The treatment paradigm of advanced renal cell carcinoma (RCC) has changed rapidly in recent years. This article reports on the cost‐effectiveness of nivolumab and ipilimumab compared with sunitinib for first‐line treatment of poor‐ to intermediate‐risk advanced RCC from the U.S. payer perspective.
Keywords: Cost effectiveness, Immunotherapy, Programmed death 1 receptor, Renal cell carcinoma, Kidney cancer
Abstract
Background.
The treatment paradigm of advanced renal cell carcinoma (RCC) has changed rapidly in recent years. In first‐line treatment of intermediate‐ to poor‐risk patients, the CheckMate 214 study demonstrated a significant survival advantage for nivolumab and ipilimumab versus sunitinib. The high cost of combined immune‐modulating agents warrants an understanding of the combination's value by considering both efficacy and cost. The objective of this study was to estimate the cost‐effectiveness of nivolumab and ipilimumab compared with sunitinib for first‐line treatment of intermediate‐ to poor‐risk advanced RCC from the U.S. payer perspective.
Materials and Methods.
A Markov model was developed to compare the costs and effectiveness of nivolumab and ipilimumab with those of sunitinib in the first‐line treatment of intermediate‐ to poor‐risk advanced RCC. Health outcomes were measured in life‐years and quality‐adjusted life‐years (QALYs). Drug costs were based on Medicare reimbursement rates in 2017. We extrapolated survival beyond the trial closure using Weibull distribution. Model robustness was addressed in univariable and probabilistic sensitivity analyses.
Results.
The total mean cost per‐patient of nivolumab and ipilimumab versus sunitinib was $292,308 and $169,287, respectfully. Nivolumab and ipilimumab generated a gain of 0.978 QALYs over sunitinib. The incremental cost‐effectiveness ratio (ICER) for nivolumab and ipilimumab was $125,739/QALY versus sunitinib.
Conclusion.
Our analysis established that the base case ICER in the model for nivolumab and ipilimumab versus sunitinib is below what some would consider the upper limit of the theoretical willingness‐to‐pay threshold in the U.S. ($150,000/QALY) and is thus estimated to be cost‐effective.
Implications for Practice.
This article assessed the cost‐effectiveness of nivolumab and ipilimumab versus sunitinib for treatment of patients with intermediate‐ to poor‐risk metastatic kidney cancer, from the U.S. payer perspective. It would cost $125,739 to gain 1 quality‐adjusted life‐year with nivolumab and ipilimumab versus sunitinib in these patients.
Introduction
Renal cell carcinoma (RCC) accounts for approximately 3.8% of newly diagnosed cancer worldwide [1]. The 5‐year overall survival (OS) rate for advanced RCC is 11.6% [1], and more effective treatments are needed. Antiangiogenesis therapy is the main first‐line therapy in advanced RCC [2], specifically the tyrosine kinase inhibitors—sunitinib and pazopanib.
The prognosis of advanced RCC is divided into favorable, intermediate, and poor risks based on well‐established clinical and laboratory factors. One commonly used and validated prognostic model is the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) model [3], [4]. Approximately 20%–25% of patients have favorable‐risk disease, 50% have intermediate‐risk disease, and 25%–30% have poor‐risk disease [3], [4]. In one trial, the median OS for patients receiving sunitinib or pazopanib in the favorable‐risk group was 43 months compared with 8–10 months in the poor‐risk group [5].
Sunitinib was approved for advanced RCC after showing a 6‐month benefit in the median progression‐free survival (PFS) compared with interferon alpha (IFN‐α) [6], [7]. Nivolumab, an immune checkpoint inhibitor, was approved for the second‐line setting on the basis of the CheckMate 025 study [8], demonstrating a survival advantage with reduced toxicity [9], [10].
Nivolumab with ipilimumab has shown high response rates and an OS benefit in the first‐line setting in advanced melanoma [9], and has been approved for this indication by the U.S. Food and Drug Administration (FDA) [10]. The CheckMate 214 trial examined this combination in previously untreated metastatic RCC compared with the standard of care, sunitinib. Patients in the combination group received nivolumab 3 mg/kg and ipilimumab 1 mg/kg every 3 weeks for four doses followed by nivolumab 3 mg/kg every 2 weeks until progression. Patients in the comparator group received sunitinib 50 mg once daily, in 6‐week cycles of 4 weeks on and 2 weeks off. Radiologic disease assessments were performed every 6 weeks during the trial. Patients were stratified according to IMDC risk score. A total of 425 out of 550 patients receiving nivolumab and ipilimumab, and 422 out of 546 receiving sunitinib, had intermediate or poor risk. Among the intermediate‐ and poor‐risk group, an OS advantage was seen with the nivolumab and ipilimumab combination over sunitinib (hazard ratio for death 0.63). Objective response was 42% with nivolumab and ipilimumab versus 27% with sunitinib, with a complete response seen in 9% versus 1%, respectively [11]. The safety profile of nivolumab and ipilimumab was consistent with that of previous reports [12], [13].
The objective of this study was to estimate the cost‐effectiveness of nivolumab and ipilimumab compared with sunitinib for first‐line treatment of poor‐to‐intermediate‐risk advanced RCC from the U.S. payer perspective.
Materials and Methods
Model Structure
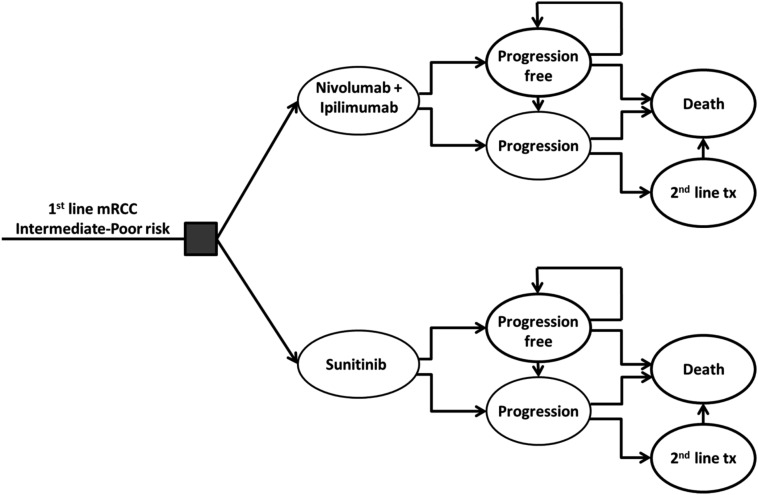
The Markov model involved an initial treatment decision with nivolumab and ipilimumab or sunitinib (Fig. 1). Patients then transitioned through different health states: stable/responsive (progression‐free) disease, progressive disease, and death. Each model cycle represented 1 month over a 10‐year time horizon. All patients started with stable, progression‐free disease and either remained at that stage or transitioned to progressive disease or death. Once in the progressive stage, patients could receive second‐line treatment or transition to death.
Figure 1.
Markov model.
Abbreviations: mRCC, metastatic renal cell carcinoma; tx, treatment.
The primary outputs of the model were cost and quality‐adjusted life years (QALYs), which were used to calculate the incremental cost‐effectiveness ratio (ICER). The Markov model was implemented in TreeAgePro 2016 software (TreeAge Software Inc., Williamstown, MA), and statistical analyses were performed in Matlab 2016‐B software (MathWorks Inc., Natick, MA).
Mortality Estimates
The probability for transition from a progression‐free state to a postprogression state was derived from the PFS curves in the CheckMate 214 trial. The probability for transition from any state to the death state was derived from the OS curves in the CheckMate 214 trial. We used the WebPlotDigitizer software (https://automeris.io/WebPlotDigitizer) to extract the data points from each PFS and OS plot from the CheckMate 214 trial, and these data points were then used to fit parametric models. Weibull distribution was used as it provided the best fit for all curves (supplemental online Figs. S1 and S2).
Utility Estimates
In order to compute the total QALYs in the Markov models, we adjusted the survival time by the health‐related quality of life. The health utility score was based on quality‐of‐life (QOL) data collected in the CheckMate 214 trial. In the trial, QOL was assessed every 4 weeks using the National Comprehensive Cancer Network Functional Assessment of Cancer Therapy–Kidney Symptom Index 19. The mean baseline score for intermediate‐ to poor‐risk patients was similar in both groups (60/76), and there was an improvement in QOL in the immunotherapy arm compared with sunitinib (p < .001) [11]. To translate these trial findings to the model, we used a previously published methodology [14]. In the model, we incorporated an average utility of 0.828 for patients in the immunotherapy arm and of 0.767 for patients in the sunitinib arm (supplemental online Table S3). We did not use disutilities, as the QOL data also reflects disutilities from disease progression and adverse events. We used ±10% as the boundaries of the range in sensitivity analyses.
Cost Estimates
Only direct medical costs were considered in the analysis including drug, administration, and adverse event (AE) costs. The cost of nivolumab and ipilimumab administration was calculated for intravenous treatment at doses of 1 mg per kg for ipilimumab and 3 mg per kg for nivolumab administered every 3 weeks for the first 3 months or until disease progression. After 3 months of treatment, cost was calculated for nivolumab administered every 2 weeks at a dose of 1 mg per kg until disease progression. To calculate doses, we used the U.S. mean body weight of 82 kg [13], [15]. The cost of sunitinib administration was calculated as 50 mg daily administered orally for 4 weeks and 2 weeks off for 6‐week cycles until disease progression.
We included in the model grade 3–4 AEs that had significantly different rates between the arms of the CheckMate 214 trial, had an incidence of at least 4%, and had an economic impact. These AEs included diarrhea and hypertension. The cost of fatigue, increased lipase, and hand‐foot syndrome were negligible and were not included. The treatment of AEs was estimated based on clinical experience and clinical guidelines [16]. We assumed that grade 3/4 diarrhea would be managed with a 3‐day hospitalization and will include a sigmoidoscopy in half of the patients, and that grade 3/4 hypertension would be managed with oral amlodipine 10 mg/day for 1 year. All costs and health outcomes were discounted by 3% annually for the U.S. We used prices that, to the best of our knowledge, account for nonconfidential discounts and rebates. Details of drug costs are available in Table 1 and in the supplemental online data.
Table 1. Model parameters: baseline values and ranges for sensitivity analysis.
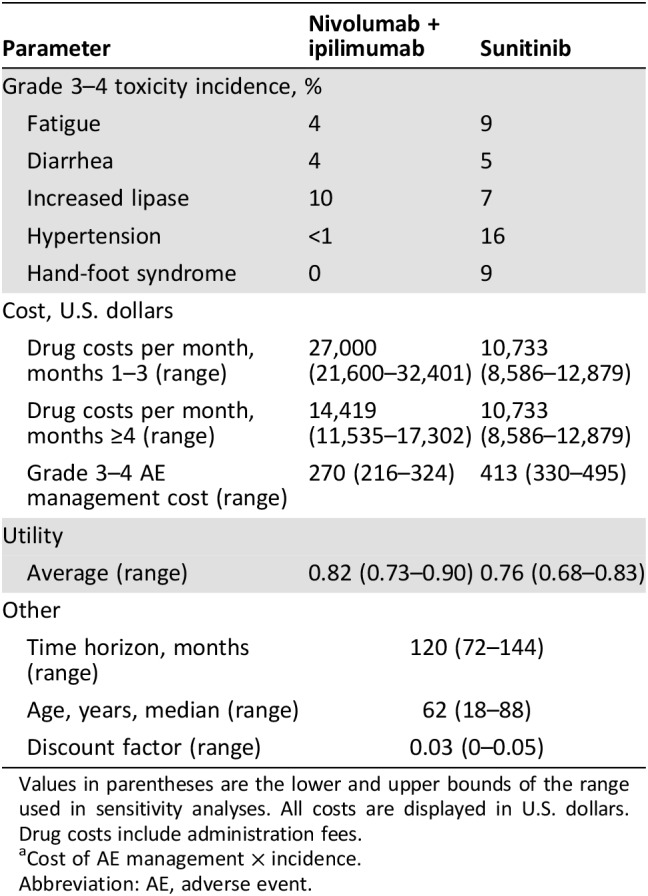
Values in parentheses are the lower and upper bounds of the range used in sensitivity analyses. All costs are displayed in U.S. dollars. Drug costs include administration fees.
Cost of AE management × incidence.
Abbreviation: AE, adverse event.
The estimated costs of treatment beyond progression (TBP) and of second‐line treatments are elaborated on in the supplemental online data.
Sensitivity Analysis
A series of sensitivity analyses was performed to evaluate the robustness of the model and to address the uncertainty in the estimation of variables as in a similar study [14] (supplemental online Appendix).
Structural Sensitivity Analysis
Wastage: To account for wastage and assuming no vial sharing, we used a fixed dose of 50 mg vials ×2 for ipilimumab, and one 240‐mg vial for nivolumab (as rounding [17] of ≤10% is a common practice and according to the FDA label) [10].
TBP: To account for differences in real‐world practice of TBP, we examined different proportions of patients continuing TBP.
Cabozantinib: To account for the use of cabozantinib for second‐line treatment in the real world [2], we examined different proportions of patients receiving second‐line cabozantinib.
Results
Base Case Results
Nivolumab and ipilimumab generated a gain of 0.978 QALYs over sunitinib in the U.S., with an incremental cost of $123,021. The ICER, meaning the additional cost of nivolumab and ipilimumab versus sunitinib, was $125,739 per QALY gained (Table 2).
Table 2. Base case results.
Abbreviations: ICER, incremental cost‐effectiveness ratio; QALY, quality‐adjusted life‐years.
Sensitivity Analyses
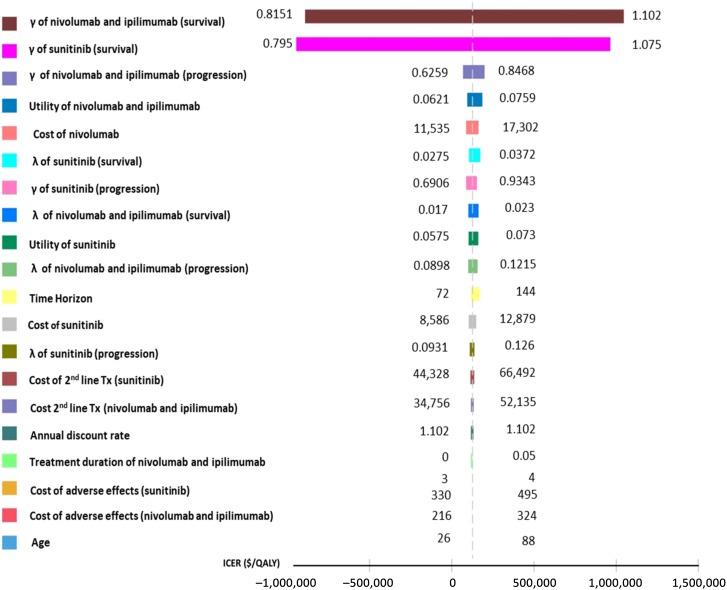
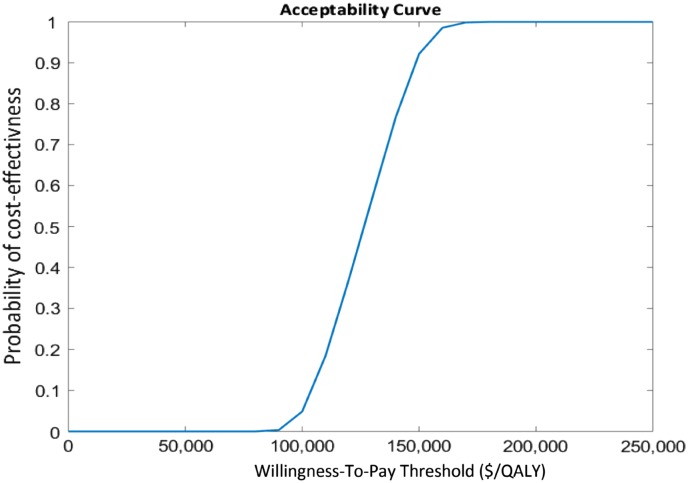
The results of univariate sensitivity analyses are presented in the tornado diagram (Fig. 2). The parameters with the greatest influence on the ICER were those of the overall survival extrapolation. The effects of other parameters were negligible. The results of the probabilistic sensitivity analyses are shown in the cost‐effectiveness acceptability curves (Fig. 3). These curves show the probability that nivolumab and ipilimumab is cost‐effective across increasing willingness‐to‐pay (WTP) thresholds. These results demonstrated >90% probability that nivolumab and ipilimumab is cost‐effective compared with sunitinib at WTP thresholds of $150,000 per QALY. Wastage had only a minor effect on the ICER (supplemental online Table S5). The proportion of patients treated beyond progression and the proportion of patients treated with second‐line cabozantinib had an effect on the ICER, which remained below the $150,000/QALY threshold (supplemental online Tables S6 and S7).
Figure 2.
Univariable sensitivity analyses.
Abbreviations: ICER, incremental cost‐effectiveness ratio; QALY, quality‐adjusted life‐year.
Figure 3.
Cost‐effectiveness acceptability curves in U.S. dollars.
Abbreviation: QALY, quality‐adjusted life‐year.
Discussion
We performed a cost‐effectiveness analysis of nivolumab and ipilimumab versus sunitinib in first‐line intermediate‐ to poor‐risk advanced RCC. The ICER for nivolumab and ipilimumab was estimated as $125,739/QALY versus sunitinib.
We performed our analysis only on the intermediate‐to‐poor‐risk group, even though the immunotherapy combination was found to have a survival benefit in the whole intention‐to‐treat population (favorable, intermediate, and poor risks) in the CheckMate 214 trial [11]. In the exploratory analysis of the favorable‐risk group in this trial, sunitinib was found to have a superior objective response rate and PFS compared with nivolumab and ipilimumab; therefore, the immunotherapy combination will not be part of common practice in this risk group.
The WTP threshold in the U.S. is considered to be in the range from $100,000 to $150,000/QALY for cancer drugs, and from $50,000 to $100,000/QALY for non‐cancer drugs [18], putting this treatment on the high end of the WTP threshold. Nevertheless, many cancer drugs, especially new biological agents, are in common use despite an ICER high above this threshold. For example, the combination of paclitaxel and ramucirumab for second‐line metastatic gastric cancer is estimated to have an ICER of $1,000,000/QALY [19].
Our approach was to only incorporate the costs where there was expected to be a difference between the two groups of patients. Therefore, some costs, such as regular follow‐up visits and regular computed tomography scans, were not incorporated, as they were considered to be the same between each arm of the model. Therefore, when reading the results, one should look only at the incremental differences in costs and ICER, and not at the total costs. The total costs in the model will not be fully representative of actual total costs in the real world.
Our analysis was limited by data availability and our assumptions. In the sensitivity analyses, we used a range for certain values to account for possible inaccuracies, as described above. The use of the ±10% boundary for the utility sensitivity analysis may not reflect the true uncertainty but nevertheless is used as an acceptable boundary in similar studies [14]. One limitation of this model is that it is based upon clinical trial patients as opposed to real‐world patients, where the outcomes may be substantially different. Ideally the model would incorporate real‐world data; however, as this regimen was only recently approved for kidney cancer, no such real‐world data currently exist.
An important issue in cost‐effective analysis is selecting the appropriate standard of care for the comparator arm in the model. According to the National Comprehensive Cancer Network guidelines, there are several preferred options for first‐line therapy in RCC, making this a challenge. The CheckMate 214 trial used sunitinib as the competitor arm as it was the standard of care at the time of the beginning of the trial.
Sunitinib has been assessed for cost‐effectiveness in the U.S. and has been found to be cost‐effective, with an ICER of $52,593 per QALY gained compared with IFN‐α [20]. An economic evaluation comparing sunitinib with newer treatments including IFN and bevacizumab and sorafenib in the U.S. and Sweden has found sunitinib to be cost‐effective [21].
Our study evaluated the cost‐effectiveness of the traditional 4 weeks on followed by 2 weeks off as performed in the CheckMate 214 trial. Alternate sunitinib schedules, such as 2 weeks on followed by 1 week off, may influence efficacy outcomes, the adverse‐event profile, and adherence to therapy [22]. Two phase II trials demonstrated less toxicity and a lower discontinuation rate with comparable efficacy [23], [24]. Whether alternative schedules change the cost‐effectiveness of sunitinib is unknown.
The cost of grade 3–4 toxicity management was lower in the nivolumab and ipilimumab arm than the sunitinib arm because of lower incidence rate, similar to a different analysis [25]. The data regarding toxicity incidence are limited by the fact that they are based on a single trial.
The long‐term efficacy of nivolumab and ipilimumab in RCC remains unknown. We await more mature data to see if there is a plateau on the tail of the survival curve, as seen in long‐term follow‐up for immunotherapy in melanoma [9]. At the 2016 American Society of Clinical Oncology congress, data of long‐term OS with second‐line nivolumab from phase I and II studies were presented. In the phase Ib CheckMate 003 trial, the 3‐ and 5‐year OS rates were 41% and 34%, and in the phase II CheckMate 010 trial, the 3‐year OS rate was 35% [26], giving some hope for long‐term survival with checkpoint inhibitor treatments in RCC.
The long‐term survival data for the model are an extrapolation, and limit our confidence in the results. Although we currently have no way of knowing the true long‐term survival data with nivolumab and ipilimumab, there are some ways to potentially hypothesize the long‐term survival with sunitinib. One study [27] reported long‐term efficacy of sunitinib pooled from six randomized controlled trials; however, the follow‐up period was only ~40 months, which was insufficient to validate our extrapolation, which has a 10‐year time horizon. A large real‐world study [28] of sunitinib had follow‐up of up to 70 months; however, as this was a real‐world study, the patients included would have been substantially different than those included in the trial. Both studies included patients with good‐risk disease, which also influences the results and makes any attempt at validation more difficult.
The CheckMate 214 trial investigators performed an exploratory analysis according to programmed death‐ligand 1 (PD‐L1) expression levels. In the intermediate‐to‐poor‐risk group, 26% of the immunotherapy group and 29% of the sunitinib group had PD‐L1 levels >1%. Among the intermediate‐to‐poor‐risk group, overall survival was longer with nivolumab and ipilimumab across PD‐L1 expression levels. The survival advantage and response rates were more pronounced for the immunotherapy arm among patients with >1% PD‐L1 levels. PFS was not different between arms in patients with PD‐L1 levels <1%, but showed a large advantage in patients with PD‐L1 > 1% (median 22.8 vs. 5.9 months) [11].
We did not perform an analysis on this subgroup, but we speculate that because of the better progression‐free survival and overall survival data the ICER for this group would potentially be lower than estimated in our study. The immunotherapy arm had an advantage across PD‐L1 expression levels, so PD‐L1 testing does not give us predictive information (according to the current data). In practice, this treatment would be given to all patients with poor‐to‐intermediate risk regardless of PD‐L1 levels.
It is common practice to continue treatment with checkpoint inhibitors beyond first progression in selected cases [29], which may affect the results of our analysis. In a previous study, up to 48% of patients were treated with nivolumab beyond progression in second line RCC, with 13% of them experiencing a subsequent response (i.e., ≥30% tumor burden reduction) [30]. This example demonstrates that there may be clinical benefit of this practice in selected patients. There are no clear guidelines regarding TBP, and the decision depends on the clinical judgement of the treating physicians. In the CheckMate 214 trial, 29% of patients in the nivolumab and ipilimumab group and 24% of those in the sunitinib group received TBP. As TBP may offer some clinical benefit only in a minority of patients, if at all, it is costly from an economic point of view. TBP was taken into account in our model, but real‐world practices may be different from those in this trial.
The CheckMate 214 trial did not have a crossover design, meaning patients in the sunitinib arm did not receive the option to be treated with nivolumab and ipilimumab upon progression or at any later stage in the study. The most common therapies given to patients in the sunitinib arm that progressed were nivolumab as a single agent (27%) and axitinib (19%), and in the nivolumab and ipilimumab arm were sunitinib (21%) and axitinib (19%). Nivolumab and ipilimumab was not evaluated in the second‐line setting, and whether dual checkpoint inhibition in the second‐line setting is cost effective is unknown.
Conclusion
Our analysis established that the base case ICER in the model for nivolumab and ipilimumab versus sunitinib is below what some would consider the upper limit of the theoretical WTP threshold in the U.S. ($150,000/QALY) and is thus estimated to be cost‐effective.
See http://www.TheOncologist.com for supplemental material available online.
Contributed equally
Author Contributions
Conception/design: Daniel Reinhorn, Michal Sarfaty, Victoria Neiman, Eli Rosenbaum, Daniel A. Goldstein
Provision of study material or patients: Daniel Reinhorn, Michal Sarfaty
Collection and/or assembly of data: Daniel Reinhorn, Michal Sarfaty, Assaf Moore, Victoria Neiman
Data analysis and interpretation: Michal Sarfaty, Moshe Leshno, Victoria Neiman, Eli Rosenbaum, Daniel A. Goldstein
Manuscript writing: Daniel Reinhorn, Michal Sarfaty, David A. Goldstein
Final approval of manuscript: Daniel Reinhorn, Michal Sarfaty, Moshe Leshno, Assaf Moore, Victoria Neiman, Eli Rosenbaum, Daniel A. Goldstein
Disclosures
The authors indicated no financial relationships.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Jonasch E, Agarwal N et al. Kidney Cancer, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:804–834. [DOI] [PubMed] [Google Scholar]
- 3.Heng DY, Xie W, Regan MM et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor‐targeted agents: Results from a large, multicenter study. J Clin Oncol 2009;27:5794–5799. [DOI] [PubMed] [Google Scholar]
- 4.Heng DY, Xie W, Regan MM, et al. External validation and comparison with other models of the International Metastatic Renal‐Cell Carcinoma Database Consortium prognostic model: A population‐based study. Lancet Oncol 2013;14:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motzer RJ, Hutson TE, McCann L et al. Overall survival in renal‐cell carcinoma with pazopanib versus sunitinib. N Engl J Med 2014;370:1769–1770. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Hutson TE, Tomczak P et al. Sunitinib versus interferon alfa in metastatic renal‐cell carcinoma. N Engl J Med 2007;356:115–124. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Hutson TE, Tomczak P et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:3584–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.FDA . Nivolumab ‐ FDA approval. FDA. 2015. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125554s058lbl.pdf
- 9.Wolchok JD, Chiarion‐Sileni V, Gonzalez R et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.FDA 2018. OPDIVO (nivolumab) injection, for intravenous use. www.fda.gov/medwatch. Accessed July 16, 2018.
- 11.Motzer RJ, Tannir NM, McDermott DF et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal‐cell carcinoma. N Engl J Med 2018;378:1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammers HJ, Plimack ER, Infante JR et al. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: The CheckMate 016 study. J Clin Oncol 2017;35:3851–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodi FS, Chesney J, Pavlick AC et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2‐year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 2016;17:1558–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarfaty M, Leshno M, Gordon N et al. Cost effectiveness of nivolumab in advanced renal cell carcinoma. Eur Urol 2018;73:628–634. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention . Body Measurements. https://www.cdc.gov/nchs/fastats/body‐measurements.htm. Accessed July 16, 2018.
- 16.Haanen JBAG, Carbonnel F, Robert C et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2017;28(suppl 4):iv119–iv142. [DOI] [PubMed] [Google Scholar]
- 17.Fahrenbruch R, Kintzel P, Bott AM et al. Dose rounding of biologic and cytotoxic anticancer agents: A position statement of the Hematology/Oncology Pharmacy Association. J Oncol Pract 2018;14:e130–e136. [DOI] [PubMed] [Google Scholar]
- 18.Bae YH, Mullins CD. Do value thresholds for oncology drugs differ from nononcology drugs? J Manag Care Pharm 2014;20:1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam SW, Wai M, Lau JE et al. Cost‐effectiveness analysis of second‐line chemotherapy agents for advanced gastric cancer. Pharmacother J Hum Pharmacol Drug Ther 2017;37:94–103. [DOI] [PubMed] [Google Scholar]
- 20.Remák E, Charbonneau C, Négrier S et al. Economic evaluation of sunitinib malate for the first‐line treatment of metastatic renal cell carcinoma. J Clin Oncol 2008;26:3995–4000. [DOI] [PubMed] [Google Scholar]
- 21.Benedict A, Figlin RA, Sandström P et al. Economic evaluation of new targeted therapies for the first‐line treatment of patients with metastatic renal cell carcinoma. BJU Int 2011;108:665–672. [DOI] [PubMed] [Google Scholar]
- 22.Kalra S, Rini BI, Jonasch E. Alternate sunitinib schedules in patients with metastatic renal cell carcinoma. Ann Oncol 2015;26:1300–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jonasch E, Slack RS, Geynisman DM et al. Phase II study of two weeks on, one week off sunitinib scheduling in patients with metastatic renal cell carcinoma. J Clin Oncol 2018;36:1588–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JL, Kim MK, Park I et al. RandomizEd phase II trial of Sunitinib four weeks on and two weeks off versus Two weeks on and One week off in metastatic clear‐cell type REnal cell carcinoma: RESTORE trial. Ann Oncol 2015;26:2300–2305. [DOI] [PubMed] [Google Scholar]
- 25.Betts K, Yang S, Du EX et al. Comparison of adverse event costs of nivolumab combined with ipilimumab versus sunitinib for previously untreated metastatic renal cell carcinoma. J Clin Oncol 2018;36(suppl 15):e16561–e16561. [Google Scholar]
- 26.McDermott DF, Motzer RJ, Atkins MB et al. Long‐term overall survival (OS) with nivolumab in previously treated patients with advanced renal cell carcinoma (aRCC) from phase I and II studies. J Clin Oncol 2016;34(suppl 15):4507a. [Google Scholar]
- 27.Motzer RJ, Escudier B, Bukowski R et al. Prognostic factors for survival in 1059 patients treated with sunitinib for metastatic renal cell carcinoma. Br J Cancer 2013;108:2470–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gore ME, Szczylik C, Porta C et al. Final results from the large sunitinib global expanded‐access trial in metastatic renal cell carcinoma. Br J Cancer 2015;113:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blumenthal GM, Theoret MR, Pazdur R. Treatment beyond progression with immune checkpoint inhibitors—known unknowns. JAMA Oncol 2017;3:1473–1474. [DOI] [PubMed] [Google Scholar]
- 30.Escudier B, Motzer RJ, Sharma P et al. Treatment beyond progression in patients with advanced renal cell carcinoma treated with nivolumab in CheckMate 025. Eur Urol 2017;72:368–376. [DOI] [PubMed] [Google Scholar]