Abstract
Purpose of Review.
Botanicals have long played a crucial role in the management of chronic and infected wounds, yet the mechanistic basis of these therapies remains largely poorly understood by modern science.
Recent Findings.
Studies have begun to unveil the mechanistic bases of botanical therapies for wound healing, but more work is necessary. Most notably, investigation into the growing conditions, postharvest treatment and pharmacological preparation of these botanicals has demonstrated their importance in terms of the chemical makeup and pharmacological activity of the final product used in pre-clinical and clinical studies.
Summary.
This work evaluates the potential safety, efficacy and mechanistic basis of some key botanical ingredients used in traditional medicine for wound care: aloe, marigold and St. John’s wort. Furthermore, perspectives on the future role that botanical natural products may play in anti-infective and wound care innovations are explored.
Keywords: Wounds, botanicals, wound healing, medicinal plants, complementary medicine, infection
Introduction
Wound healing is a multiphased process, involving the steps of hemostasis, inflammation, proliferation and remodeling. The wound healing process can be further complicated by factors such as poor circulation to the wound bed and microbial infection. Botanicals offer a number of therapeutic opportunities to promote the wound healing process and some exhibit antimicrobial, anti-inflammatory, angiogenic, antioxidant, or cell proliferation promoting activities. Phytomedicines derived from botanicals have a long history of use in the management of cutaneous disease and for the promotion of wound healing [1]. Indeed, phytotherapy is a form of medicine that has been practiced by humans for millennia. There are an estimated 390,900 species of plants on Earth, and of these, at least 28,187 species (7%) have been documented as having a medicinal use in one or more systems of traditional medicine [2]. Of these practices, the topical application of botanicals for the treatment of wounds and other dermatologic conditions is common, and can be found in Traditional Chinese Medicine, Ayruveda, Unani, Shamanic healing, European herbalism, and more systems of medicine. The pharmacological effects of botanicals derives from the biological activities of their secondary metabolites. These are natural products produced by plants for the purposes of communication with other organisms in their environment, and more specifically in the recruitment of pollinators and seed dispersers and defense against pathogens and other threats to their survival. Over time, humans have learned which species are most medicinally useful for specific diseases, and have incorporated this information into various systems of medicine. While there is no accurate number published concerning the number of species specifically used to promote wound healing in different medical traditions, there are a few species that predominate in the current literature on this topic: aloe, marigold and St. John’s wort.
The scope of this article is to review the literature on the main botanicals documented for use in wound healing. The objective of this work is to provide dermatologists, general practitioners, and scientists in the wound care field with a framework for evaluating botanical ingredients used in complementary and alternative medicine (CAM) and in general wound care strategies. Plant species were selected based on prevalence in the literature for wound healing-related reports, and only single ingredient (single plant – no mixtures) studies were included. Botanical names reported in the literature were checked for current validation on The Plant List [3], and any synonyms replaced with current accepted names. Botanical nomenclature at the family level follows current Angiosperm Phylogeny Group IV assignments [4]. Reports of bioactivity for each selected plant species as evidenced by chemical and pharmacological analyses, in vitro research, vertebrate animal studies, and human clinical trials for topical therapy is summarized by species below.
Aloe vera: Aloe vera (L.) Burm.f., Xanthorrhoeaceae
Botanical Description
Aloe vera is native to North Africa and the Mediterranean, but is now found cultivated across the globe. It is a succulent and member of the Xanthorrhoeaceae family, and has a short stem with leaves that cluster together in groups of up to 20 leaves that are 20–60 cm long, 6–7 cm wide at the base and 1–2.5 cm thick with spiny edges (Figure 1A) [5]. Formerly, it was categorized under the Liliaceae family. It has many common names, with the most common being “Aloe” in English-speaking countries, “Sabila” in Latin America, and “Ghikumar” in India. The current accepted botanical name is Aloe vera (L.) Burm.f., but there are many synonyms in the literature that may cause some confusion; this includes: A. barbadensis, A. chinensis, A. elongata, A. flava, A. indica, A. lanzae, A. maculata, A. perfoliata, A. rubescens, A. variegate, A. vulgaris [3]. All of these refer to the same species: A. vera.
Figure 1.
Images of plants with wound healing properties: A. Aloe vera (Aloe vera), B. Marigold (Calendula officinalis) and C. St. John’s Wort (Hypericum perforatum). All images obtained under CC0 Creative Commons permissions.
Traditional Uses
Aloe has a long history of use as a topical therapeutic for the treatment of burns, wounds and a variety of other skin conditions. In traditional preparations, the leaf gel is either apply fresh directly to the affected area, or extracted in hot water and topically applied [6]. One of the earliest records of A. vera use as a medicine can be found in Pedanius Dioscorides’ De Materia Medica. Dioscorides (40–90 AD) was a Greek physician, botanist and pharmacologist – widely recognized as an authority on herbal therapies of this period [5].
Chemistry and Pharmacology
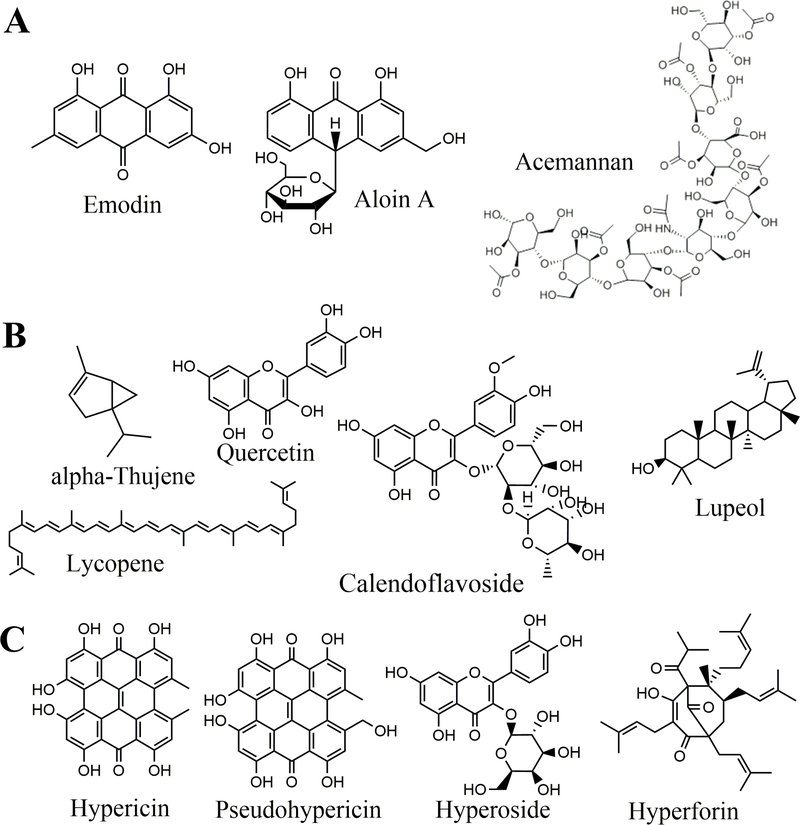
A. vera gel is composed of approximately 98.5–99.5% water, with more than 200 different components identified in the remaining solids, and polysaccharides making up the majority of that [7]. This includes acemannan - a D-isomer mucopolysaccharide found in the leaf gel an which exhibits immunomodulatory effects, acting on both macrophages and T-cells [8]. Regarding small molecules, aloe is also rich in anthraquinones – a class of phenolic anthraquinone natural products with antimicrobial properties; two examples include emodin and aloin A (Figure 2A) [9]. The composition and structural features of the acetylated mannose-rich polysaccharides and small molecules found in aloe gel can be altered by various environmental factors, including climate and soil, as well as postharvest treatments and processing, potentially leading to significant variability in different final products [10].
Figure 2.
Structures of biologically active compounds found in A. Aloe vera (Aloe vera), B. Marigold (Calendula officinalis) and C. St. John’s Wort (Hypericum perforatum).
Biological Activity: In vitro and In Vivo Studies
An in vitro study with normal primary human skin fibroblasts and keratinocytes demonstrated that solutions of A. vera (1–3%) exhibited significant stimulatory effects on cell proliferation and fibroblast and keratinocyte migration, as well as protection against preservative-induced keratinocyte death [11]. The evidence for the burn wound healing activity of aloe is conflicting; some have shown a reduction in healing times for burns, while others have been inconclusive or have shown limited effects [12]. In a study with juvenile pigs, topical application of aloe was no more efficacious than control concerning reepithelialization or scar histology. The subdermal temperature of the wound was decreased, however, which may explain the cooling and soothing properties attributed to aloe [13].
A study comparing the efficacy of Nigella sativa oil to that of Aloe vera gel in wound healing in a diabetic foot model found that the aloe treatment resulted both in improved resolution of the wound area and improved reepithelialization as compared to the N. sativa oil and control [14]. An aqueous extract of aloe was assessed for topical treatment of wounded skin surfaces in mice over seven days and compared to control (0.9% NaCl solution). While the histopathological assessment found improvements in epithelization at the 50 mg/kg dose as compared to control, some mutagenic and cytotoxic effects were also observed at this dose in peripheral blood [15]. Another study evaluated a porous wound dressing containing aloe extract versus the dressing with aloe and the salinomycin. Both dressings were found to permit cell growth and attenuated TGFβ-induced scar-forming myofibroblast formation in vitro [16]. A study on A. vera extract on oral wounds in rats found no significant difference, however, between test and control groups in the repair of mouth ulcers [17].
Clinical Studies
Similar to the contradictions seen in preclinical studies, there is conflicting evidence concerning the efficacy of topical A. vera therapy. For example, a Cochrane review on topical aloe gels for the healing of acute wounds (including lacerations, surgical incisions and burns) and chronic wounds (e.g. infected wounds, arterial and venous ulcers) examined seven trials encompassing a total of 347 participants and concluded that there was a high risk in bias due to study design, making it difficult to assess the value of topical aloe agents or dressings for acute and chronic wounds [18].
Importantly, the percent aloe in the topical formulation is generally recognized as being important to outcomes, with 100% aloe being considered most effective and anything less than 50% showing little or no effect in some studies [19]. The application of aloe for the care of outpatient burn wounds was noted to be effective in treating the pain associated with superficial burns [20]. Aside from burns, aloe has demonstrated some efficacy in clinical trials in other types of wounds. For example, a recent doubleblind, randomized, controlled trial on patients with split-thickness skin graft harvests from the thigh demonstrated that treatment with topical A. vera gel accelerated donor-site healing, but did not significantly impact pain relief [21].
Contraindications
Aloe is generally considered safe for topical use [12].
Discussion
The current research concerning the wound healing properties of topically applied A. vera formulations is conflicting and inconclusive both in the preclinical and clinical literature. One major variable in the study of A. vera has been the means by which the therapeutic is extracted and prepared. While the percent of aloe gel in the final formula is generally recognized as being important, rigor in the chemical characterization and batch-to-batch standardization of the aloe products created or purchased for the majority of aloe studies appears to be lacking.
Marigold: Calendula officinalis L., Asteraceae
Botanical Description
Marigold is native to Europe and parts of Asia, but is now broadly cultivated in home gardens. It’s English common name is “Pot Marigold”, but it is also known as “Mejorana” in Spanish, “Souci des Champs” in French, and “Ringelblume” in German, among others [22]. It is an herbaceous perennial species that grows up to 80 cm tall, has yellow inflorescences and oblong-lanceolate leaves with entire margins, sometimes waved or toothed (Figure 1B). Calendula officinalis L. is the current accepted scientific name and it is classified as a member of the Asteraceae (Compositae) family. It is also known by a number of synonyms: C. aurantiaca, C. eriocarpa, C. hydruntina, C. prolifera, C. x santamariae, C. sinuata and Caltha officinalis [3].
Traditional Uses
The inflorescence is edible, and is sometimes added as a colorful garnish to salads. It has been cultivated since at least the 12th century for medicinal use as an antiseptic, anti-inflammatory, cicatrizing agent, and antibacterial agent [23]. Ethnomedical reports demonstrate its utility in various cultures. Inflorescences are used to make ointments for wounds, ulcers and other skin eruptions in India [23]. Similarly, they are used in preparations for the treatment of inflammatory skin conditions and wounds in Italy [24], Portugal [25], and the Balkans [26] (Albania [27], Kosovo [28] and Serbia [29]), among other places. In Canada, oil of the inflorescence is used to treat wounds in livestock [30].
Chemistry and Pharmacology
The inflorescences of C. officinalis are rich in terpenoids, coumarins and flavonoids [31]. The following terpenoids have been identified in the inflorescences, and are responsible for calendula’s antiinflammatory and anti-oedematous activity: sitosterols, stigmasterols, diol diesters, taraxasterol monoesters, erythrodiol, brien, ursadiol, faradiol esters, arnidiol esters, calenduladiol esters, oleanolic acid saponins, calendulosides, calendulaglycosides, glucuronides, calenduladiol, faradiol, lupeol and cornulacic acid. The following flavonoids have been identified in the inflorescence, and are responsible for some of the antimicrobial properties: quercetin, isorhamnetin, isoquercitin, narcissin, calendoflaside, calendoflavoside, calendoflavobioside, rutin, isoquercetirin, neoheperididosides, and isohamnetin and quercetin rutinosides. The volatile oils of the inflorescence include monoterpenes and sesquiterpenes: α-thujene, α-pinene, limonene, 1,8-cineol, geraniol, α-cadinene and α-cadinol. The carotenoids, which are responsible for the yellow to orange color of the petals and some of the anti-inflammatory effects include: neoxanthins, violaxanthins, luteoxanthins, auroxanthin, favoxanthin, luteins, cryptoxanthins, lycopene, α-carotene and β-carotene [31]. Examples of a select few of these bioactive compounds are presented in Figure 2B.
Biological Activity: In vitro and In Vivo Studies
C. officinalis has been credited with a number of biological activities of relevance to wound healing, including antimicrobial, antiviral, anti-inflammatory, antioedematous, immunomodulatory, spasmolytic, spasmogenic, antidiabetic, antihyperlipidaemic, antitumor, antioxidant, and hepatoprotective effects; the specific mechanisms of action and responsible constituents for these activities remain unidentified in most cases. The anti-odematous activity of the inflorescences has been credited to the triterpendiol esters content; this is based on murine studies of ear oedema with croton oil as the irritant [32]. A study on the anti-inflammatory properties of the inflorescence found that a combination of carotenoids, flavonoids and triterpenoids were capable of mediating acute and chronic inflammation in a murine model via cytokine and macrophage inhibition, as well as free-radical scavenging effects of the antioxidants in the mixture [33]. Investigation of the wound healing properties with a gel formulation of inflorescence extract was examined in rats receiving a 2 × 2 cm skin incision; collegen production was significantly increased in the 7% calendula gel treatment group [34]. The antimicrobial effects of inflorescence extracts have been examined in a panel of pathogenic fungi and bacteria, with some broad antimicrobial activity observed [35]. Investigation of the wound healing effects of C. officinalis infloresence extracts with in vitro studies on immortalized human keratinocytes (HaCaT cells) determined that the inflammatory phase is influenced by activation of the transcription factor NF-kB activation coupled with increased levels of the chemokine IL-8. They also noted a significant but moderate decrease in collagenase activity compared to control [36].
Clinical Studies
A hydroglycolic extract of C. officinalis was examined in a prospective, descriptive study for twice-daily topical applications in the treatment of diabetic foot ulcers. At 30 weeks of treatment, 78% of patients achieved complete wound closure and no adverse events were observed [37]. A randomized study examining the therapeutic efficacy of topical applications of aloe cream versus calendula ointment on sixty-six infants with diaper dermatitis found that while both treatment groups resulted in reduced severity of disease, the calendula group had significantly fewer rash sites than the aloe group. Neither group had adverse effects reported [38]. A systematic review of twenty-eight trials examining management strategies for acute skin reactions following cancer radiation therapy concluded that calendula ointment might be effective for skin damage prevention [39]. The European Medicines Agency (EMA) has approved the aqueous alcoholic and lipophilic extracts of C. officinalis as traditional medical products for healing of minor wounds and treatment of minor skin inflammations [40].
Contraindications
C. officinalis is generally recognized as safe for consumption or topical application. However, as it is a member of the daisy family (Asteraceae; Compositae), individuals with known allergies to other members of this family should avoid using this herb. In particular, it contains sesquiterpene lactones that may trigger acute allergic reactions or delayed hypersensitivity [41].
St. John’s Wort: Hypericum perforatum L., Hypericaceae
Botanical Description
The common name of “St. John’s Wort” derives from the date of ritual harvest in Europe – June 23rd, the eve of the festival that celebrates John the Baptist. It is characterized by its distinctive yellow flowers with oblong petals that feature brown-black glandular dots, rounds leaves and a height of almost 1 meter when mature (Figure 1C). It is native to Europe and Asia, but has spread as an invasive species in Oceania and North America. Hypericum perforatum L. is the current accepted scientific name and it is classified as a member of the Hypericaceae family (formerly in the Clusiaceae family). It is also confused with a number of synonyms: H. assurgens, H. deidescheimense, H. lineolatum, H. marylandicum, H. officinale, H. officinarum, H. pseudoperforatum and H. vulgare [3].
Traditional Uses
H. perforatum has a long history of use in traditional medicine for disease indications ranging from depression (treated with consumption of teas) to topical applications in the treatment of burns, wounds and various inflammatory skin diseases. It was also used in ritual applications, to ward off evil spirits and ensure a strong harvest. For topical applications, an oil macerate of H. perforatum is made by taking dried flowering aerial parts and steeping them in vegetable oil (most commonly olive oil) and left in a clear container outdoors for sun exposure over a period of 40 days [42]. The medicament is ready after this period and when it takes on the notable color of a deep red hue. This preparation of “Oleum hyperici” is topically applied in the treatment of burn wounds and chronic ulcers throughout the Balkans and Anatolia, as evidenced by recent ethnobotanical studies in this region [43–48, 27, 26]. It has also been used in traditional medicine for the topical treatment of sunburns, myalgia, bruises, hemorrhoids and as an antiseptic in Turkey [49, 50].
Chemistry and Pharmacology
H. perforatum is rich in proanthocyanidins (catechin, epicatechin, leucocyanidin), flavonoids (hyperoside, rutin, quercitrin, isoquercitrin, quercetin and kaempferol), biflavonoids, tannins, phloroglucinol derivatives (hyperforin), phenolic acids, volatiles oil, sterols, naphthadianthrones (hypericin and pseudohypericin), vitamin C and A and xanthones [51, 52]. The most notable bioactive compounds include hypericin, pseudohypericin and hyperforin (Figure 2C). The antidepressant activity of the herb is attributed to its hyperforin contant, which acts a reuptake inhibitor for neurotransmitters such as dopamine, norepinepherine, serotonin, and glutamate [53].
Biological Activity: In vitro and In Vivo Studies
A study on the antibacterial activity of H. perforatum extracts was found to differ substantially based on the extraction method used, with aqueous and organic extracts generally exhibiting more classic antibacterial activity in growth inhibition of pathogens, whereas the oleum hyperici formulation exhibited mild quorum quenching and anti-biofilm properties against Staphylococcus aureus [54]. A study on the efficacy of oleum hyperici on palatal wound healing in rabbits found that the topical oil treatment showed no additional benefit to healing over that of the control (the olive oil base) [55]. On the other hand, a study evaluating the oral versus topical efficacy of oleum hyperici versus control (olive oil) in diabetic rats with dorsal surgical wounds determined that topical application of oleum hyperici yields a faster inflammatory response and healing than oral dosing of oleum hyperici or olive oil, or topical dosing of olive oil alone [56]. Notably, this is in line with traditional medicine practices, which entail topical applications of oleum hyperici to wounds.
Incorporation of oleum hyperici into a chitosan film based wound dressing resulted in antimicrobial activity against Staphylococcus aureus and Escherichia coli in an in vitro model, whereas no cytotoxicity was observed in either the chitosan or H. perforatum oil loaded chitosan films in a fibroblast (NIH3T3) cell viability assay [57]. A number of other studies evaluating H. perforatum extracts have demonstrated antibacterial, antiviral, antioxidant, anti-inflammatory, anticancer and keratinocyte differentiation effects; a full review of these studies is provided by Wölfle et al. [58]
Clinical Studies
Oleum hyperici was assessed for its potential efficacy in treating pressure sores (decubitus ulcer) in a case study with an 82 year man. The histopathological results showed progress in epithelialization and the ulcer wound area (cm) went from 4.55 × 6.57 to 1.39 × 2.73 following 32 days of twice daily treatments, bringing his Braden score from 10 to 21 in that time period [59]. Examination of the immunomodulatory potential of St. John’s Wort on the skin of human volunteers found that topical applications of ointment inhibited the allostimulatory capacity of epidermal cells and reduced their capacity to stimulate proliferation of allogeneic T cells in a mixed epidermal cell lymphocyte reaction following exposure to radiation, which may provide a rationale for the traditional treatment of inflammatory skin conditions with hypericum oil [60]. A study on a 70:30 mixture of H. perforatum and Calendula arvensis oil extract for a twice-daily topical application to surgical site wounds following Caesarean section demonstrated significant improvement in tissue regeneration time in comparison to control (wheat germ oil) [61].
Contraindications
The natural product hypericin can cause phototoxic skin reactions if ingested or exposed to the skin, resulting in the condition of hypericism, especially in fair skinned individuals [62]. Furthermore, UVactivated hypericin exhibits necrotic and apoptotic effects on human keratinocytes and melanocytes [63]. Notably, chemical analysis of the traditional oleum hyperici formulation used throughout the Balkans as a topical therapy revealed the absence of hypericin in the resulting product; this aligned with ethnographic data suggesting frequent users of the oil did not exhibit symptoms of hypericism [54].
Conclusions
Patients seek natural, botanical alternatives for a number of reasons, including lower cost and greater ease of accessibility relative to prescription pharmaceuticals. Botanicals have played an important role in wound care strategies throughout the history of medicine and some of the most common species used historically continue to be used in traditional and complementary medical strategies across the globe. The aim of this review was to assess the current state of knowledge concerning the chemical makeup, pharmacological potential and clinical outcomes of three botanicals with widespread use in wound care.
Pharmacological Activities of Botanicals for Wounds
For each botanical reviewed here, it was evident that their chemical complexity is also tied to a variety of pharmacological activities that may prove more effective in unison, rather than individually as single compound drugs. Specifically, these shared common features in their antimicrobial, anti-inflammatory, antioxidant, and anti-oedema activities. In some cases, they shared common chemical features in terms of flavonoid and triterpenoid content, but still remain chemically distinct in both the overall composition and relative levels of secondary metabolites in the medicinal plant tissues.
Future Study Design
Future studies on the wound healing potential of topical botanical formulations must incorporate careful consideration of the chemical makeup of the final therapeutic product as major differences in the chemical makeup can result in conflicting study results. Even when products are created following the same methodology, implementation of batch-to-batch standardization controls is critical to ensure that the same relative levels of major marker compounds are present to foster rigor and reproducibility in future studies. The composition and stability of pharmaceutical formulations derived from plants can vary greatly. For example, plants grown under different climatic and environmental factors are known exhibit variability in their chemical composition. Likewise, different means of extracting plant compounds (e.g., in aqueous versus organic solvents) also creates chemically distinct final products with differing pharmacological profiles and bioactivities. Storage parameters can also impact product quality and biological activity.
Regarding future clinical studies, in addition to the need for through consideration of characterization and batch-to-batch reproducibility of topical botanical therapeutics used in the clinic, properly designed randomized controlled trials are suggested. Trials should follow CONSORT statement guidelines and include randomization, blinded outcome assessment, appropriate sample size, baseline comparability of groups and clear inclusion and exclusion criteria.
Acknowledgements
Work in the Quave Research Group is funded by the National Institutes of Health, National Institute of Allergy and Infectious Disease (R21 AI136563, PI: CLQ). The content is solely the responsibility of the authors and does not necessarily reflect the official views of the NIH or NIAID. The funding agency had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Compliance with Ethical Standards
Conflict of Interest
The author is an inventor on patents concerning botanical inhibitors of microbial biofilm formation and quorum sensing; the author confirms that any competing interests do not alter her adherence to journal policies on ethics and sharing data or materials. The author provided consulting services to Medline during the period of writing this study. The author declares that provision of these services did not have any impact on the present study.
Human and Animal Rights
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Compliance with Ethics Guidelines
Conflict of Interest
Dr. Quave reports grants from National Institutes of Health, National Institute of Allergy and Infectious Disease, from null, during the conduct of the study; personal fees from Medline, from null, outside the submitted work; In addition, Dr. Quave has a patent Quave, C.L., M.S. Smeltzer, C.M. Compadre, H. Hendrickson. Anti-biofilm compositions and methods for using. Patent Numbers: WO2012048119-A2; US2012088671-A1; WO2012048119-A3. Derwent Primary Accession No.: 2012-E22562; Issued June 3, 2014; US 9,351,492 B2 Issued May 31, 2016 issued, a patent Quave, C.L., A.R. Horswill, J.T. Lyles. Botanical extracts and compounds from Castanea plants and methods of use. Provisional Filed June 26, 2015. Serial No. 62/185,146. Non-Provisional Filed June 24, 2016 Serial No. 15/195,514. pending, and a patent Quave, C.L. and J.T. Lyles. Botanical extracts and compounds from Schinus plants and methods of use. Provisional Filed July 10, 2015. Serial No. 62/190,802. Non-Provisional Filed July 8, 2016. Serial No. 15/205,493. pending.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors
References
Recently published papers of particular interest have been highlighted as:
● Of importance
●● Of major importance
- 1.Pazyar N, Yaghoobi R, Rafiee E, Mehrabian A, Feily A. Skin wound healing and phytomedicine: A review. Skin Pharmacology and Physiology. 2014;27(6):303–10. [DOI] [PubMed] [Google Scholar]
- 2.Willis KJ, editor. State of the World’s Plants Report. London, England: Royal Botanic Gardens, Kew; 2017. [PubMed] [Google Scholar]
- 3.The Plant List Version 1.1. Published on the Internet. 2013. http://www.theplantlist.org/. Accessed September 9 2018.
- 4.Stevens PF. Angiosperm Phylogeny Website, version 14. 2001. Onwards. http://www.mobot.org/MOBOT/research/APweb/. Accessed September 10 2018. [Google Scholar]
- 5.Carter S, Newton LE, Lavranos JJ, Walker CC. Aloes: The Definitive Guide. London: Royal Botanic Gardens, Kew; 2011. [Google Scholar]
- 6.Ross IA. Medicinal Plants of the World : Chemical Constituents, Traditional, and Modern Medicinal Uses. Humana Press; 1999. [Google Scholar]
- 7.Femenia A, Sánchez ES, Simal S, Rosselló C. Compositional features of polysaccharides from Aloe vera (Aloe barbadensis Miller) plant tissues. Carbohydrate Polymers. 1999;39(2):109–17. doi: 10.1016/S0144-8617(98)00163-5. [DOI] [Google Scholar]
- 8.Tizard IR, Ni Y. Carbohydrates, Immune Stimulating In: Delves PJ, editor. Encyclopedia of Immunology (Second Edition). Oxford: Elsevier; 1998. p. 427–31. [Google Scholar]
- 9.Wamer WG, Vath P, Falvey DE. In vitro studies on the photobiological properties of aloe emodin and aloin A. Free Radical Biology and Medicine. 2003;34(2):233–42. [DOI] [PubMed] [Google Scholar]
- 10.●●.Minjares-Fuentes R, Femenia A, Comas-Serra F, Rodríguez-González VM. Compositional and structural features of the main bioactive polysaccharides present in the Aloe vera plant. Journal of the AOAC International. 2018;101. doi: 10.5740/jaoacint.18-0119. This study examines the variability in compositional features of Aloe vera gel and provides a chemical explanation for differences in the pharmacology of aloe shown in the literature. [DOI] [PubMed] [Google Scholar]
- 11.Teplicki E, Ma Q, Castillo DE, Zarei M, Hustad AP, Chen J et al. The effects of Aloe vera on wound healing in cell proliferation, migration, and viability. Wounds. 2018;30(9):263–8. [PubMed] [Google Scholar]
- 12.Maenthaisong R, Chaiyakunapruk N, Niruntraporn S, Kongkaew C. The efficacy of aloe vera used for burn wound healing: a systematic review. Burns: Journal of the International Society for Burn Injuries. 2007;33(6):713–8. doi: 10.1016/j.burns.2006.10.384. [DOI] [PubMed] [Google Scholar]
- 13.Cuttle L, Kempf M, Kravchuk O, George N, Liu PY, Chang HE et al. The efficacy of Aloe vera, tea tree oil and saliva as first aid treatment for partial thickness burn injuries. Burns: Journal of the International Society for Burn Injuries. 2008;34(8):1176–82. doi: 10.1016/j.burns.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 14.●.Sari Y, Purnawan I, Kurniawan DW, Sutrisna E. A comparative study of the effects of Nigella sativa oil gel and Aloe vera gel on wound healing in diabetic rRats. Journal of Evidence-based Integrative Medicine. 2018;23:2515690X18772804. doi: 10.1177/2515690X18772804. This study examines two popular traditional medicines for wound care and offers comparative insight into the efficacy of Aloe vera over that of another botanical. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodrigues LlO, de Oliveira ACL, Tabrez S, Shakil S, Khan MI, Asghar MN et al. Mutagenic, antioxidant and wound healing properties of Aloe vera. Journal of Ethnopharmacology. 2018;227:191–7. doi: 10.1016/j.jep.2018.08.034. [DOI] [PubMed] [Google Scholar]
- 16.●.Woeller Collynn F, Woodroof A, Cottler Patrick S, Pollock Stephen J, Haidaris Constantine G, Phipps Richard P. In vitro characterization of variable porosity wound dressing with anti-scar properties. Eplasty. 2018;18:e21 This study offers an interesting perspective on the incorporation of botanicals into medical devices for the promotion of wound healing. [PMC free article] [PubMed] [Google Scholar]
- 17.Coelho FH, Salvadori G, Rados PV, Magnusson A, Danilevicz CK, Meurer L et al. Topical Aloe vera (Aloe barbadensis Miller) extract does not accelerate the oral wound healing in rats. Phytotherapy Research. 2015;29(7):1102–5. doi:doi: 10.1002/ptr.5352. [DOI] [PubMed] [Google Scholar]
- 18.Dat AD, Poon F, Pham KBT, Doust J. Aloe vera for treating acute and chronic wounds. Cochrane Database of Systematic Reviews. 2012(2). doi: 10.1002/14651858.CD008762.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korac RR, Khambholja KM. Potential of herbs in skin protection from ultraviolet radiation. Pharmacognosy Review. 2011;5(10):164–73. doi: 10.4103/0973-7847.91114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lloyd EC, Rodgers BC, Michener M, Williams MS. Outpatient burns: prevention and care. American family physician. 2012;85(1):25–32. [PubMed] [Google Scholar]
- 21.Burusapat C, Supawan M, Pruksapong C, Pitiseree A, Suwantemee C. Topical Aloe vera gel for accelerated wound healing of split-thickness skin graft donor sites: A double-blind, randomized, controlled trial and systematic review. Plastic and Reconstructive Surgery. 2018;142(1):217–26. [DOI] [PubMed] [Google Scholar]
- 22.Basch E, Bent S, Foppa I, Haskmi S, Kroll D, Mele M et al. Marigold (Calendula officinalis L.): An evidence-based systematic review by the natural standard research collaboration. Journal of Herbal Pharmacotherapy. 2006;6(3/4):135–59. doi: 10.1300/J157v06n03-08. [DOI] [PubMed] [Google Scholar]
- 23.Khalid KA, da Silva JAT. Biology of Calendula officinalis Linn.: Focus on pharmacology, biological activities and agronomic practices. Medicinal and Aromatic Plant Science and Biotechnology. 2012;6(1):12–27. [Google Scholar]
- 24.De Feo V, Aquino R, Menghini A, Ramundo E, Senatore F. Traditional phytotherapy in the Peninsula Sorrentina, Campania, Southern Italy. Journal of Ethnopharmacology. 1992;36(2):113–25. doi: 10.1016/0378-8741(92)90010-O. [DOI] [PubMed] [Google Scholar]
- 25.Neves JM, Matos C, Moutinho C, Queiroz G, Gomes LR. Ethnopharmacological notes about ancient uses of medicinal plants in Trás-os-Montes (northern of Portugal). Journal of Ethnopharmacology. 2009;124(2):270–83. doi: 10.1016/j.jep.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 26.●.Jarić S, Kostić O, Mataruga Z, Pavlović D, Pavlović M, Mitrović M et al. Traditional wound-healing plants used in the Balkan region (Southeast Europe). Journal of Ethnopharmacology. 2018;211:311–28. doi: 10.1016/j.jep.2017.09.018. This is a literature review of 128 plant species used in traditional remedies for wound healing in the Balkans. It highlights the medicinal applications of Calendula officinalis and Hypericum perforatum for these purposes. [DOI] [PubMed] [Google Scholar]
- 27.Mustafa B, Hajdari A, Krasniqi F, Hoxha E, Ademi H, Quave CL et al. Medical ethnobotany of the Albanian Alps in Kosovo. Journal of Ethnobiology and Ethnomedicine. 2012;8:6-. doi: 10.1186/17464269-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mustafa B, Hajdari A, Pieroni A, Pulaj B, Koro X, Quave CL. A cross-cultural comparison of folk plant uses among Albanians, Bosniaks, Gorani and Turks living in south Kosovo. Journal of Ethnobiology and Ethnomedicine. 2015;11:39. doi: 10.1186/s13002-015-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarić S, Popović Z, Mačukanović-Jocić M, Djurdjević L, Mijatović M, Karadžić B et al. An ethnobotanical study on the usage of wild medicinal herbs from Kopaonik Mountain (Central Serbia). Journal of Ethnopharmacology. 2007;111(1):160–75. doi: 10.1016/j.jep.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Lans C, Turner N, Khan T, Brauer G, Boepple W. Ethnoveterinary medicines used for ruminants in British Columbia, Canada. Journal of Ethnobiology and Ethnomedicine. 2007;3(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muley B, Khadabadi S, Banarase N. Phytochemical constituents and pharmacological activities of Calendula officinalis Linn (Asteraceae): A review. Tropical Journal of Pharmaceutical Research. 2009;8(5). [Google Scholar]
- 32.Zitterl-Eglseer K, Sosa S, Jurenitsch J, Schubert-Zsilavecz M, Della Loggia R, Tubaro A et al. Antioedematous activities of the main triterpendiol esters of marigold (Calendula officinalis L.). Journal of Ethnopharmacology. 1997;57(2):139–44. doi: 10.1016/S0378-8741(97)00061-5. [DOI] [PubMed] [Google Scholar]
- 33.Preethi KC, Kuttan G, Kuttan R. Anti-inflammatory activity of flower extract of Calendula officinalis Linn. and its possible mechanism of action. Indian Journal of Experimental Biology. 2009;47(2):113–20. [PubMed] [Google Scholar]
- 34.Naeini A, Miri R, Shafiei N, Tabandeh M, Oryan A, Nazifi S. Effects of topical application of Calendula officinalis gel on collagen and hydroxyproline content of skin in rats. Comparative Clinical Pathology. 2012;21(3):253–7. doi: 10.1007/s00580-010-1087-1. [DOI] [Google Scholar]
- 35.Efstratiou E, Hussain AI, Nigam PS, Moore JE, Ayub MA, Rao JR. Antimicrobial activity of Calendula officinalis petal extracts against fungi, as well as Gram-negative and Gram-positive clinical pathogens. Complementary Therapies in Clinical Practice. 2012;18(3):173–6. doi: 10.1016/j.ctcp.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 36.●●.Nicolaus C, Junghanns S, Hartmann A, Murillo R, Ganzera M, Merfort I. In vitro studies to evaluate the wound healing properties of Calendula officinalis extracts. Journal of Ethnopharmacology. 2017;196:94–103. doi: 10.1016/j.jep.2016.12.006. This study provides details on a useful model for in vitro evaluation of botanical extracts for potential mechanisms of wound healing. [DOI] [PubMed] [Google Scholar]
- 37.●●.Buzzi M, de Freitas F, Winter M. A prospective, descriptive study to assess the clinical benefits of using Calendula officinalis hydroglycolic extract for the topical treatment of diabetic foot ulcers. Ostomy Wound Management. 2016;62(3):8–24. This study demonstrates promising data on the topical spray application of C. officinalis extracts in diabetic foot ulcers. In addition to observations of wound closure in many patients, there was also a reduction in odorous wounds. [PubMed] [Google Scholar]
- 38.Panahi Y, Sharif MR, Sharif A, Beiraghdar F, Zahiri Z, Amirchoopani G et al. A randomized comparative trial on the therapeutic efficacy of topical Aloe vera and Calendula officinalis on diaper dermatitis in children. The Scientific World Journal. 2012;2012:810234. doi: 10.1100/2012/810234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolderston A, Lloyd NS, Wong RKS, Holden L, Robb-Blenderman L, Supportive Care Guidelines Group of Cancer Care Ontario Program in Evidence-based Care. The prevention and management of acute skin reactions related to radiation therapy: a systematic review and practice guideline. Supportive Care in Cancer. 2006;14(8):802. doi: 10.1007/s00520-006-0063-4. [DOI] [PubMed] [Google Scholar]
- 40.HMPC. Community Herbal Monograpg on Calendula officinalis L., flos. Committee on Herbal Medicinal Products (HMPC), European Medicines Agency; 2008. [Google Scholar]
- 41.Reider N, Komericki P, Hausen BM, Fritsch P, Aberer W. The seamy side of natural medicines: contact sensitization to arnica (Arnica montana L.) and marigold (Calendula officinalis L.). Contact Dermatitis. 2001;45(5):269–72. doi: 10.1034/j.1600-0536.2001.450503.x. [DOI] [PubMed] [Google Scholar]
- 42.Mattalia G, Quave CL, Pieroni A. Traditional uses of wild food and medicinal plants among Brigasc, Kyé, and Provençal communities on the Western Italian Alps. Genetic Resources and Crop Evolution. 2013;60(2):587–603. doi: 10.1007/s10722-012-9859-x. [DOI] [Google Scholar]
- 43.Kültür S Medicinal plants used in Kırklareli Province (Turkey). Journal of Ethnopharmacology. 2007;111(2). [DOI] [PubMed] [Google Scholar]
- 44.Savikin K, Zdunic G, Menkovic N, Zivkovic J, Cujic N, Terescenko M et al. Ethnobotanical study on traditional use of medicinal plants in South-Western Serbia, Zlatibor district. Journal of Ethnopharmacology. 2013;146(3):803–10. doi: 10.1016/j.jep.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Redžić SS. The ecological aspect of ethnobotany and ethnopharmacology of population in Bosnia and Herzegovina. Collegium Antropologicum. 2007;3:869–90. [PubMed] [Google Scholar]
- 46.Šarić-Kundalić B, Fritz E, Dobeš C, Saukel J. Traditional medicine in the pristine village of Prokoško Lake on Vranica Mountain, Bosnia and Herzegovina. Scientia Pharmaceutica. 2010;78(2):275–90. doi: 10.3797/scipharm.1003-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mustafa B, Hajdari A, Pieroni A, Pulaj B, Koro X, Quave CL. A cross-cultural comparison of folk plant uses among Albanians, Bosniaks, Gorani and Turks living in south Kosovo. Journal of Ethnobiology and Ethnomedicine. 2015;11(1):39. doi: 10.1186/s13002-015-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mustafa B, Hajdari A, Pajazita Q, Syla B, Quave CL, Pieroni A. An ethnobotanical survey of the Gollak region, Kosovo. Genetic Resources and Crop Evolution. 2012;59(5):739–54. doi: 10.1007/s10722-011-9715-4. [DOI] [Google Scholar]
- 49.Yeşilada E, Honda G, Sezik E, Tabata M, Goto K, Ikeshiro Y. Traditional medicine in Turkey IV. Folk medicine in the Mediterranean subdivision. Journal of Ethnopharmacology. 1993;39(1):31–8. doi: 10.1016/0378-8741(93)90048-A. [DOI] [PubMed] [Google Scholar]
- 50.Sezik E, Yeşilada E, Honda G, Takaishi Y, Takeda Y, Tanaka T. Traditional medicine in Turkey X. Folk medicine in Central Anatolia. Journal of Ethnopharmacology. 2001;75(2):95–115. doi: 10.1016/S0378-8741(00)00399-8. [DOI] [PubMed] [Google Scholar]
- 51.Hölzl J, Petersen M. Chemical constituents of Hypericum species In: Ernst E, editor. Hypericum: The Genus Hypericum. New York: Taylor & Francis; 2003. p. 77–93. [Google Scholar]
- 52.Crockett SL, Poller B, Tabanca N, Pferschy-Wenzig E-M, Kunert O, Wedge DE et al. Bioactive xanthones from the roots of Hypericum perforatum (common St John’s wort). Journal of the Science of Food and Agriculture. 2011;91(3):428–34. doi: 10.1002/jsfa.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chatterjee SS, Bhattacharya SK, Wonnemann M, Singer A, Müller WE. Hyperforin as a possible antidepressant component of Hypericum extracts. Life Sciences. 1998;63(6):499–510. doi: 10.1016/S0024-3205(98)00299-9. [DOI] [PubMed] [Google Scholar]
- 54.●●.Lyles JT, Kim A, Nelson K, Bullard-Roberts AL, Hajdari A, Mustafa B et al. The chemical and antibacterial evaluation of St. John’s Wort oil macerates used in Kosovar traditional medicine. Frontiers in Microbiology. 2017;8:1639. doi: 10.3389/fmicb.2017.01639. This study provides a detailed look at the differences in the chemical makeup and biological activity of St. John’s Wort extracts, highlighting the important role that extraction methodologies can have on study outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gunpinar S, Kilic OA, Duran I, Tosun M, Firat T, Soyler G. Evaluation of the effect of topical Hypericum perforatum oil on excisional palatal wound healing in rabbits. Journal of Investigative Surgery. 2018:1–10. doi: 10.1080/08941939.2018.1474980. [DOI] [PubMed] [Google Scholar]
- 56.Altıparmak M, Eskitaşçıoğlu T. Comparison of systemic and topical Hypericum perforatum on diabetic surgical wounds. Journal of Investigative Surgery. 2018;31(1):29–37. doi: 10.1080/08941939.2016.1272654. [DOI] [PubMed] [Google Scholar]
- 57.●.Güneş S, Tıhmınlıoğlu F. Hypericum perforatum incorporated chitosan films as potential bioactive wound dressing material. International Journal of Biological Macromolecules. 2017;102:933–43. doi: 10.1016/j.ijbiomac.2017.04.080. This study demonstrates how botanical ingredients can be incorporated into wound dressings and evaluated both for antimicrobial and human cytotoxicity in vitro. [DOI] [PubMed] [Google Scholar]
- 58.Wölfle U, Seelinger G, Schempp CM. Topical application of St. John’s wort (Hypericum perforatum). Planta Medica. 2014;80(02/03):109–20. doi: 10.1055/s-0033-1351019. [DOI] [PubMed] [Google Scholar]
- 59.Yücel A, Kan Y, Yesilada E, Akın O. Effect of St.John’s wort (Hypericum perforatum) oily extract for the care and treatment of pressure sores; a case report. Journal of Ethnopharmacology. 2017;196:23641. doi: 10.1016/j.jep.2016.12.030. [DOI] [PubMed] [Google Scholar]
- 60.Schempp CM, Winghofer B, Lüdtke R, Simon-Haarhaus B, Schöpf E, Simon JC. Topical application of St John’s wort (Hypericum perforatum L.) and of its metabolite hyperforin inhibits the allostimulatory capacity of epidermal cells. British Journal of Dermatology. 2002;142(5):979–84. doi: 10.1046/j.13652133.2000.03482.x. [DOI] [PubMed] [Google Scholar]
- 61.Lavagna SM, Secci D, Chimenti P, Bonsignore L, Ottaviani A, Bizzarri B. Efficacy of Hypericum and Calendula oils in the epithelial reconstruction of surgical wounds in childbirth with caesarean section. Il Farmaco. 2001;56(5):451–3. doi: 10.1016/S0014-827X(01)01060-6. [DOI] [PubMed] [Google Scholar]
- 62.Kamuhabwa A, Roelandts R, Witte P Skin photosensitization with topical hypericin in hairless mice. Journal of Photochemistry and Photobiology B. 1998;53:110–4. [DOI] [PubMed] [Google Scholar]
- 63.Davids LM, Kleemann B, Kacerovska D, Pizinger K, Kidson SH. Hypericin phototoxicity induces different modes of cell death in melanoma and human skin cells. Journal of Photochemistry and Photobiology B. 2008;91(2–3):67–76. doi: 10.1016/j.jphotobiol.2008.01.011. [DOI] [PubMed] [Google Scholar]