One of the most promising new areas of cancer treatment is the development of immune checkpoint inhibitors that target the CTLA4 and PD1/PD‐L1 pathways. Growing interest in these agents has translated into implementation in patients with transplant‐related malignancies. This article reviews the mechanisms of action of transplant immunosuppression and cancer immunomodulation, presenting the case of a patient with stage IV lung cancer who had undergone kidney transplant.
Keywords: Immune checkpoint inhibitors, Immunotherapy, Allograft, Transplant rejection, Cytotoxic T‐lymphocyte‐associated antigen 4, Programmed cell death protein 1, Programmed death‐ligand 1
Abstract
Background.
It is well known that the state of immune tolerance induced by broad immunosuppression to prevent allograft rejection leads to an increased risk of the development of cancer. One of the most promising new areas of cancer treatment has been the development of immune checkpoint inhibitors that target the cytotoxic T‐lymphocyte‐associated antigen 4 and programmed cell death protein 1/programmed death‐ligand 1 (PD‐L1) pathways. As a logical consequence, growing interest in these agents translated into their implementation in patients with transplant‐related malignancies. Because of overlapping and perhaps mutually exclusive mechanisms of action of transplant immunosuppression and cancer immunomodulation, it is critical to examine these interactions.
Materials and Methods.
We carried out a systematic search for review articles and case reports published between July 2014 and November 2017 using three engines: Usearch, PubMed, and Up‐to‐date.
Results.
Overall, there were 20 cases with 12 allograft rejections. The rejection rate associated with nivolumab was 73% (8/11) and with pembrolizumab it was 100% (2/2). The use of ipilimumab did not lead to rejection in any instance (0/4, 0%). Of the two patients treated with the sequential use of ipilimumab/nivolumab, one lost his allograft, yielding a rejection rate of 50%. The sequential use of ipilimumab/pembrolizumab led to a rejection rate of 100% (1/1, 100%).
Conclusion.
The use of agents that act on the PD‐L1 pathway are contraindicated in the face of solid organ allografts because of unacceptably high rates of irreversible allograft rejection. It appears that the use of ipilimumab may be tolerated as the mechanism is different from that of the PD‐L1 agents.
Implications for Practice.
Transplant rejection is a complex process that puts stress on patients and their families and can lead to tragic results. Significant advancements in the field of immunosuppression have led to the engenderment of agents devised to extend the survival of transplant recipients. The advent of immunomodulators in cancer therapy has been paradigm‐shifting; however, because of their mechanism of action, their use must be carefully considered in patients with allografts and concomitant cancer. It appears that ipilimumab can be administered safely in these patients but that agents acting on the programmed death‐ligand 1 pathway are contraindicated because of high rates of irreversible rejection.
Introduction
A 59‐year‐old Hispanic male with a history of long‐standing type 2 diabetes mellitus, essential hypertension, and a 45 pack‐year history of smoking with stage IV lung cancer and end‐stage renal disease s/p living‐donor kidney transplant (LDKT) in 2011 was transferred to the Jackson Memorial Hospital (JMH) renal transplant unit for evaluation of worsening renal function.
The patient had undergone LDKT surgery in October 2011. He received induction therapy with basiliximab (Simulect; Novartis) and thymoglobulin and was started on tacrolimus (Prograf; Astellas Pharma) and mycophenolate mofetil (Myfortic; Novartis) for maintenance immunosuppression. In March 2017, he was diagnosed with a T2N1M0 squamous cell carcinoma of the lung due to cough, reduced exercise tolerance, and orthopnea. The patient underwent left lower lobectomy later that month. Immunosuppression was stopped.
Two months after surgery, he was admitted because of worsening cough, shortness of breath, reduced exercise capacity, and orthopnea. Work‐up documented new metastasis to the ribs, heart, lung, and diaphragm. He received one cycle of carboplatin and paclitaxel in June 2017. His disease progressed after the first dose of chemotherapy, and he was given one dose of nivolumab on July 15, 2017. Nine days later, he was admitted with fever, generalized malaise, myalgia, and acute kidney injury with a creatinine of 7.39. He was transferred to JMH on June 27 for further investigation and management of suspected renal allograft rejection.
A computed tomography scan of the abdomen revealed a significantly edematous kidney. A renal ultrasound showed increased echogenicity of the parenchyma of the transplanted kidney without any accompanying perirenal collections or signs of hydronephrosis consistent with acute allograft rejection. He was treated with a high dose of steroids, but he remained oliguric. The patient refused dialysis and died of renal failure and progressive lung cancer 4 weeks after receiving one dose of nivolumab.
Background
According to the Organ Procurement and Transplantation Network, a total of 33,610 transplants were allocated in the U.S. in the calendar year 2016, 56.7% of which were kidney allografts, 23.3% liver, 9.5% heart, 6.9% lung, 2.4% dual kidney/pancreas, 0.6% pancreas alone, and 0.5% corresponding to other organs (dual heart/lung, intestine, craniofacial, and uterus) [1]. At a glance, there are 114,930 candidates in the national waiting list to receive a transplant as of March 16, 2018. Every 10 minutes, someone is added to the waiting list. On average, 20 people die each day while waiting for a transplant [2]. In the course of 2016 alone, more than 7,000 registered patients died while on the waiting list [2]. It is thus a misfortune when cases of imminent and unrecoverable allograft loss occur. The most common cause of loss is rejection of the allograft.
Immunosuppression has been extensively studied and implemented to prevent allograft rejection. It is well known that this induced state of immune tolerance leads to an increase risk in the development of malignant neoplasms, particularly non‐Hodgkin lymphomas and cancers of the lung, liver, and kidney [3]. Certain other malignancies are also seen at a higher rate, such as colorectal cancers and cancers of the urinary bladder, thyroid, skin (melanoma and nonmelanoma), and gastrointestinal tract (pancreas, esophagus, intrahepatic bile duct, small intestine), as well as hematologic malignancies (plasma cell dyscrasias, acute myelogenous leukemia, chronic myelogenous leukemia) [3]. The mechanisms behind this heightened incidence are believed to be multifactorial in nature and include infection, reactivation and proliferation of oncogenic viruses, and loss of immune surveillance in the setting of chronic immunosuppression [3].
One of the most promising new areas of cancer treatment has been the development of immunotherapy with the cytotoxic T‐lymphocyte‐associated antigen 4 (CTLA4) and the programmed cell death protein 1 (PD1)/programmed death‐ligand 1 (PD‐L1) pathways as the primary targets. The approval of ipilimumab (Yervoy; Bristol‐Myers Squibb, New York City, NY), pembrolizumab (Keytruda; Merck, Kenilworth, NJ), nivolumab (Opdivo; Bristol‐Myers Squibb), atezolizumab (Tecentriq; Genentech Oncology, San Francisco, CA), and durvalumab (Imfinzi; AstraZeneca UK Limited, Cambridge, U.K.) by the U.S. Food and Drug Administration (FDA) has added a potent weapon to the cancer treatment armamentarium.
Given the two‐ to threefold increase in cancer incidence in transplant recipients, it is not surprising that these immunomodulatory agents would come to be used in patients with transplant‐related malignancies. Because of overlapping and perhaps mutually exclusive mechanisms of action of transplant immunosuppression and cancer treatment immunomodulation, it is critical to examine these interactions.
Materials and Methods
We carried out a comprehensive systematic search for potential review articles and case reports using three main search engines: Usearch (proprietary software of the University of Miami Miller School of Medicine), PubMed, and Up‐to‐date. The search was restricted to publication dates between March 2011, the date of approval of the first licensed immune checkpoint inhibitor for cancer therapy by the FDA, and November 2017. Key words included immunotherapy, immune checkpoint inhibitors, PD‐L1, PD1, CTLA‐4, allograft, rejection, organ transplant, anti‐PD1 therapy, ipilimumab, nivolumab, and pembrolizumab.
Results
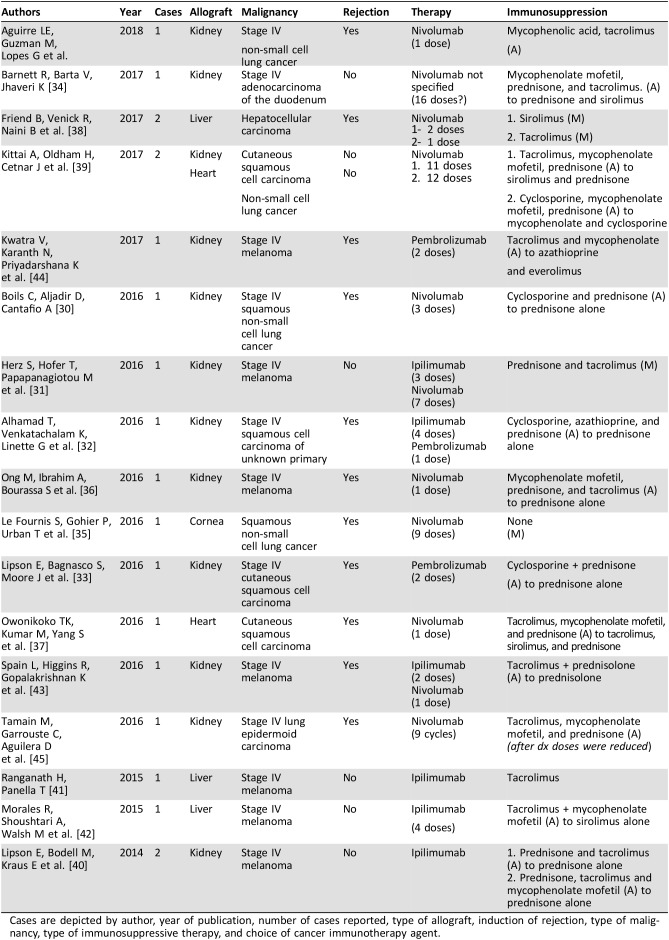
As of November 2017, there are 19 reported cases describing the use of targeted cancer immunotherapies in the setting of metastatic solid tumors in transplanted patients undergoing chronic immunosuppression in the literature (Table 1). With the addition of our case, we examined 20 instances of patients with solid organ transplants who underwent anticancer therapy with targeted immune modulators. All reported cases involved the use of nivolumab, pembrolizumab, or ipilimumab either alone or used sequentially. There are no data on the use of the newer agents atezolizumab or durvalumab.
Table 1. Summary of all cases included in this report.
Cases are depicted by author, year of publication, number of cases reported, type of allograft, induction of rejection, type of malignancy, type of immunosuppressive therapy, and choice of cancer immunotherapy agent.
The most common allograft was kidney. Sixty‐five percent of the patients had renal allografts (n = 13), followed by liver (20%, n = 4), heart (10%, n = 2), and cornea (5%, n = 1).
All patients had stage IV cancer at the time of implementation of targeted immunotherapy. The most common type of malignant neoplasm was melanoma, which was seen in 40% of the patients. This was followed by five cases of non‐small cell lung cancer (NSCLC; 25%, four squamous histology, one epidermoid), three cases of cutaneous squamous cell carcinoma (15%), two instances of hepatocellular carcinoma (10%), one case of adenocarcinoma of the duodenum (5%), and one report of squamous cell carcinoma of unknown origin (5%).
The agent most commonly used was nivolumab as a single agent (55%, n = 11), followed by ipilimumab as a single agent (20%, n = 4), pembrolizumab as a single agent (10%, n = 2), the sequential use of ipilimumab/nivolumab (10%, n = 2), and the sequential use of ipilimumab/pembrolizumab (5%, n = 1).
There were a total of 12 allograft rejections in the 20 reported cases. There were only eight cases in which the allograft was successfully maintained. Permanent and irreversible allograft loss occurred in 60% of all cases compared with 40% in which the allograft survived.
Choice of Immune Modulatory Agent and Associated Rate of Allograft Rejection
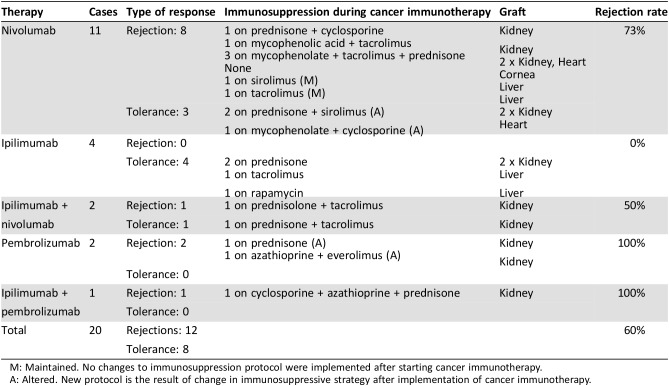
The use of nivolumab as a single agent in 11 patients yielded eight instances of allograft rejection, whereas in 3 patients, the allograft remained intact and no rejection occurred. Thus, the overall rejection rate associated with the use of nivolumab as a single agent was 73% (8/11). The majority of cases of rejection occurred in kidney allograft recipients (4/8, 50%), three of whom were on immunotherapy to treat stage IV NSCLC and one in the setting of metastatic melanoma. Two cases (2/8, 25%) of rejection were reported in liver allograft recipients who were being treated for hepatocellular carcinoma, one case (1/8, 12.5%) of rejection occurred in a heart transplant recipient receiving nivolumab for metastatic cutaneous squamous cell carcinoma, and one case of transplanted cornea rejection occurred in a patient receiving immunotherapy to treat stage IV NSCLC (1/8, 12.5%).
Two patients were treated with pembrolizumab as a single agent, and both lost their allografts (2/2, 100%). Both patients had renal allografts. One patient had metastatic melanoma and the other had stage IV cutaneous squamous cell carcinoma.
The use of ipilimumab as a single agent was not associated with the loss of allografts in four patients reported in the literature (0/4, 0%). Two patients had kidney allografts and two had liver allografts. All four patients were treated for stage IV melanoma.
There were two patients treated with the sequential use of ipilimumab and nivolumab for stage IV melanoma. One patient lost their allograft whereas the second patient‘s allograft was successfully maintained, yielding a rejection rate in this setting of 50%.
There is only one case report of the sequential use of ipilimumab followed by pembrolizumab. This patient, who was being treated for metastatic squamous cell carcinoma of unknown origin, lost their renal allograft (1/1, 100%) (Table 2).
Table 2. Outcomes classified by choice of immunotherapy agent.
M: Maintained. No changes to immunosuppression protocol were implemented after starting cancer immunotherapy.
A: Altered. New protocol is the result of change in immunosuppressive strategy after implementation of cancer immunotherapy.
Type of Cancer and Rate of Allograft Rejection
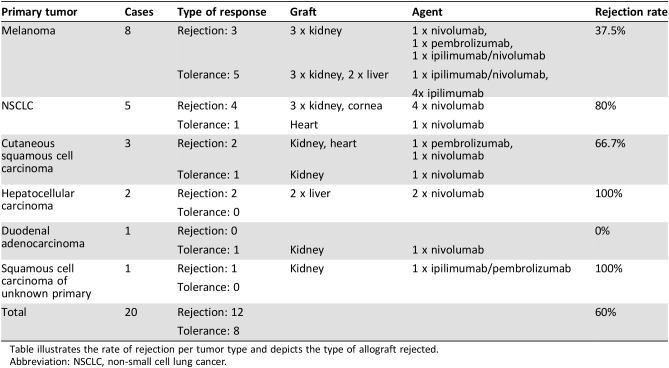
Melanoma was the most commonly occurring tumor in our series, constituting 40% of the tumors. Of the eight patients with melanoma, five did not reject their allograft (63%) and three allografts were lost (37%).
There were five patients with lung cancer (NSCLC). Four of the patients with lung cancer lost their allograft (80%). Two of the three patients with metastatic squamous cell carcinoma of the skin lost their allografts (66%), and both patients with hepatocellular carcinoma lost their liver allografts (100%). Regarding the two other cases, one of adenocarcinoma of the duodenum and another squamous cell carcinoma of unknown origin, both were associated with loss of the allograft (Table 3).
Table 3. Rejection by type of tumor.
Table illustrates the rate of rejection per tumor type and depicts the type of allograft rejected.
Abbreviation: NSCLC, non‐small cell lung cancer.
Type of Allograft and the Rate of Allograft Rejection
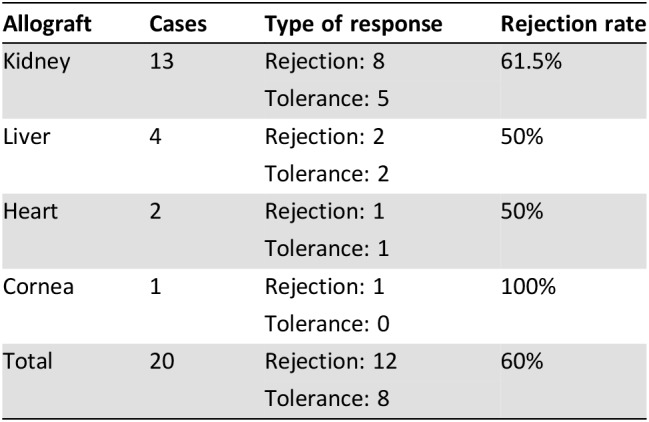
The most common type of allograft found in our series was renal, making up 65% of the allografts. Of the 13 patients with renal allografts, 8 experienced rejection (62%). One half of the four liver allografts were permanently rejected (50%). One of the two heart transplants was rejected (50%), and the one cornea transplant was rejected (100%) (Table 4).
Table 4. Rejection by type of allograft.
Discussion
Immunosuppressant drugs and immune checkpoint inhibitors have opposing effects on the immune system. In transplant recipients, immunosuppressants are used to induce tolerance to the allograft by dampening the immune response. On the other hand, in cancer therapy, immune checkpoint inhibitors are implemented to enhance cell‐mediated immunity and antigen recognition. Coadministration of these agents is fraught with peril.
According to figures obtained from the Transplant Cancer Match study conducted by Engels and colleagues and published in JAMA in 2011 using data on 175,732 out of 442,629 solid organ transplant recipients from the U.S. Scientific Registry of Transplant Recipients over a 21‐year period (1987–2008), the incidence of cancer among allograft recipients is increased by a factor of two when compared with the general population [3]. Data showed that 10,656 patients out of a total of 175,732 transplant recipients developed cancer during follow‐up (6.06%). Overall cancer incidence among transplant recipients was estimated at 1,375 cases per 100,000 person‐years [3].
Transplant rejection is a complex process that involves humoral and cell‐mediated immune responses. Antibody‐mediated rejection can occur in hyperacute, acute, or chronic settings and involves production of antibodies that target human leukocyte antigen molecules, ABO antigens on endothelial cells and erythrocytes, and other antigens expressed on the surface of endothelial cells [4]. T‐cell‐mediated rejection, on the other hand, is the most common form of acute rejection, and it involves the recognition of alloantigens expressed in the transplanted organ by the recipient's T cells once antigen presentation by antigen‐presenting cells (APCs) has occurred in lymphoid structures [4]. Primed T cells will subsequently differentiate in a process orchestrated by pro‐inflammatory cytokines arising from receptor‐ligand interactions between APCs and naïve T cells and invade the graft, leading to its destruction. Allograft rejection can thus be averted by disrupting this mechanism with agents that target critical intracellular effectors.
Immunosuppressive agents such as azathioprine and mycophenolate mofetil inhibit cell proliferation by arresting DNA replication in T and B cells [5]. Calcineurin inhibitors such as tacrolimus and cyclosporine act by blocking activation of nuclear factor of activated T cell (NFAT) via calcineurin‐mediated dephosphorylation and thus prevent its translocation to the nucleus where it would bind to DNA promoter sequences and lead to heightened expression of interleukin‐2 [6]. Reduced expression of this pro‐inflammatory cytokine results in inhibition of T‐cell growth and differentiation—a trait that is critical in the induction and maintenance of cell‐mediated immunity. In the context of transplant medicine, this effect is induced pharmacologically to safeguard the integrity of the allograft.
Malignant neoplastic clones establish an immunosuppressive microenvironment in which they thrive by successfully evading immunological eradication via different mechanisms that are deeply intertwined with the host's prototypical immune response [7]. To provide better understanding of this phenomenon, it is of paramount importance to illustrate the salient intricacies and overall significance of the PD1 pathway.
Not long ago, the interplay between B7‐CD28‐CTLA4 was considered to be the main mechanism orchestrating cell‐mediated immunity [8], [9]. The discovery of negative costimulatory signals (inhibitory signals) as regulatory components of said response helped elucidate things further and has led to a paradigm shift in the management of a variety of cancers. These signals act as immune checkpoints, providing an additional mechanism to safeguard peripheral tolerance by inducing a state of quiescence among self‐reactive T cells and in those immune effectors that remain active for prolonged periods of time [10]. The paramount example is the interaction between the PD1 receptor and its ligands PD‐L1/PD‐L2 [10], [11], [12].
Natively, the PD1 receptor is expressed on the surface of multiple immune mediators, particularly on activated mature T cells as well as B cells, primed natural killer (NK) cells, monocytes, and immature dendritic cells [10]. PD1 expression on effector T cells occurs as a consequence of NFAT translocation to the nucleus precipitated by T‐cell activation following exposure to pro‐inflammatory cytokines [13]. Consequently, expression of PD1 will always be seen in inflammatory states.
PD1 binds namely to three ligands on the surface of immune mediators: PD‐L1, PD‐L2, and B7 [12], [14]. PD‐L1 is expressed constitutively at low concentrations on the surface of professional and nonprofessional APCs. However PD‐L1 is also expressed on multiple noninflammatory cells such as in the pancreas, endothelial cells, and in immune privileged sites such as testes, eye, and placenta [12], [14]. The expression of these ligands is amplified significantly by exposure to inflammatory cytokines such as interferons, tumor necrosis factor alpha, and vascular endothelial growth factor [7].
Exhaustion of effector T cells occurs as a consequence of prolonged antigenic exposure and is characterized by a significantly damped cell‐mediated immune response due to expression of coinhibitory molecules and PD1, which act as a regulatory mechanism to suppress inflammation [15]. More importantly, PD1 expression is not exclusively associated with cell‐mediated immune exhaustion but can also occur in a pathological context where the PD1 pathway and its anti‐inflammatory properties serve as vulnerabilities that can be exploited by disease, such as cancer trying to elude recognition [11], [16]. Upregulation of PD‐L1 expression in the tumor microenvironment can also be a consequence of aberrant activation of signaling pathways driven by overactive epidermal growth factor receptor, mitogen‐activated protein kinase, and PI3K‐AKT as well as heightened production of transcription factors such as hypoxia‐inducible factor‐1 (HIF‐1) [17], [18], [19].
Cancer cells are capable of exploiting vulnerabilities in this pathway to evade cell‐mediated immunity by expressing PD‐L1 selectively. The binding of PD‐L1 ligands on cancer cells to PD1 receptors on the surface of effector T cells and activated NK cells leads to efficient blockade of cell‐mediated immunity, terminating it and perpetuating the survival of neoplastic clones. As a result of this, it is logical to understand how the implementation of therapeutic agents that evade this immune blockade is of particular interest in oncology and provides an invaluable tool in the fight against cancer.
The juxtaposition of seemingly antagonistic mechanisms of action associated with the concomitant use of immunosuppression and immune checkpoint blockade in the context of de novo malignancies in transplant recipients seems paradoxical—and at first glance detrimental—for the purpose of negatively impacting tumor biology and ensuring transplant survival. Immunosuppression can fuel tumor growth and development, whereas enhanced activation of immunity can invariably lead to rejection. Thus, maximum therapeutic benefit from these antagonistic agents cannot possibly be attained for their intended purposes without compromising one another, which would result in potential rejection or, alternatively, disease progression.
In our series, the lowest probability of allograft rejection was seen among patients receiving ipilimumab as monotherapy. All patients on ipilimumab were being treated for melanoma. Of the four patients treated with ipilimumab as monotherapy, none developed allograft rejection. Two of these cases were on maintenance immunosuppression with prednisone alone, one on tacrolimus and one on sirolimus. It appears that the use of anti‐CTLA4 antibodies in the setting of chronically induced immunosuppression does not lead to allograft rejection in transplanted patients, regardless of the immunosuppressive regimen pursued.
Ipilimumab acts by binding to CTLA‐4, a molecule expressed on the surface of activated T cells, which leads to transduction of an inhibitory signal that dampens T‐cell activation. By doing so, ipilimumab guarantees the interaction between the CD28 molecule expressed on primed T cells and the B7 antigen expressed on the surface of APCs leading to transduction of a costimulatory signal that is needed to drive and perpetuate cell‐mediated immunity. Ipilimumab appears to be safe in the context of immunosuppression because of characteristics inherent to the CTLA4 pathway. CTLA‐4 expression seems to play a critical role in peripheral effector T‐cell quiescence and as such in the induction of peripheral tolerance to solid organ allografts [20]. From what has been observed in experimental models, even though early blockade of CTLA‐4 signaling following transplantation leads to acute organ rejection, late‐stage CTLA‐4 signal disruption taking place once induction of tolerance has been achieved does not appear to affect allograft survival [21], [22]. Extrapolation of these results to a clinical context seems to corroborate these findings given that patients in this series were on chronic immunosuppression long before developing cancer and that subsequent disruption of CTLA‐4 signaling enacted by ipilimumab did not lead to rejection.
In our series, rates of allograft rejection were high among patients treated with PD‐L1 inhibitors. In patients treated with nivolumab alone or in combination with ipilimumab, 73% of allografts were rejected. All patients on pembrolizumab lost their allograft. It appears that use of anti‐PD1 agents is not safe in patients with solid organ allografts.
The PD1 pathway acts as a negative costimulatory signal (immune checkpoint), providing a mechanism to safeguard peripheral tolerance by inducing a state of quiescence among self‐reactive T cells. The anti‐PD‐L1 agents bind to the PD1 receptor on the surface of primed immune effectors, counteracting the damping of the immune response exerted by the PD1/PD‐L1 interaction and thus leading to induction of a pro‐inflammatory state, potentiation, and sustained activation of cell‐mediated immunity. The PD‐L1/PD1 pathway appears to play a major and critical role in the maintenance of immune tolerance to solid organ allografts [23]. Lack of PD1 expression is associated with a state of persistent systemic inflammation akin to what is seen in autoimmune conditions [24]. In an animal model, the early infusion of anti‐PD1‐targeted agents precluded the induction of peripheral tolerance, whereas infusion at a later stage following transplantation (i.e., once induction of self‐tolerance had been achieved) led to complete loss of the allograft [23]. Thus, it appears that the mechanism of action of the PD‐L1 agents in cancer therapy is diametrically opposed to their use in allograft maintenance.
Given these findings, we encourage the use of agents other than PD1 inhibitors in the setting of de novo malignant neoplasms in transplant recipients for the purpose of cancer therapy. Prospective studies analyzing the impact of these agents in solid transplants should not be conducted.
On the other hand, health care providers should institute enhanced cancer prevention strategies as part of the framework of proper patient care when dealing with transplant recipients. There are a number of widely‐known and certified guidelines on cancer screening recommendations for solid organ transplant individuals classified by type of allograft, such as Kidney Disease: Improving Global Outcomes (KIDGO) for kidney, American Association for the Study of Liver Diseases‐Adult and pediatric for liver, and the International Society for Heart and Lung Transplantation for dual heart/lung among others [25], [26], [27], [28]. According to a systematic review of clinical practice guidelines for post‐transplant care conducted by Acuna et al. in 2017, most of the recommendations available are targeted toward kidney recipients, with a smaller number targeted toward other transplant recipients [29]. Yearly clinical skin examination for melanoma and nonmelanoma skin cancer by either a primary care physician or dermatologist is uniformly recommended. Screening for breast, cervical, prostate, and colon cancer were noted to be often the same as for the general population, with some variation seen according to the clinical practice guideline [29]. Screening for renal and lung cancer is not actively encouraged among kidney recipients [29]. Other recommendations are too specific and vary by guideline and target allograft and as such exceed the scope of this article.
Conclusion
Transplant rejection is a complex, costly, and onerous process that puts stress on patients and their families and can lead to tragic results. Significant advancements in the field of immunosuppression over the decades have led to the engenderment of valuable and novel agents and protocols of immunosuppression devised to extend the survival of a donor allograft while preserving quality of life. The advent of immunomodulators in cancer therapy has been paradigm shifting; however, because of their mechanism of action, it appears that their use must be carefully considered in patients with allografts and concomitant cancer. It would appear that the use of ipilimumab in the face of solid organ allografts might be safely administered but that agents that act on the PD‐L1 pathway are contraindicated in this setting because of the high rate of irreversible rejection and allograft loss. Counseling patients on potential risks and benefits in this context as well as on other safer alternatives needs to be pursued. Alternatively, the institution of prevention strategies using established clinical practice guidelines for post‐transplant cancer screening should be an integral component of a framework of proper patient care and as such should be actively encouraged by the health care provider.
Author Contributions
Conception/design: Luis E. Aguirre, Maria E. Guzman, Judith Hurley
Provision of study material or patients: Luis E. Aguirre, Maria E. Guzman, Judith Hurley
Collection and/or assembly of data: Luis E. Aguirre, Maria E. Guzman
Data analysis and interpretation: Luis E. Aguirre, Gilberto Lopes, Judith Hurley
Manuscript writing: Luis E. Aguirre, Maria E. Guzman, Gilberto Lopes, Judith Hurley
Final approval of manuscript: Luis E. Aguirre, Gilberto Lopes, Judith Hurley
Disclosures
The authors indicated no financial relationships.
References
- 1.United Network for Organ Sharing online database. Available at https://unos.org/data/. Accessed March 10, 2018.
- 2.Organ Procurement and Transplantation Network . Death Removals by UNOS Status by Year. Available at https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/. Accessed March 10, 2018.
- 3.Engels EA, Pfeiffer RM, Fraumeni JF Jr et al. Spectrum of cancer risk among U.S. solid organ transplant recipients. JAMA 2011;306:1891–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nankivell BJ, Alexander SI. Rejection of the kidney allograft. N Engl J Med 2010;363:1451–1462. [DOI] [PubMed] [Google Scholar]
- 5.Clark M, Finkel R, Rey J, Whalen K. Immunosuppressants In: Harvey R, ed. Lippincott's Illustrated Reviews: Pharmacology. 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2012:514–519. [Google Scholar]
- 6.Ganong WF. Review of Medical Physiology. 22nd ed The McGraw Hill Companies, Inc., 2005:530. [Google Scholar]
- 7.Boussiotis V. Molecular and biochemical aspects of the PD‐1 checkpoint pathway. N Engl J Med 2016;375:1767–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tivol EA, Borriello F, Schweitzer AN et al. Loss of CTLA‐4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA‐4. Immunity 1995;3:541–547. [DOI] [PubMed] [Google Scholar]
- 9.Waterhouse P, Penninger JM, Timms E et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla‐4. Science 1995;270:985–988. [DOI] [PubMed] [Google Scholar]
- 10.Freeman GJ, Long AJ, Iwai Y et al. Engagement of the PD‐1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Latchman Y, Wood CR, Chernova T et al. PD‐L2 is a second ligand for PD‐1 and inhibits T cell activation. Nat Immunol 2001;2:261–268. [DOI] [PubMed] [Google Scholar]
- 12.Okazaki T, Honjo T. PD‐1 and PD‐1 ligands: From discovery to clinical application. Int Immunol 2007;19:813–824. [DOI] [PubMed] [Google Scholar]
- 13.Oestreich KJ, Yoon H, Ahmed R et al. NFATc1 regulates PD‐1 expression upon T cell activation. J Immunol 2008;181:4832–4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francisco LM, Sage PT, Sharpe AH. The PD‐1 pathway in tolerance and autoimmunity. Immunol Rev 2010;236:219–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blackburn SD, Shin H, Haining WN et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 2009;10:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong H, Strome SE, Salomao DR et al. Tumor‐associated B7‐H1 promotes T‐cell apoptosis: A potential mechanism of immune evasion. Nat Med 2002;8:793–800. [DOI] [PubMed] [Google Scholar]
- 17.Akbay EA, Koyama S, Carretero J et al. Activation of the PD‐1 pathway contributes to immune escape in EGFR‐driven lung tumors. Cancer Discov 2013;3:1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marzec M, Zhang Q, Goradia A et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD‐L1, B7‐H1). Proc Natl Acad Sci U S A 2008;105:20852–20857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noman MZ, Desantis G, Janji B et al. PD‐L1 is a novel direct target of HIF‐1α, and its blockade under hypoxia enhanced MDSC‐mediated T cell activation. J Exp Med 2014;211:781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito T, Ueno T, Clarkson MR et al. Analysis of the role of negative T cell costimulatory pathways in CD4 and CD8 T‐cell mediated alloimmune responses in vivo. J Immunol 2005;174:6648–6656. [DOI] [PubMed] [Google Scholar]
- 21.Li W, Zheng XX, Kuhr CS et al. CTLA4 engagement is required for induction of murine liver transplant spontaneous tolerance. Am J Transplant 2005;5:978–986. [DOI] [PubMed] [Google Scholar]
- 22.Judge TA, Wu Z, Zheng XG et al. The Role of CD80, CD86, and CTLA4 in alloimmune responses and the induction of long‐term allograft survival. J Immunol 1999;162:1947–1951. [PubMed] [Google Scholar]
- 23.Tanaka K, Albin MJ, Yuan X et al. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J Immunol 2007;179:5204–5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Intlekofer AM, Thompson CB. At the bench: Preclinical rationale for CTLA‐4 and PD‐1 blockade as cancer immunotherapy. J Leukoc Biol 2013;94:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group . KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009;9(suppl 3):S1–S157. [DOI] [PubMed] [Google Scholar]
- 26.Bia M, Adey DB, Bloom RD et al. KDOQI US commentary on the 2009 KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Kidney Dis 2010;56:189–218. [DOI] [PubMed] [Google Scholar]
- 27.Lucey MR, Terrault N, Ojo L et al. Long‐term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl 2013;19:3–26. [DOI] [PubMed] [Google Scholar]
- 28.Kelly DA, Bucuvalas JC, Alonso EM et al. Long‐term medical management of the pediatric patient after liver transplantation: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl 2013;19:798–825. [DOI] [PubMed] [Google Scholar]
- 29.Acuna SA, Huang JW, Scott AL et al. Cancer screening recommendations for solid organ transplant recipients: A systematic review of clinical practice guidelines. Am J Transplant 2017;17:103–114. [DOI] [PubMed] [Google Scholar]
- 30.Boils CL, Aljadir DN, Cantafio AW. Use of the PD‐1 pathway inhibitor nivolumab in a renal transplant patient with malignancy. Am J Transplant 2016;16:2496–2497. [DOI] [PubMed] [Google Scholar]
- 31.Herz S, Hofer T, Papapanagiotou M et al. Checkpoint inhibitors in chronic kidney failure and an organ transplant recipient. Eur J Cancer 2016;67:66–72. [DOI] [PubMed] [Google Scholar]
- 32.Alhamad T, Venkatachalam K, Linette G et al. Checkpoint inhibitors in kidney transplant recipients and the potential risk of rejection. Am J Transplant 2016;16:1332–1313. [DOI] [PubMed] [Google Scholar]
- 33.Lipson E, Bagnasco S, Moore J et al. Tumor regression and allograft rejection after administration of anti‐PD‐1. N Engl J Med 2016;374:896–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnett R, Barta V, Jhaveri K et al. Preserved renal‐allograft function and the PD‐1 pathway inhibitor nivolumab. N Engl J Med 2017;376:191–192. [DOI] [PubMed] [Google Scholar]
- 35.Le Fournis S, Gohier P, Urban T et al. Corneal graft rejection in a patient treated with nivolumab for primary lung cancer. Lung Cancer 2016;102:28–29. [DOI] [PubMed] [Google Scholar]
- 36.Ong M, Ibrahim A, Bourassa‐Blanchette S et al. Antitumor activity of nivolumab on hemodialysis after renal allograft rejection. J Immunother Cancer 2016;4:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Owonikoko TK, Kumar M, Yang S et al. Cardiac allograft rejection as a complication of PD‐1 checkpoint blockade for cancer immunotherapy: A case report. Cancer Immunol Immunother 2017;66:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friend BD, Venick RS, McDiarmid SV et al. Fatal orthotopic liver transplant organ rejection induced by a checkpoint inhibitor in two patients with refractory, metastatic hepatocellular carcinoma. Pediatr Blood Cancer 2017;64. [DOI] [PubMed] [Google Scholar]
- 39.Kittai AS, Oldham H, Cetnar J et al. Immune checkpoint inhibitors in organ transplant patients. J Immunother 2017;40:277–281. [DOI] [PubMed] [Google Scholar]
- 40.Lipson EJ, Bodell MA, Kraus ES et al. Successful administration of ipilimumab to two kidney transplantation patients with metastatic melanoma. J Clin Oncol 2014;32:e69–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ranganath HA, Panella TJ. Administration of ipilimumab to a liver transplant recipient with unresectable metastatic melanoma. J Immunother 2015;38:211. [DOI] [PubMed] [Google Scholar]
- 42.Morales RE, Shoushtari AN, Walsh MM et al. Safety and efficacy of ipilimumab to treat advanced melanoma in the setting of liver transplantation. J Immunother Cancer 2015;3:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spain L, Higgins R, Gopalakrishnan K et al. Acute renal allograft rejection after immune checkpoint inhibitor therapy for metastatic melanoma. Ann Oncol 2016;27:1135–1137. [DOI] [PubMed] [Google Scholar]
- 44.Kwatra V, Karanth NV, Priyadarshana K et al. Pembrolizumab for metastatic melanoma in a renal allograft recipient with subsequent graft rejection and treatment response failure: A case report. J Med Case Rep 2017;11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamain M, Garrouste C, Aguilera D et al. Mixed acute kidney allograft rejection after an antiprogrammed cell death protein 1 antibody treatment for lung epidermoid carcinoma. Transpl Int 2016;29:1247–1248. [DOI] [PubMed] [Google Scholar]