Abstract
Rationale: The identification of informative elements of the host response to infection may improve the diagnosis and management of bacterial pneumonia.
Objectives: To determine whether the absence of alveolar neutrophilia can exclude bacterial pneumonia in critically ill patients with suspected infection and to test whether signatures of bacterial pneumonia can be identified in the alveolar macrophage transcriptome.
Methods: We determined the test characteristics of alveolar neutrophilia for the diagnosis of bacterial pneumonia in three cohorts of mechanically ventilated patients. In one cohort, we also isolated macrophages from alveolar lavage fluid and used the transcriptome to identify signatures of bacterial pneumonia. Finally, we developed a humanized mouse model of Pseudomonas aeruginosa pneumonia to determine if pathogen-specific signatures can be identified in human alveolar macrophages.
Measurements and Main Results: An alveolar neutrophil percentage less than 50% had a negative predictive value of greater than 90% for bacterial pneumonia in both the retrospective (n = 851) and validation cohorts (n = 76 and n = 79). A transcriptional signature of bacterial pneumonia was present in both resident and recruited macrophages. Gene signatures from both cell types identified patients with bacterial pneumonia with test characteristics similar to alveolar neutrophilia.
Conclusions: The absence of alveolar neutrophilia has a high negative predictive value for bacterial pneumonia in critically ill patients with suspected infection. Macrophages can be isolated from alveolar lavage fluid obtained during routine care and used for RNA-Seq analysis. This novel approach may facilitate a longitudinal and multidimensional assessment of the host response to bacterial pneumonia.
Keywords: bacterial pneumonia, host response, alveolar macrophages, RNA-Seq
At a Glance Commentary
Scientific Knowledge on the Subject
Analysis of lower respiratory tract specimens in patients with suspected pneumonia has largely focused on pathogen detection. Assessment of the host response is recognized as an important yet understudied approach to aid pneumonia diagnosis and management.
What This Study Adds to the Field
Assessment of alveolar neutrophilia can help to rapidly exclude bacterial pneumonia in critically ill patients with a negative predictive value approaching 100% when an alveolar neutrophil percentage of less than 50% is paired with a negative lavage fluid gram stain. In addition, we demonstrate the feasibility of measuring changes in host gene expression to refine pneumonia diagnosis using alveolar macrophages from BAL samples obtained during routine clinical care. This novel approach may facilitate a longitudinal and multidimensional assessment of the host response to bacterial pneumonia.
Antibiotic overuse is an urgent public health problem (1, 2). The frequent use of broad-spectrum antibiotic therapy puts patients at risk for infection with multidrug-resistant pathogens and increases antimicrobial resistance within the healthcare system (3, 4). Pneumonia is a leading cause of death and the most common infection identified in patients admitted to the ICU (5–7). Diagnostic uncertainty regarding both the presence and etiology of pneumonia contributes to the frequent administration of antibiotic therapy to uninfected patients. Clinical variables such as fever, sputum production, and leukocytosis are insufficient to reliably guide management decisions (8). Improved diagnostic approaches are urgently needed to help minimize antibiotic exposure for uninfected patients.
Rapid diagnostic testing in patients with suspected pneumonia has focused primarily on pathogen detection (9). However, there is growing recognition that pneumonia pathogenesis is driven as much by host-specific factors as by the invading pathogen (10). Diagnostic tests based on the host response to infection may therefore improve pneumonia diagnosis and management.
Bacterial pneumonia is characterized by the rapid influx of neutrophils into the alveolar space in immunocompetent patients (11). The alveolar space can be safely sampled during routine clinical care using nonbronchoscopic bronchoalveolar lavage (NBBAL) (12). Assessment of alveolar neutrophilia provides a rapid and inexpensive means of assessing a key immune response to bacterial pneumonia. Although a low neutrophil percentage on cell count analysis has been reported to have a high negative predictive value for bacterial pneumonia, these data are based on a limited number of small studies (8, 13–15).
Next-generation sequencing technologies, including RNA-Seq, allow for a detailed analysis of the host response to infection. As RNA-Seq and PCR techniques are based on similar chemistry, predictive gene signatures identified in an unbiased fashion with RNA-Seq could be rapidly incorporated into PCR-based diagnostic tests currently being used for pathogen detection.
In this study, we evaluate whether the absence of alveolar neutrophilia can exclude bacterial pneumonia in retrospective and prospective cohorts of critically ill patients with suspected infection. We then pair flow-cytometry sorting of lavage fluid with RNA-Seq to investigate whether the transcriptome of a key lung immune cell, the alveolar macrophage, can be used to discriminate patients with and without bacterial pneumonia. Finally, we use a humanized mouse model of Pseudomonas aeruginosa pneumonia to determine if pathogen-specific signatures can be identified in human alveolar macrophages. Our results show that the absence of alveolar neutrophilia, especially if paired with a negative gram stain, can help to rapidly exclude the diagnosis of bacterial pneumonia with encouraging test characteristics. In addition, we demonstrate how RNA-Seq can be used to identify transcriptome-level changes in alveolar macrophages using samples obtained during routine clinical care. This approach may dramatically improve our ability to study dynamic changes in host gene expression over the course of infection. Some of the results of these studies have been previously reported in the form of abstracts (16, 17).
Methods
Patients
In a retrospective analysis, we analyzed BAL specimens obtained as part of routine care from patients undergoing evaluation for ventilator-associated pneumonia at an urban academic medical center between January 1, 2010 and June 30, 2013. Bronchoscopic BAL specimens were collected by intensivists. NBBAL specimens were collected by respiratory therapists using a 16-F sampling catheter (BALCath; Kimberly-Clark Corporation). A volume of at least 60 ml of nonbacteriostatic saline was instilled into the right or left lung as determined by the treating physician. The initial NBBAL aliquot was discarded as part of routine practice to minimize the contribution of central airway colonization. For bronchoscopic BAL, an initial aliquot is not routinely discarded by all operators. Cell counts were performed through standard automated Coulter counter technology. Cell differentials were determined manually by a clinical laboratory technologist. BAL neutrophils were recorded as a percentage of the cells in the lavage fluid.
Subsequently, in two independent prospective cohorts, we enrolled mechanically ventilated adult patients aged 18 years or older in the ICU in whom a bronchoscopic BAL or NBBAL was performed to investigate suspected pneumonia from December 1, 2015 to January 4, 2017. In one of these cohorts, a small aliquot (1–5 ml) of lavage fluid was obtained for flow-cytometry sorting as part of study protocols approved by the Northwestern University Institutional Review Board. Fluid was stored in 15-ml conical tubes (Corning) at 4°C until processing.
In all cohorts, BALs with greater than or equal to 12% bronchial epithelial cells were excluded. Patients who were neutropenic (defined as a serum absolute neutrophil count < 1,000 cells/μl) or who had bronchiectasis or cystic fibrosis were also excluded. In the prospective cohorts, patients could be enrolled more than once if each lavage was collected for a distinct episode of suspected pneumonia during the index hospitalization or if they were rehospitalized with concern for a new infection.
Pneumonia Definition
The definition of bacterial pneumonia was based on recent consensus guidelines (18, 19). Specifically, pneumonia was defined as greater than or equal to 104 cfu/ml of a bacterial species on quantitative culture in the setting of a clinical suspicion of infection (identified by the treating clinician ordering a bronchoscopic BAL or NBBAL with bacterial culture) and an abnormal chest radiograph. For patients with a recent (<48 h) change in antibiotics at the time of sampling, bacterial growth of greater than or equal to 103 cfu/ml was considered positive, similar to previous trials (20). Calibrated loop technology was used for quantitative cultures.
Lavage Fluid Processing and Cell Sorting
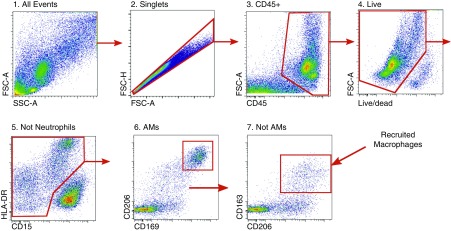
All lavage samples were processed for flow-cytometry sorting within 12 hours of collection using a modification of a previously described procedure, as detailed in the online supplement (21, 22). The gating strategy used is shown in Figure 1.
Figure 1.
Gating strategy used to identify resident and recruited macrophages from patient BAL fluid. Tissue-resident alveolar macrophages were identified using sequential gating as singlets/CD45+/live/CD15−/CD206++/CD169+/HLA−DR+/high autofluorescence; recruited macrophages were identified as singlets/CD45+/live/CD15−/CD169−/CD206++/CD163+/HLA-DR+/high autofluorescence. Data from a representative patient are shown. AMs = alveolar macrophages; CD = cluster of differentiation; FSC-A = forward scatter–area; FSC-H = forward scatter–height; SSC-A = side scatter–area.
Humanized Mouse Pneumonia Model
The Institutional Animal Care and Use Committee and Institutional Review Board (IS00000747) at Northwestern University approved all animal procedures. MISTRG mice (Jax 017712) were reconstituted with CD34+ stem cells isolated from human cord blood obtained from a commercial vendor (StemCell Technologies) (23). Fourteen-week-old mice with confirmed reconstitution were intratracheally inoculated with either a low-virulence (PABL065) or high-virulence (PABL012) clinical strain of P. aeruginosa. Four hours after infection, lungs were harvested for flow sorting of alveolar macrophages. Full details of the experimental procedure are provided in the online supplement.
RNA Isolation and Sequencing
RNA isolation and sequencing procedures are detailed in the online supplement.
Bioinformatics Analysis
Bioinformatic analyses are detailed in the online supplement.
Statistics
Data are expressed as mean and SD or median and interquartile range (IQR) according to data distribution. BAL neutrophil and procalcitonin distributions were compared between patients with and without bacterial pneumonia using the Mann-Whitney U test. Receiver operating characteristic (ROC) curves were constructed for alveolar neutrophilia and procalcitonin to discriminate between patients with and without bacterial pneumonia. The area under the curve (AUC), sensitivity, specificity, positive predictive value, negative predictive value (NPV), positive likelihood ratio, and negative likelihood ratio were calculated.
For predictive modeling, least absolute shrinkage and selection operator (LASSO) was fit to the top 50 differentially expressed genes ordered by false discovery rate (FDR) q value in the resident and recruited macrophage datasets. A summary of statistics with LASSO was calculated using the R package GLMNET (24). Tenfold cross-validation was performed to determine the most suitable parameters for the prediction model for each cell type. The average mean squared error and 95% confidence interval (CI) were reported from the most accurate model derived from 11 cross-validation runs. An ROC curve was generated for each cell type using the R package pROC and the AUC was calculated. Weighted correlation network analysis was performed using log counts per million and clinical variables (25, 26).
Analysis was performed using GraphPad Prism (version 7.02, GraphPad Software) and R (version 3.4.1).
Results
Predictive Value of BAL Neutrophilia
Clinical characteristics of patients are shown in Table 1. Bacterial pneumonia was diagnosed in more than one-third of each cohort. The majority of patients were receiving antibiotics at the time of BAL (81% in the retrospective cohort, 86% in prospective cohort A, and 100% in prospective cohort B). In-hospital mortality was 26% in the retrospective cohort, 37% prospective cohort A, and 37% in prospective cohort B.
Table 1.
Characteristics of Study Patients
| Retrospective Cohort (851 Patients) | Prospective Cohort A (73 Patients, 76 Unique Episodes of Suspected Pneumonia) | Prospective Cohort B (68 Patients, 79 Unique Episodes of Suspected Pneumonia) | |
|---|---|---|---|
| Male | 500 (58) | 42 (55) | 39 (57) |
| Age, yr | 61.4 (50–70) | 61 (54–71) | 63.5 (53–73) |
| White | 467 (55) | 33 (76) | 32 (40) |
| Pneumonia type | |||
| CAP | 0 (0) | 22 (29) | 20 (25) |
| HAP/VAP | 851 (100) | 54 (71) | 59 (75) |
| APS score on ICU admission | 67 (46–91) | 53 (33–76) | 61 (48–85) |
| On antibiotics before BAL | 692 (81) | 65 (86) | 79 (100) |
| Bacterial pneumonia | 344 (40) | 28 (37) | 29 (37) |
| ICU length of stay, d | 15.6 (7–28) | 11 (6–20) | 13.5 (7–26) |
| In-hospital mortality | 225 (26) | 28 (37) | 25 (37) |
Definition of abbreviations: APS = Acute Physiology Score; CAP = community-acquired pneumonia; HAP = hospital-acquired pneumonia; VAP = ventilator-associated pneumonia.
Data are presented as n (%) or median (interquartile range).
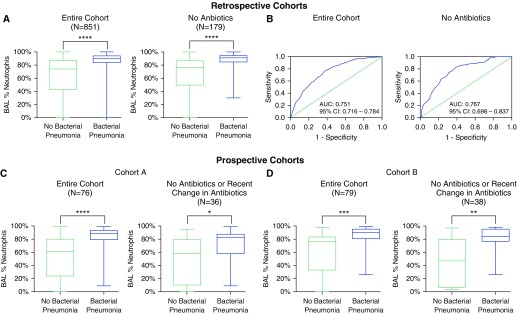
In the retrospective cohort, 1,156 BALs were screened for inclusion. After exclusion of 305 samples (53 with ≥12% bronchial epithelial cells, 126 due to repeat sampling, 104 from patients with a serum absolute neutrophil count < 1,000 cells/μl, and 22 for normal chest imaging), 851 were included in the final analysis. Of these, 344 (40.4%) met the definition for bacterial pneumonia. The most common pathogens isolated were: P. aeruginosa (90, 26.2%), methicillin-resistant Staphylococcus aureus (30, 8.7%), and Klebsiella pneumoniae (30, 8.7%). A full list of isolated pathogens from all cohorts is included in the online supplement. Receiver operating characteristics for BAL neutrophilia as a predictor of bacterial pneumonia are show in Figure 2. The AUC for BAL neutrophilia was 0.751 (95% CI, 0.719–0.784). When BAL neutrophils were less than 50%, the NPV for pneumonia was 91.5%. The operating characteristics for various thresholds of BAL neutrophil percentage are shown in Table 2.
Figure 2.
BAL neutrophil percentage has a high negative predictive value for bacterial pneumonia in critically ill patients with suspected infection. (A) Percent of neutrophils in BAL fluid in a retrospective cohort of mechanically ventilated patients with suspected pneumonia stratified by antibiotic use at the time of sampling. Boxes extend from the 25th to the 75th percentiles of recorded values, and the horizontal line is plotted at the median. Vertical lines represent ranges. (B) Receiver operating characteristics curve of BAL neutrophil percentage for the diagnosis of bacterial pneumonia stratified by antibiotic use at the time of sampling. (C and D) Percentage of neutrophils in BAL fluid in two independent prospective cohorts of mechanically ventilated patients with suspected pneumonia. Results are shown for the entire cohort and the subgroup of patients who were either not on antibiotics or did not have a change in antibiotics within 48 hours of sampling. Patients who were receiving antibiotics that did not cover the isolated pathogen were considered to be off antibiotics. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. AUC = area under the curve; CI = confidence interval.
Table 2.
Operating Characteristics of BAL Neutrophil Percentage to Identify the Presence of Bacterial Pneumonia in Mechanically Ventilated Patients with Suspected Infection
| BAL Neutrophils (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Positive LR (95% CI) | Negative LR (95% CI) |
|---|---|---|---|---|---|---|
| ≥40 | 96.8 | 23.9 | 46.2 | 91.6 | 1.27 (1.2–1.3) | 0.14 (0.1–0.3) |
| ≥50 | 95.9 | 29.8 | 48.1 | 91.5 | 1.37 (1.3–1.5) | 0.14 (0.1–0.2) |
| ≥60 | 92.7 | 35.7 | 49.4 | 87.8 | 1.44 (1.3–1.6) | 0.20 (0.1–0.3) |
| ≥70 | 88.1 | 44.4 | 51.8 | 84.6 | 1.58 (1.5–1.7) | 0.27 (0.2–0.4) |
| ≥80 | 79.1 | 59.0 | 56.7 | 80.6 | 1.93 (1.7–2.2) | 0.35 (0.3–0.4) |
| ≥90 | 48.5 | 82.6 | 65.4 | 70.3 | 2.79 (2.2–3.5) | 0.62 (0.6–0.7) |
Definition of abbreviations: CI = confidence interval; LR = likelihood ratio; NPV = negative predictive value; PPV = positive predictive value.
Retrospective cohort (n = 851).
As antibiotic administration may impact the microbiologic yield of BAL, we then restricted our analysis to the 179 patients who were not receiving antibiotics at the time of sampling. In this cohort, the AUC of alveolar neutrophilia for the diagnosis of bacterial pneumonia was 0.767 (95% CI, 0.697–0.837). A BAL neutrophil cutoff of less than 50% had a sensitivity of 86.7%, specificity of 56.5%, positive predictive value of 56.5%, and NPV of 86.7%.
In our prospective cohorts, 76 samples were included in cohort A and 79 samples in cohort B. Twenty-eight samples (36.8%) met the definition for bacterial pneumonia in cohort A and 29 (36.7%) in cohort B. A BAL neutrophil threshold of less than 50% had an NPV of 90.5% in cohort A and 90.9% in cohort B (see Table E3 in the online supplement for full operating characteristics). When the analysis was restricted to patients who were either not receiving antibiotics, receiving antibiotics that did not cover the pathogen that was ultimately isolated, or did not have a change in antibiotics within 48 hours of sampling, a BAL neutrophil threshold of less than 50% retained a high NPV: 85.7% in cohort A (40 samples) and 86.7% in cohort B (38 samples). We repeated our analysis of our prospective cohorts using a bronchial epithelial cell cutoff of greater than 5% to identify contaminated samples. This did not significantly change our results (data not shown).
We then compared the test characteristics of BAL neutrophilia to procalcitonin—a commonly used biomarker to discriminate bacterial from nonbacterial lower respiratory tract infections (Figure 3) (27, 28). This analysis included 61 patients from prospective cohort A (80% of the cohort) and 60 patients from prospective cohort B (76% of the cohort) who had a procalcitonin obtained within 6 hours of alveolar sampling. In both cohorts, procalcitonin was a poor predictor of bacterial pneumonia. There was no significant difference in procalcitonin values between patients with and without bacterial pneumonia in either cohort. The AUC for bacterial pneumonia was 0.550 (95% CI, 0.393–0.707) in cohort A and 0.566 (95% CI, 0.413–0.720) in cohort B.
Figure 3.
Procalcitonin does not discriminate between the presence or absence of bacterial pneumonia in mechanically ventilated patients with suspected infection. (A) Scatter plots showing procalcitonin values in two independent prospective cohorts of mechanically ventilated patients with suspected pneumonia. Vertical lines extend from the 25th to the 75th percentiles of recorded values, and the horizontal line is plotted at the median (P > 0.05 for both cohorts). (B) Receiver operating characteristics curves of procalcitonin for the diagnosis of bacterial pneumonia. AUC = area under the curve; CI = confidence interval; ns = not significant.
Finally, we asked whether pairing a BAL neutrophil threshold of less than 50% with a negative BAL gram stain (another inexpensive and rapidly available test) could further improve the NPV for bacterial pneumonia. This combination had an NPV for bacterial pneumonia of 100% in prospective cohort A and 95.0% in prospective cohort B.
RNA-Seq Analysis of Flow-sorted Macrophages
As no BAL neutrophil cutoff could exclude bacterial pneumonia with certainty, we explored whether gene expression profiling of alveolar macrophages might further discriminate between patients with and without pneumonia. Accordingly, we performed RNA-Seq analysis of flow-sorted resident and recruited macrophages obtained from patients in prospective cohort B.
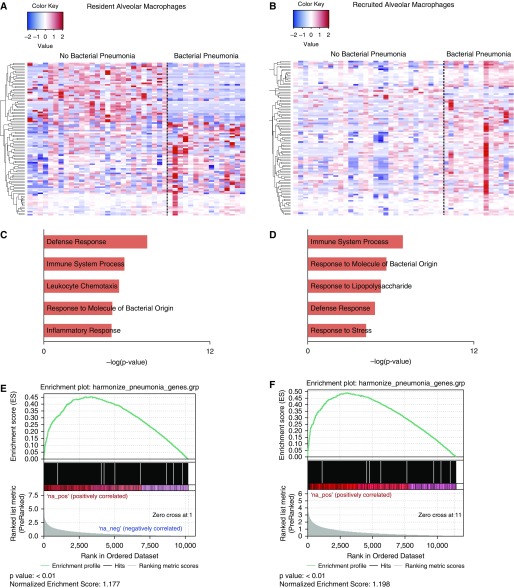
Nine samples were excluded from our analysis on the basis of our described filtering criteria. Estimation of differential gene expression was performed in a derivation cohort, which included 42 flow-sorted resident alveolar macrophage samples (15 from patients with pneumonia and 27 from patients without pneumonia) and 44 recruited macrophage samples (14 from patients with pneumonia and 30 from patients without pneumonia). Using an FDR q value less than 0.05, 33 differentially expressed genes were identified in resident alveolar macrophages and 24 differentially expressed genes were identified in recruited macrophages (Figures 4A and 4B). Functional enrichment analysis with GO Biological Processes revealed common infection-related processes including Defense Response, Immune System Process, and Response to Molecule of Bacterial Origin in both sorted-cell populations (Figures 4C and 4D).
Figure 4.
Transcriptional profiling of flow-sorted resident and recruited alveolar macrophages from critically ill patients with suspected infection reveals a signature of bacterial pneumonia. (A and B) In a derivation cohort, 49 flow-sorted resident alveolar macrophage samples and 46 recruited macrophage samples were obtained from critically ill patients with suspected infection and processed for RNA sequencing. Estimation of differential gene expression using EdgeR was performed comparing patients with and without pneumonia, and heatmaps were generated showing significantly differentially expressed genes ordered using hierarchical clustering. Columns represent individual patients, and rows represent specific genes. (C and D) Functional enrichment analysis with GO Biological Processes was performed using GOrilla. Representative GO processes upregulated in patients with pneumonia are shown. (E and F) Gene set enrichment analysis was performed using the curated Comparative Toxicogenomics Database pneumonia gene set. Enrichment plots with P values and normalized enrichment scores for both cells types are shown. GO = gene ontology.
Although functional enrichment analysis is helpful to identify broad pathophysiologic categories within a given gene set, the processes identified are not specific to lower respiratory tract infections. We therefore performed gene set enrichment analysis using the curated Comparative Toxicogenomics Database pneumonia gene set to assess whether our transcriptional data were enriched for genes known to be associated with pneumonia (29, 30). Significant enrichment for the curated gene set was found among differentially expressed genes between patients with and without pneumonia in both cell types (Figures 4E and 4F). Two previously published gene signatures associated with bacterial pneumonia, both generated from analysis of peripheral blood, were not identified in our dataset (31, 32).
We then used LASSO regression to generate a predictive model of bacterial pneumonia for both resident and recruited macrophages using our training dataset. The predictive gene signatures identified were then applied to a validation cohort, which included 20 resident macrophage samples (9 from patients with pneumonia and 11 from patients without pneumonia) and 11 recruited macrophage samples (7 from patients with pneumonia and 4 from patients without pneumonia). The AUC for the three-gene signature (TNFAIP3, WSB1, and PFKFB3) identified in resident alveolar macrophages was 0.78 (95% CI, 0.56–0.99). The AUC for the five-gene signature (MMP14, TNFAIP3, NFKBIZ, TNFAIP6, and HSP90AA1) identified in recruited macrophage samples was 0.74 (95% CI, 0.56–1.00).
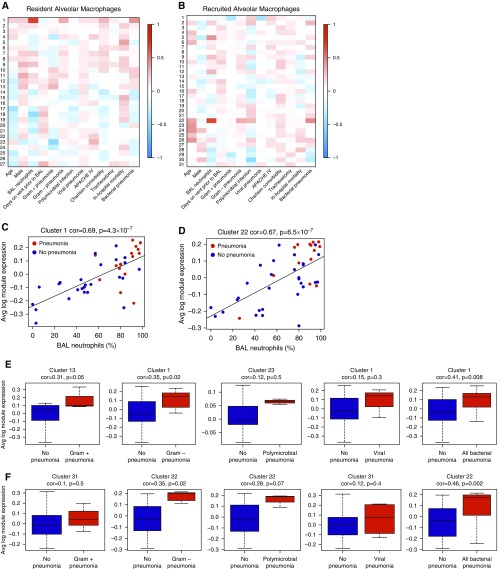
As we were underpowered to focus on any one specific pathogen, we performed correlation analysis using weighted correlation network analysis to determine whether clinical variables including subtypes of isolated pathogens might be associated with gene expression in our dataset (Figure 5). In both resident and recruited macrophages, the cluster of genes most strongly associated with bacterial pneumonia was also highly associated with BAL neutrophilia. In resident alveolar macrophages, this cluster included 319 genes strongly associated with both BAL neutrophilia (P = 4 × 10−7) and bacterial pneumonia (P = 0.008). Sixteen of these genes overlapped with those found in our differential expression analysis. Similarly, in recruited macrophages, a unique cluster of 220 genes was significantly associated with both BAL neutrophilia (P = 5 × 10−7) and bacterial pneumonia (P = 0.002). Twenty-five of these genes overlapped with genes identified in the differential expression analysis. A full list of overlapping genes is provided in the online supplement.
Figure 5.
Weighted correlation network analysis (WGCNA) identifies unique gene clusters associated with pneumonia subtypes. (A and B) WGCNA was performed for resident alveolar macrophages (A) and recruited macrophages (B). Each column represents a unique clinical variable. For resident macrophages, row 1 identifies a cluster of 391 genes strongly associated with both BAL neutrophilia and bacterial pneumonia. For recruited macrophages, cluster 22 identifies a cluster of 220 genes strongly associated with both BAL neutrophilia and bacterial pneumonia. (C and D) Average log module expression of these two clusters is plotted against the percentage of neutrophils in BAL fluid. (E and F) Average log module expression of gene clusters with the strongest positive association with pneumonia subtypes. (E) Expression for resident alveolar macrophages; (F) expression for recruited macrophages. APACHE = Acute Physiology and Chronic Health Evaluation; Avg = average; cor = correlation coefficient.
Humanized Mouse Model of P. aeruginosa Pneumonia
Macrophages exhibit remarkable plasticity, demonstrating distinct transcriptional responses to different pathogens (33). Identification of unique host response signatures might therefore facilitate the diagnosis of pneumonia caused by a specific pathogen. We compared the transcriptional response of human alveolar macrophages in a mouse lung environment (humanized mouse) during infection with two clinical strains of P. aeruginosa.
After intratracheal infection with either a low- or high-virulence strain of P. aeruginosa, human alveolar macrophages were isolated from murine whole-lung tissue and processed for RNA-Seq (Figures 6A–6D). Using k-means clustering (k = 6), several distinct gene clusters were identified, including a group of 711 genes upregulated in all infected mice, a cluster of 447 genes upregulated in the setting of infection with the low-virulence strain of P. aeruginosa, and a cluster of 279 genes unique to infection with the high-virulence strain. Overall, there were 1,925 differentially expressed genes (identified by an FDR q value < 0.05) between mice infected with the high-virulence strain of P. aeruginosa and control mice.
Figure 6.
Pseudomonas aeruginosa pneumonia produces an identifiable transcriptional signature in alveolar macrophages from humanized (MISTRG) mice. (A) Gating strategy used to identify human alveolar macrophages in humanized mice. (B) Immunohistochemistry for CD206 demonstrates CD206+ cells in the alveolar space of humanized mice (black arrows). Representative histology from each experimental group is shown. Scale bars = 200 μm; insets, scale bars = 100 μm. (C) k-means clustering (k = 6) of 3,078 differentially expressed genes between all groups. Genes were selected using an ANOVA approach and a false discovery rate (FDR) q value less than 0.05. (D) Expression of the top 100 differentially expressed genes (identified using an FDR q value) in humanized mice infected with a high-virulence strain of P. aeruginosa compared with control subjects was assessed in four patients with P. aeruginosa pneumonia and four mechanically ventilated patients without infection. Counts for mice and humans were normalized separately. Representative genes are shown to the right of the figure. Each row represents an individual gene. Each column represents an individual mouse or patient. AM = alveolar macrophage; CD = cluster of differentiation; FSC-A = forward scatter-area; FSC-H = forward scatter-height.
Of the 100 most differentially expressed genes in mice infected with the high-virulence strain, 89 were identified in alveolar macrophages isolated from a cohort of four mechanically ventilated patients with P. aeruginosa and four uninfected control subject. To approximate alveolar macrophage gene expression in the healthy human lung, we used alveolar macrophage RNA-Seq data from lung tissue obtained from four age- and sex-matched donors at the time of lung transplantation as our controls (34). Humans with P. aeruginosa pneumonia demonstrated a similar gene expression pattern to infected MISTRG mice (Figure 6D).
Discussion
We demonstrate how an assessment of the host response can aid the diagnosis of bacterial pneumonia. First, we show in both retrospective and prospective cohorts that the absence of alveolar neutrophilia is useful in ruling out bacterial pneumonia in mechanically ventilated patients with suspected infection. Our study is the largest to determine the test characteristics of BAL neutrophilia in critically ill patients (8, 13–15). Second, we show how next-generation sequencing techniques can be applied to clinical samples to identify transcriptomic signatures of infection within a key lung immune cell, the alveolar macrophage. Finally, we provide proof of concept using a humanized mouse model of pneumonia that pathogen-specific transcriptional signatures can be identified. Our results support the identification of novel host response biomarkers to aid the diagnosis and treatment of pneumonia.
Current guidelines recommend empiric broad-spectrum antibacterial therapy for patients with suspected pneumonia and respiratory failure (18, 19). Because of this emphasis on early empiric therapy, patients in the ICU receive on average more than 1.5 doses of antibiotics per day (35). Antibiotic overuse promotes the emergence of resistant pathogens and leads to adverse patient outcomes (36, 37). Accordingly, biomarkers with high sensitivity are needed to identify patients in whom antibiotics can be safely avoided or rapidly discontinued. BAL neutrophilia is an appealing diagnostic test, as it is inexpensive, widely available, and reflective of a fundamental host immune response to bacterial pneumonia.
We show that the absence of BAL neutrophilia has a high NPV for bacterial pneumonia. A BAL neutrophil percentage of less than 50% had an NPV for bacterial pneumonia exceeding 90% in all cohorts. When a BAL neutrophil threshold of less than 50% is paired with a negative BAL gram stain, the NPV for bacterial pneumonia approaches 100%. We believe the combination of these two rapid and inexpensive tests has the potential to facilitate the rapid discontinuation of empiric antibiotic therapy in many critically ill patients with suspected bacterial pneumonia. Importantly, we demonstrate that the test characteristics of BAL neutrophilia are minimally impacted by the administration of antibiotics. As the majority of critically ill patients with a suspected new pneumonia receive antibiotics before diagnostic testing, a biomarker that remains informative in this setting is particularly valuable (20, 38).
In both of our prospective cohorts, BAL neutrophilia outperformed procalcitonin, a peripheral blood biomarker used to help discriminate bacterial from nonbacterial infection (28). This observation is concordant with recent work demonstrating that disease-specific host response signatures are superior to systemic markers of inflammation (39). The results of our correlation analysis, in which the clusters of genes most strongly associated with bacterial pneumonia were also highly associated with BAL neutrophilia, further support the utility of this test.
As BAL neutrophilia is insufficient to exclude bacterial pneumonia with certainty, we asked whether a detailed assessment of the host response using next-generation sequencing might yield additional informative markers of bacterial pneumonia. Recent advances in flow cytometry allow for the unequivocal identification of myeloid cells in the human lung (21, 40, 41). When paired with BAL in patients with suspected pneumonia, these techniques enable individual immune cell populations to be isolated from the alveolus and processed for RNA-Seq.
We identified a transcriptional signature of infection in both resident and recruited macrophages from patients with bacterial pneumonia compared with those without infection. We were able to leverage this dataset to generate predictive gene signatures from both resident and recruited macrophages. These signatures identified patients with bacterial pneumonia with encouraging operating characteristics despite significant clinical heterogeneity in our study cohort, including a small number of patients infected with the same pathogen. We envision gene signatures identified with this approach being used to inform genes that could be included in PCR-based diagnostic tests—technology that is based on similar chemistry to RNA-Seq.
Although both resident and recruited macrophages exhibited a transcriptional response to bacterial infection, this response differed between cell types. We did not identify any shared differentially expressed genes between resident and recruited macrophages from patients with pneumonia using an FDR-adjusted q value less than 0.05, and only 143 shared genes when using an unadjusted P value less than 0.05. Our results are consistent with animal models describing distinct transcriptional responses to lung injury among macrophage subsets (22, 42). We found that gene expression changes in resident macrophages were more predictive of bacterial pneumonia than changes in recruited macrophages. Although monocyte-derived cells play a prominent role in disease pathogenesis, monocyte-to-macrophage differentiation is associated with dynamic remodeling of more than 60% of the transcriptome (22, 43). It is therefore not surprising that infection-specific gene signatures might be more readily identified in a fully mature cell like the tissue-resident alveolar macrophage.
We concluded by asking whether host response signatures might aid our ability to attribute pneumonia to a specific pathogen. We compared the transcriptomic response of human alveolar macrophages using humanized mice infected with two clinical strains of P. aeruginosa. Infection with P. aeruginosa was chosen as it is one of the most frequently isolated pathogens in hospital-acquired pneumonia (44, 45). Infection with a virulent strain of P. aeruginosa produced a transcriptomic response distinct from infection with a low virulence strain and from control subjects. We then compared expression of the top 100 differentially expressed genes from this gene set with expression of those same genes in patients with P. aeruginosa from our prospective cohort with P. aeruginosa pneumonia and in uninfected control subjects. Expression of these genes notably differed between the two patient groups, suggesting that humanized mice might serve as a novel translational tool to inform the identification of pathogen-specific host response signatures in patients.
Our work has several important implications. Diagnostic tests that move beyond pathogen detection and instead identify informative elements of the host response to infection are needed to drive innovation in the diagnosis and management of patients with pneumonia (10). Ideally, a multifaceted assessment of the host response will incorporate molecular characterization of host–pathogen interactions. However, the application of next-generation sequencing technologies to clinical specimens obtained from critically ill patients is challenging and understudied. We demonstrate that RNA-Seq can be used to assess transcriptome-level changes in alveolar macrophages using samples obtained as part of routine clinical care in the ICU. Furthermore, we provide data to suggest that the application of machine learning tools to larger datasets may identify biomarkers that predict causative pathogens. Importantly, we have recently shown that NBBAL is safe and easily performed by nonphysicians (12). Our approach, therefore, could readily be performed serially in informative cohorts, including those infected with high-risk pathogens or after the initiation of a targeted therapy, enabling an unprecedented evaluation of dynamic changes in the alveolar transcriptome. We believe our approach will help answer many of the key questions identified in a recent NHLBI working group report, “Future Research Directions in Pneumonia” (10).
Our study has limitations. Our definition of pneumonia required positive quantitative culture of lower respiratory tract specimens. The clinical utility of such an approach is controversial (18). We considered isolation of oropharyngeal pathogens in lower respiratory culture indicative of true pneumonia if all other clinical criteria were met. Although these bacteria may behave like typical pathogens, their clinical significance remains a source of debate (46). The yield of BAL cultures may have been impacted by antibiotic administration leading to sample misclassification. The operating characteristics of BAL neutrophilia may differ in populations where the pretest probability of pneumonia is lower. Because of sample volume and quality, not all patients in our prospective cohort had sufficient resident and recruited macrophages sorted for RNA sequencing. In addition, the delay from the time of collection to sample processing may have impacted our transcriptome analysis. Finally, although the largest series to date, sample size for our transcriptomic analysis is still too small to reliably generate and validate specific biomarkers.
In conclusion, assessment of alveolar neutrophilia can help to rapidly exclude bacterial pneumonia in critically ill patients, with an NPV approaching 100% when an alveolar neutrophil percentage of less than 50% is paired with a negative lavage fluid gram stain. Resident and recruited macrophages can be isolated from BAL fluid obtained during routine ICU care and used for RNA-Seq analysis. This approach may be useful to identify transcriptional signatures that characterize the host response to bacterial pneumonia. A combination of cellular and molecular analysis of the host and pathogen in BAL fluid shows promise for improving the diagnosis and management of pneumonia.
Footnotes
Supported by Northwestern University’s Lung Sciences Training Program grant 5T32HL076139-13 and a Dixon Translational Research Grant (J.M.W. and P.A.R.); Northwestern University’s Lung Sciences Training Program grant 1F32HL136111-01A1 (P.A.R.); NIH/National Institute of Allergy and Infectious Diseases (NIAID) grant 1 U19AI135964-01 (H.K.D., L.A.A., A.V.M., A.R.H., G.R.S.B., and R.G.W.); NIH grant HL125940 and matching funds from the Thoracic Surgery Foundation, a research grant from Society of University Surgeons, and a John H. Gibbon Jr. Research Scholarship from the American Association of Thoracic Surgery (A.B.); NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases grant AR061593, an ATS/Scleroderma Foundation Research Grant, NHLBI 1R56HL135124-01, Department of Defense grant PR141319, and a BD Bioscience Immunology Research Grant (A.V.M.); NIH grants ES013995, HL071643, AG049665, The Veterans Administration Grant BX000201, and Department of Defense grant PR141319, (G.R.S.B.); and the Office of the Assistant Secretary of Defense for Health Affairs, through the Peer Reviewed Medical Research Program under Award W81XWH-15-1-0215 (A.V.M. and G.R.S.B.). Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense. RNA-seq library preparation and sequencing were performed in the RNA-seq Center of the Division of Pulmonary and Critical Care Medicine at Northwestern. Flow Cytometry Cell Sorting was performed on a BD FACSAria SORP system, purchased through the support of NIH 1S10OD011996-01. This work was supported by the Northwestern University Pathology Core Facility and Cancer Center Support grant NCI CA060553.
Author Contributions: J.M.W. contributed to conceptualization, methodology, formal analysis, investigation, data curation, writing, visualization, and supervision. Z.R. contributed to conceptualization, methodology, software, validation, formal analysis, data curation, and visualization. T.Y. and K.R.A. contributed to methodology, software, formal analysis, data curation, and visualization. P.A.R. contributed to methodology, software, formal analysis, and visualization. R.D.S., J.P., P.H., and L.A.A. contributed to methodology, formal analysis, and data curation. H.A.-V. contributed to investigation, resources, data analysis, data curation, and project administration. K.N. and M.C. contributed to methodology, investigation, and data curation. V.K.M. contributed to methodology, validation, investigation, and data curation. N.J. contributed to methodology, formal analysis, and investigation. A.C.M.-P. contributed to methodology, validation, and formal analysis. C.-IC. and R.P.A. contributed to methodology, validation, and investigation. S.H., F.J.G.-G., S.S., S.W., and K.J.N.W. contributed to methodology, validation, formal analysis, investigation, and data curation. Z.L. contributed to methodology, investigation, and formal analysis. M.B., N. Borkowski, and H.K.D. contributed to methodology, validation, and data curation. J.P.A. contributed to conceptualization, methodology, and data curation. A.B. contributed to methodology, validation, formal analysis, and visualization. A.V.M. contributed to conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, writing, visualization, and supervision. N. Bagheri contributed to methodology, formal analysis, and visualization. A.R.H. contributed to methodology, validation, formal analysis, writing, and visualization. G.R.S.B. and R.G.W. contributed to conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, writing, visualization, supervision, project administration, and funding acquisition.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201804-0650OC on November 6, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:e51–e77. doi: 10.1093/cid/ciw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flanders SA, Saint S. Why does antimicrobial overuse in hospitalized patients persist? JAMA Intern Med. 2014;174:661–662. doi: 10.1001/jamainternmed.2014.897. [DOI] [PubMed] [Google Scholar]
- 3.Marston HD, Dixon DM, Knisely JM, Palmore TN, Fauci AS. Antimicrobial resistance. JAMA. 2016;316:1193–1204. doi: 10.1001/jama.2016.11764. [DOI] [PubMed] [Google Scholar]
- 4.Shindo Y, Ito R, Kobayashi D, Ando M, Ichikawa M, Shiraki A, et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2013;188:985–995. doi: 10.1164/rccm.201301-0079OC. [DOI] [PubMed] [Google Scholar]
- 5.Xu J, Murphy SL, Kochanek KD, Bastian BA. Deaths: final data for 2013. Natl Vital Stat Rep. 2016;64:1–119. [PubMed] [Google Scholar]
- 6.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. EPIC II Group of Investigators. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 7.Collaborators GL GBD 2015 LRI Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1133–1161. doi: 10.1016/S1473-3099(17)30396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klompas M. Does this patient have ventilator-associated pneumonia? JAMA. 2007;297:1583–1593. doi: 10.1001/jama.297.14.1583. [DOI] [PubMed] [Google Scholar]
- 9.Roisin S, Huang TD, de Mendonça R, Nonhoff C, Bogaerts P, Hites M, et al. Prospective evaluation of a high multiplexing real-time polymerase chain reaction array for the rapid identification and characterization of bacteria causative of nosocomial pneumonia from clinical specimens: a proof-of-concept study. Eur J Clin Microbiol Infect Dis. 2018;37:109–116. doi: 10.1007/s10096-017-3108-3. [DOI] [PubMed] [Google Scholar]
- 10.Dela Cruz CS, Wunderink RG, Christiani DC, Cormier SA, Crothers K, Doerschuk CM, et al. Future research directions in pneumonia: NHLBI Working Group report. Am J Respir Crit Care Med. 2018;198:256–263. doi: 10.1164/rccm.201801-0139WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Craig A, Mai J, Cai S, Jeyaseelan S. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect Immun. 2009;77:568–575. doi: 10.1128/IAI.00832-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walter JM, Helmin KA, Abdala-Valencia H, Wunderink RG, Singer BD. Multidimensional assessment of alveolar T cells in critically ill patients. JCI Insight. 2018;3:e123287. doi: 10.1172/jci.insight.123287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marquette CH, Copin MC, Wallet F, Neviere R, Saulnier F, Mathieu D, et al. Diagnostic tests for pneumonia in ventilated patients: prospective evaluation of diagnostic accuracy using histology as a diagnostic gold standard. Am J Respir Crit Care Med. 1995;151:1878–1888. doi: 10.1164/ajrccm.151.6.7767535. [DOI] [PubMed] [Google Scholar]
- 14.Kirtland SH, Corley DE, Winterbauer RH, Springmeyer SC, Casey KR, Hampson NB, et al. The diagnosis of ventilator-associated pneumonia: a comparison of histologic, microbiologic, and clinical criteria. Chest. 1997;112:445–457. doi: 10.1378/chest.112.2.445. [DOI] [PubMed] [Google Scholar]
- 15.Balthazar AB, Von Nowakonski A, De Capitani EM, Bottini PV, Terzi RG, Araújo S. Diagnostic investigation of ventilator-associated pneumonia using bronchoalveolar lavage: comparative study with a postmortem lung biopsy. Braz J Med Biol Res. 2001;34:993–1001. doi: 10.1590/s0100-879x2001000800004. [DOI] [PubMed] [Google Scholar]
- 16.Walter JM, Yacoub T, Shah RD, Reyfman PA, Joshi N, Anekella KR, et al. Predicting the diagnosis of bacterial pneumonia in mechanically ventilated patients: a comparison of bronchoalveolar lavage cell counts and a transcriptomic classifier [abstract] Am J Respir Crit Care Med. 2018;197:A107. [Google Scholar]
- 17.Shah RD, Weiss CH, Wunderink RG. The test characteristics of bronchoalveolar neutrophils for the diagnosis of bacterial pneumonia in critically ill and intubated adult patients [abstract] Am J Respir Crit Care Med. 2014;189:D50. [Google Scholar]
- 18.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT) Eur Respir J. 2017;50:1700582. doi: 10.1183/13993003.00582-2017. [DOI] [PubMed] [Google Scholar]
- 20.Canadian Critical Care Trials Group. A randomized trial of diagnostic techniques for ventilator-associated pneumonia. N Engl J Med. 2006;355:2619–2630. doi: 10.1056/NEJMoa052904. [DOI] [PubMed] [Google Scholar]
- 21.Bharat A, Bhorade SM, Morales-Nebreda L, McQuattie-Pimentel AC, Soberanes S, Ridge K, et al. Flow cytometry reveals similarities between lung macrophages in humans and mice. Am J Respir Cell Mol Biol. 2016;54:147–149. doi: 10.1165/rcmb.2015-0147LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Misharin AV, Morales-Nebreda L, Reyfman PA, Cuda CM, Walter JM, McQuattie-Pimentel AC, et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med. 2017;214:2387–2404. doi: 10.1084/jem.20162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rongvaux A, Willinger T, Martinek J, Strowig T, Gearty SV, Teichmann LL, et al. Development and function of human innate immune cells in a humanized mouse model. Nat Biotechnol. 2014;32:364–372. doi: 10.1038/nbt.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang B, Horvath S. A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol. 2005;4 doi: 10.2202/1544-6115.1128. Article17. [DOI] [PubMed] [Google Scholar]
- 26.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuetz P, Wirz Y, Sager R, Christ-Crain M, Stolz D, Tamm M, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis. 2018;18:95–107. doi: 10.1016/S1473-3099(17)30592-3. [DOI] [PubMed] [Google Scholar]
- 28.Schuetz P, Wirz Y, Sager R, Christ-Crain M, Stolz D, Tamm M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498. doi: 10.1002/14651858.CD007498.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rouillard AD, Gundersen GW, Fernandez NF, Wang Z, Monteiro CD, McDermott MG, Ma'ayan A. The harmonizome: a collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database (Oxford) 2016. 2016:baw100. [DOI] [PMC free article] [PubMed]
- 30.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhattacharya S, Rosenberg AF, Peterson DR, Grzesik K, Baran AM, Ashton JM, et al. Transcriptomic biomarkers to discriminate bacterial from nonbacterial infection in adults hospitalized with respiratory illness. Sci Rep. 2017;7:6548. doi: 10.1038/s41598-017-06738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suarez NM, Bunsow E, Falsey AR, Walsh EE, Mejias A, Ramilo O. Superiority of transcriptional profiling over procalcitonin for distinguishing bacterial from viral lower respiratory tract infections in hospitalized adults. J Infect Dis. 2015;212:213–222. doi: 10.1093/infdis/jiv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avraham R, Haseley N, Brown D, Penaranda C, Jijon HB, Trombetta JJ, et al. Pathogen cell-to-cell variability drives heterogeneity in host immune responses. Cell. 2015;162:1309–1321. doi: 10.1016/j.cell.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reyfman PA, Walter JM, Joshi N, Anekalla KR, McQuattie-Pimentel AC, Chiu S, et al. Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am J Respir Crit Care Med. doi: 10.1164/rccm.201712-2410OC. [online ahead of print] 15 Dec 2018; DOI: 10.1164/rccm.201712-2410OC. [DOI] [PMC free article] [PubMed]
- 35.Bitterman R, Hussein K, Leibovici L, Carmeli Y, Paul M. Systematic review of antibiotic consumption in acute care hospitals. Clin Microbiol Infect. 2016;22:561.e7–561.e19. doi: 10.1016/j.cmi.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 36.Baditoiu L, Axente C, Lungeanu D, Muntean D, Horhat F, Moldovan R, et al. Intensive care antibiotic consumption and resistance patterns: a cross-correlation analysis. Ann Clin Microbiol Antimicrob. 2017;16:71. doi: 10.1186/s12941-017-0251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Waele JJ, Akova M, Antonelli M, Canton R, Carlet J, De Backer D, et al. Antimicrobial resistance and antibiotic stewardship programs in the ICU: insistence and persistence in the fight against resistance. A position statement from ESICM/ESCMID/WAAAR round table on multi- drug resistance. Intensive Care Med. 2018;44:189–196. doi: 10.1007/s00134-017-5036-1. [DOI] [PubMed] [Google Scholar]
- 38.Ruiz M, Torres A, Ewig S, Marcos MA, Alcón A, Lledó R, et al. Noninvasive versus invasive microbial investigation in ventilator-associated pneumonia: evaluation of outcome. Am J Respir Crit Care Med. 2000;162:119–125. doi: 10.1164/ajrccm.162.1.9907090. [DOI] [PubMed] [Google Scholar]
- 39.Scicluna BP, Klein Klouwenberg PM, van Vught LA, Wiewel MA, Ong DS, Zwinderman AH, et al. A molecular biomarker to diagnose community-acquired pneumonia on intensive care unit admission. Am J Respir Crit Care Med. 2015;192:826–835. doi: 10.1164/rccm.201502-0355OC. [DOI] [PubMed] [Google Scholar]
- 40.Yu YR, Hotten DF, Malakhau Y, Volker E, Ghio AJ, Noble PW, et al. Flow cytometric analysis of myeloid cells in human blood, bronchoalveolar lavage, and lung tissues. Am J Respir Cell Mol Biol. 2016;54:13–24. doi: 10.1165/rcmb.2015-0146OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desch AN, Gibbings SL, Goyal R, Kolde R, Bednarek J, Bruno T, et al. Flow cytometric analysis of mononuclear phagocytes in nondiseased human lung and lung-draining lymph nodes. Am J Respir Crit Care Med. 2016;193:614–626. doi: 10.1164/rccm.201507-1376OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mould KJ, Barthel L, Mohning MP, Thomas SM, McCubbrey AL, Danhorn T, et al. Cell origin dictates programming of resident versus recruited macrophages during acute lung injury. Am J Respir Cell Mol Biol. 2017;57:294–306. doi: 10.1165/rcmb.2017-0061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med. 2010;362:1804–1813. doi: 10.1056/NEJMra0904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veesenmeyer JL, Hauser AR, Lisboa T, Rello J. Pseudomonas aeruginosa virulence and therapy: evolving translational strategies. Crit Care Med. 2009;37:1777–1786. doi: 10.1097/CCM.0b013e31819ff137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lambotte O, Timsit JF, Garrouste-Orgeas M, Misset B, Benali A, Carlet J. The significance of distal bronchial samples with commensals in ventilator-associated pneumonia: colonizer or pathogen? Chest. 2002;122:1389–1399. doi: 10.1378/chest.122.4.1389. [DOI] [PubMed] [Google Scholar]