Abstract
Objective
To investigate the effects of lifestyle-related factors on ischemic heart disease (IHD) according to body mass index (BMI) and fasting blood glucose (FBG) levels among Korean adults.
Methods
A total of 119,929 men and 89,669 women (from National Sample Cohort version 2.0, National Health Insurance Service) aged ≥20 years who were examined during 2003–2006 and had no preexisting type 2 diabetes or circulatory diseases were followed until December 2015 to confirm IHD incident cases. Data on lifestyle-related factors (BMI, FBG, diet, smoking, alcohol consumption, and physical activity) were collected at baseline. Lifestyle-related risk factors were defined as mainly vegetable/mainly meat diet, former/current smoking, alcohol consumption ≥3 times/week, and no physical activity. Associations between these factors and IHD were examined using Cox proportional hazards regression models.
Results
High BMI (≥25 kg/m2), high FBG (≥100 mg/dL), mainly meat diet, and former/current smoking were associated with increased risk for IHD. Alcohol consumption ≤twice/week and physical activity ≤twice/week were associated with lower risk of IHD. With increased lifestyle-related risk factors, the risk of IHD also increased in women (hazard ratio [HR] = 3.21, 95% confidence interval [CI] 2.18–4.73) and men (HR = 1.66, 95% CI 1.48–1.85). This increase was larger in women, with a significant sex interaction (p = 0.0001). Significant interactions between BMI and alcohol consumption (p = 0.0002) and between BMI and physical activity (p = 0.0063) were observed. Interactions were seen between FBG level and meal type in both BMI<25 kg/m2 (p = 0.0106) and BMI≥25 kg/m2 (p = 0.0281) and between FBG level and alcohol consumption in BMI ≥25 kg/m2 (p = 0.0118).
Conclusions
The impact of lifestyle-related factors on IHD was more pronounced in women than in men and may be modified by BMI and FBG level among Korean adults. This might be taken into account when planning individual interventions to reduce IHD risk.
Introduction
Ischemic heart disease (IHD), also known as coronary heart disease, is one of the leading causes of death globally [1, 2]. In South Korea (hereafter “Korea”), IHD contributes to >50% of deaths (31.0 cases in men and 26.3 cases in women per 100,000 persons) from cardiovascular diseases (CVDs), which were the second biggest causes of death (58.2 cases per 100,000 persons) after cancer in 2016 [3, 4]. Although more common among men [2, 5], IHD is one of the major causes of death in both men and women [6]. Women seem to have IHD events 7–10 years later than men, yet the outcome of IHD is worse in women [7, 8].
The morbidity and mortality rates associated with IHD among people with obesity or impaired glucose tolerance were reported to be higher than those of healthy people [9–13]. Furthermore, high body mass index (BMI) and fasting blood glucose (FBG) levels are affected by other lifestyle-related factors such as diet, smoking, alcohol consumption, and physical activity [14–22]. People who are overweight or obese were found to have a higher IHD risk than those with normal BMI. Additionally, risk factors related to unhealthy lifestyle increased IHD risk. For example, heavy smoking and sedentary lifestyle were strongly related to IHD risk in people with an increased BMI [9]. Meanwhile, a study conducted in Japan reported that high blood glucose levels increased CVD mortality more than obesity in combination with 2 or more metabolic factors, such as BMI>25 kg/m2, high blood pressure, high blood glucose, high triglyceride, and low high-density lipoprotein cholesterol (HDL-C) levels [12]. Moreover, FBG levels strongly interacted with the other lifestyle-related factors in intervention studies on IHD risk; a lower calorie intake and more vigorous physical activity decreased FBG levels [23, 24]. To our knowledge, however, there is limited evidence about the effects of the interaction between BMI or FBG levels and the other lifestyle-related factors on the incidence of IHD among Koreans. Furthermore, the association between lifestyle-related factors and IHD has not been investigated in large-scale cohorts in Korea.
Most studies on IHD considering the association between BMI or FBG levels and the other lifestyle-related factors have been conducted in Western countries [9, 10, 15, 23–25]. Hence, independent studies on IHD risk for Asian countries would be necessary considering that Asians have different ethnicity and standards for obesity as well as different lifestyle-related factors, which may affect IHD risk [11, 26–28]. Therefore, this study aimed to assess the effects of lifestyle-related factors on IHD incidence according to BMI and FBG levels among Korean adults.
Materials and methods
This study was approved by the Institutional Review Board of Seoul National University (IRB No. E1709/002-001) and the National Health Insurance Service (NHIS) (No. NHIS-2017-2-541).
Data source
The National Sample Cohort version 2.0 (NSC-v2) of the NHIS was used for this analysis. Korea established the NHIS in 1963, and all citizens have been registered under a universal health insurance system since 1989 [29]. All national health insurance records from healthcare institutions are gathered by the NHIS. In 2017, NHIS constructed NSC-v2 by extracting 1 million representative samples from the total eligible Korean population in 2006. The need for prior consent from the study population was waived because data were anonymized by NHIS before entry into the database, according to their strict rules to protect personal information. The cohort has five databases: birth and death, insurance eligibility, medical treatment, general health examination, and information on healthcare providers between 2002 and 2015. More details have been described elsewhere [29–31].
Study subjects
Among the 1 million samples from NSC-v2, 313,156 people who received a general health examination at least once between 2003 and 2006 were initially selected for analysis. We excluded subjects who had medical records of type 2 diabetes (T2D) or circulatory diseases before the health examinations (n = 90,375) based on International Classification of Diseases (ICD)-10 codes [32]: E11 for T2D and I00-I99 for circulatory diseases. We also excluded subjects aged <20 years (n = 1,013); those who could be considered as outliers (with a mean of ±3 standard deviations) [33] or had missing information on lifestyle-related factors including BMI, FBG, meal type, smoking status, alcohol consumption, and physical activity (n = 12,169); or those with no information on insurance eligibility (n = 1) at baseline. A total of 209,598 people (119,929 men and 89,669 women) were included in the final analysis.
Variables
We linked the five databases of NSC-v2 using anonymized identity numbers. Age at baseline was calculated by subtracting the year of birth from the year of health examination and was categorized into three groups: 20–39, 40–59, and ≥60 years. Income level was estimated according to the percentiles of health insurance fee and was categorized into three groups: high (upper 30%), medium (31–70%), and low (lower 30% or medical-aid beneficiaries).
Height, weight, and biochemical indices after an overnight fasting were obtained from the health examination records. The subjects were stratified according to BMI and FBG levels. BMI was calculated from the measurement of height and weight and categorized into two groups: <25.0 and ≥25.0 kg/m2 [11, 26]. FBG was stratified into two levels: normal (<100 mg/dL) and high (≥100 mg/dL) [34]. Risk status was divided into four groups according to BMI (kg/m2) and FBG (mg/dL) levels: (1) BMI<25 and FBG<100, (2) BMI<25 and FBG≥100, (3) BMI≥25 and FBG<100, and (4) BMI≥25 and FBG≥100.
Data on lifestyle-related factors were collected from self-reported questionnaires during the health examinations. The question on meal types consisted of three options: balanced consumption of vegetables and meat, consuming mainly vegetables, and consuming mainly meat. Smoking was classified based on smoking status: never, former, or current smokers. Alcohol consumption frequency was categorized into three levels: none, ≤twice/week, or ≥3 times/week [35]. Physical activity frequency was assessed on weekly basis and classified into three groups: none, ≤twice/week, or ≥3 times/week [36]. Lifestyle-related risk factors were defined as follows: mainly vegetable/mainly meat diet, former/current smoking, alcohol consumption ≥3 times/week, or no physical activity. IHD incidence was identified based on ICD-10 codes (I20-I25) and dates of the first diagnosis in the medical treatment database [32].
Statistical analysis
The subjects’ medical records were followed from the first health examination until the first diagnosis of IHD, loss of insurance eligibility, or end of follow-up (December 2015). Person-time for each subject was calculated as the number of months of follow-up divided by 12 to convert the value into a fraction of years. We calculated frequencies and percentages for each of the following variables: sex, age, income level, BMI, FBG, meal type, smoking status, alcohol consumption, and physical activity at baseline. Chi-square tests were performed to determine whether the distributions of lifestyle-related factors between men and women were significantly different. The association between lifestyle-related factors (BMI, FBG, meal type, smoking, alcohol consumption, and physical activity) and IHD incidence was estimated in hazard ratios (HR) from Cox proportional hazards regression models according to sex and risk status (BMI and FBG levels). Population attributable risk (PAR) was calculated using the equation PAR = P(HR−1)÷[1+P(HR−1)], where P is the prevalence of exposure [37]. The assumption of proportional hazards was evaluated and satisfied by examining Schoenfeld residuals [38]. Confounding variables including age, sex (for total subjects only), BMI, FBG, income level, meal type, smoking, alcohol consumption, and physical activity were adjusted. We computed Pinteraction values using a likelihood ratio test to compare Cox proportional hazard models with and without cross-product terms for IHD incidence, and each lifestyle category in the analyses was stratified by sex, or by BMI and FBG [39]. All statistical analyses were performed with SAS Enterprise Guide version 7.1 (SAS Institute, Cary, NC). P-values of <0.05 were considered statistically significant.
Results
The IHD incidence rate in men (1359.9 cases per 100,000 person-years) was significantly higher than that in women (1336.0 cases per 100,000 person-years; HR = 0.91, 95% confidence interval [CI] 0.88–0.94) during the follow-up periods. The average follow-up periods were 10.5 and 10.3 person-years in men and women, respectively.
Baseline characteristics of study subjects
Table 1 shows the general characteristics of the subjects at baseline. The distributions of the subjects were significantly different for all variables between men and women (p<0.0001). More men had a high BMI (≥25 kg/m2; 32.5%) and a high FBG level (≥100 mg/dL; 24.7%) than women (19.4% and 16.4%, respectively). Regarding risk status, more men (9.6%) had a risk status of a high BMI and a high FBG than women (4.7%). Balanced diet was the most common in both men (77.9%) and women (71.9%). The proportions of never smokers and non-drinkers were greater in women than in men. More women (65.1%) had sedentary lifestyles than men (47.9%).
Table 1. General characteristics of the subjects at the baseline.
| Variables | Total | Men | Women | P valuea | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| N | 209,598 | 119,929 | 89,669 | ||||
| Age (years) | |||||||
| 20–39 | 97,128 | 46.3 | 61,950 | 51.7 | 35,178 | 39.2 | <0.0001 |
| 40–59 | 91,334 | 43.6 | 47,168 | 39.3 | 44,166 | 49.3 | |
| ≥60 | 21,136 | 10.1 | 10,811 | 9.0 | 10,325 | 11.5 | |
| Income levelb | |||||||
| Low | 62,978 | 30.0 | 29,271 | 24.4 | 33,707 | 37.6 | <0.0001 |
| Medium | 83,786 | 40.0 | 52,091 | 43.4 | 31,695 | 35.3 | |
| High | 62,834 | 30.0 | 38,567 | 32.2 | 24,267 | 27.1 | |
| BMI (kg/m2) | |||||||
| <25 | 153,305 | 73.1 | 80,997 | 67.5 | 72,308 | 80.6 | <0.0001 |
| ≥25 | 56,293 | 26.9 | 38,932 | 32.5 | 17,361 | 19.4 | |
| FBG (mg/dL) | |||||||
| <100 | 165,265 | 78.8 | 90,330 | 75.3 | 74,935 | 83.6 | <0.0001 |
| ≥100 | 44,333 | 21.2 | 29,599 | 24.7 | 14,734 | 16.4 | |
| Risk status | |||||||
| BMI<25, FBG <100 | 124,628 | 59.5 | 62,869 | 52.4 | 61,759 | 68.9 | <0.0001 |
| BMI<25, FBG≥100 | 40,637 | 19.4 | 27,461 | 22.9 | 13,176 | 14.7 | |
| BMI≥25, FBG <100 | 28,677 | 13.7 | 18,128 | 15.1 | 10,549 | 11.8 | |
| BMI≥25, FBG≥100 | 15,656 | 7.5 | 11,471 | 9.6 | 4,185 | 4.7 | |
| Meal type | |||||||
| Balanced | 157,826 | 75.3 | 93,389 | 77.9 | 64,437 | 71.9 | <0.0001 |
| Mainly vegetables | 38,626 | 18.4 | 17,404 | 14.5 | 21,222 | 23.7 | |
| Mainly meat | 13,146 | 6.3 | 9,136 | 7.6 | 4,010 | 4.5 | |
| Smoking | |||||||
| Never | 128,409 | 61.3 | 43,168 | 36.0 | 85,241 | 95.1 | <0.0001 |
| Former | 17,658 | 8.4 | 16,151 | 13.5 | 1,507 | 1.7 | |
| Current | 63,531 | 30.3 | 60,610 | 50.5 | 2,921 | 3.3 | |
| Alcohol consumption | |||||||
| None | 97,879 | 46.7 | 35,700 | 29.8 | 62,179 | 69.3 | <0.0001 |
| ≤twice/week | 91,880 | 43.8 | 66,263 | 55.3 | 25,617 | 28.6 | |
| ≥3 times/week | 19,839 | 9.5 | 17,966 | 15 | 1,873 | 2.1 | |
| Physical activity | |||||||
| None | 115,868 | 55.3 | 57,502 | 47.9 | 58,366 | 65.1 | <0.0001 |
| ≤twice/week | 58,375 | 27.9 | 40,690 | 33.9 | 17,685 | 19.7 | |
| ≥3 times/week | 35,355 | 16.9 | 21,737 | 18.1 | 13,618 | 15.2 | |
aP values were obtained from Chi-square test.
bLow: lower 30 percentiles or medical-aid beneficiaries; medium: 31–70 percentiles; high: upper 30 percentiles
Abbreviations: BMI, body mass index; FBG, fasting blood glucose
HRs and PARs of IHD according to lifestyle-related factors
The HRs and PARs of IHD incidence among men and women according to lifestyle-related factors are presented in Table 2. Among lifestyle-related factors of IHD, high BMI (≥25 kg/m2) exhibited the highest PAR both in men (8.39%) and women (6.82%). In all subjects, those with a high BMI or a high FBG level (≥100 mg/dL) showed a significantly increased IHD risk compared to those with normal BMI or FBG levels (HR = 1.31, 95% CI 1.28–1.34, and HR = 1.10, 95% CI 1.07–1.13, respectively). Subjects with a mainly meat diet (HR = 1.15, 95% CI 1.09–1.21), former (HR = 1.08, 95% CI 1.04–1.13), and current smokers (HR = 1.09, 95% CI 1.06–1.13) had a significantly increased IHD incidence, whereas subjects who consumed alcohol ≤twice/week (HR = 0.89, 95% CI 0.87–0.92) and performed physical activity ≤twice/week (HR = 0.97, 95% CI 0.94–0.99) showed a decreased IHD risk. Among men, those with mainly meat diet showed an increased IHD risk and those with physical activity ≤twice/week had a reduced risk (HR = 1.17, 95% CI 1.10–1.24, and HR = 0.96, 95% CI 0.93–0.99, respectively).
Table 2. Hazard ratios and population attributable risk of ischemic heart disease according to lifestyle-related factors.
| Total | Men | Women | Pinteractionb | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Person-year | HRa | 95% CI | PAR % | Cases | Person-year | HRa | 95% CI | PAR % | Cases | Person-year | HRa | 95% CI | PAR % | ||
| BMI (kg/m2) | ||||||||||||||||
| <25 | 19,430 | 1,610,884 | 1.00 | Ref. | 10,705 | 856,239 | 1.00 | Ref. | 8,725 | 754,645 | 1.00 | Ref. | 0.1949 | |||
| ≥25 | 10,033 | 577,557 | 1.31 | (1.28, 1.34) | 8.06 | 6,374 | 405,232 | 1.29 | (1.25, 1.33) | 8.39 | 3,659 | 172,326 | 1.30 | (1.25, 1.35) | 6.82 | |
| FBG (mg/dL) | ||||||||||||||||
| <100 | 21,664 | 1,736,649 | 1.00 | Ref. | 11,949 | 957,509 | 1.00 | Ref. | 9,715 | 779,141 | 1.00 | Ref. | 0.2753 | |||
| ≥100 | 7,799 | 451,792 | 1.10 | (1.07, 1.13) | 2.41 | 5,130 | 303,962 | 1.10 | (1.07, 1.14) | 2.73 | 2,669 | 147,830 | 1.10 | (1.05, 1.14) | 1.96 | |
| Meal type | ||||||||||||||||
| Balanced | 21,869 | 1,649,931 | 1.00 | Ref. | 13,231 | 982,272 | 1.00 | Ref. | 8,638 | 666,659 | 1.00 | Ref. | ||||
| Mainly vegetables | 6,018 | 400,816 | 1.01 | (0.98, 1.04) | 0.20 | 2,640 | 182,935 | 0.98 | (0.94, 1.03) | -0.32 | 3,378 | 217,881 | 1.03 | (0.99, 1.07) | 0.79 | 0.0012 |
| Mainly meat | 1,576 | 138,694 | 1.15 | (1.09, 1.21) | 0.70 | 1,208 | 96,264 | 1.17 | (1.10, 1.24) | 1.03 | 368 | 42,430 | 1.09 | (0.98, 1.21) | 0.25 | |
| Smoking | ||||||||||||||||
| Never | 18,225 | 1,332,368 | 1.00 | Ref. | 6,457 | 450,451 | 1.00 | Ref. | 11,768 | 881,917 | 1.00 | Ref. | ||||
| Former | 2,748 | 184,881 | 1.08 | (1.04, 1.13) | 0.69 | 2,572 | 169,257 | 1.07 | (1.02, 1.12) | 0.99 | 176 | 15,624 | 1.19 | (1.02, 1.38) | 0.23 | 0.0174 |
| Current | 8,490 | 671,192 | 1.09 | (1.06, 1.13) | 2.38 | 8,050 | 641,763 | 1.06 | (1.03, 1.10) | 2.67 | 440 | 29,429 | 1.20 | (1.09, 1.33) | 0.59 | |
| Alcohol consumption | ||||||||||||||||
| None | 15,497 | 1,005,517 | 1.00 | Ref. | 5,781 | 369,071 | 1.00 | Ref. | 9,716 | 636,446 | 1.00 | Ref. | ||||
| ≤twice/week | 10,426 | 981,063 | 0.89 | (0.87, 0.92) | -4.37 | 8,041 | 709,587 | 0.89 | (0.86, 0.92) | -5.82 | 2,385 | 271,488 | 0.91 | (0.87, 0.96) | -1.90 | 0.0902 |
| ≥3 times/week | 3,540 | 201,850 | 1.00 | (0.96, 1.04) | -0.04 | 3,257 | 182,813 | 1.01 | (0.96, 1.05) | 0.19 | 283 | 19,037 | 1.05 | (0.93, 1.19) | 0.11 | |
| Physical activity | ||||||||||||||||
| None | 16,546 | 1,203,047 | 1.00 | Ref. | 8,525 | 598,827 | 1.00 | Ref. | 8,021 | 604,220 | 1.00 | Ref. | ||||
| ≤twice/week | 7,432 | 618,269 | 0.97 | (0.94, 0.99) | -0.78 | 5,196 | 434,438 | 0.96 | (0.93, 0.99) | -1.27 | 2,236 | 183,831 | 0.97 | (0.92, 1.01) | -0.56 | 0.6468 |
| ≥3 times/week | 5,485 | 367,125 | 0.99 | (0.96, 1.02) | -0.19 | 3,358 | 228,206 | 0.98 | (0.94, 1.02) | -0.40 | 2,127 | 138,919 | 0.98 | (0.94, 1.03) | -0.35 | |
| Total numbers of risk factorsc | ||||||||||||||||
| No risk factors | 2,560 | 236,044 | 1.00 | Ref. | 978 | 85,877 | 1.00 | Ref. | 1,582 | 150,167 | 1.00 | Ref. | ||||
| 1–2 risk factors | 18,474 | 1,452,546 | 1.14 | (1.09, 1.19) | 7.60 | 9,534 | 767,606 | 1.10 | (1.03, 1.17) | 4.94 | 8,940 | 684,941 | 1.15 | (1.09, 1.21) | 9.36 | 0.0001 |
| 3–4 risk factors | 7,927 | 476,557 | 1.35 | (1.29, 1.41) | 14.50 | 6,091 | 385,315 | 1.29 | (1.21, 1.38) | 12.96 | 1,836 | 91,242 | 1.37 | (1.28, 1.47) | 13.38 | |
| 5–6 risk factors | 502 | 23,294 | 1.74 | (1.58, 1.92) | 15.32 | 476 | 22,673 | 1.66 | (1.48, 1.85) | 14.06 | 26 | 620 | 3.21 | (2.18, 4.73) | 13.52 | |
aHRs were adjusted for age, sex (for total subjects only), income level, and additionally mutually adjusted for lifestyle-related factors (all categorical variables).
bPinteraction: p value of interaction between lifestyle-related factor and sex
cRisk factors were defined as BMI≥25 kg/m2, FBG≥100 mg/dL, mainly vegetable/mainly meat meal diet, former/current smoking, ≥3 times/week alcohol consumption, or no physical activity; and HRs were adjusted for age, sex (for total subjects only), and income level (all categorical variables).
Abbreviations: HR, hazard ratio; CI, confidence interval; PAR, population attributable risk; BMI, body mass index; FBG, fasting blood glucose
In addition, the associations between lifestyle-related factors and IHD incidence were modified by sex, with significant interactions with meal types (p = 0.0012) and smoking status (p = 0.0174). A significantly increased risk of IHD was identified in both men and women, as the numbers of lifestyle-related risk factors increased. Comparing subjects with 5–6 risk factors with subjects without any lifestyle-related risk factors, the HR for IHD incidence was 1.74 (95% CI 1.58–1.92) in total subjects. Noticeably, this association was stronger in women (HR = 3.21, 95% CI 2.18–4.73) than in men (HR = 1.66, 95% CI 1.48–1.85), with a significant sex interaction (p = 0.0001 for interaction).
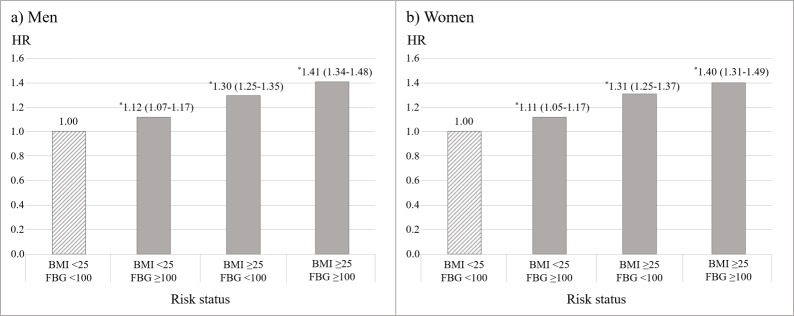
Men and women showed similar patterns of HRs of IHD incidence according to risk status of BMI and FBG levels (Fig 1). Compared to those with normal BMI and FBG levels, both men and women with high BMI and FBG levels showed the highest HRs of 1.41 (95% CI 1.34–1.48) and 1.40 (95% CI 1.31–1.49), respectively.
Fig 1. Hazard ratios of ischemic heart disease according to BMI and FBG levels.
*95% confidence interval of hazard ratio does not include the reference value (1.00). HRs of IHD were adjusted for age, income level, meal type, smoking status, alcohol consumption, and physical activity (all categorical variables). A group of BMI<25 kg/m2 and FBG<100 mg/dL was set as reference and colored as a pattern in the bar graph. Abbreviations: HR, hazard ratio; BMI, body mass index (kg/m2); FBG, fasting blood glucose (mg/dL).
HRs of IHD according to lifestyle-related factors by the status of BMI and FBG levels
HRs of IHD incidence according to individual lifestyle-related factors by the status of BMI and FBG levels are presented in Table 3. A mainly vegetable meal type was positively associated with IHD risk in subjects with BMI <25 kg/m2 and FBG <100 mg/dL (HR = 1.05, 95% CI 1.01–1.09). A meal type consisting of mainly meat was positively associated with IHD risk, except in subjects with BMI ≥25 kg/m2 and FBG <100 mg/dL. Former (HR = 1.09, 95% CI 1.03–1.16) and current (HR = 1.08, 95% CI 1.04–1.13) smoking significantly increased the IHD risk among subjects with BMI <25 kg/m2, which remained significant in subgroup analysis stratified by FBG. Alcohol consumption ≤twice/week reduced the incidence of IHD, but not significantly among subjects with BMI ≥25 kg/m2 and FBG ≥100 mg/dL. Alcohol consumption ≥3 times/week significantly increased IHD risk (HR = 1.22, 95% CI 1.09–1.36), while physical activity ≥3 times/week decreased the risk (HR = 0.90, 95% CI 0.82–0.99) among subjects with BMI ≥25 kg/m2 and FBG ≥100 mg/dL. Compared to the results of total subjects, men showed similar patterns of the association between lifestyle-related factors and IHD risk regardless BMI and FBG levels, but with a varied significance. Conversely, only women with normal BMI and FBG levels had consistent associations between IHD risk and current smoking and alcohol consumption ≤twice/week with the results of total subjects.
Table 3. Hazard ratios of ischemic heart disease according to lifestyle-related factors stratified by the status of BMI and FBG levels.
| BMI < 25 | BMI ≥ 25 | P interactiond |
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Person-year | HRa | 95% CI | FBG < 100 | FBG ≥ 100 | P interactionc |
Cases | Person-year | HRa | 95% CI | FBG < 100 | FBG ≥ 100 | P interactionc |
|||||||||||||||
| Cases | Person-year | HRb | 95% CI | Cases | Person-year | HRb | 95% CI | Cases | Person-year | HRb | 95% CI | Cases | Person-year | HRb | 95% CI | |||||||||||||
| Total | ||||||||||||||||||||||||||||
| Meal type | ||||||||||||||||||||||||||||
| Balanced | 14,084 | 1,197,496 | 1.00 | Ref. | 10,614 | 977,369 | 1.00 | Ref. | 3,470 | 220,127 | 1.00 | Ref. | 7,785 | 451,436 | 1.00 | Ref. | 5,356 | 327,728 | 1.00 | Ref. | 2,429 | 123,708 | 1.00 | Ref. | ||||
| Mainly vegetables | 4,430 | 320,896 | 1.02 | (0.99, 1.06) | 3,468 | 262,087 | 1.05 | (1.01, 1.09) | 962 | 58,809 | 0.94 | (0.88, 1.01) | 1,588 | 79,909 | 0.99 | (0.94, 1.04) | 1,101 | 58,905 | 0.97 | (0.91, 1.04) | 487 | 21,004 | 1.03 | (0.94, 1.14) | ||||
| Mainly meat | 1,916 | 92,481 | 1.20 | (1.12, 1.28) | 1,707 | 77,189 | 1.20 | (1.12, 1.30) | 209 | 15,292 | 1.17 | (1.01, 1.34) | 0.0106 | 660 | 46,212 | 1.08 | (0.999, 1.174) | 418 | 33,372 | 1.03 | (0.93, 1.14) | 242 | 12,840 | 1.19 | (1.04, 1.36) | 0.0281 | 0.0614 | |
| Smoking | ||||||||||||||||||||||||||||
| Never | 12,310 | 1,021,279 | 1.00 | Ref. | 9,667 | 854,329 | 1.00 | Ref. | 2,643 | 166,950 | 1.00 | Ref. | 5,915 | 311,090 | 1.00 | Ref. | 4,138 | 230,104 | 1.00 | Ref. | 1,777 | 80,986 | 1.00 | Ref. | ||||
| Former | 1,651 | 121,791 | 1.09 | (1.03, 1.16) | 1,171 | 94,743 | 1.08 | (1.01, 1.15) | 480 | 27,048 | 1.14 | (1.02, 1.27) | 1,097 | 63,089 | 1.07 | (0.99, 1.15) | 735 | 43,981 | 1.11 | (1.02, 1.21) | 362 | 19,108 | 0.99 | (0.88, 1.12) | ||||
| Current | 5,469 | 467,814 | 1.08 | (1.04, 1.13) | 3,951 | 367,573 | 1.07 | (1.02, 1.12) | 1,518 | 100,241 | 1.11 | (1.02, 1.20) | 0.6033 | 3,021 | 203,378 | 1.09 | (1.03, 1.15) | 2,002 | 145,921 | 1.10 | (1.03, 1.18) | 1,019 | 57,457 | 1.07 | (0.97, 1.17) | 0.1261 | 0.0925 | |
| Alcohol consumption | ||||||||||||||||||||||||||||
| None | 10,637 | 764,894 | 1.00 | Ref. | 8,331 | 637,063 | 1.00 | Ref. | 2,306 | 127,831 | 1.00 | Ref. | 4,860 | 240,622 | 1.00 | Ref. | 3,486 | 179,788 | 1.00 | Ref. | 1,374 | 60,834 | 1.00 | Ref. | ||||
| ≤twice/week | 6,569 | 709,321 | 0.87 | (0.84, 0.90) | 5,034 | 581,869 | 0.88 | (0.85, 0.92) | 1,535 | 127,452 | 0.85 | (0.79, 0.92) | 3,857 | 271,742 | 0.95 | (0.90, 0.99) | 2,637 | 197,862 | 0.94 | (0.884, 0.995) | 1,220 | 73,880 | 0.97 | (0.88, 1.06) | ||||
| ≥3 times/week | 2,224 | 136,657 | 0.96 | (0.91, 1.01) | 1,424 | 97,713 | 0.94 | (0.887, 1.003) | 800 | 38,944 | 1.00 | (0.91, 1.09) | 0.2102 | 1,316 | 65,194 | 1.08 | (1.01, 1.15) | 752 | 42,356 | 1.00 | (0.92, 1.09) | 564 | 22,838 | 1.22 | (1.09, 1.36) | 0.0118 | 0.0002 | |
| Physical activity | ||||||||||||||||||||||||||||
| None | 11,263 | 916,522 | 1.00 | Ref. | 8,554 | 752,500 | 1.00 | Ref. | 2,709 | 164,022 | 1.00 | Ref. | 5,283 | 286,513 | 1.00 | Ref. | 3,589 | 208,329 | 1.00 | Ref. | 1,694 | 78,184 | 1.00 | Ref. | ||||
| ≤twice/week | 4,649 | 437,696 | 1.01 | (0.97, 1.05) | 3,529 | 355,897 | 0.97 | (0.93, 1.01) | 1,120 | 81,799 | 0.96 | (0.89, 1.03) | 2,783 | 180,572 | 0.97 | (0.92, 1.01) | 1,911 | 131,255 | 0.98 | (0.93, 1.04) | 872 | 49,317 | 0.94 | (0.86, 1.02) | ||||
| ≥3 times/week | 3,518 | 256,653 | 0.88 | (0.85, 0.92) | 2,706 | 208,247 | 1.02 | (0.98, 1.07) | 812 | 48,406 | 0.98 | (0.90, 1.06) | 0.2655 | 1,967 | 110,473 | 0.95 | (0.899, 0.999) | 1,375 | 80,421 | 0.97 | (0.91, 1.03) | 592 | 30,052 | 0.90 | (0.82, 0.99) | 0.1213 | 0.0063 | |
| Men | ||||||||||||||||||||||||||||
| Meal type | ||||||||||||||||||||||||||||
| Balanced | 8,150 | 659,284 | 1.00 | Ref. | 5,825 | 514,819 | 1.00 | Ref. | 2,325 | 144,465 | 1.00 | Ref. | 5,081 | 322,989 | 1.00 | Ref. | 3,363 | 229,616 | 1.00 | Ref. | 1,718 | 93,373 | 1.00 | Ref. | ||||
| Mainly vegetables | 1,909 | 140,563 | 0.98 | (0.94, 1.04) | 1,431 | 109,137 | 1.03 | (0.97, 1.09) | 478 | 31,426 | 0.88 | (0.80, 0.97) | 731 | 42,361 | 0.98 | (0.91, 1.07) | 486 | 30,243 | 0.97 | (0.88, 1.07) | 245 | 12,118 | 1.01 | (0.88, 1.15) | ||||
| Mainly meat | 646 | 56,381 | 1.22 | (1.13, 1.32) | 485 | 45,269 | 1.26 | (1.14, 1.38) | 161 | 11,112 | 1.13 | (0.96, 1.32) | 0.0056 | 562 | 39,882 | 1.10 | (1.01, 1.20) | 359 | 28,425 | 1.08 | (0.96, 1.20) | 203 | 11,457 | 1.14 | (0.99, 1.33) | 0.2524 | 0.2598 | |
| Smoking | ||||||||||||||||||||||||||||
| Never | 4,048 | 304,176 | 1.00 | Ref. | 2,987 | 239,254 | 1.00 | Ref. | 1,061 | 64,922 | 1.00 | Ref. | 2,409 | 146,275 | 1.00 | Ref. | 1,586 | 104,348 | 1.00 | Ref. | 823 | 41,927 | 1.00 | Ref. | ||||
| Former | 1,525 | 108,631 | 1.08 | (1.01, 1.14) | 1,063 | 83,062 | 1.05 | (0.98, 1.13) | 462 | 25,569 | 1.14 | (1.02, 1.27) | 1,047 | 60,625 | 1.06 | (0.98, 1.14) | 695 | 41,982 | 1.10 | (1.01, 1.21) | 352 | 18,643 | 0.98 | (0.86, 1.12) | ||||
| Current | 5,132 | 443,431 | 1.05 | (1.001, 1.093) | 3,691 | 346,909 | 1.03 | (0.98, 1.09) | 1,441 | 96,522 | 1.08 | (0.99, 1.18) | 0.1138 | 2,918 | 198,331 | 1.08 | (1.02, 1.15) | 1,927 | 141,953 | 1.10 | (1.03, 1.18) | 991 | 56,378 | 1.05 | (0.95, 1.16) | 0.1719 | 0.0065 | |
| Alcohol consumption | ||||||||||||||||||||||||||||
| None | 3,832 | 256,821 | 1.00 | Ref. | 2,859 | 204,661 | 1.00 | Ref. | 973 | 52,160 | 1.00 | Ref. | 1,949 | 112,249 | 1.00 | Ref. | 1,365 | 81,944 | 1.00 | Ref. | 584 | 30,305 | 1.00 | Ref. | ||||
| ≤twice/week | 4,842 | 477,804 | 0.70 | (0.83, 0.91) | 3,605 | 379,168 | 0.88 | (0.84, 0.93) | 1,237 | 98,636 | 0.84 | (0.77, 0.92) | 3,199 | 231,771 | 0.93 | (0.87, 0.99) | 2,151 | 166,921 | 0.91 | (0.85, 0.98) | 1,048 | 64,850 | 0.97 | (0.87, 1.08) | ||||
| ≥3 times/week | 2,031 | 121,602 | 0.97 | (0.92, 1.03) | 1,277 | 85,396 | 0.95 | (0.89, 1.02) | 754 | 36,206 | 1.00 | (0.90, 1.11) | 0.1175 | 1,226 | 61,212 | 1.07 | (0.996, 1.157) | 692 | 39,419 | 0.99 | (0.90, 1.09) | 534 | 21,793 | 1.24 | (1.09, 1.40) | 0.0074 | 0.0012 | |
| Physical activity | ||||||||||||||||||||||||||||
| None | 5,640 | 420,436 | 1.00 | Ref. | 4,038 | 327,068 | 1.00 | Ref. | 1,602 | 93,368 | 1.00 | Ref. | 2,885 | 178,379 | 1.00 | Ref. | 1,875 | 126,173 | 1.00 | Ref. | 1,010 | 52,206 | 1.00 | Ref. | ||||
| ≤twice/week | 3,049 | 288,425 | 0.94 | (0.90, 0.98) | 2,210 | 227,127 | 0.94 | (0.89, 0.99) | 839 | 61,298 | 0.95 | (0.87, 1.03) | 2,147 | 146,013 | 0.99 | (0.93, 1.05) | 1,431 | 104,712 | 1.00 | (0.93, 1.07) | 716 | 41,301 | 0.98 | (0.88, 1.08) | ||||
| ≥3 times/week | 2,016 | 147,366 | 1.00 | (0.96, 1.05) | 1,493 | 115,030 | 1.02 | (0.96, 1.09) | 523 | 32,336 | 0.93 | (0.84, 1.02) | 0.0519 | 1,342 | 80,840 | 0.97 | (0.91, 1.03) | 902 | 57,399 | 1.00 | (0.92, 1.08) | 440 | 23,441 | 0.91 | (0.82, 1.02) | 0.1819 | 0.0062 | |
| Women | ||||||||||||||||||||||||||||
| Meal type | ||||||||||||||||||||||||||||
| Balanced | 5,934 | 538,212 | 1.00 | Ref. | 4,789 | 462,550 | 1.00 | Ref. | 1,145 | 75,662 | 1.00 | Ref. | 2,704 | 128,447 | 1.00 | Ref. | 1,993 | 98,112 | 1.00 | Ref. | 711 | 30,335 | 1.00 | Ref. | ||||
| Mainly vegetables | 2,521 | 180,333 | 1.04 | (0.99, 1.09) | 2,037 | 152,950 | 1.05 | (0.996, 1.110) | 484 | 27,383 | 1.01 | (0.91, 1.13) | 857 | 37,548 | 0.99 | (0.91, 1.07) | 615 | 28,662 | 0.96 | (0.88, 1.05) | 242 | 8,886 | 1.06 | (0.92, 1.23) | ||||
| Mainly meat | 270 | 36,100 | 1.13 | (0.999, 1.280) | 222 | 31,920 | 1.10 | (0.96, 1.26) | 48 | 4,180 | 1.28 | (0.95, 1.71) | 0.2524 | 98 | 6,330 | 1.00 | (0.82, 1.23) | 59 | 4,947 | 0.84 | (0.65, 1.10) | 39 | 1,383 | 1.38 | (0.997, 1.909) | 0.0125 | 0.0833 | |
| Smoking | ||||||||||||||||||||||||||||
| Never | 8,262 | 717,103 | 1.00 | Ref. | 6,680 | 615,075 | 1.00 | Ref. | 1,582 | 102,028 | 1.00 | Ref. | 3,506 | 164,815 | 1.00 | Ref. | 2,552 | 125,756 | 1.00 | Ref. | 954 | 39,059 | 1.00 | Ref. | ||||
| Former | 126 | 13,160 | 1.16 | (0.97, 1.39) | 108 | 11,681 | 1.19 | (0.98, 1.44) | 18 | 1,479 | 1.01 | (0.64, 1.62) | 50 | 2,464 | 1.26 | (0.95, 1.68) | 40 | 1,999 | 1.36 | (0.99, 1.87) | 10 | 466 | 1.00 | (0.54, 1.88) | ||||
| Current | 337 | 24,383 | 1.24 | (1.11, 1.39) | 260 | 20,664 | 1.24 | (1.09, 1.40) | 77 | 3,719 | 1.25 | (0.99, 1.58) | 0.5023 | 103 | 5,046 | 1.08 | (0.89, 1.32) | 75 | 3,967 | 1.08 | (0.85, 1.36) | 28 | 1,079 | 1.10 | (0.75, 1.62) | 0.3507 | 0.2176 | |
| Alcohol consumption | ||||||||||||||||||||||||||||
| None | 6,805 | 508,073 | 1.00 | Ref. | 5,472 | 432,402 | 1.00 | Ref. | 1,333 | 75,671 | 1.00 | Ref. | 2,911 | 128,373 | 1.00 | Ref. | 2,121 | 97,844 | 1.00 | Ref. | 790 | 30,529 | 1.00 | Ref. | ||||
| ≤twice/week | 1,727 | 231,517 | 0.88 | (0.84, 0.93) | 1,429 | 202,701 | 0.88 | (0.83, 0.94) | 298 | 28,816 | 0.89 | (0.78, 1.01) | 658 | 39,971 | 0.99 | (0.91, 1.08) | 486 | 30,941 | 1.00 | (0.91, 1.11) | 172 | 9,030 | 0.97 | (0.81, 1.15) | ||||
| ≥3 times/week | 193 | 15,055 | 1.03 | (0.89, 1.19) | 147 | 12,317 | 1.04 | (0.88, 1.23) | 46 | 2,738 | 1.00 | (0.74, 1.35) | 0.8231 | 90 | 3,982 | 1.08 | (0.88, 1.34) | 60 | 2,937 | 1.05 | (0.81, 1.36) | 30 | 1,045 | 1.17 | (0.81, 1.69) | 0.4683 | 0.0038 | |
| Physical activity | ||||||||||||||||||||||||||||
| None | 5,623 | 496,086 | 1.00 | Ref. | 4,516 | 425,432 | 1.00 | Ref. | 1,107 | 70,654 | 1.00 | Ref. | 2,398 | 108,134 | 1.00 | Ref. | 1,714 | 82,156 | 1.00 | Ref. | 684 | 25,978 | 1.00 | Ref. | ||||
| ≤twice/week | 1,600 | 149,271 | 0.99 | (0.93, 1.05) | 1,319 | 128,770 | 1.00 | (0.94, 1.06) | 281 | 20,501 | 0.95 | (0.83, 1.08) | 636 | 34,559 | 0.92 | (0.84, 1.01) | 480 | 26,543 | 0.96 | (0.87, 1.06) | 156 | 8,016 | 0.83 | (0.69, 0.99) | ||||
| ≥3 times/week | 1,502 | 109,287 | 1.02 | (0.96, 1.08) | 1,213 | 93,217 | 1.01 | (0.95, 1.08) | 289 | 16,070 | 1.06 | (0.93, 1.21) | 0.2617 | 625 | 29,633 | 0.91 | (0.836, 0.997) | 473 | 23,022 | 0.93 | (0.84, 1.03) | 152 | 6,611 | 0.87 | (0.73, 1.03) | 0.1090 | 0.0714 | |
aHRs were adjusted for age, sex (for total subjects only), and income level, and additionally mutually adjusted for lifestyle-related risk factors (all categorical variables) except BMI.
bHRs were adjusted for age, sex (for total subjects only), and income level, and additionally mutually adjusted for lifestyle-related risk factors (all categorical variables) except BMI and FBG.
cPinteraction: p value of interaction between lifestyle-related factor and FBG within BMI level
dPinteraction: p value of interaction between lifestyle-related factor and BMI level
Abbreviations: HR, hazard ratio; CI, confidence interval; BMI, body mass index; FBG, fasting blood glucose
The association between lifestyle-related factors and IHD risk was modified by BMI. Significant interactions were observed between BMI and alcohol consumption (p = 0.0002) and between BMI and physical activity (p = 0.0063). The interaction of physical activity with BMI was not significant in women. The interaction between smoking status and BMI was significant in men (p = 0.0065). Regarding the interaction between FBG level and lifestyle-related factors, the association between meal type and IHD risk was statistically different by FBG level in both BMI<25 kg/m2 (p = 0.0106) and BMI≥25 kg/m2 (p = 0.0281), particularly in men with BMI<25 kg/m2 (p = 0.0056) and women BMI≥25 kg/m2 (p = 0.0125). A statistically significant interaction between alcohol consumption and FBG level was observed in total subjects (p = 0.0118) and in men (p = 0.0074) with BMI ≥25 kg/m2.
Discussion
This study showed that lifestyle-related factors were significantly associated with IHD incidence in both men and women, and the effects varied according to BMI and FBG levels among Korean adults. Subjects with a high BMI, high FBG level, mainly meat consuming meal type, or former or current smoking showed an elevated IHD incidence, whereas those with alcohol consumption ≤twice/week or physical activity ≤twice/week had a reduced IHD risk for both sexes. As the numbers of lifestyle-related risk factors increased, the risk of IHD was also significantly increased. This increased risk was more pronounced in women than in men, with a significant sex interaction. Significant interactions between BMI and alcohol consumption (p = 0.0002) and between BMI and physical activity (p = 0.0063) were observed for IHD incidence in all subjects. Interactions between FBG level and meal type and between FBG level and alcohol consumption were observed.
A high BMI was the strongest risk factor of IHD incidence in our study. In a previous Korean study on BMI and IHD incidence, young adults with obesity grade 1 (BMI 25.0–29.9 kg/m2) showed a significant increase in IHD risk (45% in men and 52% in women) compared with the reference group (BMI 18.5–22.9 kg/m2) [40].
This study reported higher IHD incidence rates than our results (29% in men and 30% in women), potentially because they excluded only IHD patients at baseline [40], while our study excluded IHD patients and other patients with circulatory diseases or T2D who were more likely to develop IHD.
A high FBG level was also a significant risk factor of IHD in our study, which is consistent with the results of previous studies [12, 13]. T2D (FBG≥126 mg/dL) has been confirmed to increase IHD incidence [41–43]. A meta-analysis of 17 prospective studies worldwide showed a positive relationship between IHD risk and impaired fasting glucose (FBG 100–125 or 110–125 mg/dL) [13].
Previous studies in Western countries reported that high meat intake increased IHD risk in both men and women [17, 21, 44, 45]. However, in our study, women consuming mainly meat did not show a significant increase in IHD risk. The traditional Korean diet is rich in vegetables, while Western dietary patterns comprise food with more fat content [46]. Moreover, women tend to eat more fruits, vegetables, and fiber than men [47]. Considering the healthier diet of women and the Korean diet of various vegetables, we assume that the three meal types used in our study are not sufficient to fully distinguish a mainly meat diet from a balanced diet, which explains why a mainly meat diet among women did not induce a significant change in IHD risk.
In this study, women current smokers showed increased IHD risk by 20% compared with women who were never smokers, while the risk was increased by 6% among men current smokers. A previous meta-analysis reported that smoking increased IHD risk more adversely in women than in men [48]. Smoking may have more harmful effects on IHD in women because women smokers tend to have a unhealthier cholesterol status, that is, a higher low-density lipoprotein cholesterol (LDL-C) level and a lower HDL-C level, than men smokers, and thus unhealthy cholesterol status could make blood vessel narrower and aggravate IHD risk [49, 50]. Information on LDL-C and HDL-C levels was not available in our study based on the 2003–2006 health examination. Thus, further research needs to investigate associations between sex disparity in Korean smokers and IHD incidence with more various metabolic indices including LDL-C and HDL-C levels.
Our results on alcohol consumption are consistent with those of previous studies advocating that only moderate alcohol consumption is inversely related to IHD risk [16, 18]. Although some studies suggested that heavy alcohol consumption ≥5 times/week also reduced IHD incidence [17, 22], we did not observe such relationship in our study. Koreans tend to consume meat with alcohol [46, 51]; thus, the effect of alcohol consumption on IHD risk must be considered when studying dietary patterns among Koreans. High-fat-content food like meat could exacerbate IHD risk especially with heavy drinking.
Moderate physical activity has been shown to decrease IHD risk in both men and women in previous studies [21, 44, 52]. However, in our study, women with any physical activity did not show a significant decrease in IHD risk. According to a meta-analysis on the dose-response relationship between physical activity and IHD risk, physical activity in women was inversely related to IHD risk, and the advantageous effect was greater in women than in men [52]. Our results, however, showed the opposite, in that only men with physical activity ≤twice/week had a significantly decreased HR of IHD. More research is necessary on why women in our study did not show any significant relationship between physical activity and IHD risk.
In this study, subjects with any number of lifestyle-related risk factors showed a significantly increased IHD risk in both sexes. Noticeably, the difference in IHD risk with 3 or more lifestyle-related risk factors compared to no risk factors was significantly higher in women than in men. However, the opposite was seen in a Japanese study reporting that only Japanese men with a high FBG or a high BMI showed a significantly increased IHD risk, but not in women [53]. This disparity in the results of the two studies might be due to the different selection of subjects by age, and by risk factors, except high BMI and high FBG levels. Our subjects were aged over 20 years, while subjects in the Japanese study were aged between 40–69 years. To our knowledge, the result of our study has not been fully explained by previous research, and there has been no study on IHD risk considering lifestyle-related factors with BMI and FBG levels. In this regard, future studies need to clarify why women showed more adverse effects from increasing numbers of lifestyle-related risk factors.
This study has some limitations. First, the NSC-v2 of the NHIS might contain some information bias due to a possible gap between recorded diseases and actual diseases of the subjects, since the disease codes were entered for the purpose of health insurance reimbursement. Some diseases might have been overestimated considering the intention of receiving larger reimbursement. This might have affected our inclusion/exclusion of subjects. Second, there was uncertainty in selecting patients with preexisting diseases due to unavailability of information before 2002, although we considered health examination data in 2002 as part of the washout period. Third, a self-report of lifestyle-related factors might lead to response bias. We were not able to use information on amount of alcohol consumption and intensity of physical activity since these were not collected at baseline. Additionally, we did not consider changes in lifestyle-related factors during the follow-up periods. Lastly, there could be additional confounding factors such as waist circumference and blood cholesterol levels that were not available in the NSC-v2.
Despite these limitations, this study has the advantages. To our knowledge, there is no research regarding the effect of multiple lifestyle-related factors on IHD incidence between sexes according to the status of BMI and FBG. To address this research gap, our study estimated the difference in IHD risk between men and women according to lifestyle-related factors stratified by BMI and FBG levels. Furthermore, we analyzed the dose-response relationship between IHD risk and lifestyle-related risk factors and estimated PARs. Therefore, our results could provide information for developing specific lifestyle guidelines to prevent IHD among Korean men and women according to BMI and FBG levels. Our results can serve as reference for other Asian countries with similar lifestyle patterns and for Western countries with different obesity standards.
Conclusions
The current study shows that the risk of IHD is higher in people with more lifestyle-related risk factors such as mainly vegetable/mainly meat diet, former/current smoking, alcohol consumption ≥3 times/week, and no physical activity and these factors are influenced by BMI and FBG level among Korean adults. The number of lifestyle-related factors and their effect on IHD incidence was more pronounced in women than in men. This suggests that programs to prevent IHD should consider lifestyle and sex along with BMI and FBG levels. Further research is necessary to investigate the effects of other metabolic indices and lifestyle-related risk factors on the risk of IHD.
Acknowledgments
We would like to thank Dr. Byung Chul Ahn for statistical advice and proofreading of the manuscript.
Data Availability
The National Sample Cohort is a third party data provided by the Korean National Health Insurance Sharing Service (KNHISS) upon request to the review committee at KNHISS. Any researchers can request access to the data at the website (https://nhiss.nhis.or.kr/bd/ab/bdaba000eng.do). All researchers should agree to follow the research ethics and complete the application process. Data cannot be shared publicly because of confidentiality. After receiving approval from the review committee, the researchers need to pay fee for data according to period of use. Then the researchers are allowed to access the database via encoded remote access server during the period of use. The authors did not have any special access or privileges during the application process.
Funding Statement
This research was supported by Support Program for Women in Science, Engineering and Technology through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (No. 2016H1C3A1903202).
References
- 1.World Health Organization. WHO fact sheet: Cardiovascular diseases (CVDs) 2017 [cited 5 Apr 2018]. Available from: http://www.who.int/mediacentre/factsheets/fs317/en/.
- 2.Sekikawa A, Kuller L, Ueshima H, Park J, Suh I, Jee S, et al. Coronary heart disease mortality trends in men in the post World War II birth cohorts aged 35–44 in Japan, South Korea and Taiwan compared with the United States. Int J Epidemiol. 1999;28(6):1044–1049. [DOI] [PubMed] [Google Scholar]
- 3.Lee SW, Kim HC, Lee HS, Suh I. Thirty-year trends in mortality from cardiovascular diseases in Korea. Korean Circ J. 2015;45(3):202–209. 10.4070/kcj.2015.45.3.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Statistics Korea. Causes of Death Statistic in 2016 [cited 10 Apr 2018]. Available from: http://kostat.go.kr/portal/eng/pressReleases/8/10/index.board?bmode=read&bSeq=&aSeq=363695&pageNo=1&rowNum=10&navCount=10&currPg=&sTarget=title&sTxt=
- 5.Castelli WP. Epidemiology of coronary heart disease: the Framingham study. Am J Med. 1984;76(2):4–12. [DOI] [PubMed] [Google Scholar]
- 6.Cobble M. Coronary heart disease in women. J Fam Pract. 2014;63(2):S9–S9. [PubMed] [Google Scholar]
- 7.Khamis RY, Ammari T, Mikhail GW. Gender differences in coronary heart disease. Heart. 2016;102(14):1142–1149. 10.1136/heartjnl-2014-306463 [DOI] [PubMed] [Google Scholar]
- 8.Maas AHEM, van der Schouw YT, Regitz-Zagrosek V, Swahn E, Appelman YE, Pasterkamp G, et al. Red alert for women's heart: the urgent need for more research and knowledge on cardiovascular disease in women: proceedings of the workshop held in Brussels on gender differences in cardiovascular disease, 29 September 2010. Eur Heart J. 2011;32(11):1362–1368. 10.1093/eurheartj/ehr048 [DOI] [PubMed] [Google Scholar]
- 9.Jensen MK, Chiuve SE, Rimm EB, Dethlefsen C, Tjønneland A, Joensen AM, et al. Obesity, behavioral lifestyle factors, and risk of acute coronary events. Circulation. 2008;117(24):3062–3069. 10.1161/CIRCULATIONAHA.107.759951 [DOI] [PubMed] [Google Scholar]
- 10.Wilson PW D'agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162(16):1867–1872. [DOI] [PubMed] [Google Scholar]
- 11.Yang HK, Han K, Kwon H-S, Park Y-M, Cho J-H, Yoon K-H, et al. Obesity, metabolic health, and mortality in adults: a nationwide population-based study in Korea. Sci Rep. 2016;6:30329 10.1038/srep30329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadota A, Hozawa A, Okamura T, Kadowaki T, Nakmaura K, Murakami Y, et al. Relationship between metabolic risk factor clustering and cardiovascular mortality stratified by high blood glucose and obesity: NIPPON DATA90, 1990–2000. Diabetes Care. 2007;30(6):1533–1538. 10.2337/dc06-2074 [DOI] [PubMed] [Google Scholar]
- 13.Xu T, Liu W, Cai X, Ding J, Tang H, Huang Y, et al. Risk of coronary heart disease in different criterion of impaired fasting glucose: a meta-analysis. Medicine. 2015;94(40):e1740 10.1097/MD.0000000000001740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiuve SE, McCullough ML, Sacks FM, Rimm EB. Healthy lifestyle factors in the primary prevention of coronary heart disease among men: benefits among users and nonusers of lipid-lowering and antihypertensive medications. Circulation. 2006;114(2):160–167. 10.1161/CIRCULATIONAHA.106.621417 [DOI] [PubMed] [Google Scholar]
- 15.Gregg EW, Cheng YJ, Cadwell BL, Imperatore G, Williams DE, Flegal KM, et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA. 2005;293(15):1868–1874. 10.1001/jama.293.15.1868 [DOI] [PubMed] [Google Scholar]
- 16.Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ. 2011;342:d636 10.1136/bmj.d636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169(7):659–669. 10.1001/archinternmed.2009.38 [DOI] [PubMed] [Google Scholar]
- 18.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671 10.1136/bmj.d671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinstein AR, Sesso HD, Lee I, et al. The joint effects of physical activity and body mass index on coronary heart disease risk in women. Arch Intern Med. 2008;168(8):884–890. 10.1001/archinte.168.8.884 [DOI] [PubMed] [Google Scholar]
- 20.Lindström J, Peltonen M, Eriksson J, Ilanne-Parikka P, Aunola S, Keinänen-Kiukaanniemi S, et al. Improved lifestyle and decreased diabetes risk over 13 years: long-term follow-up of the randomised Finnish Diabetes Prevention Study (DPS). Diabetologia. 2013;56(2):284–293. 10.1007/s00125-012-2752-5 [DOI] [PubMed] [Google Scholar]
- 21.Yusuf S, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. 10.1016/S0140-6736(04)17018-9 [DOI] [PubMed] [Google Scholar]
- 22.Tolstrup J, Jensen MK, Anne T, Overvad K, Mukamal KJ, Grønbæk M. Prospective study of alcohol drinking patterns and coronary heart disease in women and men. BMJ. 2006;332(7552):1244 10.1136/bmj.38831.503113.7C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villareal DT, Miller BV III, Banks M, Fontana L, Sinacore DR, Klein S. Effect of lifestyle intervention on metabolic coronary heart disease risk factors in obese older adults. Am J Clin Nutr. 2006;84(6):1317–1323. 10.1093/ajcn/84.6.1317 [DOI] [PubMed] [Google Scholar]
- 24.Perreault L, Ma Y, Dagogo-Jack S, Horton E, Marrero D, Crandall J, et al. Sex differences in diabetes risk and the effect of intensive lifestyle modification in the Diabetes Prevention Program. Diabetes Care. 2008;31(7):1416–1421. 10.2337/dc07-2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yusuf S, Hawken S, Ôunpuu S, Bautista L, Franzosi MG, Commerford P, et al. Obesity and the risk of myocardial infarction in 27 000 participants from 52 countries: a case-control study. Lancet. 2005;366(9497):1640–1649. 10.1016/S0140-6736(05)67663-5 [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia; 2000. [Google Scholar]
- 27.D'agostino RB, Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286(2):180–187. [DOI] [PubMed] [Google Scholar]
- 28.Forouhi NG, Sattar N, Tillin T, McKeigue PM, Chaturvedi N. Do known risk factors explain the higher coronary heart disease mortality in South Asian compared with European men? Prospective follow-up of the Southall and Brent studies, UK. Diabetologia. 2006;49(11):2580–2588. 10.1007/s00125-006-0393-2 [DOI] [PubMed] [Google Scholar]
- 29.Lee J, Lee JS, Park S-H, Shin SA, Kim K. Cohort profile: the national health insurance service–national sample cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2016;46(2):e15–e15. [DOI] [PubMed] [Google Scholar]
- 30.Seong SC, Kim YY, Khang YH, Park JH, Kang HJ, Lee H, et al. Data Resource Profile: The National Health Information Database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017;46(3):799–800. 10.1093/ije/dyw253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Health Insurance Sharing Service Hompage [cited 22 June 2018]. Available from: https://nhiss.nhis.or.kr/bd/ab/bdaba022eng.do.
- 32.World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision 2016 [cited 5 Jan 2018]. Available from: http://apps.who.int/classifications/icd10/browse/2016/en
- 33.Clinical and Laboratory Standards Institute. Defining, establishing, and verifying reference intervals in the clinical laboratory. C28-A3. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- 34.Alberti K, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120(16):1640–1645. 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 35.Lee JK, Park BJ, Yoo KY, Ahn YO. Dietary factors and stomach cancer: a case-control study in Korea. Int J Epidemiol. 1995;24(1):33–41. [DOI] [PubMed] [Google Scholar]
- 36.Kim MK, Ko MJ, Han JT. Alcohol consumption and mortality from all-cause and cancers among 1.34 million Koreans: the results from the Korea national health insurance corporation’s health examinee cohort in 2000. Cancer Causes Control. 2010;21(12):2295–2302. 10.1007/s10552-010-9656-9 [DOI] [PubMed] [Google Scholar]
- 37.Benichou J. A review of adjusted estimators of attributable risk. Stat Methods Med Res. 2001;10(3):195–216. 10.1177/096228020101000303 [DOI] [PubMed] [Google Scholar]
- 38.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239–241. [Google Scholar]
- 39.Klein JP MM (2005) Survival Analysis: Techniques for Censored and Truncated Data. New York, NY. [Google Scholar]
- 40.Choi S, Kim K, Kim SM, Lee G, Jeong S-M, Park SY, et al. Association of obesity or weight change with coronary heart disease among young adults in South Korea. JAMA Intern Med. 2018;178(8):1060–1068. 10.1001/jamainternmed.2018.2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332(7533):73–78. 10.1136/bmj.38678.389583.7C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ko DT, Wijeysundera HC, Udell JA, Vaccarino V, Austin PC, Guo H, et al. Traditional cardiovascular risk factors and the presence of obstructive coronary artery disease in men and women. Can J Cardiol. 2014;30(7):820–826. 10.1016/j.cjca.2014.04.032 [DOI] [PubMed] [Google Scholar]
- 43.Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57(8):1542–1551. 10.1007/s00125-014-3260-6 [DOI] [PubMed] [Google Scholar]
- 44.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. New Engl J Med. 2000;343(1):16–22. 10.1056/NEJM200007063430103 [DOI] [PubMed] [Google Scholar]
- 45.Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, et al. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med. 2012;172(7):555–563. 10.1001/archinternmed.2011.2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song Y, Joung H. A traditional Korean dietary pattern and metabolic syndrome abnormalities. Nutr Metab Cardiovasc Dis. 2012;22(5):456–462. 10.1016/j.numecd.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 47.Wardle J, Haase AM, Steptoe A, Nillapun M, Jonwutiwes K, Bellisie F. Gender differences in food choice: the contribution of health beliefs and dieting. Ann Behav Med. 2004;27(2):107–116. 10.1207/s15324796abm2702_5 [DOI] [PubMed] [Google Scholar]
- 48.Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet. 2011;378(9799):1297–1305. 10.1016/S0140-6736(11)60781-2 [DOI] [PubMed] [Google Scholar]
- 49.Campbell SC, Moffatt RJ, Stamford BA. Smoking and smoking cessation—The relationship between cardiovascular disease and lipoprotein metabolism: A review. Atherosclerosis. 2008;201(2):225–235. 10.1016/j.atherosclerosis.2008.04.046 [DOI] [PubMed] [Google Scholar]
- 50.Cullen P, Schulte H, Assmann G. Smoking, lipoproteins and coronary heart disease risk: Data from the Münster Heart Study (PROCAM). Eur Heart J. 1998;19(11):1632–1641. [DOI] [PubMed] [Google Scholar]
- 51.Woo HD, Shin A, Kim J. Dietary patterns of Korean adults and the prevalence of metabolic syndrome: a cross-sectional study. PLoS One. 2014;9(11):e111593 10.1371/journal.pone.0111593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sattelmair J, Pertman J, Ding EL, Kohl HW, Haskell W, Lee I-M. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation. 2011;124(7):789–795. 10.1161/CIRCULATIONAHA.110.010710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noda H, Iso H, Saito I, Konishi M, Inoue M, Tsugane S. The impact of the metabolic syndrome and its components on the incidence of ischemic heart disease and stroke: the Japan public health center-based study. Hypertens Res. 2009;32(4):289–298. 10.1038/hr.2009.14 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The National Sample Cohort is a third party data provided by the Korean National Health Insurance Sharing Service (KNHISS) upon request to the review committee at KNHISS. Any researchers can request access to the data at the website (https://nhiss.nhis.or.kr/bd/ab/bdaba000eng.do). All researchers should agree to follow the research ethics and complete the application process. Data cannot be shared publicly because of confidentiality. After receiving approval from the review committee, the researchers need to pay fee for data according to period of use. Then the researchers are allowed to access the database via encoded remote access server during the period of use. The authors did not have any special access or privileges during the application process.