Sinonasal intestinal‐type adenocarcinomas are rare tumors with no standard treatment. This article presents a case of recurrent nasoethmoidal intestinal‐type adenocarcinoma with a mutation in the KRAS exon 2 codon 12. This case exemplifies how liquid biopsy can aid in the correct and real‐time molecular characterization of tumors even in a rare non‐metastatic cancer of the head and neck.
Abstract
Sinonasal intestinal‐type adenocarcinomas (SNS‐ITAC) are very rare tumors that resemble colorectal cancer in many of their pathological and molecular characteristics. Indeed, in most published series, 10%–14% of SNS‐ITAC harbor mutations in KRAS. There is no standard systemic treatment in recurrent or metastatic SNS‐ITAC, and there is no evidence of the use of any targeted agent in this entity. We present the case of a recurrent nasoethmoidal ITAC informed as RAS and BRAF wild‐type by standard real‐time polymerase chain reaction methods and treated with first‐line cetuximab and irinotecan without response. Circulating tumor cells coupled to highly sensitive DNA analyses unveiled a mutation in KRAS exon 2 codon 12. Subsequent studies in the primary tumor using BEAMing detected a mutation in the same codon, confirming the KRAS mutated status of the tumor, and possibly explaining the absence of treatment response. This case exemplifies how liquid biopsy can aid in the correct and real‐time molecular characterization of tumors even in a rare nonmetastatic cancer of the head and neck.
Key Points.
Sinonasal intestinal type adenocarcinomas (SNS‐ITAC) are rare tumors that commonly develop after a prolonged exposure to organic dusts (wood, leather, etc.), and that resemble colorectal cancer in some of their morphological and molecular characteristics.
KRAS mutations have been described in 10%–14% in most series. However, its predictive value for guiding treatment decisions with targeted therapies (i.e., anti‐epidermal growth factor receptor [EGFR] therapy) has not been defined.
The first case of an SNS‐ITAC treated with anti‐EGFR therapy (cetuximab) is reported. Analysis of DNA from circulating tumor cells (CTCs) unveiled a mutation in KRAS not detected by standard methods in the primary tumor. However, RAS analysis using BEAMing detected a mutation in the primary tumor in the same codon of KRAS originally detected in CTCs, altogether possibly explaining the lack of treatment response.
Liquid biopsy may allow for an accurate molecular diagnosis in rare, organ‐confined tumors where few therapeutic options exist. Highly sensitive molecular diagnostics may aid in better characterizing rare entities harboring potentially druggable targets.
Patient Story
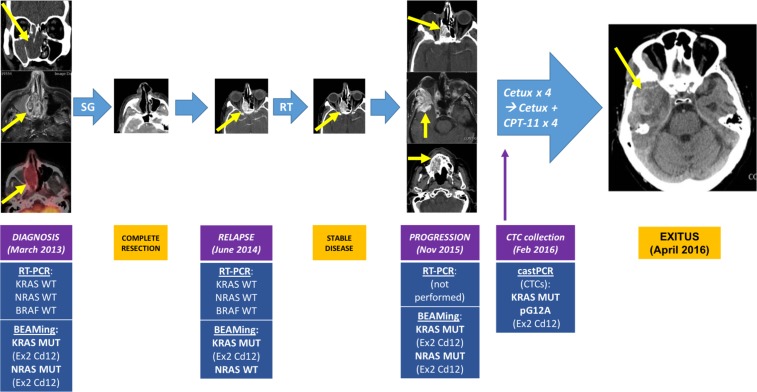
An 86‐year‐old man was diagnosed in March 2013 with a polypoid mass that obstructed the right nasal cavity and informed as an intestinal‐type adenocarcinoma (ITAC) that initially led to suspicion of a colorectal cancer (CRC) metastasis. He had worked as a cabinetmaker for more than 45 years, and had smoked 4–5 cigarettes per day for 10 years until 30 years of age. He denied exposure to other toxins or use of illicit drugs. Since 2011, he was diagnosed with a presumed prostate cancer because of raising prostate‐specific antigen levels, for which he had rejected a biopsy or any local treatment, and was receiving bicalutamide with a sustained biochemical response. Magnetic resonance imaging (MRI) showed an expansive, right nasoethmoidal, well‐delimitated, solid mass of approximately 3 cm, extending toward the sphenoid bone without infiltrating it and with low‐to‐moderate 18fluorine‐fluorodeoxyglucose (18FDG) uptake with a maximum standardized uptake value (SUVmax) of 4.4 on positron emission tomography/computed tomography (PET/CT), which also informed of an 18 × 13‐mm lesion in the sigma with high FDG uptake (SUVmax 31.7; Fig. 1). Colonoscopy confirmed the existence of a large polyp in the sigma, which was completely resected and reported as an adenomatous polyp with high‐grade dysplasia.
Figure 1.
Clinical history and molecular correlates of the patient's sinonasal intestinal‐type adenocarcinoma. Patient story: At diagnosis in March 2013, a right nasoethmoidal solid mass of 3 cm that extended toward the sphenoid sinus was seen, with low 18F‐Fluorodeoxyglucose uptake on positron emission tomography/computed tomography scan (SUVmax 4.4). In June 2014, the tumor relapsed at the right nasal fossa affecting the right maxillary sinus. Radiation therapy was given, achieving stable disease. In November 2015, the tumor progressed with intracranial extension toward the right infratemporal fossa and a second enhancing mass invading the hard palate. It was considered unresectable and RAS and BRAF studies performed using RT‐PCR informed of a KRAS, NRAS, and BRAF wild‐type tumor. Systemic treatment with cetuximab and low‐dose CPT‐11 were administered for 8 consecutive weeks without response. The patient died in April 2016 due to progression of the intracranial component of the tumor. Liquid biopsy and BEAMing studies: CTCs were collected 6 days after the first dose of cetuximab, and IsoFlux system was used for CTC detection, finding 26 CTCs. Among the seven different mutations detectable by castPCR, a mutation was found in KRAS exon 2 codon 12 (pG12A) in DNA from CTCs. Subsequently, BEAMing was performed on the different primary tumor samples, finding mutations in KRAS exon 2 codon 12, and in NRAS exon 2 codon 12. For more details, please refer to the main text and to Figure 3.Abbreviations: BEAMing, Beads, Emulsion, Amplification, and Magnetics; Cd, codon; Cetux, Cetuximab; CPT‐11: Camptothecin‐11 also known as Irinotecan; CTCs, circulating tumor cells; Ex, exon; Feb, February; MUT, mutant; Nov, November; RT, radiotherapy; RT‐PCR, real‐time polymerase chain reaction; SG, surgery; WT, wild‐type.
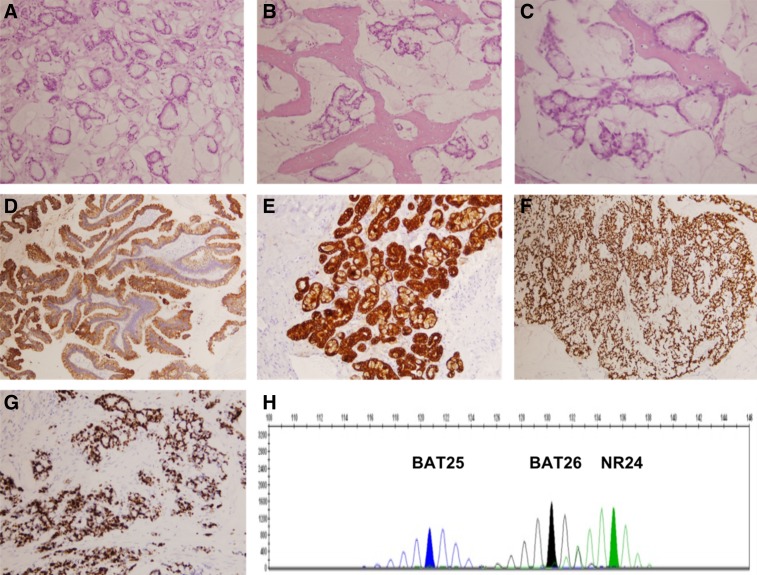
Tumor resection through a lateral rhinotomy informed of an intestinal‐type adenocarcinoma infiltrating the mucosa of the ethmoid and frontal sinuses without bone invasion, pT4 cN0 cM0, stage IVA (American Joint Committee on Cancer 7th edition). It was microsatellite stable, and neoplastic cells expressed CDX2, CK20, and CK7 and showed a high proliferative index (Ki 67 70%; Fig. 2). Adjuvant radiotherapy (RT) was disregarded because of its presumed high risk‐benefit ratio in a very elderly patient with a stage IVA tumor, and close follow‐up was initiated.
Figure 2.
Pathology of the patient's sinonasal intestinal‐type adenocarcinoma. (A–C): (hematoxylin and eosin) Malignant cells forming abnormal glands surrounded by mucinous material, resembling mucinous colorectal adenocarcinoma. The tumor infiltrates surrounding bone. (D–H): Tumor cells intensely stain for CK20 (D), CK7 (E), and CDX2 (F) and show a high proliferative index measured by Ki67 (>70%) (G). Microsatellite study shows that the tumor is “stable” (H). For more details, please refer to the main text.
18F‐FDG PET/CT in March 2014 showed a 2.6‐cm tumor relapse in the surgical bed with SUVmax 4.5. Biopsy informed of an intestinal type mucosecretory adenocarcinoma. Radical RT with a total dose of 50.4 Gy in 28 fractions was given between July and August 2014, achieving stable disease. In November 2015, MRI and 18F‐FDG PET/CT showed a new 3‐cm mass with SUVmax 6.0, invading the lateral wall of the right orbit, the ethmoid and right temporal bone, with a soft tissue component invading the right temporal fossa. In addition, a new lesion was seen extending to the right hard palate with SUVmax 6.1 (Fig. 1). An ultrasound‐guided fine‐needle aspiration of the soft tissue component in the temporal fossa informed of a well‐differentiated adenocarcinoma.
A new MRI in February 2016 informed of a substantial intracranial and extracranial tumor progression. Although already an 88‐year‐old man, the patient was in good condition, went out daily for 30‐minute walks, and only referred right eye visual loss with light orbital pain. Molecular studies in biopsy specimens from 2013 and 2014 informed the tumor as KRAS, NRAS, and BRAF wild‐type. Dexamethasone and the anti‐epidermal growth factor receptor (EGFR) agent cetuximab were started, with the patient receiving 4 weeks of treatment with excellent tolerance but no clinical improvement. Twice‐weekly reduced‐dose irinotecan was added (125 mg/m2 every 2 weeks), with the patient receiving two doses combined with weekly cetuximab, with good tolerance (grade 1 diarrhea) but no efficacy. Unfortunately, 10 weeks after starting treatment, the patient died of tumor progression with bleeding of the intracranial component.
Six days after the first dose of cetuximab and before the administration of the second dose, the patient gave consent and blood was drawn for the study of circulating tumor cells (CTCs), detecting 26 CTCs. Mutational studies of seven common KRAS mutations in CRC using castPCR on DNA from CTC unveiled a mutation in KRAS (pG12A).
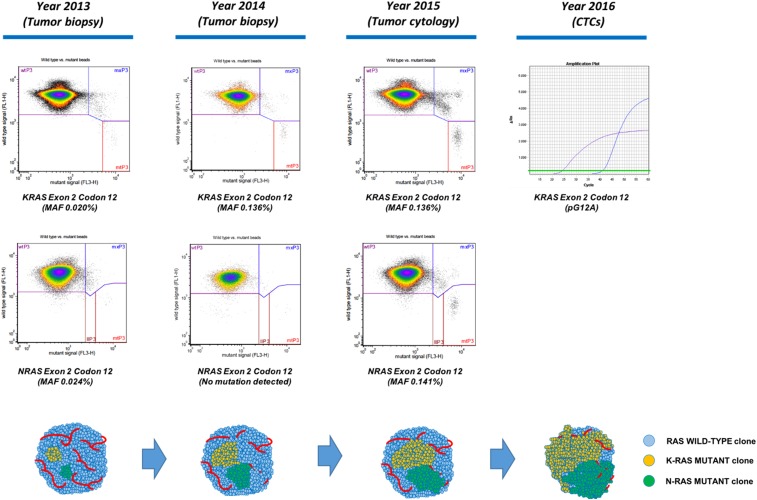
After the above results, BEAMing was used to study up to 34 KRAS and NRAS mutations common in CRC in tumor samples from 2015, 2014, and 2013, detecting mutations in KRAS exon 2 codon 12 and NRAS exon 2 codon 12 that increased in frequency along tumor evolution (Figs. 1, 3).
Figure 3.
RAS mutations detected in primary tumor and CTCs, using, respectively, BEAMing and castPCR, and proposed model of tumor evolution. Tumor biopsies from 2013 and 2014 and a tumor cytology sample from 2015 were used for RAS analysis with BEAMing. castPCR was used for the study of KRAS mutations in CTCs. Mutations in KRAS and NRAS were already detectable in the tumor biopsy from 2013, with MAF above the established limit of detection for BEAMing (MAF ≥0.01%–0.02%). MAF for KRAS and NRAS increased progressively along tumor evolution achieving MAF ≥0.1%, possibly reflecting the selection of RAS mutant clones over the wild‐type component of the tumor and allowing for a significant shedding of RAS‐mutant tumor cells to the peripheral blood. Studies in advanced colorectal cancer have shown that RAS‐mutant tumors with MAF ≥0.1% do not benefit from anti‐epidermal growth factor receptor therapy, thus possibly explaining the absence of tumor response seen in our patient. BEAMing is considerably more sensitive than castPCR. In contrast to castPCR, BEAMing informs of the mutated codon but not of the specific mutated locus. For more details, please refer to the main text.Abbreviations: CTCs, circulating tumor cells; MAF, mutated allele frequency.
Molecular Tumor Board
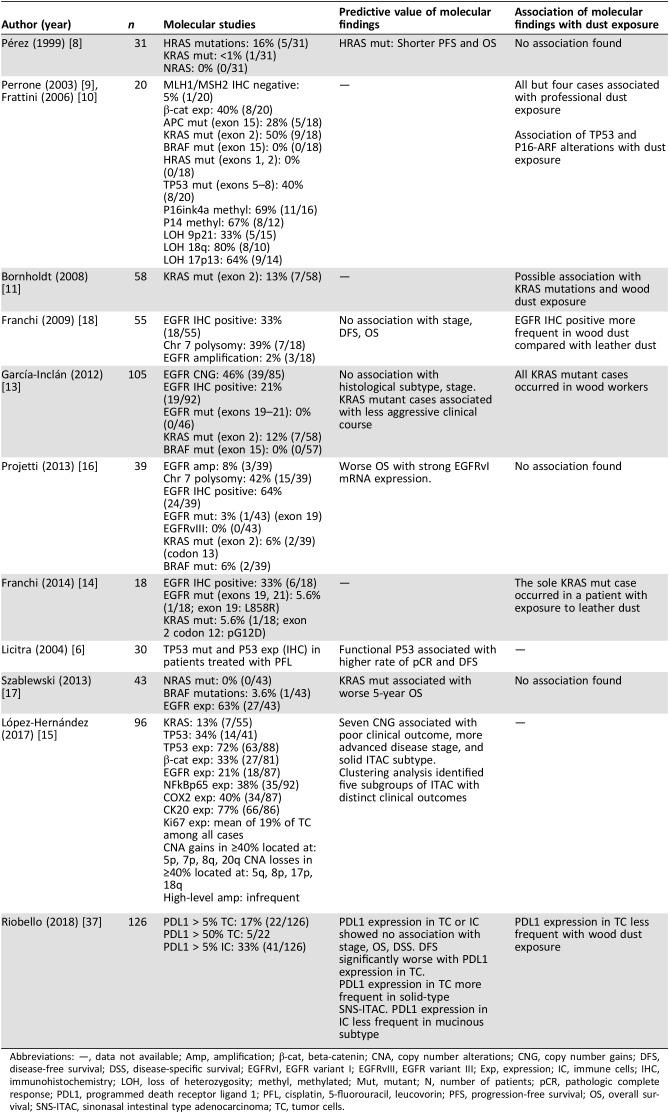
Sinonasal (SNS) adenocarcinomas occur in 1–1.5 of 100,000 persons, accounting for 13% of SNS malignancies. They are usually diagnosed with locally advanced disease, thus presenting a poor prognosis with high rates of relapse even after radical surgery and radiotherapy, with 5‐year survival rates of 25%–45%. Given the rarity of the disease, only one trial has studied, in the neoadjuvant setting, the role of systemic chemotherapy in SNS‐ITAC; therefore, data are lacking for its role in the recurrent or metastatic setting [1], [2], [3], [4], [5], [6], [7]. SNS‐ITAC resembles primary CRC, accounts for half of SNS adenocarcinomas, and is considered a professional disease. It commonly develops in the ethmoid sinus or upper part of the nasal cavity as a result of a long‐lasting exposure to wood, leather, or other organic dusts that leads to a chronic inflammatory reaction with intestinal metaplasia [1], [2], [8]. Most series report that around 10%–14% of SNS‐ITAC harbor mutations in KRAS, and up to 16% in HRAS [1], [9], [10], [11], [12], [13], [14], [15], [16]. BRAF seems to be wild‐type in most cases, although other relevant molecular markers in CRC such as microsatellite instability or NRAS mutational status have only been anecdotally studied in SNS‐ITAC [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20] (Table 1). Despite the evident similarities with CRC, there is almost no evidence of the efficacy of systemic agents used in CRC in advanced SNS‐ITAC [4], [5], [6]. Besides, contrary to CRC, where liquid biopsy studies have shown very promising results, there is no evidence of its potential role in SNS‐ITAC [21], [22], [23], [24].
Table 1. Most relevant series conducting molecular studies in SNS‐ITAC.
Abbreviations: —, data not available; Amp, amplification; β‐cat, beta‐catenin; CNA, copy number alterations; CNG, copy number gains; DFS, disease‐free survival; DSS, disease‐specific survival; EGFRvI, EGFR variant I; EGFRvIII, EGFR variant III; Exp, expression; IC, immune cells; IHC, immunohistochemistry; LOH, loss of heterozygosity; methyl, methylated; Mut, mutant; N, number of patients; pCR, pathologic complete response; PDL1, programmed death receptor ligand 1; PFL, cisplatin, 5‐fluorouracil, leucovorin; PFS, progression‐free survival; OS, overall survival; SNS‐ITAC, sinonasal intestinal type adenocarcinoma; TC, tumor cells.
Genotyping Studies and Interpretation of the Molecular Results
For CTC enumeration and recovery, peripheral blood was collected in two 10‐mL EDTA tubes (BD Vacutainer) 6 days after the first dose of cetuximab. Epithelial cell adhesion molecule (EpCAM)‐positive recovered cells were scored as CTC if they were CK+, CD45−, nucleated, and morphologically intact, as previously described [21]. Imaging was performed using a fluorescence microscope kindly provided by Izasa Scientific (WerfenLife, L'Hospitalet de Llobregat, Barcelona, Spain).
Competitive Allele Specific Technology (castPCR) was used for the study of seven codon 12 and codon 13 KRAS mutations in DNA from CTC. Analysis of purified DNA was performed by means of the TaqMan Mutation Detection Assay (ThermoFisher Scientific Inc. Waltham, MA), and the Applied Biosystems 7900HT Fast Real‐Time PCR System (Life Technologies Corporation, Foster City, CA) was used to detect mutations, as previously described [21], [25].
Real Time‐PCR (Idylla, Belgium) was used for the study of mutations in KRAS (exons 2 and 3), NRAS (exons 2, 3, and 4) and BRAF (V600E mutation) in the primary tumor biopsies from 2013 and 2014, as previously described [26], [27].
BEAMing (Sysmex Inostics) was used for the study of mutations in KRAS (exons 2 and 3) and NRAS (exons 2, 3, and 4) in the primary tumor biopsies from 2013 and 2014 and from the pre‐cetuximab tumor cytology obtained in 2015, as previously described [22], [23].
Being ineligible for further local treatment, and having no standard chemotherapy options for this type of cancer, we studied the patient's tumor for RAS and BRAF in two biopsies taken 34 and 21 months prior to cetuximab treatment. The tumor turned out to be RAS and BRAF wild‐type by both RT‐PCR (Idylla), which shows 1%–5% sensitivity for the detection of mutant alleles [26], [27]. DNA from CTCs collected 6 days after the first dose of cetuximab, and analyzed for seven different KRAS mutations using castPCR, unveiled a mutation in KRAS exon 2 codon 12 consisting of a G ➔ C transition (pG12A), not previously described in SNS‐ITAC but reported in up to 6% of CRC, where they may portend a worse prognosis [21], [25], [28], [29]. BEAMing has down to 0.01%–0.02% sensitivity to detect RAS mutations in plasma, and a cutoff ≥0.1% has been shown to reliably detect RAS mutations in solid tumor samples of patients with CRC [22], [23], [30], [31]. Therefore, we used BEAMing to study RAS status in the pre‐cetuximab tumor cytology, and found mutations in KRAS exon 2 codon 12 (mutated allele frequency [MAF] 0.136%), and NRAS exon 2 codon 12 (MAF 0.141%). Interestingly, BEAMing also detected mutations in KRAS exon 2 codon 12 (MAF 0.020%) and NRAS exon 2 codon 12 (MAF 0.024%) in the tumor biopsy taken in 2013, 28 months prior to the current relapse, as well as a KRAS mutation in exon 2 codon 2 in the biopsy from 2014 (MAF 0.136%). Altogether, these findings suggest that RAS‐mutant clones became progressively more numerous along tumor evolution, and, in part, may explain the absence of treatment benefit seen in our patient (Fig. 3). Indeed, in RAS wild‐type metastatic CRC, tumor evolution and treatment pressure may favor the emergence of RAS mutations [32], [33]. In addition, when BEAMing is used for RAS analysis in tumor samples from patients with CRC, tumors showing RAS mutations with MAF ≥0.1% do not benefit from anti‐EGFR therapy and may even be deleterious [30], [31]. Finally, as shown in our case, multiple coexisting RAS mutations have also been described in tumor samples of CRC using BEAMing, reflecting the existence of intratumor heterogeneity [30], [31].
Functional and Clinical Significance of RAS Mutations in SNS‐ITAC
No comprehensive functional studies have been undertaken in SNS‐ITAC to confirm if the EGFR/RAS pathway behaves as in CRC, and therefore, there is insufficient evidence yet to acknowledge if RAS status may have the same predictive value as in the latter [1], [30], [31], [34], [35]. However, 21%–64% of SNS‐ITAC express EGFR, and up to 46% show EGFR copy number gains. According to the existing series, KRAS mutations occur in 10%–14% of cases in most series but have been reported in up to 50% of SNS‐ITAC, and BRAF mutations are very infrequent; to our knowledge, only two groups have previously studied NRAS status in SNS cancers, finding no mutations among 31 and 43 cases, respectively [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]. Similar to CRC, where EGFR mutations occur in very rare instances, only 1 in a series of 18 cases and 2 of a series of 39 cases of SNS‐ITAC harbored a mutation in EGFR, one of them with an L858R mutation, which predicts response to tyrosine kinase inhibitors in non‐small cell lung cancer [15], [17]. Finally, other EGFR mutations that may predict resistance to cetuximab in CRC, such as the S492R, have not been reported in SNS‐ITAC [36]. Given the known mutational effects of RT, it would be of interest to compare the status of RAS, BRAF, EGFR, and other potential tumor drivers in pre‐ and post‐RT samples of SNS‐ITAC [37]. However, in a small series of 17 patients with rectal cancer, KRAS and MSI results did not change between pre‐ and post‐chemoRT biopsies [38]. In our case, RAS mutations were already detectable with BEAMing at initial diagnosis and showed increases in MAF before and after RT. Therefore, a possible contribution of RT to EGFR resistance cannot be overlooked. Unfortunately there are very few reports on the use of systemic chemotherapy in nasal or paranasal non‐maxillary sinus cancers [7], [39], [40]. In a recently published series of 743 patients with adenocarcinoma of the nasal cavity and paranasal sinuses from the Surveillance, Epidemiology, and End Results database, none had received chemotherapy, including 34 with ITAC. To our knowledge, only one trial has studied the use of systemic chemotherapy in SNS‐ITAC. Among thirty patients treated with cisplatin, infusional 5‐fluorouracil and leucovorin given every 3 weeks for five cycles, 12 patients achieved a pathologic complete response, and these responses strongly associated with a nonmutant p53 status and a better prognosis [7]. Given the important activity of platinum and 5FU combinations in p53 proficient SNS‐ITAC, other regimens active in CRC such as combinations with bevacizumab may be worth exploring because of their demonstrated activity independent of RAS status in patients with CRC [6], [34], [35].
Szablewski et al. [18] found no loss of expression of the mismatch‐repair (MMR) genes MLH1 and MSH2 in a series of 43 patients, suggesting that inherited or somatic mutations in MMR genes do not play a role in the pathogenesis of SNS‐ITAC. We found no reports on the tumor mutational burden of SNS cancers. However, it may be worth addressing this issue, given the dramatic benefit of immune checkpoint blockade in hyper‐mutated cancers regardless of their tissue of origin [41]. Interestingly, Riobello et al. [42] found expression of programmed death‐ligand 1 in tumor cells in 17% of 126 cases of SNS‐ITAC that increased to 33% when tested in immune cells, suggesting a potential benefit of PD(L)1 inhibitors in some cases of SNS‐ITAC.
There are some limitations to our study. Although there were no systemic therapies with demonstrated efficacy that could be given to our patient given his advanced age, it must be noted that anti‐EGFR therapy is not a proven treatment option in SNS‐ITAC, and RAS mutations can only theoretically be defined as a mechanism of resistance [2], [13], [14]. The patient had a clinical diagnosis of localized hormone‐sensitive prostate cancer for which he had rejected any local treatment. However, the patient was under androgen deprivation therapy showing a long‐standing biochemical response with a tumor confined to the prostate. Furthermore, although KRAS mutations in prostate cancer are rare events, in our patient, a KRAS mutation in exon 2 codon 12 was found in CTCs and in the solid tumor at different time points [43], [44], [45]. Thus, it is more likely that CTCs had stemmed from his SNS‐ITAC, an aggressive tumor with a natural history resembling that of an evolved CRC. Although clonal hematopoiesis is a concern in liquid biopsy studies, particularly in elderly patients, IsoFlux detects CTCs through EpCAM expression, an epithelial marker not present in bone marrow‐derived cells, and KRAS is not among the genes commonly mutated in this condition [46]. Finally, we did not use the same methodologies for RAS analysis in CTCs and in the tumor samples, but all are highly sensitive technologies previously used in molecular analysis and liquid biopsy studies in CRC [21], [22], [23], [25], [30], [31].
Rare cancers are defined by an incidence of fewer than six new cases per 100,000 persons, but account, as a whole, for 22% of all malignancies. Efforts should be made in promoting national and international registries and cooperative groups for conducting biomarker studies, including liquid biopsy as a diagnostic and monitoring tool. These ancillary studies should particularly be promoted during targeted therapy development for specific rare tumors [47], [48].
Conclusion
We report the first case of a recurrent nasoethmoidal ITAC treated with anti‐EGFR therapy, which was informed as RAS and BRAF wild‐type by standard methods. However, CTC analysis unveiled a KRAS mutation in exon 2 codon 12, coincident with that subsequently detected with BEAMing in the solid tumor. This case exemplifies how liquid biopsy may allow for a more accurate molecular diagnosis even in a rare cancer of the head and neck such as SNS‐ITAC.
Glossary of Genomic Terms and Nomenclature
- BEAMing
“Beads, Emulsion, Amplification, and Magnetics.” It has down to 0.01%–0.02% sensitivity for detection of common hot‐spot mutations in colorectal cancer. It does not inform of the precise locus where the mutation is located.
- castPCR
“Competitive Allele Specific Technology” PCR. It has down to 0.5%–1% sensitivity to detect somatic mutations.
- EGFR
“Epidermal growth factor receptor” is overexpressed in most colorectal cancers and in a substantial number of SNS‐ITAC.
- EpCAM‐based ISOFLUX system
an immunoaffinity‐based method for the detection of circulating tumor cells (CTCs). It detects CTCs expressing EpCAM and thus is most appropriate for studying CTCs in epithelial malignancies. It allows for the recovery of CTCs to perform molecular studies.
- KRAS, NRAS, and BRAF
“Ki‐ras2 Kirsten rat sarcoma viral oncogene homolog” (KRAS), “Neuroblastoma Ras viral oncogene homolog” (NRAS), and “v‐Raf murine sarcoma viral oncogene homolog B1” (BRAF) are crucial to the EGFR pathway. Mutation of either of these genes leads to the constitutive activation of the pathway downstream of EGFR, favoring tumor proliferation and survival and conferring resistance to EGFR‐targeted agents.
Acknowledgments
This work was carried out within the project PI16/01773 funded by Instituto de Salud Carlos III, Madrid, Spain. Reagents for CTC detection and recovery were kindly provided by Fluxion Biosciences, Inc., South San Francisco, CA, USA.
Author Contributions
Conception/design: Santiago Cabezas‐Camarero
Provision of study material or patients: Santiago Cabezas‐Camarero, Virginia de la Orden García, Vanesa García‐Barberán, Ahmad Issa Subhi‐Issa, Pedro Pérez‐Segura, Eduardo Díaz‐Rubio
Collection and/or assembly of data: Santiago Cabezas‐Camarero, Virginia de la Orden García, Vanesa García‐Barberán, Beatriz Mediero‐Valeros, Inmaculada Bando‐Polaino, Salomé Merino Menéndez
Data analysis and interpretation: Santiago Cabezas‐Camarero, Virginia de la Orden García, Vanesa García‐Barberán, Beatriz Mediero‐Valeros, Pedro Pérez‐Segura, Eduardo Díaz‐Rubio
Manuscript writing: Santiago Cabezas‐Camarero
Final approval of manuscript: Santiago Cabezas‐Camarero, Virginia De la Orden García, Vanesa García‐Barberán, Beatriz Mediero‐Valeros, Ahmad Issa Subhi‐Issa, Patricia Llovet García, Inmaculada Bando‐Polaino, Salomé Merino Menéndez, Pedro Pérez‐Segura, Eduardo Díaz‐Rubio
Disclosures
Santiago Cabezas‐Camarero: Merck KGaA (Other: travel expenses). The other authors indicated no financial relationships.
References
- 1.Hoeben A, van de Winkel L, Hoebers F et al. Intestinal‐type sinonasal adenocarcinomas: The road to molecular diagnosis and personalized treatment. Head Neck 2016;38:1564–1570. [DOI] [PubMed] [Google Scholar]
- 2.Llorente JL, López F, Suárez C et al. Sinonasal carcinoma: Clinical, pathological, genetic and therapeutic advances. Nat Rev Clin Oncol 2014;11:460–472. [DOI] [PubMed] [Google Scholar]
- 3.Choussy O, Ferron C, Védrine PO et al. Adenocarcinoma of ethmoid: A GETTEC retrospective multicenter study of 418 cases. Laryngoscope 2008;118:437–443. [DOI] [PubMed] [Google Scholar]
- 4.Bossi P, Saba NF, Vermorken JB et al. The role of systemic therapy in the management of sinonasal cancer: A critical review. Cancer Treat Rev 2015;41:836–843. [DOI] [PubMed] [Google Scholar]
- 5.Knegt PP, Ah‐See KW, vd Velden LA et al. Adenocarcinoma of the ethmoidal sinus complex: Surgical debulking and topical fluorouracil may be the optimal treatment. Arch Otolaryngol Head Neck Surg 2001;127:141–146. [DOI] [PubMed] [Google Scholar]
- 6.Licitra L, Suardi S, Bossi P et al. Prediction of TP53 status for primary cisplatin, fluorouracil, and leucovorin chemotherapy in ethmoid sinus intestinal‐type adenocarcinoma. J Clin Oncol 2004;22:4901–4906. [DOI] [PubMed] [Google Scholar]
- 7.Kiliç S, Samarrai R, Kiliç SS et al. Incidence and survival of sinonasal adenocarcinoma by site and histologic subtype. Acta Otolaryngol 2018;138:415–421. [DOI] [PubMed] [Google Scholar]
- 8.Leivo I. Sinonasal adenocarcinoma: Update on classification, immunophenotype and molecular features. Head Neck Pathol 2016;10:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pérez P, Dominguez O, González S et al. RAS gene mutations in ethmoid sinus adenocarcinoma: Prognostic implications. Cancer 1999;86:255–264. [DOI] [PubMed] [Google Scholar]
- 10.Perrone F, Oggionni M, Birindelli S et al. TP53, P14ARF, p16INK4A and H‐RAS gene molecular analysis in intestinal‐type adenocarcinoma of the nasal cavity and paranasal sinuses. Int J Cancer 2003;105;196–203. [DOI] [PubMed] [Google Scholar]
- 11.Frattini M, Perrone F, Suardi S et al. Phenotype‐genotype correlation: Challenge of intestinal‐type adenocarcinoma of the nasal cavity and paranasal sinuses. Head Neck 2006;28:909–915. [DOI] [PubMed] [Google Scholar]
- 12.Bornholdt J, Hansen J, Steiniche T et al. K‐ras mutations in sinonasal cancers in relation to wood dust exposure. BMC Cancer 2008;8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.López F, García Inclán C, Pérez‐Escudero J et al. KRAS and BRAF mutations in sinonasal cancer. Oral Oncol 2012;48:692–697. [DOI] [PubMed] [Google Scholar]
- 14.García‐Inclán C, López F, Pérez‐Escuredo J et al. EGFR status and KRAS/BRAF mutations in intestinal‐type sinonasal adenocarcinomas. Cell Oncol (Dordr) 2012;35:443–450. [DOI] [PubMed] [Google Scholar]
- 15.Franchi A, Innocenti DR, Palomba A et al. Low prevalence of K‐RAS, EGF‐R and BRAF mutations in sinonasal adenocarcinomas. Implications for anti‐EGFR treatments. Pathol Oncol Res 2014;20:571–579. [DOI] [PubMed] [Google Scholar]
- 16.López‐Hernández A, Pérez‐Escuredo J, Vivanco B et al. Genomic profiling of intestinal‐type sinonasal adenocarcinoma reveals subgroups of patients with distinct clinical outcomes. Head Neck 2018;40:259–273. [DOI] [PubMed] [Google Scholar]
- 17.Projetti F, Durand K, Chaunavel A et al. Epidermal growth factor receptor expression and KRAS and BRAF mutations: Study of 39 sinonasal intestinal‐type adenocarcinomas. Human Pathol 2013;44:2116 –2125. [DOI] [PubMed] [Google Scholar]
- 18.Szablewski V, Solassol J, Poizat F et al. EGFR expression and KRAS and BRAF mutational status in intestinal‐type sinonasal adenocarcinoma. Int J Mol Sci 2013;14:5170–5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franchi A, Fondi C, Paglierani M et al. Epidermal growth factor receptor expression and gene copy number in sinonasal intestinal type adenocarcinoma. Oral Oncol 2009;45:835–838. [DOI] [PubMed] [Google Scholar]
- 20.Sorich MJ, Wiese MD, Rowland A et al. Extended RAS mutations and anti‐EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: A meta‐analysis of randomized, controlled trials. Ann Oncol 2015;26:13–21. [DOI] [PubMed] [Google Scholar]
- 21.Harb W, Fan A, Tran T et al. Mutational analysis of circulating tumor cells using a novel microfluidic collection device and qPCR assay. Transl Oncol 2013;6:528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diehl F, Li M, Dressman D et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc Natl Acad Sci U S A 2005;102:16368–16373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grasselli J, Elez E, Caratù G et al. Concordance of blood‐ and tumor‐based detection of RAS mutations to guide anti‐EGFR therapy in metastatic colorectal cancer. Ann Oncol 2017;28:1294–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spindler KG, Boysen AK, Pallisgard N et al. Cell‐free DNA in metastatic colorectal cancer: A systematic review and meta‐analysis. The Oncologist 2017;22:1049–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Didelot A, Le Corre D, Luscan A et al. Competitive allele specific TaqMan PCR for KRAS, BRAF and EGFR mutation detection in clinical formalin fixed paraffin embedded samples. Exp Mol Pathol 2012;92:275–280. [DOI] [PubMed] [Google Scholar]
- 26.Biocartis . Idylla KRAS Mutation Assay. Available at https://biocartis.com/idylla-kras-mutation-test. Accessed September 24, 2017.
- 27.Biocartis . Idylla NRAS Mutation Assay. Available at https://biocartis.com/idylla-nras-mutation-assay. Accessed September 24, 2017.
- 28.Neumann J, Zeindl‐Eberhart E, Kirchner T et al. Frequency and type of KRAS mutations in routine diagnostic analysis of metastatic colorectal cancer. Pathol Res Pract 2009;205:858–862. [DOI] [PubMed] [Google Scholar]
- 29.Fiala O, Buchler T, Mohelnikova‐Duchonova B et al. G12V and G12A KRAS mutations are associated with poor outcome in patients with metastatic colorectal cancer treated with bevacizumab.https://www.ncbi.nlm.nih.gov/pubmed/26662311 Tumor Biol 2016;37:6823–6830. [DOI] [PubMed] [Google Scholar]
- 30.Bokemeyer C, Köhne CH, Ciardiello F et al. FOLFOX4 plus cetuximab treatment and RAS mutations in colorectal cancer. Eur J Cancer 2015;51:1243–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Cutsem E, Lenz HJ, Köhne CH et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol 2015;33:692–700. [DOI] [PubMed] [Google Scholar]
- 32.Misale S, Yaeger R, Hobor S et al. Emergence of KRAS mutations and acquired resistance to anti‐EGFR therapy in colorectal cancer. Nature 2012;486:532–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Díaz LA Jr, Williams RT, Wu J et al. The molecular evolution of acquired resistance to anti‐EGFR blockade in colorectal cancer. Nature 2012;486:537–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heinemann V, von Weikersthal LF, Decker T et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first‐line treatment for patients with metastatic colorectal cancer (FIRE‐3): A randomised, open‐label, phase 3 trial. Lancet Oncol 2014;15:1065–1075. [DOI] [PubMed] [Google Scholar]
- 35.Schwartzberg LS, Rivera F, Karthaus M et al. PEAK: A randomised, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild‐type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol 2014;32:2240–2247. [DOI] [PubMed] [Google Scholar]
- 36.Montagut C, Dalmases A, Bellosillo B et al. Identification of a mutation in the extracellular domain of the epidermal growth factor receptor conferring cetuximab resistance in colorectal cancer. Nat Med 2012;18:221–223. [DOI] [PubMed] [Google Scholar]
- 37.Kim SB, Bozeman RG, Kaisani A et al. Radiation promotes colorectal cancer initiation and progression by inducing senescence‐associated inflammatory responses. Oncogene 2016;35:3365–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ondrejka SL, Schaeffer DF, Jakubowski MA et al. Does neoadjuvant therapy alter KRAS and/or MSI results in rectal adenocarcinoma testing? Am J Surg Pathol 2011;35:1327–1330. [DOI] [PubMed] [Google Scholar]
- 39.Ock CY, Keam B, Kim TM et al. Induction chemotherapy in head and neck squamous cell carcinoma of the paranasal sinus and nasal cavity: A role in organ preservation. Korean J Intern Med 2016;31:570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen NX, Chen L, Wang JL, et al. A clinical study of multimodal treatment for orbital organ preservation in locally advanced squamous cell carcinoma of the nasal cavity and paranasal sinus. Jpn J Clin Oncol 2016;46:727–734. [DOI] [PubMed] [Google Scholar]
- 41.Le DT, Durham JN, Smith KN et al. Mismatch repair deficiency predicts response of solid tumors to PD‐1 blockade. Science 2017;357:409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riobello C, Vivanco B, Reda S et al. Programmed death ligand‐1 expression as immunotherapeutic target in sinonasal cancer. Head Neck 2018;40:818–827. [DOI] [PubMed] [Google Scholar]
- 43.Agell L, Hernández S, Salido M et al. PI3K signaling pathway is activated by PIK3CA mRNA overexpression and copy gain in prostate tumors, but PIK3CA, BRAF, KRAS and AKT1 mutations are infrequent events. Mod Pathol 2011;24:443–452. [DOI] [PubMed] [Google Scholar]
- 44.Cho NY, Choi M, Kim BH et al. BRAF and KRAS mutations in prostatic adenocarcinoma. Int J Cancer 2006;119:1858–1862. [DOI] [PubMed] [Google Scholar]
- 45.Salmaninejad A, Ghadami S, Dizaji MZ et al. Molecular characterization of KRAS, BRAF, and EGFR genes in cases with prostatic adenocarcinoma; reporting bioinformatics description and recurrent mutations. Clin Lab 2015;61:749–759. [DOI] [PubMed] [Google Scholar]
- 46.Xie M, Lu C, Wang J et al. Age‐related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med 2014;20:1472–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Casali PG, Bruzzi P, Bogaerts J et al. Rare Cancers Europe (RCE) methodological recommendations for clinical studies in rare cancers: A European consensus position paper. Ann Oncol 2015;26:300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rare Cancers Europe. Available at https://www.rarecancerseurope.org/. Accessed November 17, 2018.