J, Harwell J, Dierker D, Van Essen DC, Inder TE, Neil JJ. 2016. Comparison of cortical folding measures for evaluation of developing human brain. Neuroimage. 125:780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokouhi M, Williams JH, Waiter GD, Condon B. 2012. Changes in the sulcal size associated with autism spectrum disorder revealed by sulcal morphometry. Autism Res. 5:245–252. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. 2004. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 24:8223–8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striedter GF, Srinivasan S, Monuki ES. 2015. Cortical folding: when, where, how, and why? Annu Rev Neurosci. 38:291–307. [DOI] [PubMed] [Google Scholar]
- Su S, White T, Schmidt M, Kao CY, Sapiro G. 2013. Geometric computation of human gyrification indexes from magnetic resonance images. Hum Brain Mapp. 34:1230–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Herting MM, Goddings A-L, Meuwese R, Blakemore S-J, Dahl RE, Güroğlu B, Raznahan A, Sowell ER, Crone EA. 2017. Development of the cerebral cortex across adolescence: a multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. J Neurosci. 37:3402–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC. 1997. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 385:313. [DOI] [PubMed] [Google Scholar]
- Vijayakumar N, Allen NB, Youssef G, Dennison M, Yücel M, Simmons JG, Whittle S. 2016. Brain development during adolescence: a mixed‐longitudinal investigation of cortical thickness, surface area, and volume. Hum Brain Mapp. 37:2027–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace GL, Dankner N, Kenworthy L, Giedd JN, Martin A. 2010. Age-related temporal and parietal cortical thinning in autism spectrum disorders. Brain. 133:3745–3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace GL, Robustelli B, Dankner N, Kenworthy L, Giedd JN, Martin A. 2013. Increased gyrification, but comparable surface area in adolescents with autism spectrum disorders. Brain. 136:1956–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler D. 1999. Weschler abbreviated scale of intelligence (WASI). London: Psychological Corporation. [Google Scholar]
- White T, Su S, Schmidt M, Kao C-Y, Sapiro G. 2010. The development of gyrification in childhood and adolescence. Brain Cogn. 72:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga LM, Langen M, Oranje B, Durston S. 2014. Unique developmental trajectories of cortical thickness and surface area. Neuroimage. 87:120–126. [DOI] [PubMed] [Google Scholar]
- Williams EL, El-Baz A, Nitzken M, Switala AE, Casanova MF. 2012. Spherical harmonic analysis of cortical complexity in autism and dyslexia. Transl Neurosci. 3:36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RS, Hauser SL, Purpura DP, DeLong GR, Swisher CN. 1980. Autism and mental retardation: neuropathologic studies performed in four retarded persons with autistic behavior. Arch Neurol. 37:749–753. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. 2014. Permutation inference for the general linear model. Neuroimage. 92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DY-J, Beam D, Pelphrey KA, Abdullahi S, Jou RJ. 2016. Cortical morphological markers in children with autism: a structural magnetic resonance imaging study of thickness, area, volume, and gyrification. Mol Autism. 7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zablotsky B, Black LI, Maenner MJ, Schieve LA, Blumberg SJ. 2015. Estimated prevalence of autism and other developmental disabilities following questionnaire changes in the 2014 National Health Interview Survey. National health statistics reports; no 87. Hyattsville, MD: National Center for Health Statistics. [PubMed] [Google Scholar]
- Zielinski BA, Prigge MB, Nielsen JA, Froehlich AL, Abildskov TJ, Anderson JS, Fletcher PT, Zygmunt KM, Travers BG, Lange N. 2014. Longitudinal changes in cortical thickness in autism and typical development. Brain. 137:1799–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Armstrong E, Schleicher A, Kretschmann H-J. 1988. The human pattern of gyrification in the cerebral cortex. Anat Embryol (Berl). 179:173–179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.