Abstract
The inhibitory neurotransmitter γ-aminobutyric acid (GABA) is known to be fundamental to the neuronal processes underlying visual orientation and vibrotactile frequency and amplitude discrimination. Previous studies have demonstrated that performance on visual and vibrotactile psychophysics tasks is associated with in vivo measurements of “GABA+” levels – a measure of GABA substantially contaminated by a macromolecular (MM) signal. Here, we establish that these prior finding are indeed driven by the GABA fraction of that signal. Edited magnetic resonance spectroscopy (MRS) was used to measure GABA with and without MM suppression in the sensorimotor (SM1) and occipital cortices in fourteen healthy male adults. Volunteers also underwent psychophysical experiments to assess their performance on visual orientation discrimination and vibrotactile amplitude and frequency discrimination. We show that MM-suppressed GABA levels correlate more strongly with individual differences in vibrotactile (in the case of SM1 GABA; amplitude: r = –0.63, p = 0.03; frequency: r = –0.62, p = 0.02) and visual orientation (in the case of occipital GABA; r = –0.59, p = 0.05) discrimination thresholds than GABA levels contaminated by MM (vibrotactile amplitude: r = –0.36, p = 0.30; vibrotactile frequency: r = –0.53, p = 0.09; visual orientation: r = 0.21, p = 0.55). These findings further support the view that measurements of endogenous GABA acquired with edited MRS can usefully probe neurochemical–behavioral relationships in humans. Moreover, the more specific measurement of GABA used in this study provides increased statistical power to observe these regionally specific relationships.
Keywords: GABA, edited magnetic resonance spectroscopy, tactile processing, visual processing, orientation discrimination, vibrotactile discrimination
1. Introduction
γ-Aminobutyric-acid (GABA) is the main inhibitory neurotransmitter in the brain, and is important in shaping the neuronal responses to sensory stimulation (Bruno and Simons, 2002; Dykes et al., 1984), in plasticity, learning and memory (Heba et al., 2016; McCormick, 1989; Michels et al., 2012; Stagg et al., 2011). GABA has been implicated in a number of psychiatric, neurological and developmental disorders (Chang et al., 2003; Puts and Edden, 2012; Sanacora et al., 2006; Simister et al., 2003) and our previous work has shown a link between altered GABA and abnormal sensory function in autism spectrum disorder (Puts et al., 2016) and Tourette syndrome (Mahone et al., 2018; Puts et al., 2015).
The role of GABA in sensory encoding is well-described. Human and animal work has shown that visual orientation discrimination relies on GABA (Shapley et al., 2003; Sillito, 1975). Similarly, animal work has shown that tactile frequencies are encoded through periodic firing of neuronal ensembles which rely, at least in part, on GABA function (McLaughlin and Juliano, 2005). In the tactile domain, two simultaneously applied stimuli on the skin lead to surround-suppression through GABAergic lateral inhibition, allowing for separation between these signals, which is blocked by bicuculline, a GABA antagonist (Whitsel et al., 1989). These animal studies link GABA and sensory encoding on a cellular level, and an increasing amount of human work has focused on linking GABA levels to behavior in humans.
It is possible to measure the in vivo concentration level of GABA in the brain using edited magnetic resonance spectroscopy (MRS) (Mullins et al., 2014; Puts and Edden, 2012; Rothman et al., 1993). MRS studies of GABA have shown that individual differences in the levels of GABA are correlated with individual differences in behavioral performance. For example, we have shown that GABA in the occipital cortex correlates with performance on a visual orientation discrimination task (Edden et al., 2009) and that GABA measured in the sensorimotor cortex correlates with performance on a behavioral tactile frequency discrimination task (Puts et al., 2011). Other studies have shown correlations between in vivo GABA levels and individual differences in motor suppression (Boy et al., 2010), motor learning (Bachtiar and Stagg, 2014; Floyer-Lea et al., 2006), and the effect of peripheral modulation (Heba et al., 2016). Many of the studies also show that correlations between GABA and behavior are functionally and regionally specific. That is, occipital GABA levels correlate with visual function, sensorimotor GABA levels correlate with tactile function, but occipital GABA levels do not correlate with tactile function (Puts et al., 2011).
The J-difference editing technique used to measure GABA consists of two sub-experiments (Harris et al., 2017). In the first, a frequency-selective editing pulse is applied to the GABA spins at 1.9 ppm that are coupled to GABA spins at 3.0 ppm (edit-ON). This editing pulse is not applied (or is applied at 7.46 ppm) in the OFF experiment (edit-OFF). The difference spectrum between the ON and OFF experiments contains only those signals affected by the edit-ON pulse and results in a resolved GABA peak at 3.0 ppm, which is then used for quantification. A limitation of this J-difference technique is that the editing pulse applied at 1.9 ppm partially inverts a macromolecular (MM) signal at 1.7 ppm (Edden et al., 2012; Henry et al., 2001), which is coupled to an MM signal also at 3.0 ppm. Therefore, the edited GABA signal at 3.0 ppm is contaminated by MM and is, for this reason, often referred to as the GABA+ signal.
MM contamination limits the interpretation of the 3.0 ppm GABA+ signal as this is not a “pure” GABA measurement. Recent studies have argued that the contaminating MM signal is not functionally relevant to behavior; however, a more specific GABA measure may improve interpretation of correlational studies. The MM contribution can be suppressed by applying the editing pulses symmetrically around the MM peak at 1.7 ppm (Edden et al., 2012; Henry et al., 2001). In this symmetrical editing experiment, both the ON (at 1.9 ppm) and OFF (at 1.5 ppm) editing pulses are assumed to invert the 1.7 ppm MM signal equally, and thus the MM signal is removed from the difference spectrum.
In the current study, GABA was measured in occipital and sensorimotor regions using edited MRS with and without MM suppression. MRS-only results showing moderate associations between MM-suppressed GABA and GABA+ are reported elsewhere (Harris et al., 2015b). This study extends this finding, as well as previous studies, by asking participants to perform a visual orientation discrimination task and vibrotactile discrimination tasks. If previously observed relationships between GABA+ and behavior are driven by GABA, we hypothesized that MM-suppressed GABA levels will correlate with behavior, more strongly than GABA+ levels do. We further hypothesized that more GABA is correlated with better performance, with a dual dissociation between behavioral domains.
2. Results
2.1. Behavioral results
The results from the vibrotactile and visual orientation discrimination tasks are summarized in Table 1. Average thresholds from the vibrotactile tasks are consistent with findings in healthy adults as presented in previous work (Puts et al., 2013, 2011). Visual orientation discrimination thresholds appear to be slightly higher than those observed previously (Edden et al., 2009), but this may be due to the different visual presentation parameters (e.g., viewing distance of 30 cm here vs. 57 cm previously). Amplitude discrimination data for one participant, and visual orientation discrimination data for one participant, were rejected for non-compliance during the task. MRS data for one participant were excluded due to a poor T1 structural image (preventing adequate segmentation and tissue correction); occipital GABA+ data were excluded for one additional participant and sensorimotor GABA+ data for one participant (Table 2) due to poor quality MRS data.
Table 1.
Demographics and behavioral results (mean ± 1 standard deviation).
| Sample size | 14 |
| Age (years) | 31.3 ± 6.03 |
| Tactile amplitude discrimination threshold (μm) | 30.08 ± 14.94 |
| Tactile frequency discrimination threshold (Hz) | 4.12 ± 1.42 |
| Visual orientation discrimination threshold (deg) | 3.24 ± 1.21 |
Table 2.
Metabolite quantification results and quality assurance metrics. Linewidth is full-width half-maximum (FWHM) and SNR is signal-to-noise ratio of the GABA signal. Frequency drift is the standard deviation of frequency offsets for the 320 individual averages in each scan as estimated by spectral registration.
| Occipital | SM1 | |||||
|---|---|---|---|---|---|---|
| GABA+ (n = 12) | MM-suppressed GABA (n = 13) | GABA+ (n = 11) | MM-suppressed GABA (n = 13) | |||
| GABA levels (i.u.) | 4.52 ± 0.64 | 2.29 ± 0.42 | p < 0.001 | 3.11 ± 0.53 | 1.75 ± 0.58 | p < 0.001 |
| Linewidth (Hz) | 18.89 ± 0.94 | 17.44 ± 1.73 | p = 0.016 | 17.95 ± 2.88 | 16.62 ± 2.63 | p = 0.24 |
| Fit error (%) | 2.99 ± 0.01 | 5.34 ± 0.01 | p < 0.001 | 5.09 ± 0.43 | 9.14 ± 4.09 | p < 0.001 |
| SNR | 20.41 ± 2.03 | 10.55 ± 2.0 | p < 0.001 | 18.21 ± 3.23 | 8.80 ± 2.58 | p < 0.001 |
| Frequency drift (Hz) | 1.78 ± 1.91 | 1.03 ± 1.06 | p = 0.23 | 1.61 ± 1.29 | 1.28 ± 1.05 | p = 0.49 |
2.2. MRS results
Thorough analyses between GABA+ and MM-suppressed acquisitions have been reported previously (Harris et al., 2015b) but are briefly reported here due to analysis with the most recent version of the Gannet software (version 3.0). Edited spectra are shown in Figure 1C. Differences in GABA+ and MM-suppressed GABA levels showed MM-contributions of 50 ± 9.6% in the occipital cortex and 57 ± 16.8% in the sensorimotor cortex. MM-suppressed and GABA+ levels were not correlated for the occipital cortex (r = 0.21, p = 0.49) but are moderately for the sensorimotor cortex (r = 0.56, p = 0.04). GABA linewidth, signal-to-noise ratio (SNR) and model fit error significantly differed between MM-suppressed and GABA+ acquisitions for both voxels, as detailed in Table 2. There were no differences in frequency drift between the acquisitions, but MM-suppressed measurements significantly correlated with frequency drift in the occipital, but not SM1, region (occipital: r = –0.60, p = 0.03; SM1: r = 0.03; p = 0.92).
Figure 1.
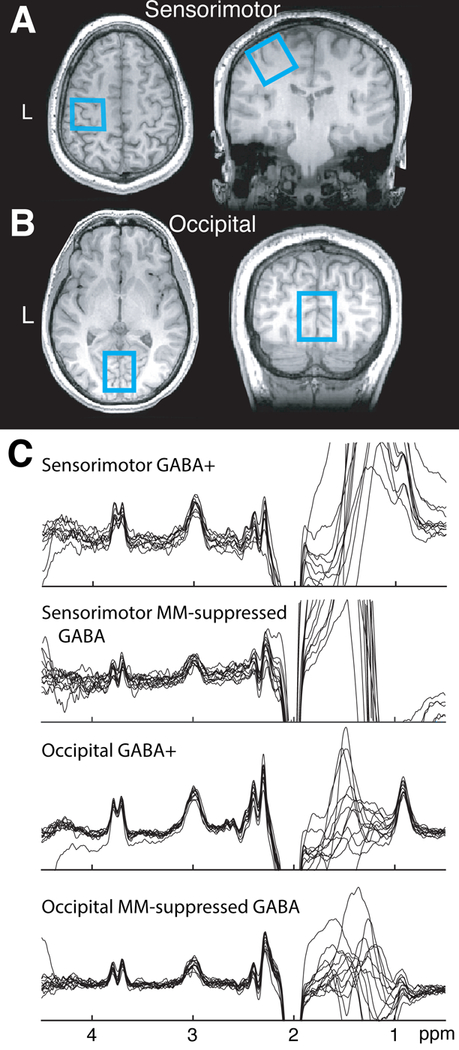
A–B) Example placement of the MRS voxel in the sensorimotor and occipital cortices. C) Difference-edited GABA spectra acquired in each volunteer with and without MM suppression. The smaller amplitude of the MM-suppressed GABA peak at 3.0 ppm indicates removal of the contaminating MM signal.
2.3. SM1 GABA associations with tactile performance
SM1 MM-suppressed GABA levels correlated with amplitude discrimination (r = –0.63, p = 0.03) but SM1 GABA+ levels did not (r = –0.36, p = 0.30; Figure 2A). A bootstrapped 95% confidence interval (CI) showed that the former correlation was robust (CI = [–0.90, –0.42]). The two correlations did not significantly differ, however (z = 0.75, p = 0.45). SM1 MM-suppressed GABA levels showed a significant correlation with frequency discrimination threshold (r = –0.62, p = 0.02) but GABA+ levels did not, although are at trend level with alpha = 0.1 (r = –0.53, p = 0.09; Figure 2B). Both of these correlations were robust (MM-suppressed: CI = [–0.89, –0.27]; GABA+: CI = [–0.83, –0.10]), and they did not significantly differ (z = 0.28, p = 0.77). There were no associations between SM1 GABA and visual orientation discrimination (GABA+: r = –0.34, p = 0.37; MM-suppressed: r = 0.25, p = 0.46).
Figure 2.
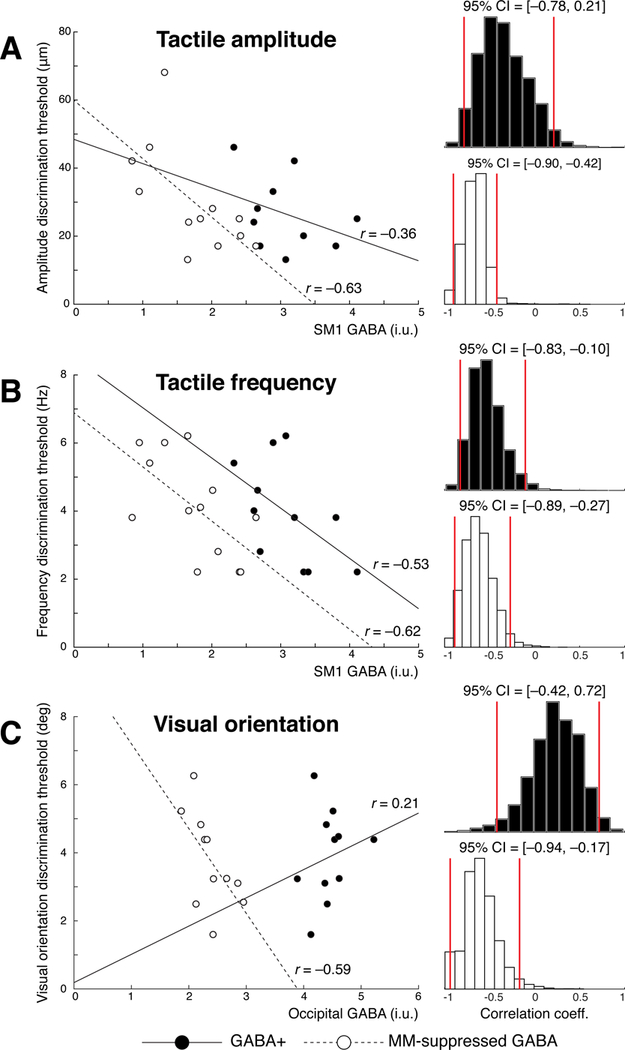
Scatterplots showing the relationships between GABA levels in SM1 and the occipital cortex and discrimination thresholds for vibrotactile amplitude (A), vibrotactile frequency (B) and visual orientation (C). The association between GABA+ (filled circles, solid line) and MM-suppressed GABA (empty circles, dotted lines) are overlaid.
2.4. Occipital GABA associations with visual performance
Occipital GABA+ did not show a significant correlation with visual orientation discrimination (r = 0.21, p = 0.55), while MM-suppressed GABA showed a trend-level correlation (r = –0.59, p = 0.05), as displayed in Figure 2C. The association with MM-suppressed GABA was robust, however (CI = [–0.94, –0.17]), and the correlations were significantly different at alpha = 0.1 but not at alpha = 0.05 (z = 1.78, p = 0.07). Occipital GABA+ showed a significant association with tactile amplitude (r = –0.60, p = 0.05, CI = [–0.87, –0.21]) and frequency (r = –0.59, p = 0.04, CI = [–0.85, –0.10]) discrimination thresholds. MM-suppressed occipital GABA showed no such correlations (amplitude: r = 0.01, p = 0.98; frequency: r = 0.12 p = 0.69).
3. Discussion
The results presented in this study of a modestly sized cohort show significant and robust correlations between MM-suppressed GABA concentration in the sensorimotor region and tactile amplitude and frequency discrimination thresholds, and a robust, near-significant correlation between MM-suppressed GABA levels in the occipital cortex and visual orientation discrimination thresholds that is consistent with previous work. Although the directions of the correlations between GABA+ and amplitude and frequency discrimination are also negative, they are not significant. These results suggest that MM-suppressed GABA has increased power to detect a correlation with these measures than GABA+ measures do, in spite of its greater technical challenges.
We show that while GABA+ measurements in SM1 correlate weakly and not significantly with amplitude and frequency discrimination, MM-suppressed GABA measurements correlate significantly with both of these tasks. In previous studies showing these correlations, measurements of GABA+, not MM-suppressed GABA, were used, and thus our replication is thematic but not exact. There are a number of possible reasons for the absence of the correlations between GABA+ and behavior. Firstly, the range in amplitude and frequency discrimination thresholds between individuals is smaller than that reported in our previous work (Puts et al., 2013, 2011), and we may lack the necessary variability among individuals to detect a significant correlation. Similarly, in our relatively tightly controlled cohort of adults, both visual GABA and visual orientation discrimination have very limited ranges. A smaller effect size requires larger numbers of participants to elucidate this correlation and it is possible that larger participant numbers would show this correlation with GABA+ as well. GABA+ measures contain both GABA and macromolecules and it may be that the variability in GABA+ across our population is limited, or that there is unknown variability in MM, therefore not providing the power necessary to detect a correlation. In contrast, MM-suppressed GABA levels are thought to be purer assessments of GABA levels and may therefore more accurately reflect differences in GABA levels across the population and provide increased power to detect such correlation. Therefore, from this data we conclude that MM-suppressed GABA levels, at least in SM1, might be more sensitive to individual differences in discrimination performance.
The MM signal that contaminates the edited GABA+ signal is thought to originate from the amino acid lysine (Behar and Ogino, 1993; Henry et al., 2001). Additionally, MM tend to have quite short T2 relaxation times (Behar et al., 1994), and since GABA-edited MRS experiments are normally performed at medium TE (68–80 ms), it has been suggested that the MM signal arises from a mobile form of lysine rather than a bound MM pool (Choi et al., 2007). Some studies have reported regional and tissue-type differences in the MM baseline (Považan et al., 2018, 2015; Schaller et al., 2014), but this is contradicted by other reports (Giapitzakis et al., 2018; Snoussi et al., 2015). Whatever the source of the contaminating MM signal or its heterogeneity across the brain, our results demonstrate that its removal from the edited GABA signal improves the discriminative power of correlational analyses of GABA measurements and behavioral metrics.
Studies have shown the importance of inhibitory function in encoding sensory information, in part, by tuning neuronal ensembles allowing for increased contrast in stimulus encoding. In the somatosensory domain, blocking GABA leads to reduced capacity to discriminate spatial and temporal information (Juliano et al., 1989; McLaughlin and Juliano, 2005; Whitsel et al., 2003, 1989) in somatosensory cortex. The directionality of our correlation is consistent with previous work; participants with higher GABA levels are better able to discriminate spatial and temporally different stimuli in both somatosensory and visual domains. Along similar lines, GABA plays an important role in orientation tuning, with the application of the GABA antagonist bicuculline reducing orientation selectivity (Sillito, 1975; Sillito et al., 1980; Tsumoto et al., 1979; Wolf et al., 1986) and the application of GABA increasing orientation tuning (Li et al., 2008). This is also consistent with our correlation between occipital GABA and visual orientation thresholds where participants with more occipital GABA were better at visual discrimination.
There are several limitations to this work. It is important to acknowledge that the MM-suppressed GABA signal is approximately 50% smaller than the GABA+ signal and is thus a noisier measurement; indeed, model fit error and SNR are significantly higher and lower, respectively, in the MM-suppressed measurement. On the other hand, the MM-suppressed measurements provide us with a purer measurement of GABA, addressing some of the limitations of GABA+ editing. While the GABA+ measurement is often over-interpreted as if it were a pure GABA signal, the MM-suppressed measurements allow for a more valid interpretation. Due to the reduction in SNR, longer scan times or more participants are recommended for this technique. Additionally, as shown here and in previous work, MM-suppressed GABA levels correlate to some degree with frequency drift (Edden et al., 2016; Mikkelsen et al., 2017). Frequency drift occurs due to heating or cooling of the gradients (El-Sharkawy et al., 2006; Oeltzschner et al., 2018), and as the editing pulses used for MM-suppression are more selective, they are more sensitive to drift in this B0 field.
Finally, the sample size used in the current study is small, especially considering exclusion of data due to poor quality. As such, the correlations would not pass correction for multiple comparisons. That said, we conducted this work on the basis of prior findings, with clear hypotheses regarding what correlations may be expected, the size of these correlations, and their directionality. In addition, we have previously shown that GABA+ and MM-suppressed GABA (Harris et al., 2015b; Mikkelsen et al., 2017), as well as behavioral metrics (Puts et al., 2014, 2013), tend to correlate, somewhat mitigating the need for multiple comparison corrections. Finally, we performed robust correlation analyses to gauge the reliability of these correlations and showed that MM-suppressed GABA provided more robust correlations with the behavioral metrics compared to GABA+ despite the small sample size. Nevertheless, although MRI and MRS studies are often limited in sample size due to financial constraints, studies with larger sample sizes are necessary to further elucidate the relationships between GABA and behavior. Future GABA-edited MRS experiments should be designed with measurement variability and predicted effect size(s) in mind (Mikkelsen et al., 2018), perhaps explaining why previous efforts have failed to replicate findings between GABA and metrics of behavior and physiology (Cousijn et al., 2014; Harris et al., 2015a). In addition, only right-handed male participants in a small age range were tested to exclude effects of menstrual cycle (Epperson et al., 2005) and age (Gao et al., 2013; Porges et al., 2017). However, studying sex and age as covariates is an important avenue of research; the addition of female and older participants may have also increased variability to detect correlations more strongly, especially considering that if sex and age are associated with changes in GABA level, and GABA levels are associated with behavior, such correlations should continue to exist in healthy populations.
In summary, we have thematically replicated earlier findings correlating vibrotactile performance and SM1 GABA levels and show a trend towards significance showing a relationship between visual orientation discrimination and GABA levels. In this study we showed that MM-suppressed GABA-pure measurements, at least over SM1, correlate more strongly with behavior than GABA+. MM-suppressed GABA-MRS may be sensitive to different GABAergic mechanisms in discrimination and adaptation.
4. Experimental procedure
4.1. Participants
Fourteen male participants (all right-handed; age: 31.3 ± 6.03 years) participated in the experiment. Written, informed consent was obtained from each participant under the approval of the local Institutional Review Board prior to testing.
4.2. Behavioral testing
4.2.1. Tactile discrimination
All participants performed tactile amplitude and frequency discrimination tasks as described in previous work (Puts et al., 2013). Trial numbers were increased compared to previous work to establish a more robust threshold for each task. A CM4 four-digit tactile stimulator (Cortical Metrics) was used for tactile stimulation. All stimuli were delivered to the glabrous skin of left digit 2 (LD2) and digit 3 (LD3) using a cylindrical probe (5 mm in diameter). All stimuli were presented within the flutter range (25–50 Hz). Visual feedback, task responses, and data collection were performed on an Acer Onebook Netbook computer running CM4 software.
4.2.1.1. Amplitude discrimination
In the amplitude discrimination task (Figure 3A), participants were asked to judge which of two simultaneously applied supra-threshold stimuli was the most intense. Two supra-threshold stimuli were simultaneously delivered on LD2 and LD3. One of the stimuli had higher amplitude (both stimuli were 25 Hz; 500 ms; standard stimulus amplitude: 100 µm; initial comparison stimulus amplitude: 200 µm; interstimulus interval (ISI) = 5 s; 30 trials). 2-up–1-down staircase tracking was used for the first 10 trials and 1-up–1-down for the remainder. Amplitude discrimination thresholds were determined by the mean comparison stimulus amplitude over the last five trials.
Figure 3.

Schematics displaying the experimental paradigms for the vibrotactile and visual psychophysics tasks. A–B) Paradigms for assessing amplitude and frequency discrimination thresholds. C) Paradigm for assessing visual orientation discrimination threshold.
4.2.1.2. Frequency discrimination
Participants were asked which of two sequentially applied supra-threshold stimuli had a higher frequency (“which one felt faster against your finger”) by indicating on which finger the highest frequency stimulus was applied (Figure 3B). The stimuli were presented with ISI of 500 ms. The standard stimulus (unchanged) was 25 Hz, the initial comparison (variable) stimulus was 40 Hz (both stimuli = 500 ms, 200 µm; ISI = 5 s; 40 trials). 2-up–1-down staircase tracking was used for the first 10 trials and 1-up–1-down for the remainder. Frequency discrimination thresholds were determined by the mean frequency of the comparison stimulus over the last five trials. Amplitude was kept constant for both standard and comparison stimulus, based on the report (Harris et al., 2001) which states that the accuracy of participants in comparing frequencies is not affected by shifts in the amplitudes of the vibration.
4.2.2. Visual orientation discrimination
A visual orientation discrimination task with standard stimulus of 45 degree was performed as described previously (Edden et al., 2009). Participants were asked to fixate on a small circle in the center of the screen. In each trial two visual gratings were presented sequentially (stimulus duration = 0.25 s; diameter = 4 degree; spatial frequency = 3 cycles/degree; contrast = 80%; interstimulus interval = 400–600 ms) in the left visual field. Participants were asked to determine whether the second grating appeared clockwise or counterclockwise with respect to the first grating (Figure 3C). They clicked left or right on a mouse using their index and middle fingers to indicate a counterclockwise or clockwise rotation, respectively. Stepwise tracking was used to track orientation discrimination threshold; the difference in angulation between the first and second stimulus decreased for correct trials and increased for incorrect trials using a one-up–two-down staircase. The fixation point turned green for correct answers and remained black for incorrect answers to provide feedback. The task ended when 12 reversals were reached, and each participant performed the task two consecutive times. Data for the first run were discarded due to training effects. Data were analyzed by averaging over the last 10 trials for the second run and taken as an individual’s orientation discrimination threshold. All stimuli were presented on a ViewSonic 20-in CRT monitor. Participants were seated 30 cm from the monitor and head location was restricted using a chin and forehead rest. The room was completely dark, and a circular frame was placed over the monitor to remove external orientation cues. All responses were acquired by a mouse click with the right hand.
4.3. MRI/MRS acquisition and analysis
All data were acquired on a Philips 3T Achieva scanner (Philips Healthcare, Best, The Netherlands) using a 32-channel phased-array head coil for receive and body coil for transmit. For each participant, a 1-mm3 T1-weighted structural image (MPRAGE; TE/TR = 53.76/57.99 ms; flip angle = 8°) was acquired for voxel localization and subsequent voxel segmentation. GABA-edited MRS data were acquired in two regions: a 3 × 3 × 3 cm3 voxel was placed in the right sensorimotor cortex (SM1) and was centered on the central sulcus posterior to the hand-knob (Yousry et al., 1997) in the axial plane and rotated to align with the cortical surface as shown in Figure 1A; and a 3 × 3 × 3 cm3 voxel was placed on the midline occipital cortex and aligned with the cerebellar tentorium as shown in Figure 1B. For each voxel, two GABA-edited scans were acquired: a standard GABA-edited MEGA-PRESS acquisition (Mescher et al., 1998) with TE = 68 ms and editing pulse length of 14 ms placed at 1.9 ppm (ON) and 7.46 ppm (OFF); and an MM-suppressed MEGA-PRESS acquisition with TE = 80 ms to accommodate editing pulse length of 20 ms, with editing pulses symmetrically placed around the MM signal at 1.7 ppm at 1.9 ppm (ON) and 1.5 ppm (OFF). Both acquisitions had the following common parameters: TR = 2000 ms; 320 averages (~11-min scan duration); 2048 data points; 2 kHz spectral width; VAPOR water suppression (Tkáč et al., 1999). The acquisition order was counterbalanced across participants. The unsuppressed water signal was acquired from the SM1 and occipital voxels as a quantification reference. All data were analyzed in Gannet 3.0 (Edden et al., 2014) using spectral registration (Near et al., 2015) for frequency-and-phase correction. The GABA signal at 3.0 ppm was modeled using a single Gaussian function with linear baseline parameters (GABA+Glx fitting mode; fit range: 2.79–4.1 ppm) and the unsuppressed water signal was modeled using a Lorentzian-Gaussian function. GABA was quantified relative to the unsuppressed water signal. Subsequently, Gannet was used in conjunction with SPM12 for voxel co-registration to the T1 structural image and subsequent segmentation. GABA estimates were tissue-corrected as per Harris et al. (2015c), including full correction for tissue-related factors assuming a 2:1 GABA ratio between grey and white matter (α = 0.5). Tissue correction was performed in the same manner for both the standard GABA+ and MM-suppressed data. Moreover, a correction factor for the assumed contribution of MM to the GABA+ signal that is applied in Gannet by default was also applied to the MM-suppressed GABA data to show clearly that these estimates are ~50% smaller than the GABA+ estimates. Finally, GABA levels were normalized to the average voxel composition. For quality assurance purposes, we report GABA signal linewidth, SNR, model fit error and the estimated degree of frequency drift as calculated by Gannet (Edden et al., 2014; Mikkelsen et al., 2017).
4.4. Statistical analysis
Paired sample t-tests were used to compare GABA levels and quality assurance metrics between GABA+ and MM-suppressed MRS acquisitions. Pearson skipped correlation coefficients (Pernet et al., 2013) were calculated to test for robust correlations between GABA+, MM-suppressed GABA, and behavioral metrics. Skipped correlations test the strength of statistical relationships between two random variables by taking into account the bivariate distribution of the data and ignoring potential outliers that may drive observed correlations (Wilcox, 2004). Robustness was further assessed by calculating 95% confidence intervals (CIs) by percentile bootstrap for each correlation (10,000 simulations). The alpha level was set to 0.05 as a threshold for significance, while not correcting for multiple comparisons as some metrics (MM-suppressed GABA/GABA+, tactile behavior) may be correlated. Differences between correlation coefficients were tested using Fisher r-to-z transformation. We report (but do not infer) on alpha < 0.1, which we consider trend-level, as we have clear a priori hypotheses regarding the size and direction of correlations, based on prior work. In addition, these data were acquired in a small sample and will be used to validate previous work.
Acknowledgements
This work was supported by NIH grants K99/R00 MH107719 and a Perovitch award from the Division of Neuroradiology, Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine. This work applies tools developed under NIH grants R01 EB016089 and P41 EB015909.
References
- Bachtiar V, Stagg CJJ, 2014. The role of inhibition in human motor cortical plasticity. Neuroscience 278, 93–104. doi: 10.1016/j.neuroscience.2014.07.059 [DOI] [PubMed] [Google Scholar]
- Behar KL, Ogino T, 1993. Characterization of macromolecule resonances in the 1H NMR spectrum of rat brain. Magn. Reson. Med 30, 38–44. doi: 10.1002/mrm.1910300107 [DOI] [PubMed] [Google Scholar]
- Behar KL, Rothman DL, Spencer DD, Petroff OAC, 1994. Analysis of macromolecule resonances in 1H NMR spectra of human brain. Magn. Reson. Med 32, 294–302. doi: 10.1002/mrm.1910320304 [DOI] [PubMed] [Google Scholar]
- Boy F, Evans CJ, Edden RAE, Singh KD, Husain M, Sumner P, 2010. Individual Differences in Subconscious Motor Control Predicted by GABA Concentration in SMA. Curr. Biol 20, 1779–1785. doi: 10.1016/j.cub.2010.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno RM, Simons DJ, 2002. Feedforward mechanisms of excitatory and inhibitory cortical receptive fields. J. Neurosci 22, 10966–10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Cloak CC, Ernst T, 2003. Magnetic resonance spectroscopy studies of GABA in neuropsychiatric disorders. J. Clin. Psychiatry 64 Suppl 3, 7–14. [PubMed] [Google Scholar]
- Choi C, Bhardwaj PP, Kalra S, Casault CA, Yasmin US, Allen PS, Coupland NJ, 2007. Measurement of GABA and contaminants in gray and white matter in human brain in vivo. Magn. Reson. Med 58, 27–33. doi: 10.1002/mrm.21275 [DOI] [PubMed] [Google Scholar]
- Cousijn H, Haegens S, Wallis G, Near J, Stokes MG, Harrison PJ, Nobre AC, 2014. Resting GABA and glutamate concentrations do not predict visual gamma frequency or amplitude. Proc. Natl. Acad. Sci. U. S. A 111, 9301–9306. doi: 10.1073/pnas.1321072111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykes RW, Landry P, Metherate R, Hicks TP, 1984. Functional role of GABA in cat primary somatosensory cortex: shaping receptive fields of cortical neurons. J. Neurophysiol 52, 1066–1093. [DOI] [PubMed] [Google Scholar]
- Edden RAE, Muthukumaraswamy SD, Freeman TCA, Singh KD, 2009. Orientation Discrimination Performance Is Predicted by GABA Concentration and Gamma Oscillation Frequency in Human Primary Visual Cortex. J. Neurosci 29, 15721–15726. doi: 10.1523/JNEUROSCI.4426-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RAE, Oeltzschner G, Harris AD, Puts NAJ, Chan KL, Boer VO, Schär M, Barker PB, 2016. Prospective frequency correction for macromolecule-suppressed GABA editing at 3T. J. Magn. Reson. Imaging 44, 1474–1482. doi: 10.1002/jmri.25304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RAE, Puts NAJ, Barker PB, 2012. Macromolecule-suppressed GABA-edited magnetic resonance spectroscopy at 3T. Magn. Reson. Med 68, 657–661. doi: 10.1002/mrm.24391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RAE, Puts NAJ, Harris AD, Barker PB, Evans CJ, 2014. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J. Magn. Reson. Imaging 40, 1445–1452. doi: 10.1002/jmri.24478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sharkawy AM, Schär M, Bottomley PA, Atalar E, 2006. Monitoring and correcting spatio-temporal variations of the MR scanner’s static magnetic field. Magn. Reson. Mater. Physics, Biol. Med 19, 223–236. doi: 10.1007/s10334-006-0050-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, O’Malley S, Czarkowski KA, Gueorguieva R, Jatlow P, Sanacora G, Rothman DL, Krystal JH, Mason GF, 2005. Sex, GABA, and nicotine: The impact of smoking on cortical GABA levels across the menstrual cycle as measured with proton magnetic resonance spectroscopy. Biol. Psychiatry 57, 44–48. doi: 10.1016/j.biopsych.2004.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyer-Lea A, Wylezinska M, Kincses T, Matthews PM, 2006. Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. J. Neurophysiol 95, 1639–1644. doi: 10.1152/jn.00346.2005 [DOI] [PubMed] [Google Scholar]
- Gao F, Edden RA, Li M, Puts NA, Wang G, Liu C, Zhao B, Wang H, Bai X, Zhao C, Wang X, Barker PB, 2013. Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage 78, 75–82. doi: 10.1016/j.neuroimage.2013.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giapitzakis I-A, Avdievich N, Henning A, 2018. Characterization of macromolecular baseline of human brain using metabolite cycled semi-LASER at 9.4T. Magn. Reson. Med 80, 462–473. doi: 10.1002/mrm.27070 [DOI] [PubMed] [Google Scholar]
- Harris AD, Puts NAJ, Anderson BA, Yantis S, Pekar JJ, Barker PB, Edden RAE, 2015a. Multi-Regional Investigation of the Relationship between Functional MRI Blood Oxygenation Level Dependent (BOLD) Activation and GABA Concentration. PLoS One 10, e0117531. doi: 10.1371/journal.pone.0117531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AD, Puts NAJ, Barker PB, Edden RAE, 2015b. Spectral-editing measurements of GABA in the human brain with and without macromolecule suppression. Magn. Reson. Med 74, 1523–1529. doi: 10.1002/mrm.25549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AD, Puts NAJ, Edden RAE, 2015c. Tissue correction for GABA-edited MRS: Considerations of voxel composition, tissue segmentation, and tissue relaxations. J. Magn. Reson. Imaging 42, 1431–1440. doi: 10.1002/jmri.24903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AD, Saleh MG, Edden RAE, 2017. Edited 1H magnetic resonance spectroscopy in vivo: Methods and metabolites. Magn. Reson. Med 77, 1377–1389. doi: 10.1002/mrm.26619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Harris IM, Diamond ME, 2001. The topography of tactile learning in humans. J. Neurosci 21, 1056–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heba S, Puts NAJ, Kalisch T, Glaubitz B, Haag LM, Lenz M, Dinse HR, Edden RAE, Tegenthoff M, Schmidt-Wilcke T, 2016. Local GABA Concentration Predicts Perceptual Improvements After Repetitive Sensory Stimulation in Humans. Cereb. Cortex 26, 1295–1301. doi: 10.1093/cercor/bhv296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry P-G, Dautry C, Hantraye P, Bloch G, 2001. Brain GABA editing without macromolecule contamination. Magn. Reson. Med 45, 517–520. doi: [DOI] [PubMed] [Google Scholar]
- Juliano SL, Whitsel BL, Tommerdahl M, Cheema SS, 1989. Determinants of patchy metabolic labeling in the somatosensory cortex of cats: a possible role for intrinsic inhibitory circuitry. J. Neurosci 9, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Yang Y, Liang Z, Xia J, Yang Y, Zhou Y, 2008. GABA-mediated inhibition correlates with orientation selectivity in primary visual cortex of cat. Neuroscience 155, 914–922. doi: 10.1016/j.neuroscience.2008.06.032 [DOI] [PubMed] [Google Scholar]
- Mahone EM, Puts NA, Edden RAE, Ryan M, Singer HS, 2018. GABA and glutamate in children with Tourette syndrome: A 1H MR spectroscopy study at 7 T. Psychiatry Res. Neuroimaging 273, 46–53. doi: 10.1016/j.pscychresns.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, 1989. GABA as an inhibitory neurotransmitter in human cerebral cortex. J. Neurophysiol 62, 1018–1027. [DOI] [PubMed] [Google Scholar]
- McLaughlin DF, Juliano SL, 2005. Disruption of layer 4 development alters laminar processing in ferret somatosensory cortex. Cereb. Cortex 15, 1791–1803. doi: 10.1093/cercor/bhi056 [DOI] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R, 1998. Simultaneous in vivo spectral editing and water suppression. NMR Biomed 11, 266–272. doi: [DOI] [PubMed] [Google Scholar]
- Michels L, Martin E, Klaver P, Edden R, Zelaya F, Lythgoe DJ, Lüchinger R, Brandeis D, O’Gorman RL, 2012. Frontal GABA Levels Change during Working Memory. PLoS One 7, e31933. doi: 10.1371/journal.pone.0031933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen M, Barker PB, Bhattacharyya PK, Brix MK, Buur PF, Cecil KM, Chan KL, Chen DY-T, Craven AR, Cuypers K, Dacko M, Duncan NW, Dydak U, Edmondson DA, Ende G, Ersland L, Gao F, Greenhouse I, Harris AD, He N, Heba S, Hoggard N, Hsu T, Jansen JFA, Kangarlu A, Lange T, Lebel RM, Li Y, Lin CE, Liou J, Lirng J-F, Liu F, Ma R, Maes C, Moreno-Ortega M, Murray SO, Noah S, Noeske R, Noseworthy MD, Oeltzschner G, Prisciandaro JJ, Puts NAJ, Roberts TPL, Sack M, Sailasuta N, Saleh MG, Schallmo M, Simard N, Swinnen SP, Tegenthoff M, Truong P, Wang G, Wilkinson ID, Wittsack H-J, Xu H, Yan F, Zhang C, Zipunnikov V, Zöllner HJ, Edden RAE, 2017. Big GABA: Edited MR spectroscopy at 24 research sites. Neuroimage 159, 32–45. doi: 10.1016/j.neuroimage.2017.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen M, Loo RS, Puts NAJ, Edden RAE, Harris AD, 2018. Designing GABA-edited magnetic resonance spectroscopy studies: Considerations of scan duration, signal-to-noise ratio and sample size. J. Neurosci. Methods 303, 86–94. doi: 10.1016/j.jneumeth.2018.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins PG, McGonigle DJ, O’Gorman RL, Puts NAJ, Vidyasagar R, Evans CJ, Cardiff Symposium on MRS of GABA, Edden RAE, 2014. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage 86, 43–52. doi: 10.1016/j.neuroimage.2012.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Near J, Edden R, Evans CJ, Paquin R, Harris A, Jezzard P, 2015. Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magn. Reson. Med 73, 44–50. doi: 10.1002/mrm.25094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeltzschner G, Snoussi K, Puts NA, Mikkelsen M, Harris AD, Pradhan S, Tsapkini K, Schär M, Barker PB, Edden RAE, 2018. Effects of eddy currents on selective spectral editing experiments at 3T. J. Magn. Reson. Imaging 47, 673–681. doi: 10.1002/jmri.25813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet CR, Wilcox R, Rousselet GA, 2013. Robust Correlation Analyses: False Positive and Power Validation Using a New Open Source Matlab Toolbox. Front. Psychol 3, 1–18. doi: 10.3389/fpsyg.2012.00606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges EC, Woods AJ, Edden RAE, Puts NAJ, Harris AD, Chen H, Garcia AM, Seider TR, Lamb DG, Williamson JB, Cohen RA, 2017. Frontal Gamma-Aminobutyric Acid Concentrations Are Associated With Cognitive Performance in Older Adults. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2, 38–44. doi: 10.1016/j.bpsc.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Považan M, Hangel G, Strasser B, Gruber S, Chmelik M, Trattnig S, Bogner W, 2015. Mapping of brain macromolecules and their use for spectral processing of 1 H-MRSI data with an ultra-short acquisition delay at 7 T. Neuroimage 121, 126–135. doi: 10.1016/j.neuroimage.2015.07.042 [DOI] [PubMed] [Google Scholar]
- Považan M, Strasser B, Hangel G, Heckova E, Gruber S, Trattnig S, Bogner W, 2018. Simultaneous mapping of metabolites and individual macromolecular components via ultra-short acquisition delay 1 H MRSI in the brain at 7T. Magn. Reson. Med 79, 1231–1240. doi: 10.1002/mrm.26778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts NA, Wodka EL, Harris AD, Crocetti D, Tommerdahl M, Mostofsky SH, Edden RA, 2016. Reduced GABA and altered somatosensory function in children with autism spectrum disorder. Autism Res doi: 10.1002/aur.1691 [DOI] [PMC free article] [PubMed]
- Puts NAJ, Edden RAE, 2012. In vivo magnetic resonance spectroscopy of GABA: A methodological review. Prog. Nucl. Magn. Reson. Spectrosc 60, 29–41. doi: 10.1016/j.pnmrs.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts NAJ, Edden RAE, Evans CJ, McGlone F, McGonigle DJ, 2011. Regionally Specific Human GABA Concentration Correlates with Tactile Discrimination Thresholds. J. Neurosci 31, 16556–16560. doi: 10.1523/JNEUROSCI.4489-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts NAJ, Edden RAE, Wodka EL, Mostofsky SH, Tommerdahl M, 2013. A vibrotactile behavioral battery for investigating somatosensory processing in children and adults. J. Neurosci. Methods 218, 39–47. doi: 10.1016/j.jneumeth.2013.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts NAJ, Harris AD, Crocetti D, Nettles C, Singer HS, Tommerdahl M, Edden RAE, Mostofsky SH, 2015. Reduced GABAergic inhibition and abnormal sensory symptoms in children with Tourette syndrome. J. Neurophysiol 114, 808–817. doi: 10.1152/jn.00060.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts NAJ, Wodka EL, Tommerdahl M, Mostofsky SH, Edden RAE, 2014. Impaired tactile processing in children with autism spectrum disorder. J. Neurophysiol 111, 1803–1811. doi: 10.1152/jn.00890.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman DL, Petroff OA, Behar KL, Mattson RH, 1993. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc. Natl. Acad. Sci. U. S. A 90, 5662–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Fenton LR, Fasula MK, Rothman DL, Levin Y, Krystal JH, Mason GF, 2006. Cortical γ-Aminobutyric Acid Concentrations in Depressed Patients Receiving Cognitive Behavioral Therapy. Biol. Psychiatry 59, 284–286. doi: 10.1016/j.biopsych.2005.07.015 [DOI] [PubMed] [Google Scholar]
- Schaller B, Xin L, Gruetter R, 2014. Is the macromolecule signal tissue-specific in healthy human brain? A 1H MRS study at 7 Tesla in the occipital lobe. Magn. Reson. Med 72, 934–940. doi: 10.1002/mrm.24995 [DOI] [PubMed] [Google Scholar]
- Shapley R, Hawken M, Ringach DL, 2003. Dynamics of orientation selectivity in the primary visual cortex and the importance of cortical inhibition. Neuron 38, 689–699. doi: 10.1016/S0896-6273(03)00332-5 [DOI] [PubMed] [Google Scholar]
- Sillito AM, 1975. The contribution of inhibitory mechanisms to the receptive field properties of neurones in the striate cortex of the cat. J. Physiol 250, 305–329. doi: 10.1113/jphysiol.1975.sp011056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito AM, Kemp JA, Milson JA, Berardi N, 1980. A re-evaluation of the mechanisms underlying simple cell orientation selectivity. Brain Res 194, 517–520. doi: 10.1016/0006-8993(80)91234-2 [DOI] [PubMed] [Google Scholar]
- Simister RJ, McLean MA, Barker GJ, Duncan JS, 2003. Proton MRS reveals frontal lobe metabolite abnormalities in idiopathic generalized epilepsy. Neurology 61, 897–902. doi: 10.1212/01.WNL.0000086903.69738.DC [DOI] [PubMed] [Google Scholar]
- Snoussi K, Gillen JS, Horska A, Puts NAJ, Pradhan S, Edden RAE, Barker PB, 2015. Comparison of brain gray and white matter macromolecule resonances at 3 and 7 Tesla. Magn. Reson. Med 74, 607–613. doi: 10.1002/mrm.25468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, Johansen-Berg H, 2011. The Role of GABA in Human Motor Learning. Curr. Biol 21, 480–484. doi: 10.1016/j.cub.2011.01.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkáč I, Starčuk Z, Choi I-Y, Gruetter R, 1999. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn. Reson. Med 41, 649–656. doi: [DOI] [PubMed] [Google Scholar]
- Tsumoto T, Eckart W, Creutzfeldt OD, 1979. Modification of orientation sensitivity of cat visual cortex neurons by removal of GABA-mediated inhibition. Exp. Brain Res 34, 351–363. doi: 10.1007/BF00235678 [DOI] [PubMed] [Google Scholar]
- Whitsel BL, Favorov O, Tommerdahl M, Diamond M, Juliano SJ, Kelly D, Lund EJS, 1989. Dynamic processes govern the somatosensory cortical response to natural stimulation, in: Sensory Processing in the Mammalian Brain: Neural Substrates and Experimental Strategies Oxford University Press, pp. 84–116. [Google Scholar]
- Whitsel BL, Kelly EF, Quibrera M, Tommerdahl M, Li Y, Favorov OV, Xu M, Metz CB, 2003. Time-dependence of SI RA neuron response to cutaneous flutter stimulation. Somatosens. Mot. Res 20, 45–69. doi: 10.1080/0899022031000083834 [DOI] [PubMed] [Google Scholar]
- Wilcox R, 2004. Inferences Based on a Skipped Correlation Coefficient. J. Appl. Stat 31, 131–143. doi: 10.1080/0266476032000148821 [DOI] [Google Scholar]
- Wolf W, Hicks T, Albus K, 1986. The contribution of GABA-mediated inhibitory mechanisms to visual response properties of neurons in the kitten’s striate cortex. J. Neurosci 6, 2779–2795. doi: 10.1523/JNEUROSCI.06-10-02779.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P, 1997. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 120, 141–157. doi: 10.1093/brain/120.1.141 [DOI] [PubMed] [Google Scholar]


