Abstract
Lessons Learned.
Modification of FOLFIRINOX significantly improves safety and tolerability in Chinese patients with locally advanced pancreatic cancer.
Patients with locally advanced pancreatic cancer benefit from neoadjuvant therapy and experience a much better survival than patients with upfront surgery.
Background.
The objective of this study was to evaluate the efficacy of modified‐FOLFIRINOX (mFOLFIRINOX) regimens in Chinese patients with locally advanced pancreatic cancer (LAPC) and to compare outcomes between patients with LAPC treated with mFOLFIRINOX‐based neoadjuvant therapy (LAPC‐N) and patients with LAPC who underwent upfront surgery (LAPC‐S).
Methods.
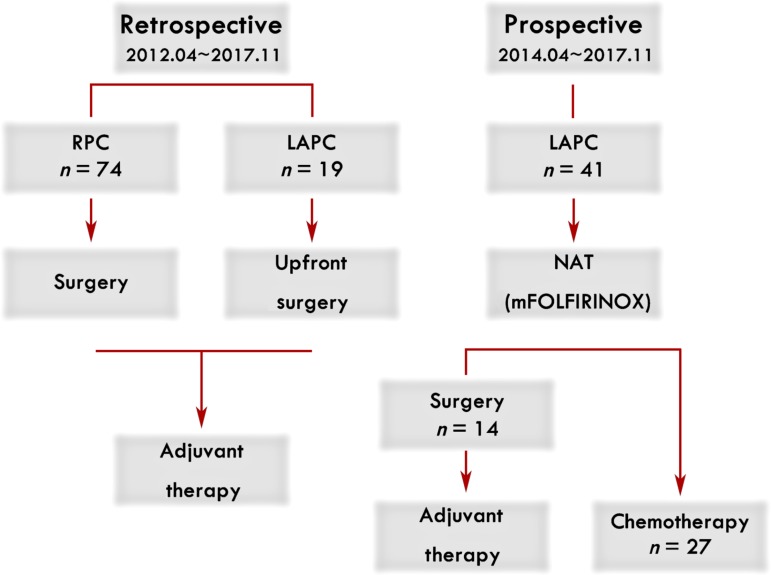
Forty‐one patients with LAPC‐N were enrolled prospectively. Imaging features, chemotherapy response, adverse events, perioperative complications, histology, and survival were analyzed. Seventy‐four patients with resectable pancreatic cancer (RPC) (from April 2012 to November 2017) and 19 patients with LAPC‐S (from April 2012 to March 2014) were set as observational cohorts, and data were collected retrospectively. LAPC‐N patients with adequate response underwent surgical treatment, whereas continuous chemotherapy was given to LAPC‐N patients who were not deemed resectable after treatment, and the response was re‐evaluated every 2 months.
Results.
Forty‐one patients with LAPC received mFOLFIRINOX with a response rate of 37.1%. The most common severe adverse events were neutropenia and anemia. mFOLFIRINOX‐based neoadjuvant therapy contributed to a remarkable decrease in CA19‐9 level and tumor diameter. Fourteen LAPC‐N patients underwent surgery (LAPC‐N‐S) after downstaging. Compared with LAPC‐N‐S cases, LAPC‐S patients had longer operative time, more blood loss, and a higher risk of grade 5 complications. The median overall survival (OS) and progression‐free survival (PFS) of LAPC‐N‐S patients were 27.7 months and 19.3 months, respectively, which were similar to those of patients with RPC (30.0 months and 23.0 months) and much longer than those of patients with LAPC‐S (8.9 months and 7.6 months), respectively.
Conclusion.
Neoadjuvant chemotherapy such as the mFOLFIRINOX regimen can be recommended for Chinese patients with LAPC after dose modification. Patients with LAPC‐N who underwent surgery obtained significantly improved survival compared with patients in the observational LAPC‐S cohort, who did not undergo neoadjuvant therapy.
Abstract
经验教训
• FOLFIRINOX 的改良显著提高了中国局部晚期胰腺癌患者的安全性和耐受性。
• 局部晚期胰腺癌患者从新辅助治疗中获益,并且比前期手术患者的生存率更高。
摘要
背景。本研究的目的是评估改良 FOLFIRINOX (mFOLFIRINOX) 方案对中国局部晚期胰腺癌 (LAPC) 患者的疗效,并将采用基于 mFOLFIRINOX 的新辅助治疗 (LAPC‐N) 进行治疗的 LAPC 患者的预后与接受了前期手术 (LAPC‐S) 的 LAPC 患者的预后进行比较。
方法。41例LAPC‐N 患者前瞻性入组。对影像特征、化疗反应、不良事件、围手术期并发症、组织学和生存率进行了分析。将 74例可切除的胰腺癌 (RPC) 患者(2012 年 4 月至 2017 年 11 月)和 19例LAPC‐S 患者(2012 年 4 月至 2014 年 3 月)作为观察队列,并回顾性收集数据。具有适当缓解的 LAPC‐N 患者接受了手术治疗,而对于在治疗后认为不可进行切除手术的 LAPC‐N 患者进行连续化疗,并且每 2 个月重新评估一次缓解。
结果。接受了 mFOLFIRINOX 的 41 例 LAPC 患者的缓解率为 37.1%。最常见的严重不良事件是中性粒细胞减少和贫血。基于 mFOLFIRINOX 的新辅助治疗有助于 CA19‐9 水平和肿瘤直径的显著降低。14例LAPC‐N 患者在指标下降后接受了手术 (LAPC‐N‐S)。与 LAPC‐N‐S 病例相比,LAPC‐S 患者的手术时间更长,失血更多,5 级并发症风险更高。LAPC‐N‐S 患者的中位总生存期 (OS) 和无进展生存期 (PFS) 分别为 27.7 个月和 19.3 个月,与 RPC 患者(30.0 个月和 23.0 个月)相似,且远远长于 LAPC‐S 患者(8.9 个月和 7.6 个月)。
结论。推荐中国 LAPC 患者采用调整剂量后的新辅助化疗,如 mFOLFIRINOX 方案。与未接受新辅助治疗的观察性 LAPC‐S 队列患者相比,接受手术的 LAPC‐N 患者的生存率显著提高。
Discussion
The promotion of preoperative neoadjuvant treatment (NAT) for patients with LAPC in China has been a challenge, and limited data have been reported. At our institution, a modified FOLFIRINOX regimen has been adopted for patients with metastatic pancreatic cancer, and promising results have been obtained. This modification drops the fluorouracil 400 mg/m2 bolus and reduces the dose of irinotecan from 180 mg/m2 to 135 mg/m2 and the dose of oxaliplatin from 85 mg/m2 to 68 mg/m2. The modification represents a greater reduction in dose intensity than the mFOLFIRINOX adopted by several Western groups. Encouraged by observed responses, we further evaluated the efficacy of mFOLFIRINOX in patients with LAPC with the goal of surgical resection in the event of an adequate response.
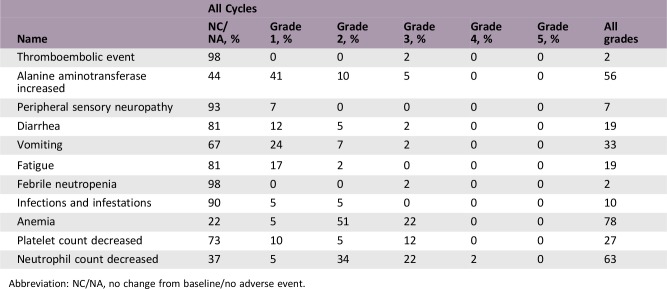
Here, we enrolled patients with LAPC who underwent preoperative therapy with mFOLFIRINOX (LAPC‐N) prospectively and compared the outcomes among patients with RPC or LAPC with upfront surgery (LAPC‐S) whose data were collected retrospectively. mFOLFIRINOX was applied in 41 LAPC cases with an objective response rate of 37.1%, which was similar to that in published studies. In our cohort, 29.3% patients had grade 3 or 4 toxicities, with 10 (24.4%) suffering neutropenia and 9 (22.0%) having anemia. No severe fatigue, diarrhea, or vomiting was observed in our study. Our modification resulted in better tolerability without attenuating the efficacy of full‐dose FOLFIRINOX.
Patients with LAPC benefited from tumor downstaging with mFOLFIRINOX‐based NAT. Fourteen (34.1%) LAPC‐N patients met the criteria for conversion to surgery after treatment (LAPC‐N‐S). According to histology, LAPC‐S patients endured later stages of disease, and the incidence of lymph‐node metastasis was much higher than that in the LAPC‐N‐S group. Moreover, as many as 58.3% LAPC‐N‐S patients achieved grade 3 (<10% viable tumor cells) histology responses. Although the R0 resection rates of LAPC‐N‐S and LAPC‐S were similar (78.6% vs. 73.7%), mFOLFIRINOX‐based NAT maintained surgical safety. LAPC‐S patients suffered longer operation time, more blood transfusion, and higher prevalence of complications. Two (12.5%) cases of grade 5 complication were observed in the LAPC‐S group, and three (15.8%) patients died within 90 days after surgery.
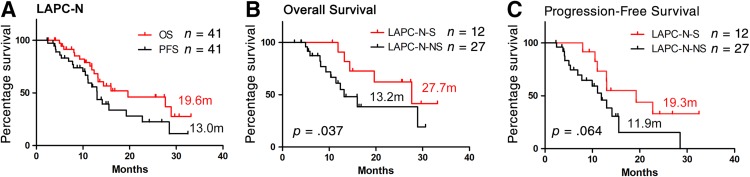
Survival was also evaluated (patients who were lost to follow‐up or who had grade 5 complications were excluded; Fig. 1). The median OS and PFS of LAPC‐N‐S patients were 27.7 months and 19.3 months, respectively, which were similar to those of patients with RPC (30.0 months and 23.0 months) and were significantly longer than those of LAPC‐S patients (8.9 months and 7.6 months). Patient selection and downstaging through preoperative treatment were the reasons for better outcomes. However, NAT did not reduce the risk of local or distal recurrence of patients with LAPC undergoing tumor resection in local or distal disease. The survival of LAPC‐S patients was even poorer than that of LAPC‐N patients without surgery (13.2 months and 11.9 months). Surgical complications or injury and irregular or delayed adjuvant therapy may be the causes of compromised survival. Regular and effective systemic treatment, including NAT and adjuvant therapy, is strongly recommended for all patients with LAPC.
Figure 1.
Overall survival and progression‐free survival of patients with pancreatic cancer. The overall survival and progression survival of LAPC patients with neoadjuvant therapy (LAPC‐N) (A). The overall survival and progression survival of LAPC‐N patients with surgical resection (LAPC‐N‐S) and LAPC‐N patients with nonsurgical treatment (LAPC‐N‐NS) (B and C). Two patients were considered surgically unresectable intraoperatively, and they were not included in survival analysis of subgroups of LAPC‐N with tumor resection and LAPC‐N without surgical treatment.Abbreviations: LAPC, locally advanced pancreatic cancer; LAPC‐N, LAPC with neoadjuvant therapy; LAPC‐N‐S, LAPC‐N with surgical resection; LAPC‐S, LAPC with upfront surgery; LAPC‐NS, LAPC‐N with nonsurgical treatment; RPC, resectable pancreatic cancer.
Trial Information
- Disease
Pancreatic cancer
- Stage of Disease/Treatment
Neoadjuvant
- Prior Therapy
One prior regimen
- Type of Study – 1
Phase II
- Type of Study – 2
Single arm
- Primary Endpoint
Overall survival
- Primary Endpoint
Progression‐free survival
- Secondary Endpoint
Overall response rate
- Secondary Endpoint
Toxicity
- Additional Details of Endpoints or Study Design
- Patients with LAPC who underwent preoperative therapy with mFOLFIRINOX were enrolled and results, including imaging features, chemotherapy response, chemotherapy‐related adverse events, perioperative complications, histology, median OS, and PFS, compared with results in an observational cohort of patients with RPC or LAPC who underwent surgery alone.
- Investigator's Analysis
Active and should be pursued further
Drug Information
- Drug 1
- Generic/Working Name
Oxaliplatin
- Trade Name
Eloxatin
- Company Name
Cenexi‐Laboratoires Thissen SA
- Drug Type
Small molecule
- Drug Class
Platinum compound
- Dose
68 milligrams (mg) per squared meter (m2)
- Route
IV
- Schedule of Administration
Twice‐weekly schedule
- Drug 2
- Generic/Working Name
Leucovorin
- Trade Name
Tong Ao
- Company Name
Jiangsu Heng Rui Medicine Co., Ltd
- Drug Type
Small molecule
- Drug Class
Antimetabolite
- Dose
400 milligrams (mg) per squared meter (m2)
- Route
IV
- Schedule of Administration
Twice‐weekly schedule
- Drug 3
- Generic/Working Name
Irinotecan
- Trade Name
Ai Li
- Company Name
Jiangsu Heng Rui Medicine. Co., Ltd
- Drug Type
Small molecule
- Drug Class
Apoptosis ‐ other
- Dose
135 milligrams (mg) per squared meter (m2)
- Route
IV
- Schedule of Administration
Twice‐weekly schedule
- Drug 4
- Generic/Working Name
Fluorouracil
- Trade Name
Hai Pu
- Company Name
Shanghai Xu Dong Hai Pu Pharmaceutical Co., Ltd
- Drug Type
Small molecule
- Drug Class
Antimetabolite
- Dose
2,400 milligrams (mg) per squared meter (m2)
- Route
Continuous intravenous infusion (CIV)
- Schedule of Administration
5‐FU CIV administered over 42 hours beginning after infusion of other agents, on a twice‐weekly schedule
Patient Characteristics
- Number of Patients, Male
22
- Number of Patients, Female
19
- Age
Median (range): 62 (44–80)
- Number of Prior Systemic Therapies
Median: 0
- Performance Status: ECOG
-
0 — 28
1 — 12
2 — 1
3 — 0
Unknown — 0
- Observational Cohorts
- Patients with RPC and patients with LAPC with upfront surgery (LAPC‐S) were selected from patients who had undergone surgery and been diagnosed histologically with pancreatic adenocarcinoma from April 2012 to November 2017 in our department. RPC and LAPC were defined according to National Comprehensive Cancer Center guidelines by a multidisciplinary team; LAPC‐S and LAPC‐N shared the same definition, staging system, and surgeon teams. The 74 patients with RPC and 19 with LAPC‐S were set as observational cohorts, which served as benchmarks for the prospective cohort.
Primary Assessment Method
- Title
Total Patient Population
- Number of Patients Screened
41
- Number of Patients Enrolled
41
- Number of Patients Evaluable for Toxicity
41
- Number of Patients Evaluated for Efficacy
35
- Evaluation Method
RECIST 1.1
- Response Assessment CR
n = 0 (0%)
- Response Assessment PR
n = 13 (37.1%)
- Response Assessment SD
n = 14 (40.0%)
- Response Assessment PD
n = 8 (22.9%)
- Response Assessment OTHER
n = 0 (0%)
- (Median) Duration Assessments PFS
13.0 months
- (Median) Duration Assessments OS
19.6 months
- Outcome Notes
- A total of 41 patients were enrolled, and the number of patients who presented with complete response, partial response, stable disease, and progressive disease after NAT treatment was 0, 13, 14, and 8, respectively (35 in total). We did not evaluate the treatment response of the other six patients, because they received fewer than four cycles of mFOLFIRINOX treatment (response was evaluated after a patient finished at least four cycles of treatment). Hence, these six patients were not included in further evaluation for efficacy, and the objective response rate was 37.1% (13/35). These six patients did not undergo surgical treatment; however, they were included in the survival analysis of LAPC‐N groups that did and did not undergo surgery.
Adverse Events
Abbreviation: NC/NA, no change from baseline/no adverse event.
Assessment, Analysis, and Discussion
- Completion
Study completed
- Investigator's Assessment
Active and should be pursued further
Pancreatic cancer is a malignant disease with an extremely poor prognosis [1], largely because only 15%–20% of patients are eligible for potentially curative surgery at diagnosis [2]. Systemic therapy is required for pancreatic cancer in all stages, and surgery is the only approach to significantly improve survival and outcomes [3]. Neoadjuvant therapy (NAT) may be recommended for patients with locally advanced pancreatic cancer (LAPC) [4], [5], and FOLFIRINOX regimens are first‐line neoadjuvant chemotherapies for LAPC worldwide [6]. However, the promotion of NAT for Chinese patients with LAPC has been a challenge.
Recently, modification of FOLFIRINOX have been adopted worldwide by omitting bolus 5‐FU and decreasing the dose of irinotecan [7], [8], [9]. Based on the published data, we further reduced oxaliplatin to 80% of the full dose. We used a further modified FOLFIRINOX (mFOLFIRINOX) regimen (oxaliplatin 68 mg/m2, leucovorin 400 mg/m2, irinotecan 135 mg/m2, and fluorouracil 2,400 mg/m2) in patients with metastatic pancreatic cancer and obtained promising results [10], suggesting that mFOLFIRINOX may be useful in patients with LAPC. Hence, we adopted the mFOLFIRINOX regimen in patients with LAPC to evaluate surgical resectability, surgical morbidity and mortality, and long‐term survival.
Forty‐one patients with LAPC were enrolled as a prospective cohort and received NAT with mFOLFIRINOX from April 2014 to November 2017 in our institution. Patients who were histologically confirmed as pancreatic adenocarcinoma and diagnosed as LAPC by a multidisciplinary team according to the National Comprehensive Cancer Network definitions were eligible for inclusion. Exclusion criteria were (a) Eastern Cooperative Oncology Group performance score higher than 2; (b) inadequate bone marrow, liver or renal function; (c) with additional malignancies; (d) more than 80 years of age. Moreover, data from 74 patients with resectable pancreatic cancer (RPC; from April 2012 to November 2017) and 19 patients with LAPC with upfront surgery (LAPC‐S; from April 2012 to March 2014) were collected retrospectively, forming two retrospective, observational cohorts that served as benchmarks to evaluate the treatment outcome of patients with LAPC with neoadjuvant mFOLFIRINOX.
A phase II trial conducted by Conroy et al. was the first to demonstrate the effectiveness of FOLFIRINOX in LAPC [11]. Moreover, the overall response rate was 26% with progression‐free survival (PFS) and overall survival (OS) of 7.6 months and 15.7 months, respectively [11]. Confirmatory studies found a comparable response rate of 30% with median OS of 15.7–26 months [7], [12], [13]. In our study, 41 patients received mFOLFIRINOX‐based NAT, and 35 of them were restaged after treatment. The prevalence of a relative response and disease control was 37.1% and 77.1%, respectively, which was similar to the data obtained from the studies mentioned above. A median OS of 19.6 months was obtained in the entire group of patients with LAPC with mFOLFIRINOX‐based NAT (LAPC‐N). Our modification did not appear to attenuate the efficacy of full‐dose FOLFIRINOX; however, this would need to be assessed by a prospective randomized study.
During treatment, the most common grade 3 or 4 adverse effects reported by Suker et al. in a meta‐analysis of 490 patients with LAPC were neutropenia (27%), fatigue (14%), diarrhea (10%), and vomiting (8%) [14]. Using our dose‐modified FOLFIRINOX regimen, the prevalence of neutropenia was reduced to 24.4%. No grade 3 or 4 fatigue, diarrhea, or vomiting were observed. However, our cohort experienced a higher prevalence of anemia (22.0%) and thrombocytopenia (12.2%) despite the use of granulocyte colony‐stimulating factor, interleukin‐11, or thrombopoietin during treatment. Hence, our modification resulted in better safety and tolerability. However, more attention should be given to hematologic adverse events during mFOLFIRINOX use.
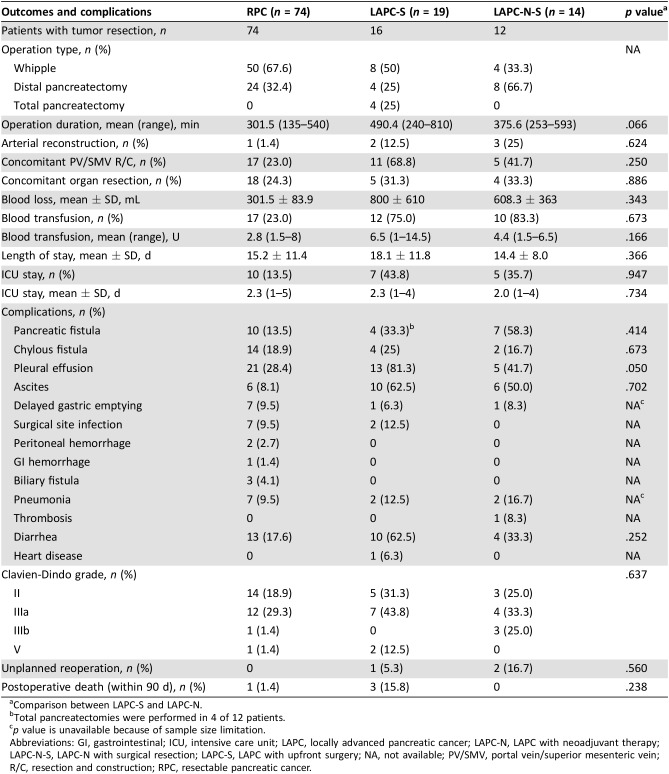
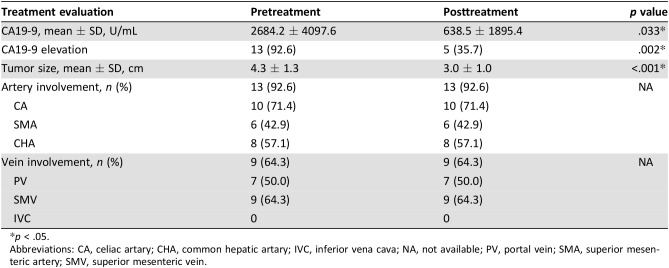
According to recent research, NAT can result in better surgical resectability in patients with LAPC after downstaging [11], [15], [16]. Fourteen patients with LAPC‐N underwent surgery in our study (LAPC‐N‐S), and the rate of conversional surgery achieved 34.1%, which was similar to that of patients with LAPC with modified or full‐dose FOLFIRINOX regimens [14]. NAT contributed to a remarkable decrease in CA19‐9 level and tumor diameter, but vascular involvement persisted, findings that are similar to those of Donahue et al. [17]. The imaging evidence of changes in vascular involvement is not considered an essential criterion for conversion to surgery [17]. We set these retrospective observational cohorts of RPC and LAPC‐S as control groups to evaluate the differences regarding surgical resectability and safety. Unexpectedly, we did not observe improved surgical resectability in the LAPC‐N group compared with that in LAPC‐S cases (R0: 78.6% vs. 73.7%). Also, 15.8% of LAPC‐S and 14.3% of LAPC‐N‐S patients continued to have surgically unresectable tumors intraoperatively. To achieve complete resection, a much more aggressive approach was needed, so total pancreatectomy was done in 25.0% of LAPC‐S patients. As a result, LAPC‐S patients suffered a longer operative time and greater blood loss compared with LAPC‐N‐S patients. Moreover, two LAPC‐S patients suffered a grade 5 complication after surgery. Hence, patients with LAPC could benefit from NAT with regard to surgical safety.
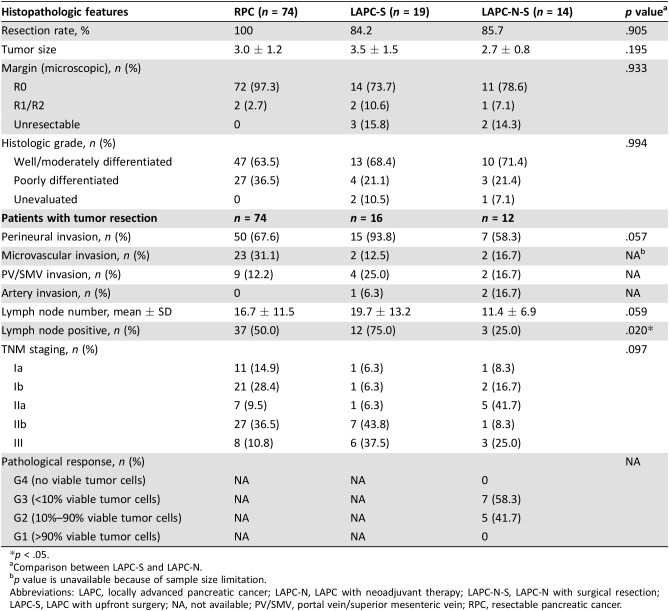
According to our data, patients with LAPC without NAT were more likely to exhibit advanced pathologic staging at surgery than those in the LAPC‐N‐S group (IIb: 43.8% vs. 8.3%; III: 37.5% vs. 25.0%), which was strongly correlated with poor survival. NAT contributed to a significantly reduced prevalence of lymph‐node positivity in LAPC‐N cases and achieved a grade 3 pathologic response of 58.3%. Investigators have reported that patients with negative lymph nodes and pathologic response have better outcomes [18], [19].
We further analyzed perioperative complications and survival in cohorts of RPC, LAPC‐S, and LAPC‐N‐S cases. LAPC‐N‐S patients suffered a higher risk of pancreatic fistulae than that of LAPC‐S. The difference in the prevalence of pancreatic fistulae could partially be because four LAPC‐S patients underwent total pancreatectomy. The prevalence of grade 2 or 3 complications was otherwise similar between LAPC‐S and LAPC‐N‐S patients. Two LAPC‐N‐S patients and one LAPC‐S patient underwent unplanned reoperation. LAPC‐N patients may be at a higher risk of perioperative complications because of the increased complexity of surgery caused by NAT and NAT‐related toxicity [20].
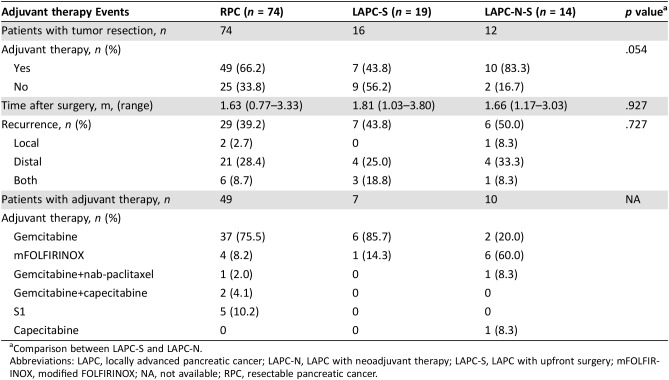
The contribution of NAT to survival in patients with LAPC was also evaluated. The median OS and PFS of 12 LAPC‐N‐S patients with tumor resection were 27.7 months and 19.3 months, respectively. Also, 27 LAPC‐N patients who did not undergo surgery (LAPC‐N‐NS) had a median OS and PFS of 13.2 months and 11.9 months. Interestingly, the median OS of LAPC‐N‐S patients was similar to that of patients with RPC (27.7 m vs. 30.0 m). Patient selection and downstaging through NAT may be reasons for better outcomes. However, NAT did not reduce the prevalence of recurrence of patients with LAPC undergoing tumor resection in local or distal disease. The median OS and PFS of LAPC‐S patients was limited to 8.9 m and 7.6 m, respectively, which was shorter than those of LAPC‐N‐NS patients. Irregular adjuvant therapy may be the main reason for compromised survival. Only 43.8% of LAPC‐S patients received chemotherapy postoperatively, whereas the prevalence was 66.2% for patients with RPC and 83.3% for LAPC‐N‐S patients. Moreover, 85.7% of LAPC‐S patients underwent gemcitabine therapy, which is not as intense as that of mFOLFIRINOX or nab‐paclitaxel plus gemcitabine [15]. Compared with LAPC‐S patients, LAPC‐N‐NS patients underwent continuous treatment with mFOLFIRINOX or nab‐paclitaxel plus gemcitabine, and they did not have surgical complications or injury. Taken together, LAPC‐N‐NS patients showed longer survival than LAPC‐S patients.
Figures and Tables
Figure 2.
Overall survival and progression‐free survival of patients with pancreatic cancer. The overall survival (A) and progression‐free survival (B) of patients with RPC, patients with LAPC‐S, and patients with LAPC‐N. The data of RPC and LAPC‐S were collected retrospectively, separately from the prospective cohort of LAPC‐N.
Abbreviations: LAPC, locally advanced pancreatic cancer; LAPC‐N, LAPC with neoadjuvant therapy; LAPC‐S, LAPC with surgery only; RPC, resectable pancreatic cancer.
Figure 3.
Enrollment and treatment schema in our institution.Abbreviations: LAPC, LAPC, locally advanced pancreatic cancer; mFOLFIRINOX, modified FOLFIRINOX; NAT, neoadjuvant therapy; RPC, resectable pancreatic cancer.
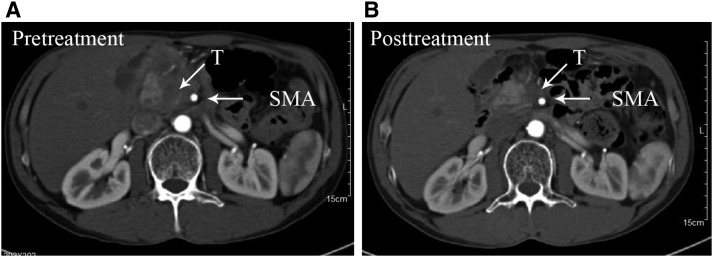
Figure 4.
Example of before treatment (A) and after treatment (B) with mFOLFIRINOX with partial response.Abbreviations: SMA, superior mesenteric artery; T, tumor.
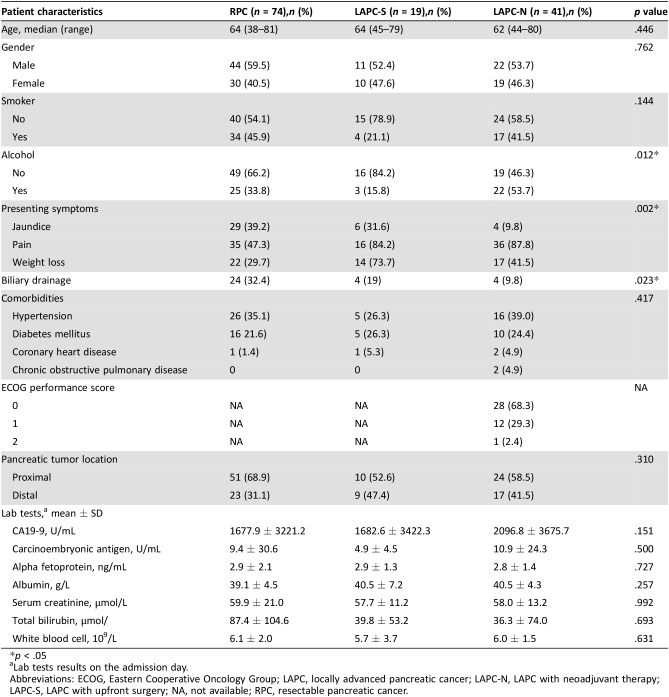
Table 1. Patient characteristics at baseline.
p < .05
Lab tests results on the admission day.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; LAPC, locally advanced pancreatic cancer; LAPC‐N, LAPC with neoadjuvant therapy; LAPC‐S, LAPC with upfront surgery; NA, not available; RPC, resectable pancreatic cancer.
Table 2. Response to modified‐FOLFIRINOX regimens.
The objective response rate was defined as the percentage of patients with CR or PR.
The rate of disease control was defined as the percentage of patients with CR, PR, or SD.
Abbreviations: CR, complete response; LAPC‐N, LAPC with neoadjuvant therapy; PD, progressive disease; PR, partial response; SD, stable disease.
Table 3. Operation outcomes and perioperative complications of patients with tumor resection.
Comparison between LAPC‐S and LAPC‐N.
Total pancreatectomies were performed in 4 of 12 patients.
p value is unavailable because of sample size limitation.
Abbreviations: GI, gastrointestinal; ICU, intensive care unit; LAPC, locally advanced pancreatic cancer; LAPC‐N, LAPC with neoadjuvant therapy; LAPC‐N‐S, LAPC‐N with surgical resection; LAPC‐S, LAPC with upfront surgery; NA, not available; PV/SMV, portal vein/superior mesenteric vein; R/C, resection and construction; RPC, resectable pancreatic cancer.
Table 4. Histopathologic features.
p < .05.
Comparison between LAPC‐S and LAPC‐N.
p value is unavailable because of sample size limitation.
Abbreviations: LAPC, locally advanced pancreatic cancer; LAPC‐N, LAPC with neoadjuvant therapy; LAPC‐N‐S, LAPC‐N with surgical resection; LAPC‐S, LAPC with upfront surgery; NA, not available; PV/SMV, portal vein/superior mesenteric vein; RPC, resectable pancreatic cancer.
Table 5. Radiological features.
Comparison between LAPC‐S and LAPC‐N.
Abbreviations: CA, celiac artary; CHA, common hepatic artary; IVC, inferior vena cava; LAPC, locally advanced pancreatic cancer; LAPC‐N, LAPC with neoadjuvant therapy; LAPC‐S, LAPC with upfront surgery; NA, not available; PV, portal vein; RPC, resectable pancreatic cancer; SMV, superior mesenteric vein.
Table 6. Treatment evaluation.
p < .05.
Abbreviations: CA, celiac artary; CHA, common hepatic artary; IVC, inferior vena cava; NA, not available; PV, portal vein; SMA, superior mesenteric artery; SMV, superior mesenteric vein.
Table 7. Adjuvant therapy and recurrence.
Comparison between LAPC‐S and LAPC‐N.
Abbreviations: LAPC, locally advanced pancreatic cancer; LAPC‐N, LAPC with neoadjuvant therapy; LAPC‐S, LAPC with upfront surgery; mFOLFIRINOX, modified FOLFIRINOX; NA, not available; RPC, resectable pancreatic cancer.
Acknowledgments
The authors thank Jian‐Feng Wang for his assistance with the information collection. This work was supported by the National High Technology Research and Development Program of China (grant number 2015AA020405), the National Natural Science Foundation of China (grant number 81672337), the National Natural Science Foundation of China (grant number 81702332, 81871925, and 81702376), the Key Program of the National Natural Science Foundation of China (grant number 81530079), the Training Program of the Key Program of the National Natural Science Foundation of China (No. 91442115) the Key Research and Development Project of Zhejiang Province (grant number 2015C03044), and the Zhejiang Provincial Program for the Cultivation of High‐Level Innovative Health Talents.
Footnotes
ClinicalTrials.gov Identifier: NCT03469375
Sponsor(s): The Second Affiliated Hospital, Zhejiang University School of Medicine
Principal Investigator: Tingbo Liang
IRB Approved: Yes
Contributor Information
Tingbo Liang, Email: liang-tingbo@zju.edu.cn.
Xueli Bai, Email: shirleybai@zju.edu.cn.
Disclosures
The authors indicated no financial relationships.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 2.Bockhorn M, Uzunoglu FG, Adham M et al. Borderline resectable pancreatic cancer: A consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014;155:977–988. [DOI] [PubMed] [Google Scholar]
- 3.Malik NK, May KS, Chandrasekhar R et al. Treatment of locally advanced unresectable pancreatic cancer: A 10‐year experience. J Gastrointest Oncol 2012;3:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang F, Kumar P. The role of radiotherapy in management of pancreatic cancer. J Gastrointest Oncol 2011;2:157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huguet F, André T, Hammel P et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol 2007;25:326–331. [DOI] [PubMed] [Google Scholar]
- 6.Sirohi B, Singh A, Dawood S et al. Advances in chemotherapy for pancreatic cancer. Indian J Surg Oncol 2015;6:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahaseth H, Brutcher E, Kauh J et al. Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma. Pancreas 2013;42:1311–1315. [DOI] [PubMed] [Google Scholar]
- 8.Blazer M, Wu C, Goldberg RM et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol 2015;22:1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghorani E, Wong HH, Hewitt C et al. Safety and efficacy of modified FOLFIRINOX for advanced pancreatic adenocarcinoma: A UK single‐centre experience. Oncology 2015;89:281–287. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Ma T, Zhang Q et al. Modified‐FOLFIRINOX in metastatic pancreatic cancer: A prospective study in Chinese population. Cancer Lett 2017;406:22–26. [DOI] [PubMed] [Google Scholar]
- 11.Conroy T, Paillot B, François E et al. Irinotecan plus oxaliplatin and leucovorin‐modulated fluorouracil in advanced pancreatic cancer‐‐A Groupe Tumeurs Digestives of the Federation Nationale des Centres de Lutte Contre le Cancer study. J Clin Oncol 2005;23:1228–1236. [DOI] [PubMed] [Google Scholar]
- 12.Faris JE, Blaszkowsky LS, McDermott S et al. FOLFIRINOX in locally advanced pancreatic cancer: The Massachusetts General Hospital Cancer Center experience. The Oncologist 2013;18:543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadot E, Doussot A, O'Reilly EM et al. FOLFIRINOX induction therapy for stage 3 pancreatic adenocarcinoma. Ann Surg Oncol 2015;22:3512–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suker M, Beumer BR, Sadot E et al. A patient‐level meta‐analysis of FOLFIRINOX for locally advanced pancreatic cancer. Lancet Oncol 2016;17:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan K, Shah K, Lien K et al. A Bayesian meta‐analysis of multiple treatment comparisons of systemic regimens for advanced pancreatic cancer. PLoS One 2014;9:e108749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gresham GK, Wells GA, Gill S et al. Chemotherapy regimens for advanced pancreatic cancer: A systematic review and network meta‐analysis. BMC Cancer 2014;14:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donahue TR, Isacoff WH, Hines OJ et al. Downstaging chemotherapy and alteration in the classic computed tomography/magnetic resonance imaging signs of vascular involvement in patients with pancreaticobiliary malignant tumors: Influence on patient selection for surgery. Arch Surg 2011;146:836–843. [DOI] [PubMed] [Google Scholar]
- 18.Christians KK, Heimler JW, George B et al. Survival of patients with resectable pancreatic cancer who received neoadjuvant therapy. Surgery 2016;159:893–900. [DOI] [PubMed] [Google Scholar]
- 19.Chuong MD, Frakes JM, Figura N et al. Histopathologic tumor response after induction chemotherapy and stereotactic body radiation therapy for borderline resectable pancreatic cancer. J Gastrointest Oncol 2016;7:221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allendorf JD, Lauerman M, Bill A et al. Neoadjuvant chemotherapy and radiation for patients with locally unresectable pancreatic adenocarcinoma: Feasibility, efficacy, and survival. J Gastrointest Surg. 2008;12:91–100. [DOI] [PubMed] [Google Scholar]