The antidiabetic compound metformin has emerged as a promising antineoplastic drug. Type 2 diabetes mellitus is associated with increased risk of colon cancer, against which metformin use seems to be protective. This article is a timely report on the association between metformin use and survival of diabetic patients with colon cancer.
Abstract
Background.
Type 2 diabetes mellitus (T2DM) is associated with increased risk of colon cancer (CC), whereas metformin use seems to be protective. However, the impact of metformin use on the risk of death or disease recurrence after radical surgery for CC remains uncertain.
Materials and Methods.
This is a substudy conducted in patients with high‐risk stage II or stage III CC randomized in the TOSCA trial, which compared 3 versus 6 months of fluoropyrimidine‐oxaliplatin adjuvant chemotherapy. Objective of the study was to investigate the impact of metformin exposure during adjuvant chemotherapy on overall survival (OS) and relapse‐free survival (RFS). We also evaluated the impact of T2DM or metformin dosage on clinical outcomes.
Results.
Out of 3,759 patients enrolled in the TOSCA trial, 133 patients with diabetes (9.2%) and 1,319 without diabetes (90.8%) were recruited in this study. After excluding 13 patients with diabetes without information on metformin exposure, 76 patients with T2DM (63.3%) were defined as metformin users and 44 (36.7%) as metformin nonusers. After a median follow‐up of 60.4 months, 26 (21.7%) patients relapsed and 16 (13.3%) died. Metformin use was neither associated with OS (adjusted hazard ratio [HR], 1.51; 95% confidence interval [CI], 0.48–4.77; p = .4781) nor with RFS (HR, 1.56; 95% CI, 0.69–3.54; p = .2881). Similarly, we found no association between T2DM or metformin dosage and OS or RFS.
Conclusions.
Metformin use and T2DM did not impact on OS or RFS in patients with resected CC treated with adjuvant fluoropyrimidine‐oxaliplatin chemotherapy. Larger studies and longer follow‐up are required to clarify the potential efficacy of metformin in improving the prognosis of patients with CC.
Implications for Practice.
The role of the antidiabetic drug metformin in colon cancer prevention and treatment is highly debated. While low‐dose metformin reduced the incidence of colorectal adenomas in two prospective studies, its effect in patients with already established colon cancer remains unclear. In this study, the potential impact of metformin on the survival of resected colon cancer patients who received adjuvant chemotherapy was investigated in the context of the TOSCA study. We did not find any association between metformin use or dosages and patient survival. Prospective studies are required to draw definitive conclusions about metformin impact on colon cancer recurrence and survival.
摘要
背景。2 型糖尿病 (T2DM) 与结肠癌 (CC) 风险增加有关,而二甲双胍的使用似乎具有保护作用。然而,二甲双胍的使用对 CC 根治性手术后死亡或疾病复发风险的影响仍不确定。
材料和方法。这是在 TOSCA 试验中随机分组的高风险 II 期或 III 期 CC 患者中进行的亚组研究,比较了 3 个月和 6 个月的氟尿嘧啶 ‐ 奥沙利铂辅助化疗。本研究的目的是调查辅助化疗期间二甲双胍暴露对总生存 (OS) 和无复发生存 (RFS) 的影响。我们还评估了 T2DM 或二甲双胍剂量对临床预后的影响。
结果。在参加 TOSCA 试验的 3 759 名患者中,本研究纳入了 133 名糖尿病患者(9.2%)和 1 319 名非糖尿病患者(90.8%)。排除 13 例无二甲双胍暴露信息的糖尿病患者后,76 例 T2DM 患者(63.3%)被定义为二甲双胍使用者,44 例(36.7%)为非二甲双胍使用者。 60.4 个月中位随访后,26 例(21.7%)患者复发,16 例(13.3%)死亡。二甲双胍的使用既不与 OS 相关 [校正风险比 (HR),1.51;95% 置信区间 (CI),0.48‐4.77;p = 0.478 1],也不与 RFS 相关(HR,1.56;95% CI,0.69‐3.54;p = 0.288 1)。同样,我们发现 T2DM 或二甲双胍剂量与 OS 或 RFS 之间没有关联。
结论。在使用氟尿嘧啶 ‐ 奥沙利铂辅助化疗治疗已切除 CC 的患者中二甲双胍的使用和 T2DM 对 OS 或 RFS 没有影响。需要更大规模的研究和更长时间的随访来阐明二甲双胍在改善 CC 患者预后方面的潜在功效。
实践意义:抗糖尿病药物二甲双胍在结肠癌预防和治疗中的作用备受争议。虽然低剂量二甲双胍在两项前瞻性研究中降低了结直肠腺瘤的发病率,但其对已经确诊的结肠癌患者的影响尚不明确。本研究在 TOSCA 研究的背景下调查了二甲双胍对接受辅助化疗的已切除结肠癌患者存活率的潜在影响。我们未发现二甲双胍的使用或剂量与患者存活率之间存在任何关联。需要前瞻性研究以得出关于二甲双胍对结肠癌复发和生存率的影响的确切结论。
Introduction
In observational studies, hyperglycemia and diabetes have been associated with increased colorectal cancer incidence and mortality [1], [2]. Increased plasma glucose levels can sustain tumor cell bioenergetics and anabolic requirements, thus stimulating tumor growth and proliferation [3]. Moreover, type 2 diabetes mellitus (T2DM) is associated with insulin resistance and hyperinsulinemia, which can stimulate the insulin‐like growth factor receptor 1‐PI3K/AKT/mammalian target of rapamycin (mTOR) axis, a crucial orchestrator of cancer cell growth, proliferation, and survival [4], [5].
In recent years, the antidiabetic compound metformin has emerged as a promising antineoplastic drug in both preclinical and clinical studies [3], [6], [7]. Proposed mechanisms of metformin activity include (a) systemic metabolic effects, such as a reduction of plasma glucose and serum insulin/IGF‐1 levels, and (b) cell autonomous antitumor effects resulting from impaired mitochondrial metabolism, activation of the AMP‐activated protein kinase, and the consequent inhibition of mTOR and anabolic functions (i.e., fatty acid and cholesterol biosynthesis) in cancer cells [8]. In preclinical studies, metformin inhibited the growth of in vitro and in vivo models of colon cancer (CC) [9], [10] and also synergized with oxaliplatin [11]. In the clinical context, the role of metformin in CC prevention is supported by observational studies reporting on lower tumor incidence in patients with diabetes taking metformin [12] and also by two prospective interventional trials, in which low‐dose metformin reduced the occurrence of colorectal adenomas in patients without diabetes [13], [14].
Conversely, the efficacy of metformin in reducing recurrences and/or improving survival in patients with resected CC is more uncertain. Although a recent meta‐analysis showed an association between metformin use and better overall survival (OS) in patients with early‐stage CC [15], the five studies included were highly heterogeneous in terms of design and patient characteristics; moreover, in two studies there was no evidence of a positive effect of metformin [16], [17]. Longer OS in metformin users could result from improved cancer‐related outcomes or from the reduction of other causes of death in patients with diabetes, such as cardiovascular, cerebrovascular, or renal disease. The fact that metformin use has also been associated with better relapse‐free survival (RFS) in some studies suggests an anti‐CC activity [15].
The TOSCA trial is an open‐label, phase III, multicenter noninferiority trial that randomized patients with high‐risk stage II or stage III CC to receive 3 or 6 months of FOLFOX‐4/CAPOX adjuvant chemotherapy (ChT). The trial failed to demonstrate a formal noninferiority of 3 months versus 6 months of treatment [18].
Based on available evidences, we hypothesized that metformin use in combination with adjuvant chemotherapy may be associated with better prognosis in patients with CC. In particular, we evaluated the association between metformin use and OS or RFS of patients with diabetes with CC enrolled in the TOSCA trial. As exploratory analyses, we also investigated the potential impact of diabetic status and metformin dosage on clinical outcomes.
Material and Methods
Study Population and Objectives of the Study
Our study was a preplanned analysis of the TOSCA population, although the participation in this subanalysis was not mandatory but at the discretion of each center. Main inclusion criteria were as follows: (a) histologically confirmed, high‐risk stage II or stage III colon adenocarcinoma (high‐risk stage II disease was defined as T4 stage; grade greater than or equal to 3; clinical presentation with bowel obstruction or perforation; histological evidence of vascular, lymphatic, or perineural invasion), (b) diagnosis of T2DM, (c) age ≥18 years, (d) Eastern Cooperative Oncology Group performance status (ECOG PS) ≤1, and (e) having undergone curative surgery no less than 3 and no more than 10 weeks prior to randomization.
Patients with diabetes were defined as patients with a diagnosis of T2DM at the time of enrollment in the study or during the course of adjuvant ChT. The diagnosis of T2DM had to be made by a physician specialist in diabetes management after excluding other causes of hyperglycemia or other diabetes types. Among patients with diabetes, we collected information on the use of antidiabetic therapies, including metformin and insulin. Metformin use and dosages were assessed at diabetes diagnosis, during ChT, and at the end of ChT. Patients were defined as metformin users if they took metformin at least during ChT, independently from its use in the pre‐ and/or post‐ChT period.
The primary objective of this study was to investigate the impact of metformin use on patient OS, as defined as the time interval between randomization and death from any cause. Secondary objective was to study the association between metformin use and RFS, as defined as the time between randomization and disease relapse or death from any cause. Patients who had not relapsed or died while on study were censored at the date of the last disease assessment. Explorative analyses were performed to evaluate the impact of diabetic status or metformin dosage on OS and RFS.
Statistical Analysis
Sample size was calculated a priori based on an expected prevalence of T2DM of at least 11% in the whole TOSCA population. Assuming that 43% of patients with diabetes received metformin, a death rate of 43% (i.e., 173 deaths out of 413 patients) should be observed and a hazard ratio (HR) for OS of at least 0.65 associated with metformin administration, with a power of 80% and type‐I error of 5% for bilateral test. Because 4 years of accrual and 3 years of follow‐up were planned for the TOCA trial, the 43% death rate seemed reasonable by considering that a recently published randomized phase III trial reported a death rate of 34.1% after a median follow‐up of 6.5 years in patients with diabetes with stage III CC [16].
The effect of metformin use and the impact of potential confounders on OS and RFS was explored by Cox proportional hazard models. Variables significantly associated with OS or RFS were included in a multivariable model to test their independent effect on clinical outcomes. Results of the analysis were expressed as HRs and 95% confidence intervals (CIs). Chi‐square (or Fisher's exact test as appropriate) and Wilcoxon tests were performed to compare the distributions of categorical and continuous variable, respectively. Statistical significance was set at p < .05 for bilateral tests. Analysis was carried out using the SAS Version 9.4 (SAS Institute; Cary, NC) software.
Results
Patients’ and Tumor Characteristics
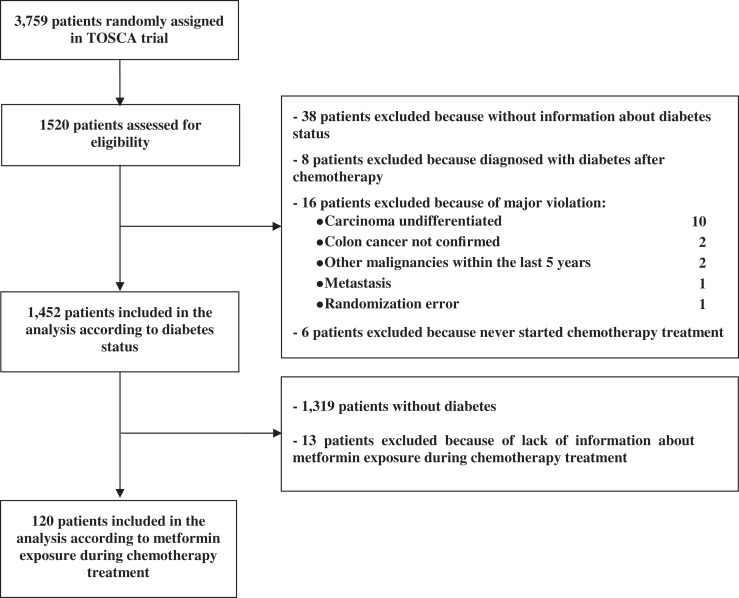
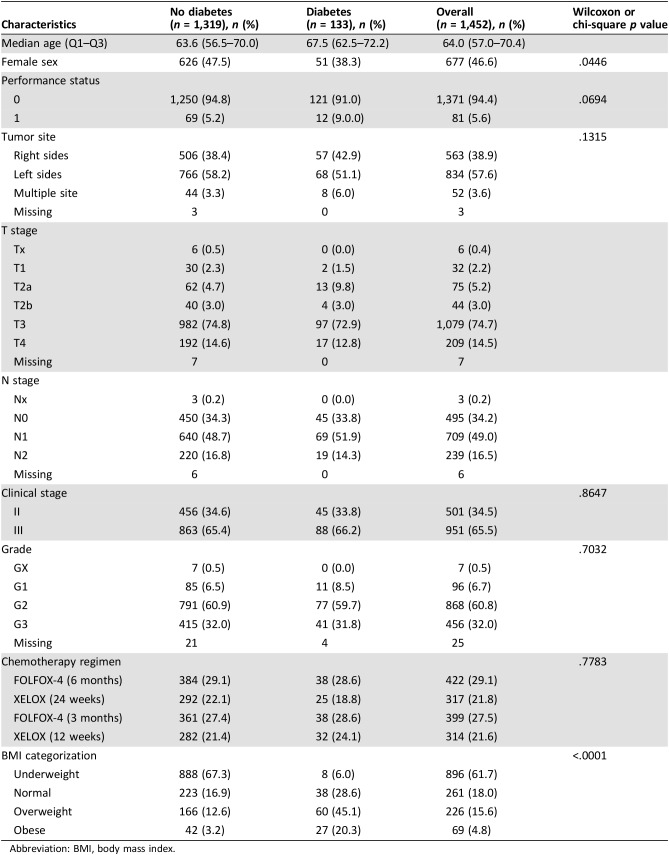
Out of 3,759 patients enrolled in the TOSCA trial from 130 centers, 1,520 patients from 37 centers were assessed for eligibility in our study. 68 patients were excluded because of unavailability of information on diabetic status. Finally, 133 patients with T2DM and 1,319 patients without T2DM were included (Fig. 1). Patient and tumor characteristics are summarized in Table 1. Briefly, patients with diabetes were older than those without diabetes (67.5 vs. 63.6 years, p < .0001) and were more frequently obese (45.1% vs. 12.6%, p < .0001) or overweight (20.3% vs. 3.2%, p < .0001); moreover, there was a higher proportion of women among patients without diabetes than among patients with diabetes (47.5% vs. 38.3%; p = .0446). ECOG PS, tumor site, clinical stage, tumor grade, type (FOLFOX vs. XELOX), and duration (6 vs. 3 months) of adjuvant ChT did not significantly differ between patients with and without diabetes.
Figure 1.
Study Flowchart.
Table 1. Patient and tumor characteristics in patients with versus without diabetes.
Abbreviation: BMI, body mass index.
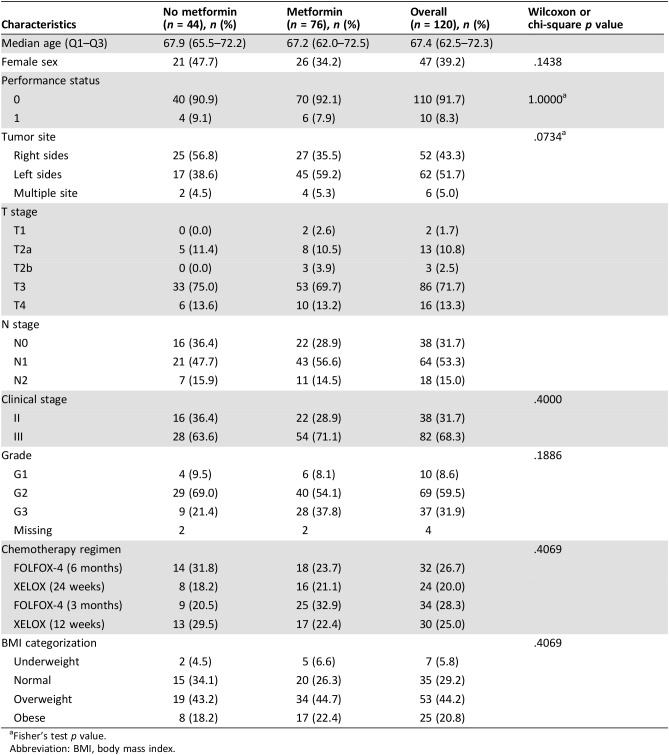
Among 133 patients with T2DM, data on metformin use were available for 120 patients, 76 of whom were metformin users and 44 were nonusers (Table 2). Metformin users were less likely to be women (34.2% vs. 47.7%) and more likely to have left‐sided tumors (59.2% vs. 38.6%). Details on metformin exposure are summarized in supplemental online Table 1.
Table 2. Patient and tumor characteristics in metformin users versus nonuser.
Fisher's test p value.
Abbreviation: BMI, body mass index.
Impact of Metformin Use on Clinical Outcomes in Patients with Diabetes
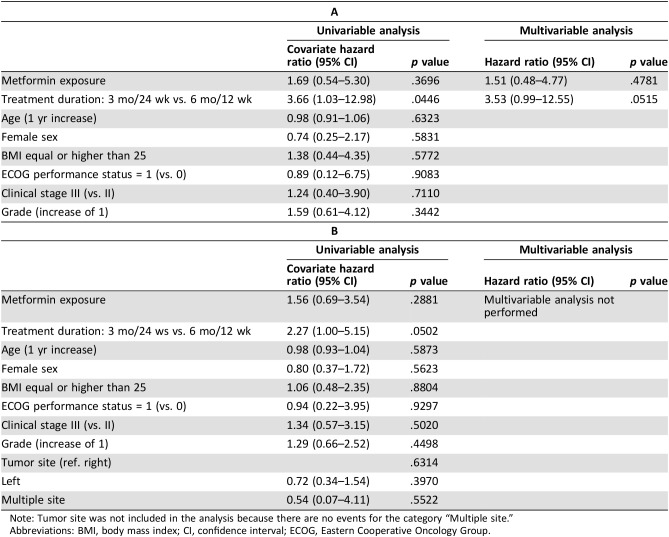
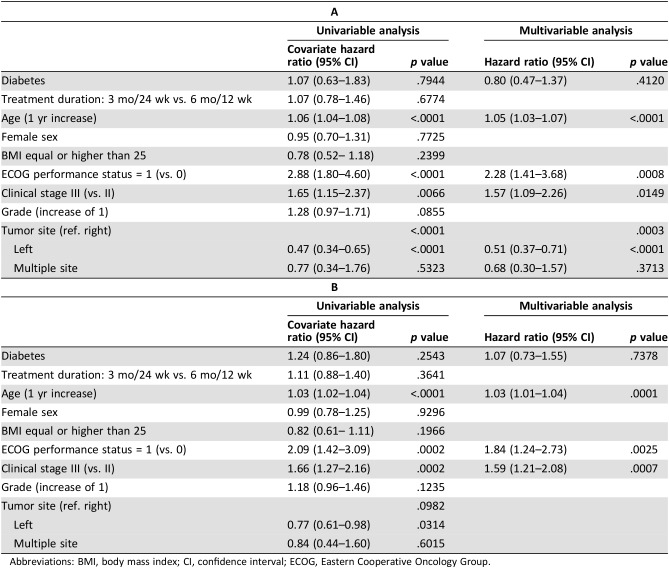
During a median follow‐up of 60.3 months, 26 (21.7%) patients with T2DM relapsed, 16 (13.3%) patients died, and 29 (24.2%) patients relapsed and/or died. Metformin exposure was associated neither with OS (adjusted HR [aHR], 1.51; 95% CI, 0.48–4.77; p = .4781; Table 3A) nor with RFS (HR, 1.56; 95% CI, 0.69–3.54; p = .2881; Table 3B). At univariable analysis, shorter duration of adjuvant ChT (3 vs. 6 months) was associated with worse lower OS, but this association was not confirmed at multivariable analysis.
Table 3. Effect of metformin exposure on overall survival (A) or relapse‐free survival (B) in diabetic patients. Univariable and multivariable Cox proportional hazard models.
Note: Tumor site was not included in the analysis because there are no events for the category “Multiple site.”
Abbreviations: BMI, body mass index; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group.
Although the HR of RFS for patients receiving 3 months versus 6 months of adjuvant ChT was equal to 2.27, this association did not meet statistical significance (p = .0502). Because no significant impact of other variables on RFS was detected, we did not perform multivariable analysis.
Exploratory Analysis on Impact of Diabetes on Clinical Outcomes
During a median follow‐up of 62.3 months, 243 (16.7%) patients relapsed, 162 (11.2%) died, and 287 (19.8%) relapsed and/or died. We found no effect of T2DM on OS after adjusting for other covariates (aHR, 0.80; 95% CI, 0.47–1.37; p = .4120; Table 4A). At both univariable and multivariable analysis, older age, worse ECOG PS (1 vs. 0), and stage III (vs. II) correlated with shorter OS, whereas left‐sided tumors were associated with better OS. Similarly, T2DM did not impact on RFS after adjusting for age, ECOG PS, and stage (aHR, 1.07; 95% CI, 0.73–1.55; p = .7378). Older age, worse ECOG PS (1 vs. 0), and stage III (vs. II) were associated with shorter RFS at both univariable and multivariable analysis (Table 4B).
Table 4. Effect of diabetic status on overall survival (A) or relapse‐free survival (B). Univariable and multivariable Cox proportional hazard models.
Abbreviations: BMI, body mass index; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group.
Exploratory Analysis on Metformin Dosage
Data on metformin dosages were available for 95 patients. Overall, 22 (23.2%) patients relapsed, 13 (13.7%) patients died, and 24 (23.3%) patients relapsed and/or died. After adjusting for ChT duration, we did not find a significant association between metformin dosages and OS (aHR for 100 mg increase, 1.74; 95% CI, 0.54–5.67; p = .3555) or RFS (aHR, 1.03; 95% CI, 0.98–1.08; p = .2680; data not shown).
Discussion
Although metformin seems to reduce the incidence of preneoplastic lesions in the colon (polyps, adenomas) of patients without diabetes as well, its impact on the prognosis of patients with already established CC remains uncertain. In this study we evaluated the impact of metformin use in patients with resected CC enrolled in the TOSCA trial. We found no significant association between metformin use and the survival of patients with diabetes with resected high‐risk stage II or stage III CC who received FOLFOX‐4/XELOX adjuvant ChT.
Our results are in contrast with the conclusions of a recent meta‐analysis of five studies, which demonstrated a significantly lower risk of death in metformin users compared with nonusers [15]. However, studies included in this meta‐analysis were highly heterogeneous in terms of design, number of patients evaluated, and inclusion criteria; moreover, in two of these studies, OS was not significantly different between metformin users and nonusers [16], [17], whereas only one study showed remarkably better OS in metformin users [19].
Different hypotheses can explain the negative results of our study: (a) the lack of a real antitumor effect of metformin against CC, (b) the type of ChT used, and (c) an insufficient number of death/relapse events.
Regarding the first hypothesis, metformin displays in vitro antitumor effects at concentrations that are in the order of millimolars (i.e. by far superior to those that can be safely reached in the blood of patients with diabetes, in the order of nanomolars) [10]. Although metformin dosages commonly used for T2DM treatment are active in more precocious phases of CC tumorigenesis [13], [14], they could be ineffective in inhibiting the growth or preventing recurrences of already established neoplasms. In retrospective studies, a dose‐response relationship is one of the strongest arguments in favor of a possible anticancer effect of metformin [20]. In our study, metformin dosages were not associated with OS or RFS. However, the low number of patients and death/relapse events limits the possibility to draw definitive conclusions.
As for the second hypothesis, a potential antitumor effect of metformin may have been masked by the fact that all patients in the TOSCA trial received highly oxaliplatin‐fuoropyrimidine ChT, which may provide the highest protection against CC recurrences. Conversely, metformin could add antitumor efficacy to ChT regimens containing only fuoropyrimidines. Our findings are consistent with results reported by Singh et al., who did not find a significant association between metformin use and the OS or RFS of patients with stage III CC receiving FOLFOX plus/minus cetuximab adjuvant ChT [16]. Our analysis and the study by Singh et al. are the only two studies that explored the impact of metformin in a homogeneous cohort of patients with stage II–III CC treated in the context of phase III randomized trials. In contrast, those studies that found an impact of metformin on better outcome considered patients with CC with stage I to IV disease, and treated with different types of ChT regimens, including patients with low‐stage cancers who did not receive ChT and patients with advanced disease receiving multiple treatment lines [19], [20], [21]. Although the effect of metformin on clinical outcomes was balanced for tumor stage, the type of ChT was not considered as a potential confounder, thus precluding the possibility to test our hypothesis [21].
The third hypothesis is related the low number of patients included and clinical events (death/relapse) observed. Unfortunately, only 37 out of 130 centers involved in the TOSCA trial took part in this substudy. Moreover, there was a much lower than expected frequency of death events in the population of patients with diabetes (13.3 vs. 43%), which was unexpected, especially if we consider that the study by Singh et al. reported a 34.1% death rate after 6.5 years of follow‐up in patients with diabetes with stage III CC cancer receiving oxaliplatin‐containing ChT. These factors have probably precluded the possibility of finding small OS differences between metformin users and nonusers.
One possible explanation for the discordant results among different studies is the definition of “metformin users.” In most published studies, they were defined as patients taking metformin at ChT initiation or within 1 year before ChT [16]. In our study, metformin users took metformin at least during the whole course of adjuvant ChT, independently from its use before and/or after ChT; this definition is based on the hypothesis of a synergistic anticancer activity between metformin and fluoropyrimidines plus oxaliplatin [11], [22]. Despite these discrepancies, results of our analysis were confirmed when we considered as metformin users those patients taking metformin before ChT initiation (not shown); these results make our findings comparable with those of previous studies.
We also failed to demonstrate an association between diabetes mellitus and RFS or OS. This result contradicts the findings of recent meta‐analyses of observational studies, which demonstrated an increased risk of death in patients with versus without diabetes with resected CRC [23], [24]. However, our study was not powered to test the hypothesis of reduced OS or RFS in patients with diabetes. Moreover, published studies are characterized by remarkable heterogeneity in statistical design and type of patients included (e.g., disease stages, type of treatment administered). Finally, differences in the time of diabetes diagnosis (e.g., before, during, or after adjuvant chemotherapy) between different analyses may in part explain the apparently contrasting findings.
Strengths of this study consist of its prospective design, a priori calculation of sample size, preplanned evaluation of glycemic status and metformin use, and a relatively homogeneous cohort of patients included in a phase III, randomized trial. Limitations consist of the lower than expected number of patients included and short follow‐up period, with consequently low number of relapse and death events. Because adequate follow‐up duration is important to reliably assess the potential role of metformin in affecting cancer recurrence, these limitations may have contributed to the negative findings of our study [15].
Conclusion
The impact of metformin on OS and RFS in patients with resected CC remains uncertain. Larger prospective studies in homogeneous patient populations—possibly in the context of randomized phase III trials to guarantee regular follow‐up and data collection—and longer follow‐up are necessary to clarify the role of metformin in preventing CC recurrence and death.
See http://www.TheOncologist.com for supplemental material available online.
Footnotes
For Further Reading: Preet Paul Singh, Qian Shi, Nathan R. Foster et al. Relationship Between Metformin Use and Recurrence and Survival in Patients With Resected Stage III Colon Cancer Receiving Adjuvant Chemotherapy: Results From North Central Cancer Treatment Group N0147 (Alliance). The Oncologist 2016;21:1509–1521.
Implications for Practice: The present study did not find any relationship between metformin use or its duration and disease‐free survival, time to recurrence, and overall survival in a large cohort of patients with resected stage III colon cancer receiving adjuvant FOLFOX (folinic acid, fluorouracil, oxaliplatin)‐based chemotherapy. This relationship was not modified by KRAS or BRAF mutation or DNA mismatch repair status. Metformin use did not increase or decrease the likelihood of chemotherapy‐related grade 3 or higher adverse events.
Contributor Information
Claudio Vernieri, Email: claudio.vernieri@istitutotumori.mi.it.
Maria Di Bartolomeo, Email: maria.dibartolomeo@istitutotumori.mi.it.
Author Contributions
Conception/design: Claudio Vernieri, Fabio Galli, Maria Di Bartolomeo
Provision of study material or patients: Claudio Vernieri, Fabio Galli, Laura Ferrari, Paolo Marchetti, Sara Lonardi, Evaristo Maiello, Rosario V. Iaffaioli, Maria G. Zampino, Alberto Zaniboni, Sabino De Placido, Maria Banzi, Azzurra Damiani, Daris Ferrari, Gerardo Rosati, Roberto F. Labianca, Paolo Bidoli, Giovanni L. Frassineti, Mario Nicolini, Lorenzo Pavesi, Maria C. Tronconi, Angela Buonadonna, Sabrina Ferrario, Giovanni Lo Re, Vincenzo Adamo, Emiliano Tamburini, Mario Clerico, Paolo Giordani, Francesco Leonardi, Sandro Barni, Andrea Ciarlo, Luigi Cavanna, Stefania Gori, Saverio Cinieri, Marina Faedi, Massimo Aglietta, Maria Antista, Katia F. Dottif, Francesca Galli, Maria Di Bartolomeo
Collection and/or assembly of data: Claudio Vernieri, Fabio Galli, Katia F. Dotti, Francesca Galli, Maria Di Bartolomeo
Data analysis and interpretation: Claudio Vernieri, Fabio Galli, Francesca Galli, Maria Di Bartolomeo
Manuscript writing: Claudio Vernieri, Fabio Galli, Maria Di Bartolomeo
Final approval of manuscript: Claudio Vernieri, Fabio Galli, Laura Ferrari, Paolo Marchetti, Sara Lonardi, Evaristo Maiello, Rosario V. Iaffaioli, Maria G. Zampino, Alberto Zaniboni, Sabino De Placido, Maria Banzi, Azzurra Damiani, Daris Ferrari, Gerardo Rosati, Roberto F. Labianca, Paolo Bidoli, Giovanni L. Frassineti, Mario Nicolini, Lorenzo Pavesi, Maria C. Tronconi, Angela Buonadonna, Sabrina Ferrario, Giovanni Lo Re, Vincenzo Adamo, Emiliano Tamburini, Mario Clerico, Paolo Giordani, Francesco Leonardi, Sandro Barni, Andrea Ciarlo, Luigi Cavanna, Stefania Gori, Saverio Cinieri, Marina Faedi, Massimo Aglietta, Maria Antista, Katia F. Dottif, Francesca Galli, Maria Di Bartolomeo
Disclosures
The authors indicated no financial relationships.
References
- 1.Giovannucci E, Harlan DM, Archer MC et al. Diabetes and cancer: A consensus report. Diabetes Care 2010;33:1674–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vigneri P, Frasca F, Sciacca L et al. Diabetes and cancer. Endocr Relat Cancer 2009;16:1103–1123. [DOI] [PubMed] [Google Scholar]
- 3.Vernieri C, Casola S, Foiani M et al. Targeting cancer metabolism: Dietary and pharmacologic interventions. Cancer Discov 2016;6:1315–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding XZ, Fehsenfeld DM, Murphy LO et al. Physiological concentrations of insulin augment pancreatic cancer cell proliferation and glucose utilization by activating MAP kinase, PI3 kinase and enhancing GLUT‐1 expression. Pancreas 2000; 21: 310‐320. [DOI] [PubMed] [Google Scholar]
- 5.Wolpin BM, Meyerhardt JA, Chan AT et al. Insulin, the insulin‐like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J Clin Oncol 2009;27:176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeCensi A, Puntoni M, Gandini S et al. Differential effects of metformin on breast cancer proliferation according to markers of insulin resistance and tumor subtype in a randomized presurgical trial. Breast Cancer Res Treat 2014;148:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pusceddu S, Vernieri C, Di Maio M et al. Metformin use is associated with longer progression‐free survival of patients with diabetes and pancreatic neuroendocrine tumors receiving everolimus and/or somatostatin analogues. Gastroenterology 2018;155:479–489.e7. [DOI] [PubMed] [Google Scholar]
- 8.Zhou G, Myers R, Li Y et al. Role of AMP‐activated protein kinase in mechanism of metformin action. J Clin Invest 2001;108:1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Algire C, Amrein L, Zakikhani M et al. Metformin blocks the stimulative effect of a high‐energy diet on colon carcinoma growth in vivo and is associated with reduced expression of fatty acid synthase. Endocr Relat Cancer 2010;17:351–360. [DOI] [PubMed] [Google Scholar]
- 10.He J, Wang K, Zheng N et al. Metformin suppressed the proliferation of LoVo cells and induced a time‐dependent metabolic and transcriptional alteration. Sci Rep 2015;5:17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richard SM, Martinez Marignac VL. Sensitization to oxaliplatin in HCT116 and HT29 cell lines by metformin and ribavirin and differences in response to mitochondrial glutaminase inhibition. J Cancer Res Ther 2015;11:336–340. [DOI] [PubMed] [Google Scholar]
- 12.Liu F, Yan L, Wang Z et al. Metformin therapy and risk of colorectal adenomas and colorectal cancer in type 2 diabetes mellitus patients: A systematic review and meta‐analysis. Oncotarget 2017;8:16017–16026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosono K, Endo H, Takahashi H et al. Metformin suppresses colorectal aberrant crypt foci in a short‐term clinical trial. Cancer Prev Res (Phila) 2010;3:1077–1083. [DOI] [PubMed] [Google Scholar]
- 14.Higurashi T, Hosono K, Takahashi H et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post‐polypectomy patients without diabetes: A multicentre double‐blind, placebo‐controlled, randomised phase 3 trial. Lancet Oncol 2016;17:475–483. [DOI] [PubMed] [Google Scholar]
- 15.Coyle C, Cafferty FH, Vale C et al. Metformin as an adjuvant treatment for cancer: A systematic review and meta‐analysis. Ann Oncol 2016;27:2184–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh PP, Shi Q, Foster NR et al. relationship between metformin use and recurrence and survival in patients with resected stage III colon cancer receiving adjuvant chemotherapy: Results from North Central Cancer Treatment Group N0147 (Alliance). The Oncologist 2016;21:1509–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zanders MM, van Herk‐Sukel MP, Vissers PA et al. Are metformin, statin and aspirin use still associated with overall mortality among colorectal cancer patients with diabetes if adjusted for one another? Br J Cancer 2015;113:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobrero A, Lonardi S, Rosati G et al.; TOSCA Investigators. FOLFOX or CAPOX in Stage II to III Colon Cancer: Efficacy Results of the Italian Three or Six Colon Adjuvant Trial. J Clin Oncol 2018;36:1478–1485. [DOI] [PubMed] [Google Scholar]
- 19.Lee GE, Aung T, Lim KH et al. Examining the effects of metformin on survival outcome in stage II/III colorectal cancer patients with diabetes mellitus. J Clin Oncol 2012;30(suppl 15):3589a. [Google Scholar]
- 20.Spillane S, Bennett K, Sharp L et al. A cohort study of metformin exposure and survival in patients with stage I‐III colorectal cancer. Cancer Epidemiol Biomarkers Prev 2013;22:1364–1373. [DOI] [PubMed] [Google Scholar]
- 21.Lee JH, Kim TI, Jeon SM et al. The effects of metformin on the survival of colorectal cancer patients with diabetes mellitus. Int J Cancer 2012;131:752–759. [DOI] [PubMed] [Google Scholar]
- 22.Nangia‐Makker P, Yu Y, Vasudevan A et al. Metformin: S potential therapeutic agent for recurrent colon cancer. PloS One 2014;9:e84369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mills KT, Bellows CF, Hoffman AE et al. Diabetes mellitus and colorectal cancer prognosis: A meta‐analysis. Dis Colon Rectum 2013;56:1304–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu B, Wu X, Wu B et al. The relationship between diabetes and colorectal cancer prognosis: A meta‐analysis based on the cohort studies. PloS One 2017;12:e0176068. [DOI] [PMC free article] [PubMed] [Google Scholar]