Abstract
Rationale: There is currently no effective pharmacological treatment for obstructive sleep apnea (OSA). Recent investigations indicate that drugs with noradrenergic and antimuscarinic effects improve genioglossus muscle activity and upper airway patency during sleep.
Objectives: We aimed to determine the effects of the combination of a norepinephrine reuptake inhibitor (atomoxetine) and an antimuscarinic (oxybutynin) on OSA severity (apnea–hypopnea index [AHI]; primary outcome) and genioglossus responsiveness (secondary outcome) in people with OSA.
Methods: A total of 20 people completed a randomized, placebo-controlled, double-blind, crossover trial comparing 1 night of 80 mg atomoxetine plus 5 mg oxybutynin (ato–oxy) to placebo administered before sleep. The AHI and genioglossus muscle responsiveness to negative esophageal pressure swings were measured via in-laboratory polysomnography. In a subgroup of nine patients, the AHI was also measured when the drugs were administered separately.
Measurements and Main Results: The participants’ median (interquartile range) age was 53 (46–58) years and body mass index was 34.8 (30.0–40.2) kg/m2. ato–oxy lowered AHI by 63% (34–86%), from 28.5 (10.9–51.6) events/h to 7.5 (2.4–18.6) events/h (P < 0.001). Of the 15/20 patients with OSA on placebo (AHI > 10 events/hr), AHI was lowered by 74% (62–88%) (P < 0.001) and all 15 patients exhibited a ≥50% reduction. Genioglossus responsiveness increased approximately threefold, from 2.2 (1.1–4.7)%/cm H2O on placebo to 6.3 (3.0 to 18.3)%/cm H2O on ato–oxy (P < 0.001). Neither atomoxetine nor oxybutynin reduced the AHI when administered separately.
Conclusions: A combination of noradrenergic and antimuscarinic agents administered orally before bedtime on 1 night greatly reduced OSA severity. These findings open new possibilities for the pharmacologic treatment of OSA.
Clinical trial registered with www.clinicaltrials.gov (NCT02908529).
Keywords: pharmacologic treatment, antimuscarinic, norepinephrine reuptake inhibitors, upper airway
At a Glance Commentary
Scientific Knowledge on the Subject
Increasing the activity of the upper airway dilator muscles, such as the genioglossus, and thereby preventing sleep-related hypotonia, is considered a promising strategy to treat obstructive sleep apnea (OSA). Some preclinical works identified the main cause of sleep-related hypotonia of the pharyngeal muscles to be the central reduction of norepinephrine from wakefulness to sleep. In addition, an inhibitory effect of acetylcholine through muscarinic receptors was found to be responsible for the REM-related hypotonia.
What This Study Adds to the Field
Our trial investigated, for the first time, the efficacy of the combination of a noradrenergic (atomoxetine) and an antimuscarinic (oxybutynin) on OSA severity and on the responsiveness to esophageal pressure swings of the genioglossus muscle versus placebo. The combination of drugs administered for 1 night highly reduced the number of obstructive events, improved the overnight oxygen desaturation, and enhanced the genioglossus muscle activity in this group of unselected patients with OSA. The data we collected in this proof-of-concept trial show, for the first time, that it is possible to improve or abolish OSA using drugs with specific neurotransmitter profiles administered systemically and warrants further investigations for the clinical application of this discovery.
Obstructive sleep apnea (OSA) is a common disorder that occurs in 6% of women and 13% of men in the United States (1). In the presence of an anatomical predisposition, a reduced responsiveness of the upper airway dilator muscles—including the genioglossus—during sleep promotes the collapse of pharyngeal structures, with ensuing episodes of reduced ventilation, intermittent hypoxia, hypercapnia, and arousals from sleep (2).
Few treatment options are available for OSA, with most patients being treated with continuous positive airway pressure (CPAP) (3). Although CPAP is virtually always efficacious, because it mechanically splints the airway open, limited adherence to this treatment remains a major problem (4). Although mandibular advancement devices are better tolerated than CPAP, residual events and long-term dental side effects are common, and can limit their real-world effectiveness (5, 6). Currently, the only OSA intervention that targets the upper airway dilator muscles is hypoglossal nerve stimulation; although this approach is promising, it also has limited applicability (7). To date, a pharmacologic intervention capable of augmenting genioglossus muscle activity for the treatment of OSA remains elusive (8).
Until recently, withdrawal of endogenous serotonin was considered the key mechanism for the loss of genioglossus electromyographic activity (EMGGG) during sleep; however, these data were based on vagally denervated animal experiments (9, 10), and it now appears that serotonergic mechanisms have minimal effects on genioglossus activity in intact animals (11, 12) and in humans (13–15). Two key contributors are now thought to be important: sleep-related withdrawal of endogenous noradrenergic drive is a major cause of genioglossus hypotonia, particularly during non-REM (NREM) sleep (16), and active muscarinic inhibition mediates pharyngeal hypotonia, particularly during REM (17). Indeed, our study in humans showed that the administration of the noradrenergic agent, desipramine (a tricyclic antidepressant [TCA]) modestly improved genioglossus activity and upper airway collapsibility during NREM sleep, and reduced OSA severity in a subgroup of patients (18). Another TCA with noradrenergic properties, protriptyline, was previously investigated in observational studies (19, 20) and randomized controlled trials (21, 22) for the treatment of OSA. Results were inconsistent, with some patients experiencing objective and subjective improvements and others no change in OSA severity. Notably, these TCAs have a wide, nonspecific spectrum of activity, including dopaminergic, serotonergic, antimuscarinic, and antihistaminergic actions. It remains unclear whether an agent with a specific noradrenergic profile, combined with an antimuscarinic, may lead to a substantial increase in genioglossus activity and improve OSA severity.
Accordingly, we performed a randomized, placebo-controlled, double-blind crossover trial to assess the effect of a potent, selective norepinephrine reuptake inhibitor (atomoxetine) administered in combination with an antimuscarinic drug (oxybutynin) on OSA severity and genioglossus responsiveness.
Methods
Subjects
Individuals with a diagnosis of OSA based on a previously performed sleep study were eligible to participate in the study. Both treated and untreated subjects were included in the study (see Table 1), and treated patients were asked to stop the treatment only during the study nights. Exclusion criteria included any comorbid medical condition other than well-controlled hypertension, diabetes and hyperlipidemia, use of respiratory stimulants or depressants, hypnotics, central nervous system stimulants, central sleep apnea, claustrophobia, inability to sleep supine, pregnancy, history of benign prostatic hyperplasia or urinary retention, which can be exacerbated by antimuscarinic medications, and known allergy to lidocaine, atomoxetine, or oxybutynin. Participants were enrolled by an experienced investigator who collected the informed consent, and approval was granted by the Partners’ Institutional Review Board. Patients were a convenience sample recruited through our sleep clinic (Brigham and Women’s Hospital, Boston, MA), the community (advertisements), and a database of previous research participants. The trial ended when 20 enrolled participants completed both study nights. In a supplementary, open-label investigation, 9 of the 20 patients who completed the primary study performed an adjunctive study in which the single drugs (80 mg atomoxetine, 5 mg oxybutynin) were administered alone in random order over two nonconsecutive nights.
Table 1.
Characteristics of the Patients
| Characteristics | Values |
|---|---|
| Sex, female, n (%) | 4 (20) |
| Age, yr | 53 (46.5–57.8) |
| BMI, kg/m2 | 34.8 (30.0–40.2) |
| Neck circumference, cm | 42.3 (39.9–44.6) |
| Waist circumference, cm | 108.9 (103.8–125.5) |
| OSA treatment, n (%) | |
| CPAP compliant | 5 (25) |
| CPAP noncompliant | 5 (25) |
| Oral appliance | 1 (5) |
| None | 9 (45) |
| Comorbidities, n (%) | |
| Hypertension | 7 (35) |
| Diabetes | 2 (10) |
| Hypercholesterolemia | 3 (15) |
| Medications, n (%) | |
| ACE-I/ARB | 5 (25) |
| Ca2+ antagonists | 3 (15) |
| β-Blockers | 2 (10) |
| Spironolactone | 2 (10) |
| HCTZ | 1 (5) |
| Antilipidemic | 3 (15) |
| Antihistaminic | 2 (10) |
| PPI | 2 (10) |
Definition of abbreviations: ACE-I = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; BMI = body mass index; CPAP = continuous positive airway pressure; HCTZ = hydrochlorothiazide; OSA = obstructive sleep apnea; PPI = protonic pump inhibitor.
Data are presented as median (interquartile range) unless otherwise specified.
Protocol
The study was performed at the Center for Clinical Investigation at the Brigham and Women’s Hospital. Two overnight sleep studies were performed approximately 1 week apart: a placebo condition (two pills) and an 80 mg atomoxetine plus 5 mg oxybutynin (ato–oxy) condition, in a double-blind, randomized design (clinical trial NCT02908529). For each night, the subjects arrived at the sleep laboratory at approximately 7:00 p.m. Placebo or ato–oxy was administered approximately 30 minutes before lights out. Time of lights out was based on the patients’ usual bedtime and kept constant between the two study nights.
The predefined primary outcome endpoint was the apnea–hypopnea index (AHI; events/h); secondary outcome was the genioglossus muscle responsiveness to esophageal pressure (Pes) swings (%/cm H2O).
Randomization and Masking
Study medications were prepared by the Brigham and Women’s Hospital Investigational Drug Service and were placed in identical capsules that could not be identified by study personnel or participants. Participant, care provider, investigators, and outcomes assessors (including the polysomnography technologist) were blinded to the treatment allocation (quadruple blinding). Sequential randomization for the active treatment and placebo order was performed by the Investigational Drug Service using a pseudorandom number generator with two randomly permuted blocks of two; all data analyses were performed before unblinding of the intervention allocation.
Measurements and Equipment
In addition to the standard clinical montage for polysomnography (23), subjects breathed through a sealed oronasal mask attached to a pneumotachometer (Hans-Rudolph; Validyne) to make accurate airflow measurements. To quantify respiratory effort, Pes was measured with a small, flexible, pressure-tipped catheter (Millar Instruments) that was advanced through an anesthetized (4% lidocaine) nostril until the tip of the catheter was in the lower third of the esophagus. Oxygen saturation was measured with an ear oximeter. EMGGG was measured using two intramuscular electrodes to create a bipolar recording (24). After all the equipment was in place, the patient was asked to sleep all night in the supine position for AHI calculation and EMGGG measurement. Subjective sleep quality and side effects were assessed in the morning. Sleep quality was evaluated with a visual analog scale that asked patients to rate sleep quality from 0 (very bad) to 10 (very good) (25). Blood pressure was tested in the morning with a single measurement in sitting position as part of routine assessment by a nurse blinded to treatment allocation.
Data Analysis
Apneas, hypopneas, and arousals were scored using standard American Academy of Sleep Medicine guidelines (26) by a registered polysomnography technologist blinded to the treatment allocation. Hypopneas were defined as reduction in flow of 30% or more from baseline, lasting at least 10 seconds, and were associated with an arousal from sleep or an oxyhemoglobin desaturation 3% or greater.
Respiratory effort–related arousals were defined as arousals from sleep after a sequence of breaths lasting 10 seconds or longer that did not meet the criteria for hypopneas (reduction in flow <30%), but were characterized by increasing respiratory effort (Pes swings) and flattening of the inspiratory portion of the flow signal. The respiratory disturbance index was calculated as the sum of apneas, hypopneas, and respiratory effort–related arousals.
In addition, we calculated the OSA-specific hypoxic burden (respiratory event–related oxygen desaturation area under preevent baseline curve, per hour), a measure recently shown to predict cardiovascular mortality in two large community cohorts (27).
The spontaneous respiratory-related activation of the genioglossus during sleep was assessed as follows: for all artifact-free breaths during non-REM sleep, we measured 1) the peak EMGGG (based on rectified moving-time average) in units of % baseline (28) during sleep, % wakefulness (24), and % maximum (24, 29); and 2) Pes swings (end-expiratory minus peak negative pressure [cm H2O]). Genioglossus muscle responsiveness was calculated as the slope of a linear regression of peak EMGGG against Pes swings (%/cm H2O) (13).
Heart rate was measured as the mean heart rate over the entire night derived from electrocardiogram interbeat (RR) intervals.
Statistical Analysis
Subjects were prospectively enrolled until 20 of them completed both study nights. Sample size was chosen to facilitate detection of a clinically important 50 (±75)% reduction in AHI (primary outcome; equivalent to a reduction by 15 ± 22.5 events/h from 30 events/h) and a 100 (±150)% increase in genioglossus responsiveness, secondary outcome (80% power, α = 0.05). Calculations were based on a previous study with the noradrenergic drug, desipramine, conducted in our laboratory (18), and were assumed to hold for nonparametric analyses. Variables were compared using a Wilcoxon matched-pairs signed-rank test, with a P value less than 0.05 considered statistically significant. Data are presented as median (interquartile range). To compare the effect of placebo, ato–oxy, atomoxetine alone, and oxybutynin alone in the nine participants who performed 4 study nights, we used the Friedman’s test with a post hoc analysis (Wilcoxon) in which each treatment was compared with placebo; the post hoc analysis was conducted with Bonferroni correction (P threshold = 0.017). The effect of the combination on AHI was also compared with the effect of the single drugs. Because EMGGG muscle responsiveness was compared between nights after normalization for sleep baseline values, quiet wakefulness values, and maximum EMGGG activity, P threshold was set at 0.017 after Bonferroni correction. Statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software).
Results
Participants
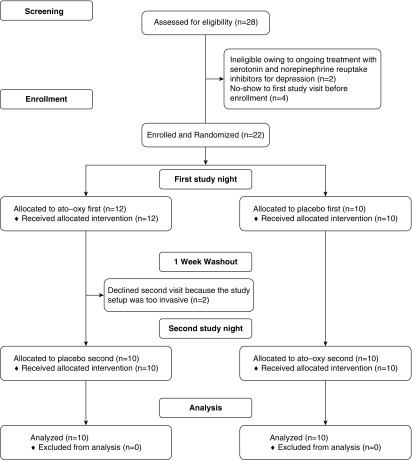
A total of 22 patients was enrolled in the study. Two patients who completed the first study night did not return for the second study night, citing the invasive nature of the study equipment. Therefore, data from 20 participants were analyzed for OSA severity on both nights. The characteristics of these individuals are described in Table 1 (see Figure 1 for the Consort diagram).
Figure 1.
Consolidated Standards of Reporting Trials diagram of the clinical trial.
EMGGG data from 16 participants were analyzed, as 4 did not agree to perform intramuscular electromyographic measurement.
The following side effects were reported during the study on the ato–oxy night: difficulty in initiating micturition (n = 2 males); dry mouth in the morning (n = 1); headache in the morning (n = 1); and insomnia symptoms (difficulty initiating and maintaining sleep; n = 1). On placebo, three patients reported insomnia symptoms. No patient experienced severe side effects or adverse events on either night.
Despite the previous diagnosis of OSA, 5 out of 20 participants exhibited an absence of OSA on placebo (AHI < 10 events/h). Primary analysis is based on all participants. We also performed an analysis that was limited to patients who had OSA on placebo (AHI > 10 events/h).
After the unblinding of the trial, nine patients (AHI > 10 events/h) agreed to come back to perform studies in which the drugs, atomoxetine and oxybutynin, were administered alone in random order. Six additional patients who had AHI greater than 10 on placebo night declined to participate in this adjunctive study. The experimental setup was identical to the original study, except for the absence of EMGGG measurement.
Effect of ato–oxy on AHI, Oxygen Saturation, Sleep Architecture, and Subjective Sleep Quality
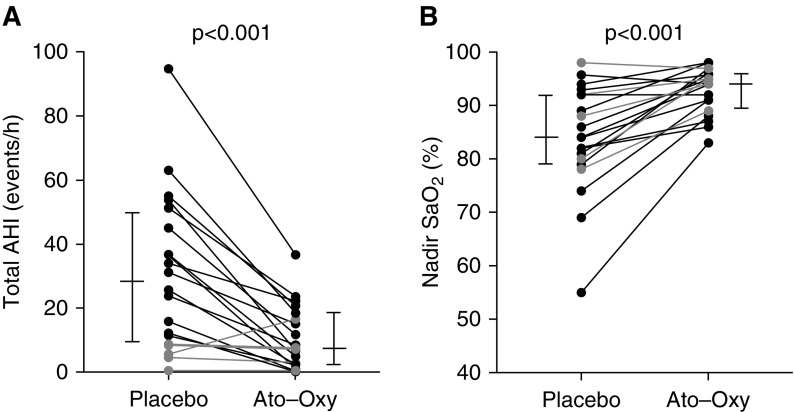
Overall, ato–oxy reduced the AHI by roughly 16 events/h, or 63%, compared with placebo (Table 2; see Figure 2A for individual data). Effects of the intervention on AHI specific to REM and NREM sleep stages, oxygen desaturation index, arousal index, sleep architecture, and subjective sleep quality are shown in Table 2.
Table 2.
Obstructive Sleep Apnea Severity and Sleep Architecture on and off the Drugs for All the Participants (n = 20)
| Placebo | ato–oxy | Median Change | P Value | |
|---|---|---|---|---|
| AHI total, events/h | 28.5 (10.9 to 51.6) | 7.5 (2.4 to 18.6) | −15.9 (−35.3 to −7.3) | <0.001 |
| % change | −63 (−86 to −33) | |||
| AHI supine, events/h | 30.8 (9.6 to 53.6) | 7.5 (2.3 to 18.3) | −22.1 (−39.8 to −3.4) | <0.001 |
| % change | −64 (−88 to −43) | |||
| AHI NREM*, events/h | 29.1 (5.4 to 53.7) | 7.5 (2.4 to 20.5) | −16.8 (−40.3 to −1.8) | <0.001 |
| % change | −63 (−86 to −32) | |||
| AHI REM*†, events/h | 30.6 (26.5 to 54.7) | 2.9 (0 to 14.4) | −24.3 (−53.2 to −14.4) | 0.004 |
| % change | −88 (−95 to −54) | |||
| Apnea index, events/h | 4.3 (1.3 to 10.7) | 0.3 (0 to 1.6) | 1.5 (0.5 to 9) | <0.001 |
| ODI 3%, events/h | 12.9 (4.6 to 35.0) | 3.4 (0.3 to 12.2) | −5.4 (−14.3 to −2.5) | <0.001 |
| Nadir SaO2, % | 84 (79.3 to 92.0) | 94 (89.5 to 95.9) | 8.0 (3.3 to 15.0) | <0.001 |
| RDI, events/h | 35.6 (12.8 to 55.0) | 16.5 (8.3 to 29.1) | −16.3 (−32.8 to −1.6) | <0.001 |
| Arousal index, events/h | 44.4 (18.4 to 62.1) | 40.7 (25.7 to 50.0) | −5.1 (−16.1 to 8.5) | 0.30 |
| Total sleep time, min | 257 (172 to 304) | 287 (240 to 317) | 22 (−41 to 80) | 0.30 |
| Sleep efficiency, %TIB | 67 (45 to 86) | 71 (63 to 84) | 3 (−7 to 18) | 0.16 |
| N1, %TST | 20 (12 to 52) | 30 (20 to 37) | 3 (−20 to 13) | 0.78 |
| N2, %TST | 32 (16 to 40) | 36 (26 to 46) | 9 (−5 to 16) | 0.05 |
| N3, %TST | 5 (0 to 17) | 0 (0 to 13) | −2 (−9 to 0) | 0.18 |
| REM, %TST | 9 (1 to 15) | 5 (0 to 13) | −2 (−8 to 6) | 0.44 |
| Subjective sleep quality, VAS score | 6 (4.0 to 8.8) | 7 (4.3 to 8.0) | 0.5 (0.0 to 2.0) | 0.10 |
| Heart rate, beats/min | 69.2 (62.0 to 77.5) | 73.8 (66.5 to 79.8) | 2.6 (−0.5 to 7) | 0.003 |
| Systolic blood pressure, mm Hg | 135.5 (123.3 to 146.5) | 139.5 (124.5 to 149.0) | 3 (−8.5 to 14) | 0.605 |
| Diastolic blood pressure, mm Hg | 82.5 (70.8 to 91.0) | 85.5 (71.8 to 91.5) | 3.5 (−14 to 9.8) | 0.677 |
Definition of abbreviations: AHI = apnea–hypopnea index; ato–oxy = atomoxetine plus oxybutynin; N1-2-3 = non-REM stage 1-2-3; NREM = non-REM sleep; ODI = oxygen desaturation index; ODI 3% = ODI, 3% desaturation; RDI = respiratory disturbance index; TIB = time in bed; TST = total sleep time; VAS = visual analog scale.
Data are presented as median (interquartile range).
AHI is calculated in supine position.
REM AHI was calculated only in nine patients in which at least 10 minutes of REM sleep were available in both nights.
Figure 2.
Individual data showing the effect of atomoxetine plus oxybutynin (ato–oxy) on (A) apnea–hypopnea index (AHI) and (B) nadir SaO2. Longer horizontal lines indicate median values, and shorter lines indicate 25th and 75th percentiles. A total of 19/20 subjects had a reduction in obstructive sleep apnea severity. Gray lines indicate patients with an AHI less than 10 on placebo night.
Nadir oxygen saturation was increased with ato–oxy versus placebo (Figure 2B). Baseline saturation was 98.8 (98.1–99.4)% on placebo versus 99.5 (99.3–99.7)% on ato–oxy (P = 0.048), average desaturation associated with respiratory events during NREM sleep was 3% (1.7–4.8%) on placebo versus 1.9% (1.5–3.7%) on ato–oxy (P = 0.005), and average desaturation associated with respiratory events in REM was 3.8% (2.1–7.7%) on placebo versus 1.5% (1–2.1%) on ato–oxy (P = 0.02). The time spent with oxygen saturation below 90% (T90) was 0.2 (0–2.3)% TST on placebo versus 0 (0 to 0)% TST on ato–oxy (P = 0.001). The OSA-specific hypoxic burden (27) was 28.7 (16.7–56.3) (% min)/h on placebo versus 4.6 (1.4 to 15.2) (% min)/h on ato–oxy (P < 0.001).
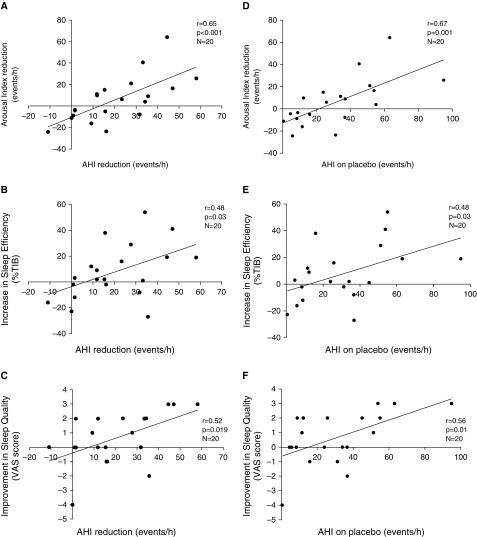
Although the arousal index was not different in the ato–oxy compared with placebo night, there was a strong, direct relationship between the reduction in AHI and the reduction in the arousal index (Figure 3A). The proportion of respiratory arousals was higher on placebo compared with ato–oxy night (76.6% [50–91%] vs. 43% [20–70%], respectively; P < 0.001). The patients with the highest AHI on placebo had the best improvement in arousal index, sleep efficiency, and subjective sleep quality (Figure 3). The change in subjective sleep quality from placebo to ato–oxy was directly related to the change in sleep efficiency (P = 0.008; r = 0.58). Hypnograms from placebo and ato–oxy nights of a representative patient are shown in the online supplement (Figure E1). The time spent supine was similar between the 2 nights: 91 (±16)% of total sleep time on placebo versus 96 (±7)% on ato–oxy (P = 0.15).
Figure 3.
(A–C) Relationship between apnea–hypopnea index (AHI) reduction and arousal index reduction (A), sleep efficiency increase (B), and sleep quality improvement (C) on atomoxetine plus oxybutynin (ato–oxy) compared with placebo. (D–F) The improvements in arousal index (D), sleep efficiency (E), and subjective sleep quality (F) from placebo to ato–oxy night were all related to the severity of obstructive sleep apnea on placebo night, so that the patients with the highest AHI on placebo had the best improvement in sleep parameters and, on the contrary, those with mild or no obstructive sleep apnea had less or no improvement in objective and subjective sleep quality. TIB = time in bed; VAS = visual analog scale.
When analysis was limited to the 15 patients with OSA on placebo (AHI ≥ 10 events/h), ato–oxy reduced sleep apnea severity by approximately 28 events/h or 74% (P < 0.001; see Table 3). In this subgroup, the objective and subjective sleep quality improved: median total sleep time increased by 45 minutes (P = 0.049), sleep efficiency increased from 56% to 69% (P = 0.018), and subjective sleep quality scores were significantly higher after ato–oxy rather than placebo administration. All 15 patients exhibited a 50% or greater reduction in AHI, and 7/15 patients also exhibited an AHI on treatment below 10 events/h (i.e., complete response).
Table 3.
Obstructive Sleep Apnea Severity and Sleep Architecture on and off the Drugs in Patients with Apnea–Hypopnea Index Greater Than 10 on the Placebo Night (n = 15)
| Placebo | ato–oxy | Median Change | P Value | |
|---|---|---|---|---|
| Total AHI, events/h | 36.7 (23.9 to 53.8) | 8.5 (2.3 to 20.8) | −27.7 (−35.6 to −15.4) | <0.001 |
| % change | −74 (−88 to −62) | |||
| AHI supine, events/h | 34.1 (29.5 to 57.5) | 8.5 (2.3 to 22.1) | −29.3 (−44.0 to −15.4) | <0.001 |
| % change | −78 (−88 to −63) | |||
| NREM*, events/h | 34.6 (22.3 to 57.5) | 8.8 (2.3 to 22.0) | −29.9 (−43.9 to −10.2) | <0.001 |
| % change | −74 (−88 to −57) | |||
| REM*†, events/h | 41.0 (24.6 to 63.2) | 2.2 (0 to 14.3) | −34.0 (−61.0 to −13.2) | 0.004 |
| % change | −88 (−96 to −61) | |||
| Apnea index, events/h | 5.8 (1.8 to 15.0) | 0.3 (0 to 1.3) | −5.5 (−12.9 to −1) | <0.001 |
| ODI 3%, events/h | 21.4 (6.1 to 39.3) | 3.7 (0.3 to 26.2) | −6.2 (−17.7 to −2.4) | <0.001 |
| Nadir SaO2, % | 84 (79 to 92) | 94 (88 to 96) | 9 (4 to 17) | <0.001 |
| RDI, events/h | 42.4 (28.3 to 57.4) | 22.4 (9.2 to 31.0) | −20.1 (−38.0 to −12.8) | <0.001 |
| Arousal index, events/h | 49.1 (35.0 to 63.7) | 46.0 (35.5 to 55.5) | −9.9 (−21 to 4.9) | 0.064 |
| Total sleep time, min | 212 (155 to 264) | 289 (221 to 307) | 45 (−6 to 97) | 0.0496 |
| Sleep efficiency, %TIB | 56 (38 to 72) | 69 (63 to 81) | 12 (1 to 29) | 0.018 |
| N1, % TST | 29 (19 to 53) | 32 (25 to 38) | 2 (−25 to 13) | 0.323 |
| N2, % TST | 20 (15 to 38) | 33 (24 to 47) | 12 (4 to 17) | 0.0175 |
| N3, % TST | 3 (0 to 16) | 0 (0 to 5) | −2 (−9 to 0) | 0.21 |
| REM, % TST | 5 (0 to 10) | 6 (0 to 12) | 0 (−6 to 7) | 0.85 |
| Subjective sleep quality, VAS score | 5 (2 to 8) | 7 (4 to 8) | 1 (0 to 2) | 0.036 |
| Heart rate, beats/min | 70 (62 to 77) | 74 (69 to 80) | 6 (1 to 7) | 0.003 |
| Systolic blood pressure, mm Hg | 140 (130 to 149) | 143 (144 to 148) | 1 (−7 to 7) | 0.83 |
| Diastolic blood pressure, mm Hg | 84 (75 to 91) | 86 (77.5 to 92) | 2 (−15 to 10.5) | 0.99 |
For definition of abbreviations, see Table 2.
Data are presented as median (interquartile range).
AHI is calculated in supine position.
REM AHI was calculated only in six patients in which at least 10 minutes of REM sleep were available in both nights.
Effect of ato–oxy on Heart Rate and Blood Pressure
Heart rate (mean from overnight electrocardiogram) and blood pressure (morning) values are reported in Table 2. Heart rate was 2.6 beats higher on ato–oxy compared with placebo, and there was no significant effect on blood pressure (systolic or diastolic).
Effect of ato–oxy on EMGGG
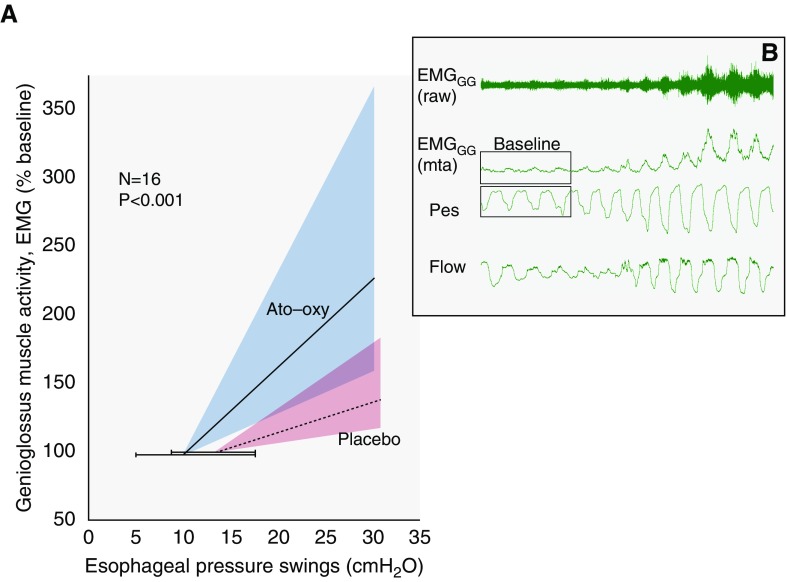
Genioglossus muscle responsiveness was greater on ato–oxy compared with placebo (6.3 [3.0–18.3] vs. 2.2 [1.1–4.7] % baseline/cm H2O; P < 0.001; n = 16; see Figure 4 for details and explanations). ato–oxy did not significantly increase genioglossus responsiveness when presented in units of % wakefulness (P = 0.065) or % maximum (P = 0.43) (see the online supplement).
Figure 4.
(A) Group data showing the effect of atomoxetine plus oxybutynin (ato–oxy) on genioglossus muscle responsiveness. Muscle responsiveness reflects the change in genioglossus muscle electromyographic activity (% of baseline) per change in esophageal pressure (Pes) swings during spontaneous breathing in non-REM sleep (see example in B for physiological context). Note that the median responsiveness on ato–oxy (slope of solid line) is greater than the responsiveness on placebo (slope of dashed line). Shaded areas represent interquartile range of the slopes. Horizontal error bars illustrate interquartile range of baseline Pes; baseline values (genioglossus electromyographic activity [EMGGG] = 100%) are offset vertically to facilitate visualization of error bars. (B) Example raw data are shown to provide context. Signals illustrate a spontaneous increase in genioglossus muscle activity with increasing Pes swings during sleep. Note the restoration of airflow (Flow) accompanying increasing muscle activity. mta = moving time average.
Single-Drug Trial
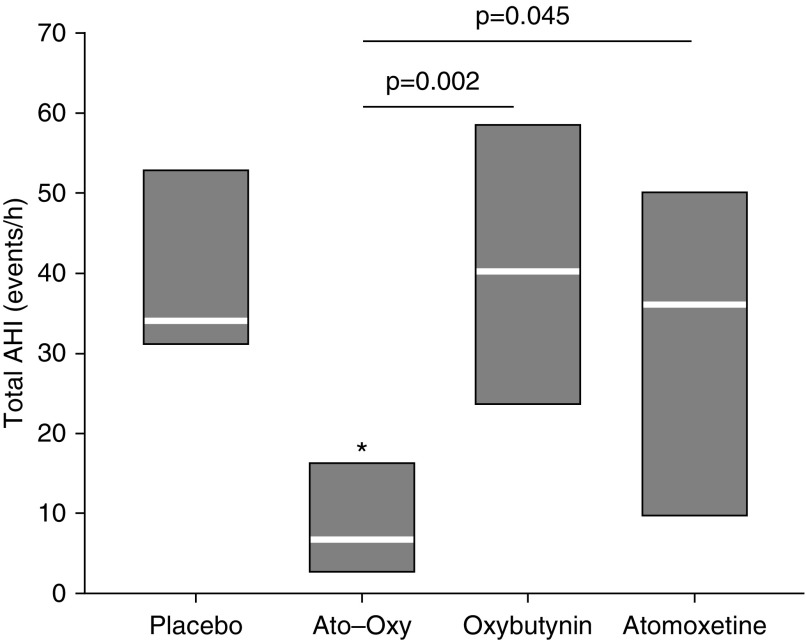
In the subset of nine patients who performed two adjunctive sleep studies to assess the impact of atomoxetine or oxybutynin alone on OSA severity, there was a statistically significant difference in AHI depending on which type of treatment was administered (P = 0.012, Friedman’s test). Median AHI values for each treatment group are reported in Figure 5. There were no significant effects on AHI of atomoxetine alone or oxybutynin alone versus placebo, despite the significant effect of ato–oxy versus placebo (P = 0.011). AHI values of placebo, atomoxetine alone, and oxybutynin alone, were not different, even when analyzed in REM and NREM sleep separately (P > 0.7).
Figure 5.
Group data showing apnea–hypopnea index (AHI) on placebo, atomoxetine plus oxybutynin (ato–oxy), oxybutynin alone, and atomoxetine alone in nine patients. White lines indicate medians; boxes indicate 25th (bottom) and 75th (top) percentiles. *P = 0.01 versus placebo.
Discussion
Our study shows that the combination of atomoxetine and oxybutynin administered before bedtime can acutely reduce or abolish OSA in patients with a wide range of severity. To date, no other pharmacologic therapy has demonstrated such an important effect on OSA.
Atomoxetine is a selective norepinephrine reuptake inhibitor approved in United States for the treatment of attention deficit hyperactivity disorder in adults and children. As previously stated, increasing the concentration of norepinephrine during sleep in the brainstem could stimulate the upper airway motoneurons to similar levels as seen during wakefulness (24). In addition to its high affinity and specificity for the norepinephrine transporter, in vitro experiment showed that atomoxetine also blocks G-coupled inwardly rectifying potassium channels, which play an important role in pharyngeal hypotonia during sleep by reducing hypoglossal motoneuron excitability (29, 30). Atomoxetine, at doses varying from 40 to 80 mg, was tested previously by Bart Sangal and colleagues (31) in a 4-week prospective observational study on 15 patients with mild OSA. The authors showed that the administration of atomoxetine before sleep did not reduce the AHI and respiratory disturbance index, but nevertheless improved subjective sleepiness. The data collected in our adjunctive study confirm the findings from Bart Sangal and colleagues in respect to OSA severity, and extend their observations to the patients with moderate to severe OSA, because atomoxetine did not reduce the AHI when administered alone.
Oxybutynin is an antimuscarinic, which has high affinity for all muscarinic receptors (M1–M5) and is approved in the United States for the treatment of overactive bladder. Work by Liu and colleagues (32) suggested that acetylcholine has mixed effects on the hypoglossal motornucleus, with muscarinic-mediated genioglossus suppression normally exceeding nicotinic excitation. Muscarinic blockade may prevent the inhibitory effect of acetylcholine on upper airway muscle tone during REM sleep, and increase the concentration of acetylcholine available for nicotinic receptors (33), thus cooperating with norepinephrine in the stimulation of upper airway dilator muscles. Interestingly, it has been shown that the inhibitory muscarinic receptor, M2, which is antagonized by oxybutynin, is one of the few G protein–coupled receptors differentially expressed in both the premotor and motor areas of the hypoglossal motor nucleus compared with the rest of the brain, suggesting that this receptor has an important role in the modulation of hypoglossal nerve activity (34). The original hypothesis, derived from animal studies by Horner’s group, that noradrenergic drugs could increase upper airway muscle activity preferentially during NREM sleep (16), whereas antimuscarinic administration can reverse REM sleep atonia (17), is challenged by our findings. Specifically, no improvement in AHI was observed over the entire night or during NREM or REM sleep when the drugs were administered alone. However, the combination was clearly quite effective in both states. One possibility is that the two drugs combined may have synergistic effects on upper airway dilator muscles: aside from preventing genioglossus REM-related atonia, the antimuscarinic scopolamine significantly increased genioglossus respiratory-related activity during NREM sleep in animal model (17). Another possibility is that the two drugs combined may target different pathophysiologic traits other than the muscle activity, reducing, for example the respiratory control instability or increasing sleep consolidation by reducing arousability (2). Accordingly, we assessed the effects of the agents administered together and separately on the respiratory arousal threshold (Pes triggering arousals), and found that atomoxetine caused a reduction in the arousal threshold by 6.2 cm H2O (P = 0.03) when administered alone, and this effect was probably related to the alerting effects associated with the central increase in norepinephrine. Interestingly, in the same patients, the reduction in arousal threshold on ato–oxy was a nonsignificant 2.6 cm H2O (P = 0.79). These data suggest that the administration of oxybutynin could attenuate the alerting effect of atomoxetine through mechanisms that need to be investigated (see Figure E2). Interestingly, previous animal data showed that the administration of the antimuscarinic atropine could abolish the fast, low-amplitude EEG activity induced by an adrenergic stimulant of the central nervous system (amphetamine) and induce low-frequency, high-amplitude brainwaves typical of NREM sleep (35). It is still uncertain if this mechanism is at work in our combination therapy, and more studies need to be performed. The possibility that the simultaneous administration of these two drugs can both increase upper airway muscle activity and consolidate sleep would be desirable for the treatment of OSA (34).
Limitations
Given that our study was a proof-of-concept, physiological trial, it is premature to employ the combination of atomoxetine and oxybutynin as a treatment option for OSA. Longer and larger studies are needed to confirm efficacy and safety of these two compounds in the greater population of patients with OSA. To confirm our findings and test the durability of these drugs, we collected pilot data on six patients who took the drug combination for 1 week after a baseline study in a prospective, observational, open-label study. We again found a similar reduction in AHI (63 [53–70]%; P < 0.001; see the online supplement for more details), suggesting that the effects of the drugs in the long term may be similar to those of the first night.
Although the combination of atomoxetine and oxybutynin had a significant favorable effect on AHI and oxygenation, the effects on sleep consolidation were not evident in the overall population studied. A possible explanation is that the invasive nature of the study equipment may have prohibited improvements in sleep that were expected to accompany improvements in respiration. Alternatively, beneficial effects on sleep may be limited to patients with OSA. Indeed, when we limit analysis to patients with OSA on placebo (AHI > 10), we observed an increase in sleep efficiency, and total sleep time, and a trend for a reduction in arousal index (this trend becomes significant in the 13 patients with AHI greater than 15 on placebo; see the online supplement). Moreover, Figure 3 shows that the improvement in sleep parameters depends on the baseline OSA status. In this study, we found no difference in sleep architecture, except for a trend for a 9% increase in N2 (P = 0.05) on ato–oxy compared with placebo. Although the proportion of REM sleep was similar between nights, it is known that the administration of adrenergic and antimuscarinic drugs could reduce the time spent in REM sleep. Accordingly, pilot data collected after 1 week of therapy in six patients showed an 11% reduction in REM sleep on ato–oxy compared with placebo (P = 0.03). It remains to be determined if this reduction will be confirmed over time in a wider population of patients with OSA, and if it may have clinically meaningful consequences.
It is important to note that atomoxetine is an adrenergic drug contraindicated in patients with severe cardiovascular conditions, such as heart failure or cardiomyopathies, and oxybutynin could cause severe, dose-dependent, anticholinergic side effects. In our trial, we tested only one dose of ato–oxy, and it remains unknown if lower doses of the combination could have similar efficacy, fewer side effects, and higher safety profile.
Finally, although all 15 individuals with OSA on placebo (AHI > 10) showed a clear reduction in OSA severity (>50%), eight of these patients still had a residual AHI of 10 or more events/h on the drug combination, suggesting that a subgroup of patients may be resistant to complete OSA resolution using this potential therapy. Because the trial was designed as a proof-of-concept, one-night physiology study, patients were not fully characterized clinically; for example, we did not systematically evaluate the baseline subjective or objective sleepiness. Moreover, in the attempt to reduce the equipment burden on the patients, we did not measure limb movements during sleep. Thus, more data are needed to identify specific clinical or physiological phenotypic traits predicting success or failure (2). Nonetheless, the current study found a major reduction in AHI and improvement in oxygen levels across a wide range of OSA severity. Further investigation into long-term therapeutic efficacy is warranted.
Conclusions
In summary, this trial showed for the first time that pharmacological resolution of OSA is possible using a combination of noradrenergic and antimuscarinic drugs with specific neurotransmitter receptor binding profiles that activate the upper airway dilator muscles during sleep. This discovery opens new possibilities for the future treatment of OSA.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Lauren Hess, Nicole Calianese, Rebecca Salant, and Kevin Zinchuk for their technical support on this project.
Footnotes
Supported by Fan Hongbing, President of OMPA Corporation, Kaifeng, China, and NIH grants R01HL102321, P01HL095491, and UL1RR025758-01; by American Heart Association grant 17POST33410436 (L.T.-M.); by a University of Brescia scholarship in respiratory medicine (L.M.); by National Health and Medical Research Council (NHMRC) of Australia’s C. J. Martin Overseas Biomedical Fellowship 1035115 and Heart Foundation of Australia Future Leader Fellowship 101167 (B.A.E.); by American Heart Association grant 15SDG25890059, NHMRC Early Career Fellowship 1053201, the Menzies Foundation, and the American Thoracic Society Foundation (S.A.S.); by the Fundacão de Amparo à Pesquisa do Estado de São Paulo, Ministry of Education of Brazil (M.M.); and by NHMRC Senior Research Fellowship 1116942 (D.J.E.).
Author Contributions: L.T.-M. contributed to study design, data collection, data analysis and interpretation, and drafting and review of the manuscript for important intellectual content; L.M. contributed to data collection, data analysis and interpretation, and review of the manuscript; S.A.S. contributed to the study design, data analysis and interpretation, and review of the manuscript; A.A. and M.M. contributed to data analysis and review of the manuscript; B.A.E., D.J.E., and D.P.W. contributed to data interpretation and review of the manuscript; A.W. contributed to the study design, data analysis and interpretation, and drafting and review of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201808-1493OC on November 5, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea: identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–865. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 4.Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15:343–356. doi: 10.1016/j.smrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips CL, Grunstein RR, Darendeliler MA, Mihailidou AS, Srinivasan VK, Yee BJ, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187:879–887. doi: 10.1164/rccm.201212-2223OC. [DOI] [PubMed] [Google Scholar]
- 6.Hammond RJ, Gotsopoulos H, Shen G, Petocz P, Cistulli PA, Darendeliler MA. A follow-up study of dental and skeletal changes associated with mandibular advancement splint use in obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 2007;132:806–814. doi: 10.1016/j.ajodo.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 7.Strollo PJ, Jr, Soose RJ, Maurer JT, de Vries N, Cornelius J, Froymovich O, et al. STAR Trial Group. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370:139–149. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 8.Mason M, Welsh EJ, Smith I. Drug therapy for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2013;(5):CD003002. doi: 10.1002/14651858.CD003002.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubin L, Davies RO, Pack AI. Control of upper airway motoneurons during REM sleep. News Physiol Sci. 1998;13:91–97. doi: 10.1152/physiologyonline.1998.13.2.91. [DOI] [PubMed] [Google Scholar]
- 10.Sood S, Liu X, Liu H, Nolan P, Horner RL. 5-HT at hypoglossal motor nucleus and respiratory control of genioglossus muscle in anesthetized rats. Respir Physiol Neurobiol. 2003;138:205–221. doi: 10.1016/j.resp.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Sood S, Morrison JL, Liu H, Horner RL. Role of endogenous serotonin in modulating genioglossus muscle activity in awake and sleeping rats. Am J Respir Crit Care Med. 2005;172:1338–1347. doi: 10.1164/rccm.200502-258OC. [DOI] [PubMed] [Google Scholar]
- 12.Fenik VB, Davies RO, Kubin L. REM sleep–like atonia of hypoglossal (XII) motoneurons is caused by loss of noradrenergic and serotonergic inputs. Am J Respir Crit Care Med. 2005;172:1322–1330. doi: 10.1164/rccm.200412-1750OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berry RB, Yamaura EM, Gill K, Reist C. Acute effects of paroxetine on genioglossus activity in obstructive sleep apnea. Sleep. 1999;22:1087–1092. doi: 10.1093/sleep/22.8.1087. [DOI] [PubMed] [Google Scholar]
- 14.Marshall NS, Yee BJ, Desai AV, Buchanan PR, Wong KK, Crompton R, et al. Two randomized placebo-controlled trials to evaluate the efficacy and tolerability of mirtazapine for the treatment of obstructive sleep apnea. Sleep. 2008;31:824–831. doi: 10.1093/sleep/31.6.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraiczi H, Hedner J, Dahlöf P, Ejnell H, Carlson J. Effect of serotonin uptake inhibition on breathing during sleep and daytime symptoms in obstructive sleep apnea. Sleep. 1999;22:61–67. [PubMed] [Google Scholar]
- 16.Chan E, Steenland HW, Liu H, Horner RL. Endogenous excitatory drive modulating respiratory muscle activity across sleep–wake states. Am J Respir Crit Care Med. 2006;174:1264–1273. doi: 10.1164/rccm.200605-597OC. [DOI] [PubMed] [Google Scholar]
- 17.Grace KP, Hughes SW, Horner RL. Identification of the mechanism mediating genioglossus muscle suppression in REM sleep. Am J Respir Crit Care Med. 2013;187:311–319. doi: 10.1164/rccm.201209-1654OC. [DOI] [PubMed] [Google Scholar]
- 18.Taranto-Montemurro L, Sands SA, Edwards BA, Azarbarzin A, Marques M, de Melo C, et al. Desipramine improves upper airway collapsibility and reduces OSA severity in patients with minimal muscle compensation. Eur Respir J. 2016;48:1340–1350. doi: 10.1183/13993003.00823-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conway WA, Zorick F, Piccione P, Roth T. Protriptyline in the treatment of sleep apnoea. Thorax. 1982;37:49–53. doi: 10.1136/thx.37.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith PL, Haponik EF, Allen RP, Bleecker ER. The effects of protriptyline in sleep-disordered breathing. Am Rev Respir Dis. 1983;127:8–13. doi: 10.1164/arrd.1983.127.1.8. [DOI] [PubMed] [Google Scholar]
- 21.Brownell LG, West P, Sweatman P, Acres JC, Kryger MH. Protriptyline in obstructive sleep apnea: a double-blind trial. N Engl J Med. 1982;307:1037–1042. doi: 10.1056/NEJM198210213071701. [DOI] [PubMed] [Google Scholar]
- 22.Whyte KF, Gould GA, Airlie MA, Shapiro CM, Douglas NJ. Role of protriptyline and acetazolamide in the sleep apnea/hypopnea syndrome. Sleep. 1988;11:463–472. doi: 10.1093/sleep/11.5.463. [DOI] [PubMed] [Google Scholar]
- 23.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research: the report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 24.Taranto-Montemurro L, Edwards BA, Sands SA, Marques M, Eckert DJ, White DP, et al. Desipramine increases genioglossus activity and reduces upper airway collapsibility during non-REM sleep in healthy subjects. Am J Respir Crit Care Med. 2016;194:878–885. doi: 10.1164/rccm.201511-2172OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholson AN, Stone BM, Clarke CH. Effect of diazepam and fosazepam (a soluble derivative of diazepam) on sleep in man. Br J Clin Pharmacol. 1976;3:533–541. doi: 10.1111/j.1365-2125.1976.tb04872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. American Academy of Sleep Medicine; Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azarbarzin A, Sands SA, Stone KL, Taranto-Montemurro L, Messineo L, Terrill PI, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J. 2019;40:1149–1157. doi: 10.1093/eurheartj/ehy624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malhotra A, Trinder J, Fogel R, Stanchina M, Patel SR, Schory K, et al. Postural effects on pharyngeal protective reflex mechanisms. Sleep. 2004;27:1105–1112. doi: 10.1093/sleep/27.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taranto-Montemurro L, Sands SA, Azarbarzin A, Marques M, de Melo CM, Edwards BA, et al. Effect of 4-aminopyridine on genioglossus muscle activity during sleep in healthy adults. Ann Am Thorac Soc. 2017;14:1177–1183. doi: 10.1513/AnnalsATS.201701-006OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grace KP, Hughes SW, Horner RL. Identification of a pharmacological target for genioglossus reactivation throughout sleep. Sleep (Basel) 2014;37:41–50. doi: 10.5665/sleep.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bart Sangal R, Sangal JM, Thorp K. Atomoxetine improves sleepiness and global severity of illness but not the respiratory disturbance index in mild to moderate obstructive sleep apnea with sleepiness. Sleep Med. 2008;9:506–510. doi: 10.1016/j.sleep.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 32.Liu X, Sood S, Liu H, Horner RL. Opposing muscarinic and nicotinic modulation of hypoglossal motor output to genioglossus muscle in rats in vivo. J Physiol. 2005;565:965–980. doi: 10.1113/jphysiol.2005.084657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quirion R, Richard J, Wilson A. Muscarinic and nicotinic modulation of cortical acetylcholine release monitored by in vivo microdialysis in freely moving adult rats. Synapse. 1994;17:92–100. doi: 10.1002/syn.890170205. [DOI] [PubMed] [Google Scholar]
- 34.Horner RL, Grace KP, Wellman A. A resource of potential drug targets and strategic decision-making for obstructive sleep apnoea pharmacotherapy. Respirology. 2017;22:861–873. doi: 10.1111/resp.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White RP, Daigneault EA. The antagonisms of atropine to the EEG effects of adrenergic drugs. J Pharmacol Exp Ther. 1959;125:339–346. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.