Abstract
The production of force and power are inherent properties of skeletal muscle, and regulated by contractile proteins within muscle fibers. However, skeletal muscle integrity and function also require strong connections between muscle fibers and their extracellular matrix (ECM). A well-organized and pliant ECM is integral to muscle function and the ability for many different cell populations to efficiently migrate through ECM is critical during growth and regeneration. For many neuromuscular diseases, genetic mutations cause disruption of these cytoskeletal-ECM connections, resulting in muscle fragility and chronic injury. Ultimately, these changes shift the balance from myogenic pathways toward fibrogenic pathways, culminating in the loss of muscle fibers and their replacement with fatty-fibrotic matrix. Hence a common pathological hallmark of muscular dystrophy is prominent fibrosis. This review will cover the salient features of muscular dystrophy pathogenesis, highlight the signals and cells that are important for myogenic and fibrogenic actions, and discuss how fibrosis alters the ECM of skeletal muscle, and the consequences of fibrosis in developing therapies.
Keywords: Muscle regeneration, Satellite cells, Transforming growth factor beta, Mechanosensing
Fibrosis in muscular dystrophies
Muscular dystrophies share a common pathology of progressive fibrosis and a replacement of functional muscle with scar tissue. There are nine major categories of muscular dystrophy; Duchenne muscular dystrophy (DMD) is one of the most well-known and common forms occurring in approximately 1 in 5000 live male births [1], caused by mutations in the x-linked dystrophin gene [2]. The prevalence of the more than 30 muscular dystrophies as a whole is much higher, approximately 1 in 1000 births [3]. The wide array of gene mutations now known to cause muscular dystrophy lead to a spectrum of severity, age of onset, and specification of affected muscle groups [4]. A predominant site of mutations involves proteins that provide a link from the muscle cell to the extracellular matrix (ECM). Skeletal muscle has a unique and prominent link to the ECM through the dystrophin glycoprotein complex (DGC) that maintains integrity during high contractile forces of muscle fibers [5]. Dystrophin is central to the DGC in that without it, the entire complex is unstable and disappears from the membrane. However, mutations of other DGC proteins also lead to muscular dystrophy, including those in the sarcoglycan subcomplex causing Limb Girdle Muscular Dystrophy (LGMD), and dystroglycan causing Congenital muscular dystrophy (CMD) [4].
Not only is the DGC itself important in this mechanical link, but additional intracellular and extracellular proteins are also necessary. The membrane localization of the DCG provides a mechanical linkage via the cytoskeleton all the way to nuclear structural elements, including lamins, of which mutations are also known to cause muscular dystrophy [6]. Extending into the ECM, dystroglycan directly interacts with laminins in the basal lamina, where mutations in LAMA2 result in loss of functional laminin 2 and a form of CMD [7]. The basal lamina is connected to more load bearing fibrous ECM, of which collagen is the primary component. Mutations that eliminate collagen VI, which connects the basal lamina to the fibrillar matrix, result in Ulrich CMD, while patients with mutations that retain some functionality of collagen VI develop the more mild Bethlem myopathy [8].
The recognition of the need for robust muscle-ECM association was clear early in the hunt for the cause of DMD. In fact, one of the gene candidates was collagen [9], indicating the pathological fibrotic hallmarks of the disease. However, fibrosis, per se, is considered a consequence, rather than a primary cause of neuromuscular disease. When the mechanical link to the ECM is broken, as in many muscular dystrophies, there can be increased muscle fiber fragility leading to rupture of the plasma membrane, or sarcolemma, during contraction. Muscle can reseal small sarcolemmal tears within seconds, and the importance of this acute repair is highlighted by the fact that mutations in key components of resealing machinery, including dysferlin and annexins, cause LGMD and modify the severity of dystrophy, respectively [10]. More substantial injury triggers a degeneration/regeneration response that repairs and/or forms new muscle fibers. As in other tissues, damaged muscle elicits an inflammatory response with infiltration of immune cells that assist in orchestrating the subsequent regeneration. The muscle resident stem cell population, called satellite cells, is largely responsible for the replacement of damaged muscle fibers as they proliferate, differentiate, migrate to the site of injury, and then fuse with existing fibers or themselves to create new myonuclei [11]. In healthy muscle the regenerative process is quite robust and after injury the muscle can grow back even stronger, as is the case following the micro-damage of resistance training [12]. However, the increased fragility of dystrophic muscle can result in a chronic state of damage and the well-orchestrated regeneration begins to fray. The process of fibrosis is progressive in these states: successive rounds of degeneration and regeneration incompletely remodel the ECM and consequently, matrix components accumulate. Thus, a vicious feed-forward cycle ensues, where the increase of ECM replaces functional muscle, and as such there is less muscle to generate force, leading to more damage, and so on, ultimately resulting in a “muscle” that is predominantly fibrosis and scar rather than contractile tissue.
Several therapies directed at cures for the primary mutations in muscular dystrophy are under evaluation, clinical trials, and have reached FDA approval [13–16]. Even so, none, to date provide complete reversal of disease. Further, depending upon the age of the patient and the stage of disease, these potential treatments will not remove the existent secondary fibrotic pathology that has accumulated. Thus, understanding the basis for fibrosis, and finding ways to effectively prevent its progression and potentially reverse its course, remain important therapeutic targets.
Fibrogenic signals in muscular dystrophy
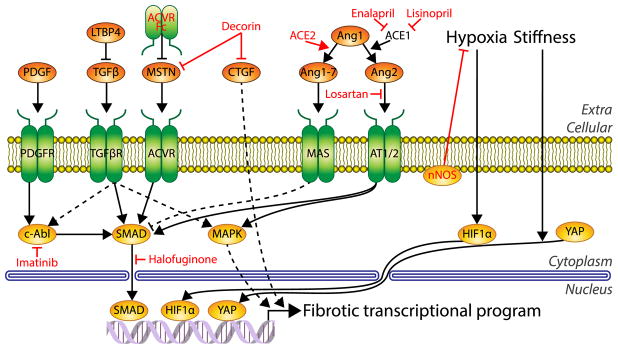
There are many signaling pathways that modulate fibrotic progression in skeletal muscle (Fig. 1). The primary pro-fibrotic signal in skeletal muscle is transforming growth factor-beta (TGFβ) [17], similar to other tissues. High expression of TGFβ is evident in dystrophic muscle [18], and it is considered a major therapeutic target. TGFβ is stored in the ECM as a complex with latent TGFβ-binding proteins (LTBP)s, and activation requires its release from the complex by proteases. Once TGFβ is released, it can act through its canonical pathway of binding to the TGFβ receptor to mediate SMAD signaling, which upon nuclear entry leads to activation of a pro-fibrotic transcriptional program that includes the production of collagen I [19]. TGFβ has also been shown to activate non-canonical MAPK pathways and c-abl that, in turn, enhance transcription of pro-fibrotic signals, including TGFβ itself, as part of the positive feedback in fibrosis. Hence, by stopping TGFβ, one could limit a critical cog in the fibrotic cascade. Halofuginone can block TGFβ-mediated SMAD activation, and results in reduced collagen 1 production. As such, it is efficacious in slowing fibrosis associated with radiation treatments in cancer, and in scleroderma [20]. Therapeutic potential as an anti-fibrotic for muscular dystrophy was demonstrated in mouse models for DMD and CMD, where there was marked functional improvement [21,22]. A clinical trial for DMD using a delayed release halofuginone (HT-100) was halted due to safety issues, but permission has been granted to resume Phase 1b/2a trials, potentially laying the groundwork for establishing this as a viable anti-fibrotic therapy for neuromuscular disease.
Fig. 1.
Common fibrotic signaling pathways in skeletal muscle. Diagram of key signaling molecules and mechanisms involved in supporting the fibrotic transcriptional program. Nodes with red text indicate molecules with anti-fibrotic potential under investigation. Nodes shaded in orange depict extracellular molecules, green depict transmembrane molecules, and yellow indicate intracellular molecules. Dashed lines indicate indirect effects. PDGF, Platelet derived growth factor; PDGFR, PDGF receptor; TGFβ, Transforming growth factor beta; LTBP4, latent TGFβ bonding protein 4; TGFβR, TGFβ receptor; MSTN, myostatin; ACVR, activin type 2 receptor; CTGF, connective tissue growth factor; Ang1–7, angiotensin 1–7; Ang1, angiotensin 1; Ang2, angiotensin 2; AT1/2, Angiotensin II receptor type I and II (AT1/2); ACE2, angiotensin converting enzyme 2; ACE1, angiotensin converting enzyme 1. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Another modulator of TGFβ activity occurs in the initial latent complex. LTBP4 contains polymorphisms that predispose the protein to cleavage, leading to enhanced TGFβ activity [23]. Initially identified in dystrophic mice that exhibited more fibrosis, a similar relationship has been demonstrated in boys with DMD [24]. Thus, blocking the cleavage of LTBP4 is a potential therapeutic target for preventing excessive release of active TGFβ [25].
Among the profibrotic signals downstream of TGFβ in skeletal muscle is the renin-angiotensin system (RAS). Angiotensin I is converted into Angiotensin II by ACE1, which then binds to its receptor AT1 or AT2, which can again activate SMAD or MAPK pathways to further the pro-fibrotic response [26]. An anti-fibrotic axis of RAS also exists, the ACE2/Angiotensin-(1–7)/Mas pathway, and it represents an untapped potential for muscular dystrophy therapy. ACE2 catalyzes the conversion of Angiotensin II to Angiotensin-(1–7), thereby reducing the activation of the AT1 receptor by Angiotensin II. In contrast to the AT1 receptor, which drives fibrosis, Angiotensin-(1–7) activation of the Mas receptor has been attributed with many beneficial actions, including reduction of oxidative stress and fibrosis as well as stabilization of glucose transport [27]. Therefore, boosting the activity of the beneficial arm of RAS may provide protection against fibrosis in DMD. Indeed, reports of the prospects of increasing Angiotensin-(1–7) activity in the mdx mouse are quite promising for reducing fibrosis [28,29]. ACE1 is highly expressed in muscular dystrophy with corresponding fibrosis, so the dual role of the angiotensin system makes it an attractive therapeutic target for anti-fibrotics. This includes the use of Losartan to block activation of AT1/AT2 [30] and the use of Enalapril and Lisinopril to inhibit ACE1 [31,32] and the profibrotic pathway. Of note is that some of the perceived benefit to skeletal muscle in DMD may be secondary to the effects on cardiac function [33,34]. Thus, while the RAS system contributes to fibrosis, it is likely that the profibrotic actions are eclipsed by TGFβ.
The fibrotic transcriptional program produces not only structural components of the ECM such as collagen 1, but also many growth factors that coordinate and amplify the actions of TGFβ. Connective tissue growth factor (CTGF/CCN2) is another regulator of fibrotic signaling. CTGF is up-regulated in dystrophic models and patients [35] and induces expression of ECM components in fibroblasts more prominently than TGFβ [36]. Interestingly, adenoviral overexpression of CTGF alone is sufficient to induce strong fibrosis in skeletal muscle [37]. Alternatively, neutralizing CTGF by an antibody has been shown to slow the progression of fibrosis in mdx muscles and preserve their function [38], with antibody therapies toward muscle CTGF progressing in clinical trials for DMD [39]. Enalapril has also been shown to reduce the expression of CTGF, suggesting that its expression is dually regulated by RAS and TGFβ [29]. Decorin has been shown to bind to and inhibit CTGF in addition to TGFβ, potentially enhancing decorin's efficacy as an anti-fibrotic for muscular dystrophy [40]. The platelet derived growth factor (PDGF) family is also induced by TGFβ and is common in many fibrotic conditions including upregulation in patients with DMD [41]. PDGFs are non-canonical activators of c-abl activity similar to TGFβ [42]. Blocking c-abl with imatinib reduced fibrosis the mdx mouse model [43], although not without concerning side effects of weight loss [44].
While the TGFβ axis is common to fibrosis in many tissues, fibrotic signaling pathways specific to skeletal muscle also exist. Myostatin is a member of the TGFβ superfamily and notable as a dramatic negative regulator of muscle mass [45]. As opposed to TGFβ, myostatin primarily signals through the activin type 2 receptors (ActRIIB), but in both cases the signals converge onto SMAD signaling to elicit their effects [46]. Given the overlap in these pathways, myostatin can promote fibrosis in skeletal muscle; injection of myostatin coated beads into mouse muscle inducing a dramatic fibrotic response [47]. Myostatin inhibition is a prime therapeutic strategy to counter muscle weakness and build muscle mass in a number of conditions, including DMD and sarcopenia [48,49], and it has the additional potential benefit of countering fibrosis. Strategies to counter myostatin activity include expression of decoy receptors (soluble ActRIIB) and expression of the myostatin propeptide, both of which act through binding of circulating ligands, thereby preventing them from activation of the ActIIB receptors on muscle [50,51]. The decoy receptors are more potent at driving muscle growth, but also bind to additional members of the activin family [52]. This has led to off-target effects in clinical trials, raising safety concerns for this strategy [53]. On the other hand, the myostatin propeptide, which associates with myostatin and its highly related GDF-11, inhibits activity with reduced off-target effects [50].
Hypoxia is a well-known inducer of the fibrotic program primarily through HIF1α, which under hypoxic conditions escapes degradation and acts as a potent transcription factor for fibrotic genes [54]. While hypoxia following injury is considered an important fibrotic mechanism in multiple tissues, muscle is a major site of oxygen utilization, particularly during exercise [55]. Muscle from patients with DMD may be particularly susceptible to hypoxia since the sarcolemmal localization of neuronal nitric oxide synthase (nNOS) is dependent on the DGC [56]. nNOS plays a critical role in preventing vasoconstriction during exercise, and in its absence patients with DMD can experience local regions of ischemia during muscle activity. Ischemia is known to contribute to skeletal muscle fibrosis [57], and thus may be a factor in the profibrotic pathways induced in muscular dystrophy. Tellingly, overexpression of nNOS in a severe mouse model of muscular dystrophy demonstrated a dramatic prevention of fibrosis along with improved lifespan [58]. It is important to consider the localization however, as expression of nNOS modified for membrane targeting in the absence of the DCG provided a distinct advantage over untargeted nNOS in improving function of mdx mouse muscles [59].
Aside from soluble molecular signaling, stiffness has been shown to be a potent fibrogenic signal in multiple tissues [60,61]. The process of matrix mechanosensing is increasingly being appreciated as a regulator of multiple cellular functions, and skeletal muscle is a known mechanosensitive tissue [62], given the mechanical nature of its primary function. While mechanosensitivity of skeletal muscle has primarily been related to sensitivity of ion channels in which the DCG plays an important role, the DCG also is a factor in MAPK pathway in muscle [63]. Extensive pathways of mechanotransduction in skeletal muscle are not well understood, however, the well-known mechanosensitive transcription factor yes-associated protein (YAP), which translocates to the nucleus in response to stiff substrates, is misregulated in muscular dystrophy [64]. Prominent target genes of YAP include CTGF and other components of the fibrogenic program [64], such that constitutively active YAP leads to expression of the fibrotic program in healthy muscle and results in fibrosis with a transcriptional profile similar to muscle of mdx mice [65]. As with the feed-forward progression of fibrotic replacement of diseased muscle, the mechanosensitive pathways may also be altered in dystrophic muscle, both within the muscle fibers due to signaling protein mislocalization, and in the ECM, where fibrosis can affect its physical properties. Ultimately, matrix mechanosensing combined with the soluble factors can impact the variety of cells in the muscle milieu in distinct ways to support the fibrogenic program.
Fibrogenic cells in muscular dystrophy
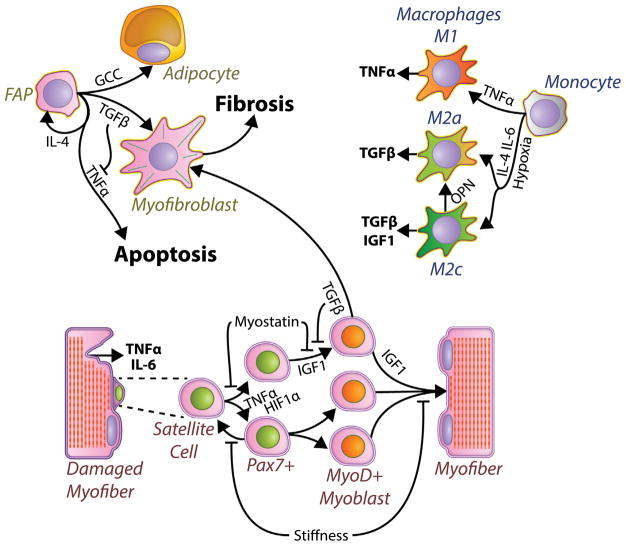
Muscle tissue provides a niche for a variety of cells in addition to the contractile muscle fibers, which respond to secreted factors and their ECM to help resolve damage (Fig. 2). The satellite cells are a Pax7+ population that resides adjacent to muscle fibers and upon injury, proliferates, differentiates, migrates, and fuses to form new myofibers, and thus are the basis for muscle regeneration [66]. This process is affected by factors that generally either promote the differentiation of myoblasts (potentially depleting the muscle progenitor pool) or block differentiation to enhance proliferation (potentially limiting muscle regeneration). In muscular dystrophy the fragility of fibers and chronic damage lead to persistent regeneration with myoblasts in all states of activation and differentiation among a myriad of inflammatory and fibrotic signals that may tilt the proper balance of proliferation vs. differentiation. Damaged muscle is known to secrete many inflammatory factors including TNFα and IL-6 [67], and TNFα is a signal supporting satellite cell activation and proliferation while inhibiting differentiation [68]. In terms of fibrotic factors, TGFβ has also demonstrated an ability to block myoblast differentiation while enabling proliferation [69]. Critically however, chronic elevation of TGFβ can also act on myoblasts to induce apoptosis [70] or to even transdifferentiate into myofibroblasts that further exacerbate fibrosis [71]. Furthermore, TGFβ can cause myoblasts to increase expression of myostatin which may interact with TGFβ to block differentiation [72]. Further, CTGF pro-fibrotic actions are compounded by CTGF mediated inhibition of muscle differentiation [73], thereby tipping the balance even further toward fibrosis. The role of hypoxia on satellite cell differentiation has been more controversial; with reports of HIF1α accumulation under hypoxic conditions blocking myoblast differentiation [74] in contrast to reports that HIF1α knockdown inhibiting myoblast differentiation in normoxia [75]. Recently, a satellite cell specific deletion of HIF1α and HIF2α showed an inhibition of self-renewal and promotion of differentiation, implicating hypoxia for driving proliferation at the expense of differentiation [76]. On the other hand, the increased stiffness of muscle in fibrosis can have opposing effects. Primary satellite cells grown on rigid tissue culture plastic are known to quickly lose their stemness, whereas growing satellite cells on soft substrates suppresses differentiation and allows them to maintain a more quiescent phenotype and even repopulate the stem cell niche when reinjected into muscle [77]. Myoblasts also possess a fine-tuned mechanosensitive differentiation, with substrates much softer or stiffer than healthy muscle reducing their ability to differentiate and form contractile sarcomeres [78]. Chronic exposure to these fibrotic signals disrupts the proper satellite cell based regeneration in muscular dystrophy, but they also disrupt the proper organization of the ECM to further propagate fibrosis in muscular dystrophy.
Fig. 2.
Diagram of critical cell types in the fibrotic response. Damaged muscle fibers express inflammatory cytokines that activate satellite cells and trigger monocytes into macrophages. Macrophages secrete a number of molecules that regulate muscle repair, fibrosis (Fig. 1) and fibro/adipogenic progenitors (FAP) state. Mis-regulated FAPs develop into myofibroblasts that produce fibrosis. Bold text includes factors secreted/released by cells and large bold font indicates processes of fibrosis and apoptosis. Italic text indicates cell states.
Satellite cells act in a dynamic environment with many other cell types that mediate removal of cellular debris and ECM remodeling so that muscle returns to a healthy functional state. The primary cellular source of fibrotic components in thought to be the myofibroblast [79], however the lineage of skeletal muscle myofibroblasts is not completely understood. Recent results show that in both healthy and fibrotic muscle, fibroblasts and muscle progenitors express collagen I, but that the predominant collagen I producing cell is the fibro/adipogenic progenitors (FAP) [80]. FAPs are a muscle resident population of mesenchymal stem cell-like cells shown to have the potential to differentiate into either a myofibroblast or an adipocyte depending on their environment [81]. In healthy muscle, FAPs respond to IL-4 to proliferate following injury in support of the regenerative program before undergoing coordinated apoptosis to return to basal levels, but importantly do not themselves undergo myogenic differentiation [81,82]. FAPs also respond to signals from muscle progenitors, for when muscle progenitors are removed, FAPs fail to expand appropriate following muscle injury [83]. Further, when FAPs are injected into damaged muscle that lacks satellite cells, FAPs are tightly associated with fibrotic regions, further implicating their role as fibrogenic cells [84]. Importantly, the orchestrated support for myogenesis by FAPs is disrupted in muscular dystrophies, as the FAP population does not undergo apoptosis, and instead is chronically elevated in dystrophies and other models of chronic injury [85], with progressive differentiation into myofibroblasts [86]. Recent studies have shown that the inflammatory factor TNFα plays a critical role in driving apoptosis of FAPs following their rapid proliferation phase, but that this process is blocked in dystrophic muscle by the simultaneous high expression of TGFβ [86]. This illustrates the importance of timing in regeneration and its disruption in fibrosis, where instead of having distinct phases of high TNFα followed by high TGFβ there is high expression of both in chronic injury. Doses of nilotinib, a kinase inhibitor with anti-TGFβ activity, was able to permit TNFα directed FAP clearance and result in decreased fibrosis in the mdx mouse model [86]. Similarly, to TGFβ, myostatin was also shown to promote expansion of FAPs and their conversion into myofibroblasts in muscle fibrosis secondary to chronic kidney disease [87]. While rare in mouse models of muscular dystrophy, muscles from dystrophic patients commonly involve both fibrosis and fatty infiltration [88]. The source of this fatty infiltration is also likely from FAPs, and unfortunately the most commonly used treatment for muscular dystrophies, glucocorticoids, can also enhance the differentiation of FAPs into adipocytes [89].
Inflammatory cells play a clear role in directing the response of FAPs to muscle injury, as well as coordinating the regenerative response of satellite cells. T cells, eosinophils, and macrophages infiltrate the muscle of dystrophic patients [90]. While immune-compromised mouse models of muscular dystrophy lacking T cells and B cells result in reduced TGFβ and fibrosis, implying a role for the adaptive immune system [91], the absence of a functioning immune system limits the ability of muscle to regenerate [92]. Macrophages of the innate immune system have been the most well studied in dystrophic muscle, as macrophages have the ability to both promote (so-called M1 macrophages) and suppress the immune system (M2 macrophages), however the spectrum of macrophage phenotype in vivo is complex [93]. M1, pro-inflammatory, macrophages are stimulated by IFNγ and TNFα and are present during the early phases of regeneration employing their phagocytic functions to clear damage muscle [94]. M1 macrophages also secrete pro-inflammatory cytokines including TNFα, which play a critical role in the clearance of FAPs following their robust expansion [86]. However, their persistent presence in dystrophic muscle can also promote chronic inflammation and thus contribute to fibrosis. This is demonstrated by M1 macrophage genetic depletion improving fibrosis and muscle histopathology of mdx mice [95]. Alternatively, M2 macrophages are induced by IL-4 and IL-13, and while being considered “pro-regenerative” due to their critical role in wound healing are also considered to be “pro-fibrotic” as the major source of TGFβ in skeletal muscle [96]. The rise of M2 macrophages typically follows M1 macrophages after acute muscle injury and regeneration [97]; however, M2 macrophages are also chronically elevated in dystrophic muscle [98]. A role for myostatin in macrophage polarization is unclear, however myostatin has been shown to increase macrophage infiltration in damaged muscle [99]. Hypoxia can polarize macrophages toward the M2 pro-fibrotic phenotype [100]. Accordingly, transgenic expression of nNOS reduces the M2 population within dystrophic muscle and improves fibrosis [101]. The role that the stiff ECM of fibrosis plays in polarizing macrophages is gaining increased attention with early studies pointing toward shifting cells toward a more M1 phenotype [102,103], yet how this impacts fibrosis in muscular dystrophy is yet to be explored. Osteopontin is another a highly expressed immunomodulator in dystrophic muscle involved in macrophage polarization [104,105]. Interestingly, ablation of osteopontin shifts the phenotype within M2 macrophages, not to reduce TGFβ expression, but to enhance IGF1 expression [106], which is capable of counteracting SMAD signaling of TGFβ as well as being a potent inducer of muscle growth [107,108]. Ultimately, the regenerative and fibrotic pathways in skeletal muscle are governed by multiple cell types responding to cues from damaged muscle, and the balance between these actions results in resolution of injury, or dissolution of muscle.
Fibrosis and cell therapy in muscular dystrophy
Cumulative fibrosis not only prevents the endogenous myogenic cell population to appropriately regenerate, but also impedes the successful delivery of cell-based therapies to damaged/diseased tissue [109]. There is a physical barrier that blocks efficient migration of cells to muscle, and the fibrotic environment can inhibit myogenic differentiation of exogenous cells just it does for the endogenous cell population. Previous studies have shown that engraftment of donor satellite cells are dependent on the satellite cell niche, with improved engraftment when that niche is preserved [110]. Thus, a critical prerequisite for successful cell-based therapies is the dissolution of fibrosis to enable successful delivery and engraftment of myogenic cells. Reducing fibrosis through neutralizing CTGF also led to more efficient transplantation of satellite cells into mdx mice subsequent dystrophin positive fibers [38]. MMP-1 has also been used as an anti-fibrotic due to its collagenase activity [111] and transplantation of myoblasts into mdx mice was dramatically improved following MMP-1 treatment leading to enhanced dystrophin expression in vivo [112]. On the other hand, inhibiting MMP-9 was shown to also increase engraftment of myoblasts into the mdx mouse, however the MMP-9 inhibition also reduced fibrosis, likely through modulating inflammation [113]. Other studies have also shown that anti-inflammatory treatment improves the efficacy of cell therapy in mdx mice through inhibition of fibrosis [114]. Furthermore, the antifibrotic drug losartan was able to enhance the number of human dystrophin positive fibers after transplantation of human myoblasts into immunocompromised mdx mice [115].
A more sophisticated strategy is to equip the cells to be transferred with the ability to produce anti-fibrotic agents. This approach was taken using placenta growth factor (PlGF) and MMP-9 overexpression in tendon fibroblasts delivered into dystrophic muscles, which provided a reduction in fibrosis of α-sarcoglycan null mice [116]. This pretreatment made the subsequent systemic injection of myogenic capable mesoangioblasts able to engraft, leading to α-sarcoglycan positive muscle fibers. In a more concise manner, myoblasts themselves were transfected with an MMP-1 expression vector which permitted more robust engraftment and expression of dystrophin in an immunocompromised mdx mouse [117]. Together these studies emphasize the reality that fibrosis presents a barrier to donor cell delivery and engraftment into diseased muscle that in certain instances can be overcome by providing anti-fibrotic treatment in conjunction with cell therapy.
Fibrotic extracellular matrix in muscular dystrophy
The multitude of cells present in muscle is also responsible for synthesizing and organizing the ECM matrix, a process gone awry in fibrotic diseases. Fibrosis is commonly defined as a pathologic increase in ECM, however the properties that make it pathologic are not well understood. Fibrosis is commonly quantified by collagen content either biochemically or histologically [118,119]. The most abundant type of collagen in muscle is fibrillar type I, and indeed in the whole body [120]. Patients with DMD have dramatic increases in collagen content [88]. The fibrillar collagens are the primary mechanical load bearing structures in ECM and over a log scale, collagen I expression tracks with tissue stiffness [120]. The increase in stiffness of fibrotic muscle is often attributed to the increased collagen content, however stiffness and collagen content are not well correlated [121]. While elastic stiffness is the common mechanical parameter measured in matrix mechanosensing, other viscoelastic properties can also impact mechanosensing [122] and are altered in fibrosis in mouse models of muscular dystrophy [121,123]. The congruence of these mechanical factors can itself limit muscle function directly and develop into debilitating joint contractures [124].
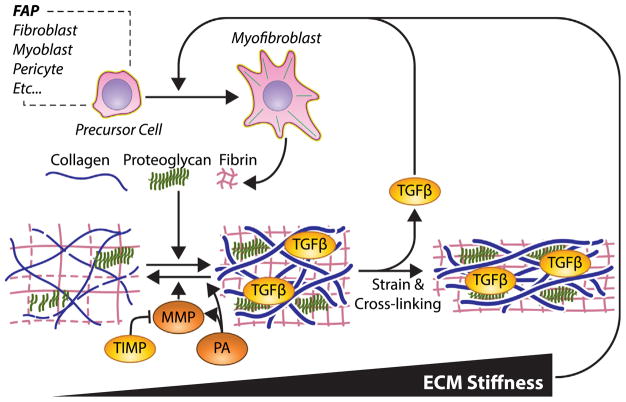
The architecture of collagen plays an important role in determining its mechanical properties as well (Fig. 3). The extent of collagen cross-linking is known to scale with stiffness [125], and dystrophic muscle from mice, dogs, and humans have demonstrated increased collagen cross-linking [126]. More heavily cross-linked collagen fibrils and fibers lead to more densely packed collagen networks, which have been shown to scale with stiffness more readily than collagen content alone in mdx mouse muscles [121]. However, the correlation reported was small and studies of other fibrotic models that show an insignificant relationship between stiffness and cross-linking [127]. This may implicate larger scale architectural changes, such as those in the liver, where formation of fibrotic septa connecting portal regions is a key feature of disease progression and tissue stiffness [128]. Recent reports of longitudinal collagen cables that are increased in fibrotic muscle may be a key architectural feature [129], but continued efforts are needed to decipher the relationship between the fibrotic ECM and its mechanical properties.
Fig. 3.
Properties of the fibrotic matrix in skeletal muscle. Myofibroblasts are the primary ECM secreting cells, which includes the secretion of collagens, proteoglycans, and small mesh forming proteins like lamin, fibronectin, and fibrin. A variety of precursor cells can be transformed into myofibroblasts, but the major sources are FAPs. Matrix degradation is controlled by MMPs, which are inhibited by TIMPs, and activated by Plasminogen activators (PA). PAs support matrix degradation directly and indirectly. Increased strain in the ECM releases TGFβ, stiffens the matrix, and blocks protease susceptibility. Enhanced collagen cross-linking also contributes to the stability of the ECM. Matrix stiffness likely supports the transformation of precursor cells into myofibroblasts to strengthen the fibrotic feedback loop.
A variety of other ECM matrix components aside from collagen may influence the matrix stiffness as well as mediating fibrotic signaling. Muscle ECM is rich in proteoglycans that hold water and contribute to the viscous mechanical properties of the ECM [130]. Patients with DMD have an increased level of biglycan and decorin proteoglycans [131]. Decorin is particularly pertinent as it is considered an anti-fibrotic agent through blocking TGFβ activity, and decorin overexpression has been shown to block TGFβ signaling and fibrosis in cardiac muscle [132] and skeletal muscle regeneration following laceration [133]. Proteoglycans interact with a variety of other matrix components and growth factors as well as playing an important role in collagen fibrillogenesis and untangling their contribution to fibrosis in muscular dystrophy requires further study [134]. Fibronectin is a serum soluble protein that also forms provisional fibrous networks for cell adhesion in wound healing [135]. Fibronectin is dramatically overexpressed in muscular dystrophy, and has even been suggested as a serum biomarker for progression of DMD [136]. However, targeting fibronectin expression for therapy may not be advisable as it supports satellite cell expansion [137], and loss of fibronectin with age is associated with the loss of satellite cell function [138]. Fibrinogen is cleaved into fibrin to form a fibrous network in wound healing as well [139]. Along with other ECM components fibrinogen is up-regulated in fibrotic dystrophic muscles and is purported to induce TGFβ expression by muscle macrophages to further stimulate the fibrotic program [140]. All of these ECM components interact and when properly regulated support muscle regeneration mechanically, by growth factor regulation, and/or direct adhesion to cells; however, in fibrosis this support of regeneration is compromised.
The accumulation of ECM components in fibrosis can result not only from increased expression of matrix components, but also from the decreased degradation of the ECM. The primary enzymes responsible for matrix degradation are the matrix metalloproteinases (MMP)s, which includes collagenases, gelatinases, stromelysins, and membrane-type metalloproteinases [141]. MMPs are secreted by resident muscle cells and inflammatory cells in order to remodel the ECM and allow cells to efficiently migrate through the ECM [142]. While there is an accumulation of ECM in fibrosis, there is also a general increase in MMPs in DMD [142]. Whether MMPs are supportive or deleterious to muscle regeneration may depend on the type of MMP. MMPs are expressed by myoblasts and broad inhibition of MMPs blocks myoblast migration and differentiation [143]. Specific inhibitors have shown that individual MMPs play important roles in myoblast migration with collagenase MMP-13 in vitro [144], collagenase MMP-1 in vivo [117], and membrane-type MMP14 through collagen matrices [145]. The use of genetic tools to ablate specific MMPs in dystrophic mouse models reveals the exacerbation of the disease phenotype and fibrosis for gelatinase MMP-2 [146] and stromelysin MMP-10 [147]. However, global deletion of gelatinase MMP-9 was found to improve fibrosis [141], which may be due to MMP-9 being expressed primarily by infiltrating immune cells and promoting chronic inflammation in dystrophy. Defining the precise mechanisms by which these MMPs elicit their effect on fibrosis is challenging due to the high degree of overlap in substrate and multiple layers of regulation. MMPs are secreted in a pro-form that is activated upon cleavage, but the active form is blocked by another class of matrix proteins, the tissue inhibitors of metalloproteinases (TIMP)s. TIMPs are commonly cited fibrotic factors that favor ECM accumulation over degradation and are up-regulated in muscles and plasma from patients with DMD [148,149]. Importantly TIMPs expression is downstream of TGFβ signaling and a critical component of the fibrotic program [150]. Activation of MMPs can occur through multiple enzymes including other MMPs and the plasminogen activating system [151]. The canonical role of plasminogen activators is to convert plasminogen into plasmin, which then degrades the fibrin matrix, and when the urokinase plasminogen activator is ablated, mdx muscle fibrosis is exacerbated [152]. Conversely, genetic ablation of plasminogen activator inhibitor 1 increases plasminogen activator and fibrin degradation, but also leads to increased fibrosis in mdx muscle, potentially by increasing TGFβ bioavailability [153]. The action of MMPs and matrix degrading enzymes is dependent on access to cleavage sites. When the ECM and collagen fibrils are under strain, access to the cleavage sites is blocked [154] and degradation is impaired [155]. This confers a built in “use it or lose it” mechanism for collagen, with the high strains of muscle and stiffness in fibrosis blocking the ability to degrade collagen. These ECM degrading enzymes are thus able to degrade and remodel the matrix, but the disorganization of ECM in muscular dystrophy impairs enzymatic degradation.
In this review we assert that fibrosis is a secondary consequence of muscular dystrophy, and that inhibition or reversal of fibrosis is a central therapeutic target. However, we would be remiss in considering that scar tissue is part of the wound healing process and provides an efficient way to restore mechanical integrity of damaged muscle. Looking at muscular dystrophies that arise directly from mutations in ECM proteins highlights the importance of a healthy ECM in preventing muscle damage. Mutations in laminin (LAMA2), collagen VI (COL6), and collagen XII (Col12A1) [156] demonstrate that disruption of the muscle-ECM linkage eliminates or reduces mechanical signaling, even though the muscle fibers initially remain intact. Hence, some stiffness of ECM is critical to mediate appropriate load to muscle fibers. On the other hand, the reduced stiffness of ECM causing joint hyperlaxity also reduces stiffness within the muscle. Muscles from a mouse model lacking Col12A1 revealed a correlation between passive stiffness and susceptibility to contractile damage [156], where reduced stiffness led to less muscle damage. This emphasizes that for fibrotic muscle, some preservation of connective tissue will be important to enable the muscle to withstand potentially damaging contractions. While there is evidence that fibrosis may be reversible [48], the structure that remains following its removal may determine whether or not anti-fibrotic therapies are functionally beneficial. For instance, if an anti-fibrotic therapy removes the abundant ECM except the extensively cross-linked ECM, then ECM stiffness would largely persist to maintain heightened susceptibility of contractile damage.
In conclusion, the pathways mediating fibrosis in muscular dystrophy share many features with those in other tissues, and as therapeutic candidates arise for fibrotic disease, they may also provide benefit to skeletal muscle.
Acknowledgments
The authors were supported by the US National Institutes of Health through NIAMS under K99AR067867 (LRS), R01AR057363 (ERB), and U54 AR052646 (ERB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other granting agencies.
References
- 1.Romitti PA, Zhu Y, Puzhankara S, James KA, Nabukera SK, Zamba GK, Ciafaloni E, Cunniff C, Druschel CM, Mathews KD, Matthews DJ, Meaney FJ, Andrews JG, Conway KM, Fox DJ, Street N, Adams MM, Bolen J M. STARnet. Prevalence of Duchenne and Becker muscular dystrophies in the United States. Pediatrics. 2015;135:513–521. doi: 10.1542/peds.2014-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffman EP, Brown RH, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 3.Theadom A, Rodrigues M, Roxburgh R, Balalla S, Higgins C, Bhattacharjee R, Jones K, Krishnamurthi R, Feigin V. Prevalence of muscular dystrophies: a systematic literature review. Neuroepidemiology. 2014;43:259–268. doi: 10.1159/000369343. [DOI] [PubMed] [Google Scholar]
- 4.Mercuri E, Muntoni F. Muscular dystrophies. Lancet. 2013;381:845–860. doi: 10.1016/S0140-6736(12)61897-2. [DOI] [PubMed] [Google Scholar]
- 5.Ervasti JM, Campbell KP. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991;66:1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- 6.Maggi L, Carboni N, Bernasconi P. Skeletal muscle laminopathies: a review of clinical and molecular features. Cell. 2016;5 doi: 10.3390/cells5030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyagoe-Suzuki Y, Nakagawa M, Takeda S. Merosin and congenital muscular dystrophy. Microsc Res Tech. 2000;48:181–191. doi: 10.1002/(SICI)1097-0029(20000201/15)48:3/4<181::AID-JEMT6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 8.Bonnemann CG. The collagen VI-related myopathies: muscle meets its matrix. Nat Rev Neurol. 2011;7:379–390. doi: 10.1038/nrneurol.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duance VC, Stephens HR, Dunn M, Bailey AJ, Dubowitz V. A role for collagen in the pathogenesis of muscular dystrophy? Nature. 1980;284:470–472. doi: 10.1038/284470a0. [DOI] [PubMed] [Google Scholar]
- 10.Demonbreun AR, Allen MV, Warner JL, Barefield DY, Krishnan S, Swanson KE, Earley JU, McNally EM. Enhanced muscular dystrophy from loss of dysferlin is accompanied by impaired annexin A6 translocation after sarcolemmal disruption. Am J Pathol. 2016;186:1610–1622. doi: 10.1016/j.ajpath.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dumont NA, Bentzinger CF, Sincennes MC, Rudnicki MA. Satellite cells and skeletal muscle regeneration. Compr Physiol. 2015;5:1027–1059. doi: 10.1002/cphy.c140068. [DOI] [PubMed] [Google Scholar]
- 12.Snijders T, Verdijk LB, van Loon LJ. The impact of sarcopenia and exercise training on skeletal muscle satellite cells. Ageing Res Rev. 2009;8:328–338. doi: 10.1016/j.arr.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Kole R, Krieg AM. Exon skipping therapy for Duchenne muscular dystrophy. Adv Drug Deliv Rev. 2015;87:104–107. doi: 10.1016/j.addr.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Bajek A, Porowinska D, Kloskowski T, Brzoska E, Ciemerych MA, Drewa T. Cell therapy in Duchenne muscular dystrophy treatment: clinical trials overview. Crit Rev Eukaryot Gene Expr. 2015;25:1–11. doi: 10.1615/critreveukaryotgeneexpr.2015011074. [DOI] [PubMed] [Google Scholar]
- 15.Al-Zaidy S, Rodino-Klapac L, Mendell JR. Gene therapy for muscular dystrophy: moving the field forward. Pediatr Neurol. 2014;51:607–618. doi: 10.1016/j.pediatrneurol.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falzarano MS, Scotton C, Passarelli C, Ferlini A. Duchenne muscular dystrophy: from diagnosis to therapy. Molecules. 2015;20:18168–18184. doi: 10.3390/molecules201018168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ceco E, McNally EM. Modifying muscular dystrophy through transforming growth factor-β. FEBS J. 2013;280:4198–4209. doi: 10.1111/febs.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernasconi P, Torchiana E, Confalonieri P, Brugnoni R, Barresi R, Mora M, Cornelio F, Morandi L, Mantegazza R. Expression of transforming growth factor-beta 1 in dystrophic patient muscles correlates with fibrosis. Pathogenetic role of a fibrogenic cytokine. J Clin Invest. 1995;96:1137–1144. doi: 10.1172/JCI118101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 20.Flanders KC. Smad3 as a mediator of the fibrotic response. Int J Exp Pathol. 2004;85:47–64. doi: 10.1111/j.0959-9673.2004.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pines M, Halevy O. Halofuginone and muscular dystrophy. Histol Histopathol. 2011;26:135–146. doi: 10.14670/HH-26.135. [DOI] [PubMed] [Google Scholar]
- 22.Nevo Y, Halevy O, Genin O, Moshe I, Turgeman T, Harel M, Biton E, Reif S, Pines M. Fibrosis inhibition and muscle histopathology improvement in laminin-alpha2-deficient mice. Muscle Nerve. 2010;42:218–229. doi: 10.1002/mus.21706. [DOI] [PubMed] [Google Scholar]
- 23.Heydemann A, Ceco E, Lim JE, Hadhazy M, Ryder P, Moran JL, Beier DR, Palmer AA, McNally EM. Latent TGF-beta-binding protein 4 modifies muscular dystrophy in mice. J Clin Invest. 2009;119:3703–3712. doi: 10.1172/JCI39845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flanigan KM, Ceco E, Lamar KM, Kaminoh Y, Dunn DM, Mendell JR, King WM, Pestronk A, Florence JM, Mathews KD, Finkel RS, Swoboda KJ, Gappmaier E, Howard MT, Day JW, McDonald C, McNally EM, Weiss RB, Project UD. LTBP4 genotype predicts age of ambulatory loss in Duchenne muscular dystrophy. Ann Neurol. 2013;73:481–488. doi: 10.1002/ana.23819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ceco E, Bogdanovich S, Gardner B, Miller T, DeJesus A, Earley JU, Hadhazy M, Smith LR, Barton ER, Molkentin JD, McNally EM. Targeting latent TGFβ release in muscular dystrophy. Sci Transl Med. 2014;6:259ra144. doi: 10.1126/scitranslmed.3010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simões e Silva AC, Silveira KD, Ferreira AJ, Teixeira MM. ACE2, angiotensin-(1-7) and Mas receptor axis in inflammation and fibrosis. Br J Pharmacol. 2013;169:477–492. doi: 10.1111/bph.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santos R, Ferreira Anderson J, Verano-Braga Thiago, Bader Michael. Angiotensin-converting enzyme 2, angiotensin-(1–7) and Mas: new players of the renin–angiotensin system. J Endocrinol. 2013;216:R1–R17. doi: 10.1530/JOE-12-0341. [DOI] [PubMed] [Google Scholar]
- 28.Acuña MJ, Pessina P, Olguin H, Cabrera D, Vio CP, Bader M, Muñoz-Canoves P, Santos RA, Cabello-Verrugio C, Brandan E. Restoration of muscle strength in dystrophic muscle by angiotensin-1-7 through inhibition of TGF-β signalling. Hum Mol Genet. 2014;23:1237–1249. doi: 10.1093/hmg/ddt514. [DOI] [PubMed] [Google Scholar]
- 29.Morales MG, Cabrera D, Céspedes C, Vio CP, Vazquez Y, Brandan E, Cabello-Verrugio C. Inhibition of the angiotensin-converting enzyme decreases skeletal muscle fibrosis in dystrophic mice by a diminution in the expression and activity of connective tissue growth factor (CTGF/CCN-2) Cell Tissue Res. 2013;353:173–187. doi: 10.1007/s00441-013-1642-6. [DOI] [PubMed] [Google Scholar]
- 30.Israili ZH. Clinical pharmacokinetics of angiotensin II (AT1) receptor blockers in hypertension. J Hum Hypertens. 2000;14(Suppl 1):S73–86. doi: 10.1038/sj.jhh.1000991. [DOI] [PubMed] [Google Scholar]
- 31.Tyralla K, Adamczak M, Benz K, Campean V, Gross ML, Hilgers KF, Ritz E, Amann K. High-dose enalapril treatment reverses myocardial fibrosis in experimental uremic cardiomyopathy. PLoS One. 2011;6:e15287. doi: 10.1371/journal.pone.0015287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation. 2000;102:1388–1393. doi: 10.1161/01.cir.102.12.1388. [DOI] [PubMed] [Google Scholar]
- 33.Spurney CF, Sali A, Guerron AD, Iantorno M, Yu Q, Gordish-Dressman H, Rayavarapu S, van der Meulen J, Hoffman EP, Nagaraju K. Losartan decreases cardiac muscle fibrosis and improves cardiac function in dystrophin-deficient mdx mice. J Cardiovasc Pharmacol Ther. 2011;16:87–95. doi: 10.1177/1074248410381757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bish LT, Yarchoan M, Sleeper MM, Gazzara JA, Morine KJ, Acosta P, Barton ER, Sweeney HL. Chronic losartan administration reduces mortality and preserves cardiac but not skeletal muscle function in dystrophic mice. PLoS One. 2011;6:e20856. doi: 10.1371/journal.pone.0020856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun G, Haginoya K, Wu Y, Chiba Y, Nakanishi T, Onuma A, Sato Y, Takigawa M, Iinuma K, Tsuchiya S. Connective tissue growth factor is overexpressed in muscles of human muscular dystrophy. J Neurol Sci. 2008;267:48–56. doi: 10.1016/j.jns.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 36.Frazier K, Williams S, Kothapalli D, Klapper H, Grotendorst GR. Stimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. J Invest Dermatol. 1996;107:404–411. doi: 10.1111/1523-1747.ep12363389. [DOI] [PubMed] [Google Scholar]
- 37.Morales MG, Cabello-Verrugio C, Santander C, Cabrera D, Goldschmeding R, Brandan E. CTGF/CCN-2 over-expression can directly induce features of skeletal muscle dystrophy. J Pathol. 2011;225:490–501. doi: 10.1002/path.2952. [DOI] [PubMed] [Google Scholar]
- 38.Morales MG, Gutierrez J, Cabello-Verrugio C, Cabrera D, Lipson KE, Goldschmeding R, Brandan E. Reducing CTGF/CCN2 slows down mdx muscle dystrophy and improves cell therapy. Hum Mol Genet. 2013;22:4938–4951. doi: 10.1093/hmg/ddt352. [DOI] [PubMed] [Google Scholar]
- 39.Trial of pamrevlumab (FG-3019), Non-Ambulatory Subjects With Duchenne Muscular Dystrophy (DMD) - Full Text View, 2018ClinicalTrials.gov.
- 40.Vial C, Gutiérrez J, Santander C, Cabrera D, Brandan E. Decorin interacts with connective tissue growth factor (CTGF)/CCN2 by LRR12 inhibiting its biological activity. J Biol Chem. 2011;286:24242–24252. doi: 10.1074/jbc.M110.189365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Y, Haginoya K, Sun G, Dai H, Onuma A, Iinuma K. Platelet-derived growth factor and its receptors are related to the progression of human muscular dystrophy: an immunohistochemical study. J Pathol. 2003;201:149–159. doi: 10.1002/path.1414. [DOI] [PubMed] [Google Scholar]
- 42.Daniels CE, Wilkes MC, Edens M, Kottom TJ, Murphy SJ, Limper AH, Leof EB. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004;114:1308–1316. doi: 10.1172/JCI19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang P, Zhao XS, Fields M, Ransohoff RM, Zhou L. Imatinib attenuates skeletal muscle dystrophy in mdx mice. FASEB J. 2009;23:2539–2548. doi: 10.1096/fj.09-129833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bizario JC, Cerri DG, Rodrigues LC, Oliveira GL, Nomizo A, de Araujo DD, Fukuhara PS, Ribeiro JC, de Castro FA, Costa MC. Imatinib mesylate ameliorates the dystrophic phenotype in exercised mdx mice. J Neuroimmunol. 2009;212:93–101. doi: 10.1016/j.jneuroim.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 45.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 46.Zhu X, Topouzis S, Liang LF, Stotish RL. Myostatin signaling through Smad2, Smad3 and Smad4 is regulated by the inhibitory Smad7 by a negative feedback mechanism. Cytokine. 2004;26:262–272. doi: 10.1016/j.cyto.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 47.Li ZB, Kollias HD, Wagner KR. Myostatin directly regulates skeletal muscle fibrosis. J Biol Chem. 2008;283:19371–19378. doi: 10.1074/jbc.M802585200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bo Li Z, Zhang J, Wagner KR. Inhibition of myostatin reverses muscle fibrosis through apoptosis. J Cell Sci. 2012;125:3957–3965. doi: 10.1242/jcs.090365. [DOI] [PubMed] [Google Scholar]
- 49.Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015;14:58–74. doi: 10.1038/nrd4467. [DOI] [PubMed] [Google Scholar]
- 50.Wolfman NM, McPherron AC, Pappano WN, Davies MV, Song K, Tomkinson KN, Wright JF, Zhao L, Sebald SM, Greenspan DS, Lee SJ. Activation of latent myostatin by the BMP-1/tolloid family of metalloproteinases. Proc Natl Acad Sci U S A. 2003;100:15842–15846. doi: 10.1073/pnas.2534946100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bogdanovich S, Perkins KJ, Krag TO, Whittemore LA, Khurana TS. Myostatin propeptide-mediated amelioration of dystrophic pathophysiology. FASEB J. 2005;19:543–549. doi: 10.1096/fj.04-2796com. [DOI] [PubMed] [Google Scholar]
- 52.Lee SJ, Reed LA, Davies MV, Girgenrath S, Goad ME, Tomkinson KN, Wright JF, Barker C, Ehrmantraut G, Holmstrom J, Trowell B, Gertz B, Jiang MS, Sebald SM, Matzuk M, Li E, Liang LF, Quattlebaum E, Stotish RL, Wolfman NM. Regulation of muscle growth by multiple ligands signaling through activin type II receptors. Proc Natl Acad Sci U S A. 2005;102:18117–18122. doi: 10.1073/pnas.0505996102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campbell C, McMillan HJ, Mah JK, Tarnopolsky M, Selby K, McClure T, Wilson DM, Sherman ML, Escolar D, Attie KM. Myostatin inhibitor ACE-031 treatment of ambulatory boys with Duchenne muscular dystrophy: results of a randomized, placebo-controlled clinical trial. Muscle Nerve. 2017;55:458–464. doi: 10.1002/mus.25268. [DOI] [PubMed] [Google Scholar]
- 54.Ruthenborg RJ, Ban JJ, Wazir A, Takeda N, Kim JW. Regulation of wound healing and fibrosis by hypoxia and hypoxia-inducible factor-1. Mol Cells. 2014;37:637–643. doi: 10.14348/molcells.2014.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mason S, Johnson RS. The role of HIF-1 in hypoxic response in the skeletal muscle. Adv Exp Med Biol. 2007;618:229–244. doi: 10.1007/978-0-387-75434-5_18. [DOI] [PubMed] [Google Scholar]
- 56.Sander M, Chavoshan B, Harris SA, Iannaccone ST, Stull JT, Thomas GD, Victor RG. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2000;97:13818–13823. doi: 10.1073/pnas.250379497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghaly A, Marsh DR. Ischaemia-reperfusion modulates inflammation and fibrosis of skeletal muscle after contusion injury. Int J Exp Pathol. 2010;91:244–255. doi: 10.1111/j.1365-2613.2010.00708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tidball JG, Wehling-Henricks M. Expression of a NOS transgene in dystrophin-deficient muscle reduces muscle membrane damage without increasing the expression of membrane-associated cytoskeletal proteins. Mol Genet Metab. 2004;82:312–320. doi: 10.1016/j.ymgme.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 59.Rebolledo DL, Kim MJ, Whitehead NP, Adams ME, Froehner SC. Sarcolemmal targeting of nNOSμ improves contractile function of mdx muscle. Hum Mol Genet. 2016;25:158–166. doi: 10.1093/hmg/ddv466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hinz B. Tissue stiffness, latent TGF-beta1 activation, and mechanical signal transduction: implications for the pathogenesis and treatment of fibrosis. Curr Rheumatol Rep. 2009;11:120–126. doi: 10.1007/s11926-009-0017-1. [DOI] [PubMed] [Google Scholar]
- 61.Wells RG. Tissue mechanics and fibrosis. Biochim Biophys Acta. 2013;1832:884–890. doi: 10.1016/j.bbadis.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith L, Cho S, Discher DE. Mechanosensing of matrix by stem cells: from matrix heterogeneity, contractility, and the nucleus in pore-migration to cardiogenesis and muscle stem cells in vivo. Semin Cell Dev Biol. 2017;71:84–98. doi: 10.1016/j.semcdb.2017.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moorwood C, Philippou A, Spinazzola J, Keyser B, Macarak EJ, Barton ER. Absence of γ-sarcoglycan alters the response of p70S6 kinase to mechanical perturbation in murine skeletal muscle. Skelet Muscle. 2014;4:13. doi: 10.1186/2044-5040-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fischer M, Rikeit P, Knaus P, Coirault C. YAP-mediated mechanotransduction in skeletal muscle. Front Physiol. 2016;7:41. doi: 10.3389/fphys.2016.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Judson RN, Gray SR, Walker C, Carroll AM, Itzstein C, Lionikas A, Zammit PS, De Bari C, Wackerhage H. Constitutive expression of Yes-associated protein (Yap) in adult skeletal muscle fibres induces muscle atrophy and myopathy. PLoS One. 2013;8:e59622. doi: 10.1371/journal.pone.0059622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ciaraldi TP, Ryan AJ, Mudaliar SR, Henry RR. Altered myokine secretion is an intrinsic property of skeletal muscle in type 2 diabetes. PLoS One. 2016;11:e0158209. doi: 10.1371/journal.pone.0158209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alter J, Rozentzweig D, Bengal E. Inhibition of myoblast differentiation by tumor necrosis factor alpha is mediated by c-Jun N-terminal kinase 1 and leukemia inhibitory factor. J Biol Chem. 2008;283:23224–23234. doi: 10.1074/jbc.M801379200. [DOI] [PubMed] [Google Scholar]
- 69.Schabort EJ, van der Merwe M, Loos B, Moore FP, Niesler CU. TGF-beta's delay skeletal muscle progenitor cell differentiation in an isoform-independent manner. Exp Cell Res. 2009;315:373–384. doi: 10.1016/j.yexcr.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 70.Cencetti F, Bernacchioni C, Tonelli F, Roberts E, Donati C, Bruni P. TGFβ1 evokes myoblast apoptotic response via a novel signaling pathway involving S1P4 transactivation upstream of Rho-kinase-2 activation. FASEB J. 2013;27:4532–4546. doi: 10.1096/fj.13-228528. [DOI] [PubMed] [Google Scholar]
- 71.Cencetti F, Bernacchioni C, Nincheri P, Donati C, Bruni P. Transforming growth factor-beta1 induces transdifferentiation of myoblasts into myofibroblasts via up-regulation of sphingosine kinase-1/S1P3 axis. Mol Biol Cell. 2010;21:1111–1124. doi: 10.1091/mbc.E09-09-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu J, Li Y, Shen W, Qiao C, Ambrosio F, Lavasani M, Nozaki M, Branca MF, Huard J. Relationships between transforming growth factor-beta1, myostatin, and decorin: implications for skeletal muscle fibrosis. J Biol Chem. 2007;282:25852–25863. doi: 10.1074/jbc.M704146200. [DOI] [PubMed] [Google Scholar]
- 73.Vial C, Zúñiga LM, Cabello-Verrugio C, Cañón P, Fadic R, Brandan E. Skeletal muscle cells express the profibrotic cytokine connective tissue growth factor (CTGF/CCN2), which induces their dedifferentiation. J Cell Physiol. 2008;215:410–421. doi: 10.1002/jcp.21324. [DOI] [PubMed] [Google Scholar]
- 74.Majmundar AJ, Lee DS, Skuli N, Mesquita RC, Kim MN, Yodh AG, Nguyen-McCarty M, Li B, Simon MC. HIF modulation of Wnt signaling regulates skeletal myogenesis in vivo. Development. 2015;142:2405–2412. doi: 10.1242/dev.123026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ono Y, Sensui H, Sakamoto Y, Nagatomi R. Knockdown of hypoxia-inducible factor-1alpha by siRNA inhibits C2C12 myoblast differentiation. J Cell Biochem. 2006;98:642–649. doi: 10.1002/jcb.20804. [DOI] [PubMed] [Google Scholar]
- 76.Yang X, Yang S, Wang C, Kuang S. The hypoxia-inducible factors HIF1α and HIF2α are dispensable for embryonic muscle development but essential for postnatal muscle regeneration. J Biol Chem. 2017;292:5981–5991. doi: 10.1074/jbc.M116.756312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 2004;166:877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Serrano AL, Mann CJ, Vidal B, Ardite E, Perdiguero E, Munoz-Canoves P. Cellular and molecular mechanisms regulating fibrosis in skeletal muscle repair and disease. Curr Top Dev Biol. 2011;96:167–201. doi: 10.1016/B978-0-12-385940-2.00007-3. [DOI] [PubMed] [Google Scholar]
- 80.Chapman MA, Mukund K, Subramaniam S, Brenner D, Lieber RL. Three distinct cell populations express extra-cellular matrix proteins and increase in number during skeletal muscle fibrosis. Am J Physiol Cell Physiol. 2017;312:C131–C143. doi: 10.1152/ajpcell.00226.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, Chawla A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376–388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, Yamaguchi M, Ogawa R, Matev MM, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Tsuchida K, Yamamoto H, Fukada S. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci. 2011;124:3654–3664. doi: 10.1242/jcs.086629. [DOI] [PubMed] [Google Scholar]
- 85.Contreras O, Rebolledo DL, Oyarzún JE, Olguín HC, Brandan E. Connective tissue cells expressing fibro/adipogenic progenitor markers increase under chronic damage: relevance in fibroblast-myofibroblast differentiation and skeletal muscle fibrosis. Cell Tissue Res. 2016;364:647–660. doi: 10.1007/s00441-015-2343-0. [DOI] [PubMed] [Google Scholar]
- 86.Lemos DR, Babaeijandaghi F, Low M, Chang CK, Lee ST, Fiore D, Zhang RH, Natarajan A, Nedospasov SA, Rossi FM. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med. 2015;21:786–794. doi: 10.1038/nm.3869. [DOI] [PubMed] [Google Scholar]
- 87.Dong J, Dong Y, Chen Z, Mitch WE, Zhang L. The pathway to muscle fibrosis depends on myostatin stimulating the differentiation of fibro/adipogenic progenitor cells in chronic kidney disease. Kidney Int. 2017;91:119–128. doi: 10.1016/j.kint.2016.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Klingler W, Jurkat-Rott K, Lehmann-Horn F, Schleip R. The role of fibrosis in Duchenne muscular dystrophy. Acta Myol. 2012;31:184–195. [PMC free article] [PubMed] [Google Scholar]
- 89.Dong Y, Silva KA, Zhang L. Glucocorticoids increase adipocytes in muscle by affecting IL-4 regulated FAP activity. FASEB J. 2014;28:4123–4132. doi: 10.1096/fj.14-254011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Arahata K, Engel AG. Monoclonal antibody analysis of mononuclear cells in myopathies. I: quantitation of subsets according to diagnosis and sites of accumulation and demonstration and counts of muscle fibers invaded by T cells. Ann Neurol. 1984;16:193–208. doi: 10.1002/ana.410160206. [DOI] [PubMed] [Google Scholar]
- 91.Farini A, Meregalli M, Belicchi M, Battistelli M, Parolini D, D'Antona G, Gavina M, Ottoboni L, Constantin G, Bottinelli R, Torrente Y. T and B lymphocyte depletion has a marked effect on the fibrosis of dystrophic skeletal muscles in the scid/mdx mouse. J Pathol. 2007;213:229–238. doi: 10.1002/path.2213. [DOI] [PubMed] [Google Scholar]
- 92.Grabowska I, Mazur MA, Kowalski K, Helinska A, Moraczewski J, Stremińska W, Hoser G, Kawiak J, Ciemerych MA, Brzoska E. Progression of inflammation during immunodeficient mouse skeletal muscle regeneration. J Muscle Res Cell Motil. 2015;36:395–404. doi: 10.1007/s10974-015-9433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mills CD. Anatomy of a discovery: m1 and m2 macrophages. Front Immunol. 2015;6:212. doi: 10.3389/fimmu.2015.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Novak ML, Weinheimer-Haus EM, Koh TJ. Macrophage activation and skeletal muscle healing following traumatic injury. J Pathol. 2014;232:344–355. doi: 10.1002/path.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mojumdar K, Liang F, Giordano C, Lemaire C, Danialou G, Okazaki T, Bourdon J, Rafei M, Galipeau J, Divangahi M, Petrof BJ. Inflammatory monocytes promote progression of Duchenne muscular dystrophy and can be therapeutically targeted via CCR2. EMBO Mol Med. 2014;6:1476–1492. doi: 10.15252/emmm.201403967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saclier M, Yacoub-Youssef H, Mackey AL, Arnold L, Ardjoune H, Magnan M, Sailhan F, Chelly J, Pavlath GK, Mounier R, Kjaer M, Chazaud B. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells. 2013;31:384–396. doi: 10.1002/stem.1288. [DOI] [PubMed] [Google Scholar]
- 98.Desguerre I, Christov C, Mayer M, Zeller R, Becane HM, Bastuji-Garin S, Leturcq F, Chiron C, Chelly J, Gherardi RK. Clinical heterogeneity of Duchenne muscular dystrophy (DMD): definition of sub-phenotypes and predictive criteria by long-term follow-up. PLoS One. 2009;4:e4347. doi: 10.1371/journal.pone.0004347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McCroskery S, Thomas M, Platt L, Hennebry A, Nishimura T, McLeay L, Sharma M, Kambadur R. Improved muscle healing through enhanced regeneration and reduced fibrosis in myostatin-null mice. J Cell Sci. 2005;118:3531–3541. doi: 10.1242/jcs.02482. [DOI] [PubMed] [Google Scholar]
- 100.Leblond MM, Gérault AN, Corroyer-Dulmont A, MacKenzie ET, Petit E, Bernaudin M, Valable S. Hypoxia induces macrophage polarization and re-education toward an M2 phenotype in U87 and U251 glioblastoma models. Oncoimmunology. 2016;5:e1056442. doi: 10.1080/2162402X.2015.1056442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wehling-Henricks M, Tidball JG. Neuronal nitric oxide synthase-rescue of dystrophin/utrophin double knockout mice does not require nNOS localization to the cell membrane. PLoS One. 2011;6:e25071. doi: 10.1371/journal.pone.0025071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Blakney AK, Swartzlander MD, Bryant SJ. The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)-based hydrogels. J Biomed Mater Res A. 2012;100:1375–1386. doi: 10.1002/jbm.a.34104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Friedemann M, Kalbitzer L, Franz S, Moeller S, Schnabelrauch M, Simon JC, Pompe T, Franke K. Instructing human macrophage polarization by stiffness and glycosaminoglycan functionalization in 3D collagen networks. Adv Healthc Mater. 2017;6 doi: 10.1002/adhm.201600967. [DOI] [PubMed] [Google Scholar]
- 104.Zanotti S, Gibertini S, Di Blasi C, Cappelletti C, Bernasconi P, Mantegazza R, Morandi L, Mora M. Osteopontin is highly expressed in severely dystrophic muscle and seems to play a role in muscle regeneration and fibrosis. Histopathology. 2011;59:1215–1228. doi: 10.1111/j.1365-2559.2011.04051.x. [DOI] [PubMed] [Google Scholar]
- 105.Lund SA, Giachelli CM, Scatena M. The role of osteopontin in inflammatory processes. J Cell Commun Signal. 2009;3:311–322. doi: 10.1007/s12079-009-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vetrone SA, Montecino-Rodriguez E, Kudryashova E, Kramerova I, Hoffman EP, Liu SD, Miceli MC, Spencer MJ. Osteopontin promotes fibrosis in dystrophic mouse muscle by modulating immune cell subsets and intramuscular TGF-beta. J Clin Invest. 2009;119:1583–1594. doi: 10.1172/JCI37662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tidball JG, Welc SS. Macrophage-derived IGF-1 is a potent coordinator of myogenesis and inflammation in regenerating muscle. Mol Ther. 2015;23:1134–1135. doi: 10.1038/mt.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barton ER, Morris L, Musaro A, Rosenthal N, Sweeney HL. Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J Cell Biol. 2002;157:137–148. doi: 10.1083/jcb.200108071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wilschut KJ, Ling VB, Bernstein HS. Concise review: stem cell therapy for muscular dystrophies. Stem Cells Transl Med. 2012;1:833–842. doi: 10.5966/sctm.2012-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boldrin L, Neal A, Zammit PS, Muntoni F, Morgan JE. Donor satellite cell engraftment is significantly augmented when the host niche is preserved and endogenous satellite cells are incapacitated. Stem Cells. 2012;30:1971–1984. doi: 10.1002/stem.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kaar JL, Li Y, Blair HC, Asche G, Koepsel RR, Huard J, Russell AJ. Matrix metalloproteinase-1 treatment of muscle fibrosis. Acta Biomater. 2008;4:1411–1420. doi: 10.1016/j.actbio.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 112.Wang W, Pan H, Murray K, Jefferson BS, Li Y. Matrix metalloproteinase-1 promotes muscle cell migration and differentiation. Am J Pathol. 2009;174:541–549. doi: 10.2353/ajpath.2009.080509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hindi SM, Shin J, Ogura Y, Li H, Kumar A. Matrix metalloproteinase-9 inhibition improves proliferation and engraftment of myogenic cells in dystrophic muscle of mdx mice. PLoS One. 2013;8:e72121. doi: 10.1371/journal.pone.0072121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cabrera D, Gutierrez J, Cabello-Verrugio C, Morales MG, Mezzano S, Fadic R, Casar JC, Hancke JL, Brandan E. Andrographolide attenuates skeletal muscle dystrophy in mdx mice and increases efficiency of cell therapy by reducing fibrosis. Skelet Muscle. 2014;4:6. doi: 10.1186/2044-5040-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fakhfakh R, Lamarre Y, Skuk D, Tremblay JP. Losartan enhances the success of myoblast transplantation. Cell Transplant. 2012;21:139–152. doi: 10.3727/096368911X576045. [DOI] [PubMed] [Google Scholar]
- 116.Gargioli C, Coletta M, De Grandis F, Cannata SM, Cossu G. PlGF-MMP-9-expressing cells restore microcirculation and efficacy of cell therapy in aged dystrophic muscle. Nat Med. 2008;14:973–978. doi: 10.1038/nm.1852. [DOI] [PubMed] [Google Scholar]
- 117.Pan H, Vojnits K, Liu TT, Meng F, Yang L, Wang Y, Huard J, Cox CS, Lally KP, Li Y. MMP1 gene expression enhances myoblast migration and engraftment following implanting into mdx/SCID mice. Cell Adhes Migr. 2015;9:283–292. doi: 10.4161/19336918.2014.983799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225–229. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 119.Street JM, Souza AC, Alvarez-Prats A, Horino T, Hu X, Yuen PS, Star RA. Automated quantification of renal fibrosis with Sirius Red and polarization contrast microscopy. Physiol Rep. 2014;2 doi: 10.14814/phy2.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, Rehfeldt F, Speicher DW, Discher DE. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Smith LR, Barton ER. Collagen content does not alter the passive mechanical properties of fibrotic skeletal muscle in mdx mice. Am J Physiol Cell Physiol. 2014;306:C889–898. doi: 10.1152/ajpcell.00383.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee HP, Lippens E, Duda GN, Mooney DJ. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater. 2016;15:326–334. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hakim CH, Grange RW, Duan D. The passive mechanical properties of the extensor digitorum longus muscle are compromised in 2- to 20-mo-old mdx mice. J Appl Physiol. 2011;110:1656–1663. doi: 10.1152/japplphysiol.01425.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Skalsky AJ, McDonald CM. Prevention and management of limb contractures in neuromuscular diseases. Phys Med Rehabil Clin N Am. 2012;23:675–687. doi: 10.1016/j.pmr.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Buehler MJ. Nature designs tough collagen: explaining the nanostructure of collagen fibrils. Proc Natl Acad Sci U S A. 2006;103:12285–12290. doi: 10.1073/pnas.0603216103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Smith LR, Hammers DW, Sweeney HL, Barton ER. Increased collagen cross-linking is a signature of dystrophin-deficient muscle. Muscle Nerve. 2016;54:71–78. doi: 10.1002/mus.24998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chapman MA, Pichika R, Lieber RL. Collagen cross-linking does not dictate stiffness in a transgenic mouse model of skeletal muscle fibrosis. J Biomech. 2015;48:375–378. doi: 10.1016/j.jbiomech.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wells RG, Schwabe RF, Schwabe R. Origin and function of myofibroblasts in the liver. Semin Liver Dis. 2015;35:97–106. doi: 10.1055/s-0035-1550061. [DOI] [PubMed] [Google Scholar]
- 129.Gillies AR, Chapman MA, Bushong EA, Deerinck TJ, Ellisman MH, Lieber RL. High resolution three-dimensional reconstruction of fibrotic skeletal muscle extracellular matrix. J Physiol. 2017;595:1159–1171. doi: 10.1113/JP273376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hardingham TE, Muir H, Kwan MK, Lai WM, Mow VC. Viscoelastic properties of proteoglycan solutions with varying proportions present as aggregates. J Orthop Res. 1987;5:36–46. doi: 10.1002/jor.1100050107. [DOI] [PubMed] [Google Scholar]
- 131.Fadic R, Mezzano V, Alvarez K, Cabrera D, Holmgren J, Brandan E. Increase in decorin and biglycan in Duchenne muscular dystrophy: role of fibroblasts as cell source of these proteoglycans in the disease. J Cell Mol Med. 2006;10:758–769. doi: 10.1111/j.1582-4934.2006.tb00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yan W, Wang P, Zhao CX, Tang J, Xiao X, Wang DW. Decorin gene delivery inhibits cardiac fibrosis in spontaneously hypertensive rats by modulation of transforming growth factor-beta/Smad and p38 mitogen-activated protein kinase signaling pathways. Hum Gene Ther. 2009;20:1190–1200. doi: 10.1089/hum.2008.204. [DOI] [PubMed] [Google Scholar]
- 133.Fukushima K, Badlani N, Usas A, Riano F, Fu F, Huard J. The use of an antifibrosis agent to improve muscle recovery after laceration. Am J Sports Med. 2001;29:394–402. doi: 10.1177/03635465010290040201. [DOI] [PubMed] [Google Scholar]
- 134.Brandan E, Gutierrez J. Role of proteoglycans in the regulation of the skeletal muscle fibrotic response. FEBS J. 2013;280:4109–4117. doi: 10.1111/febs.12278. [DOI] [PubMed] [Google Scholar]
- 135.Lenselink EA. Role of fibronectin in normal wound healing. Int Wound J. 2015;12:313–316. doi: 10.1111/iwj.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cynthia Martin F, Hiller M, Spitali P, Oonk S, Dalebout H, Palmblad M, Chaouch A, Guglieri M, Straub V, Lochmüller H, Niks EH, Verschuuren JJ, Aartsma-Rus A, Deelder AM, van der Burgt YE, 't Hoen PA. Fibronectin is a serum biomarker for Duchenne muscular dystrophy. Proteomics Clin Appl. 2014;8:269–278. doi: 10.1002/prca.201300072. [DOI] [PubMed] [Google Scholar]
- 137.Bentzinger CF, Wang YX, von Maltzahn J, Soleimani VD, Yin H, Rudnicki MA. Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell. 2013;12:75–87. doi: 10.1016/j.stem.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lukjanenko L, Jung MJ, Hegde N, Perruisseau-Carrier C, Migliavacca E, Rozo M, Karaz S, Jacot G, Schmidt M, Li L, Metairon S, Raymond F, Lee U, Sizzano F, Wilson DH, Dumont NA, Palini A, Fässler R, Steiner P, Descombes P, Rudnicki MA, Fan CM, von Maltzahn J, Feige JN, Bentzinger CF. Loss of fibronectin from the aged stem cell niche affects the regenerative capacity of skeletal muscle in mice. Nat Med. 2016;22:897–905. doi: 10.1038/nm.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Clark RA. Fibrin and wound healing. Ann N Y Acad Sci. 2001;936:355–367. doi: 10.1111/j.1749-6632.2001.tb03522.x. [DOI] [PubMed] [Google Scholar]
- 140.Vidal B, Serrano AL, Tjwa M, Suelves M, Ardite E, De Mori R, Baeza-Raja B, Martinez de Lagran M, Lafuste P, Ruiz-Bonilla V, Jardi M, Gherardi R, Christov C, Dierssen M, Carmeliet P, Degen JL, Dewerchin M, Munoz-Canoves P. Fibrinogen drives dystrophic muscle fibrosis via a TGFbeta/alternative macrophage activation pathway. Genes Dev. 2008;22:1747–1752. doi: 10.1101/gad.465908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li H, Mittal A, Makonchuk DY, Bhatnagar S, Kumar A. Matrix metalloproteinase-9 inhibition ameliorates pathogenesis and improves skeletal muscle regeneration in muscular dystrophy. Hum Mol Genet. 2009;18:2584–2598. doi: 10.1093/hmg/ddp191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen X, Li Y. Role of matrix metalloproteinases in skeletal muscle: migration, differentiation, regeneration and fibrosis. Cell Adhes Migr. 2009;3:337–341. doi: 10.4161/cam.3.4.9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bellayr I, Holden K, Mu X, Pan H, Li Y. Matrix metalloproteinase inhibition negatively affects muscle stem cell behavior. Int J Clin Exp Pathol. 2013;6:124–141. [PMC free article] [PubMed] [Google Scholar]
- 144.Lei H, Leong D, Smith LR, Barton ER. Matrix metalloproteinase 13 is a new contributor to skeletal muscle regeneration and critical for myoblast migration. Am J Physiol Cell Physiol. 2013;305:C529–538. doi: 10.1152/ajpcell.00051.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lund DK, Mouly V, Cornelison D. MMP-14 is necessary but not sufficient for invasion of three-dimensional collagen by human muscle satellite cells. Am J Physiol Cell Physiol. 2014:C140–149. doi: 10.1152/ajpcell.00032.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Miyazaki D, Nakamura A, Fukushima K, Yoshida K, Takeda S, Ikeda S. Matrix metalloproteinase-2 ablation in dystrophin-deficient mdx muscles reduces angiogenesis resulting in impaired growth of regenerated muscle fibers. Hum Mol Genet. 2011;20:1787–1799. doi: 10.1093/hmg/ddr062. [DOI] [PubMed] [Google Scholar]
- 147.Bobadilla M, Sainz N, Rodriguez JA, Abizanda G, Orbe J, de Martino A, Garcia Verdugo JM, Paramo JA, Prosper F, Perez-Ruiz A. MMP-10 is required for efficient muscle regeneration in mouse models of injury and muscular dystrophy. Stem Cells. 2014;32:447–461. doi: 10.1002/stem.1553. [DOI] [PubMed] [Google Scholar]
- 148.von Moers A, Zwirner A, Reinhold A, Bruckmann O, van Landeghem F, Stoltenburg-Didinger G, Schuppan D, Herbst H, Schuelke M. Increased mRNA expression of tissue inhibitors of metalloproteinase-1 and -2 in Duchenne muscular dystrophy. Acta Neuropathol. 2005;109:285–293. doi: 10.1007/s00401-004-0941-0. [DOI] [PubMed] [Google Scholar]
- 149.Sun G, Haginoya K, Chiba Y, Uematsu M, Hino-Fukuyo N, Tanaka S, Onuma A, Iinuma K, Tsuchiya S. Elevated plasma levels of tissue inhibitors of metalloproteinase-1 and their overexpression in muscle in human and mouse muscular dystrophy. J Neurol Sci. 2010;297:19–28. doi: 10.1016/j.jns.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 150.Arpino V, Brock M, Gill SE. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015;44–46:247–254. doi: 10.1016/j.matbio.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 151.Nagamine Y, Medcalf RL, Muñoz-Cánoves P. Transcriptional and posttranscriptional regulation of the plasminogen activator system. Thromb Haemost. 2005;93:661–675. doi: 10.1160/TH04-12-0814. [DOI] [PubMed] [Google Scholar]
- 152.Suelves M, Vidal B, Serrano AL, Tjwa M, Roma J, López-Alemany R, Luttun A, de Lagrán MM, Díaz-Ramos A, Díaz MA, Jardí M, Roig M, Dierssen M, Dewerchin M, Carmeliet P, Muñoz-Cánoves P. uPA deficiency exacerbates muscular dystrophy in MDX mice. J Cell Biol. 2007;178:1039–1051. doi: 10.1083/jcb.200705127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ardite E, Perdiguero E, Vidal B, Gutarra S, Serrano AL, Munoz-Canoves P. PAI-1-regulated miR-21 defines a novel age-associated fibrogenic pathway in muscular dystrophy. J Cell Biol. 2012;196:163–175. doi: 10.1083/jcb.201105013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dittmore A, Silver J, Sarkar SK, Marmer B, Goldberg GI, Neuman KC. Internal strain drives spontaneous periodic buckling in collagen and regulates remodeling. Proc Natl Acad Sci U S A. 2016;113:8436–8441. doi: 10.1073/pnas.1523228113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Flynn BP, Bhole AP, Saeidi N, Liles M, Dimarzio CA, Ruberti JW. Mechanical strain stabilizes reconstituted collagen fibrils against enzymatic degradation by mammalian collagenase matrix metalloproteinase 8 (MMP-8) PLoS One. 2010;5:e12337. doi: 10.1371/journal.pone.0012337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zou Y, Zwolanek D, Izu Y, Gandhy S, Schreiber G, Brockmann K, Devoto M, Tian Z, Hu Y, Veit G, Meier M, Stetefeld J, Hicks D, Straub V, Voermans NC, Birk DE, Barton ER, Koch M, Bönnemann CG. Recessive and dominant mutations in COL12A1 cause a novel EDS/myopathy overlap syndrome in humans and mice. Hum Mol Genet. 2014;23:2339–2352. doi: 10.1093/hmg/ddt627. [DOI] [PMC free article] [PubMed] [Google Scholar]