Abstract
Study Objectives
The Accreditation Council for Graduate Medical Education (ACGME) recently reinstated extended-duration (24–28 hr) work shifts (EDWS) for postgraduate year 1 (PGY-1) resident physicians. This study examined the relationship between overnight sleep duration during EDWS and subsequent “post-call” performance in PGY-1 resident physicians.
Methods
Thirty-four PGY-1 resident physicians (23 males; 24–32 years) were studied between 2002 and 2004 during 3-week Q3 “on-call” rotation schedules in the Medical and Cardiac Intensive Care Units at Brigham and Women’s Hospital in Boston. Daily sleep logs (validated by ambulatory polysomnography) were collected and the 10 min psychomotor vigilance task (PVT) was administered every ~6 hr during each EDWS. Generalized estimating equations were used to examine the relationship between overnight sleep duration and PVT performance “post-call” (0500–1900 hr). Postcall performance during EDWS was compared with sessions matched for time-of-day and weeks-into-schedule in the same resident physician during an intervention schedule that eliminated EDWS.
Results
Resident physicians obtained an average of 1.6 ± 1.5 hr cumulative sleep overnight during EDWS (<4 hr on 92% of nights). PVT attentional failures were significantly reduced only after >4 hr sleep (p = 0.027 versus no sleep). Despite this apparent improvement, the odds of incurring >1 attentional failure were 2.72 times higher during postcall following >4 hr sleep compared with matched sessions during non-EDWS.
Conclusions
Even with >4 hr sleep overnight (8% of EDWS), performance remained significantly impaired. These findings suggest that even “strategic napping,” a recommendation recently removed from ACGME guidelines, is insufficient to mitigate severe performance impairment introduced by extending duty beyond 16 hr.
Keywords: sleep deprivation, resident physicians, patient care, medical errors, medical training
Statement of Significance.
The present study examined the amount of overnight spontaneous sleep that postgraduate year 1 resident physicians obtained during extended-duration work shifts and its impact on next-day performance. Resident physicians obtained less than 4 hr of spontaneous overnight sleep on 92% of extended-duration work shifts, an amount insufficient to prevent serious neurobehavioral performance impairment. Even resident physicians who obtained more than 4 hr of sleep overnight during extended-duration work shifts remained significantly impaired compared with their performance during an intervention schedule with no extended-duration work shifts. These findings have important implications for the development of public policies related to the health and safety of resident physicians and their patients.
Introduction
The Accreditation Council for Graduate Medical Education (ACGME) in the United States recently increased the permitted shift duration for postgraduate year 1 (PGY-1) resident physicians, reinstating extended-duration (24–28 hr) work shifts (EDWS) [1]. These changes were made in part due to a recent cluster-randomized noninferiority trial that reported noninferior patient outcomes and no significant difference in resident well-being and quality of educational training between resident physicians in programs subject to the then-standard work policy (≤16 hr for PGY 1 resident physicians, ≤28 hr for PGY 2–5 resident physicians) and those who adhered to a flexible policy that included EDWS [2], despite previous findings to the contrary [3]. In addition, without explanation, the ACGME removed their prior recommendation for strategic napping during EDWS, the intent of which was to mitigate the negative impact of long continuous duty hours on performance. Given the recent increase in permitted shift duration, we revisited existing data from resident physicians studied under the recently reinstated traditional Q3 schedule to examine the amount of spontaneous sleep obtained overnight during EDWS and its subsequent impact on postcall performance [4].
Methods
The original study was approved by the human research committee of Partners Healthcare and Brigham and Women’s Hospital (2001P000814), and all participants provided written informed consent. Thirty-four PGY-1 resident physicians (23 males; mean ± standard deviation 28.0 ± 1.83 years old) were studied for 3 weeks on a Q3 schedule (24–30 hr on-call EDWS every other shift, as previously described) between 2002 and 2004 [5]. Residents completed daily sleep and work logs (validated by ambulatory polysomnography 3–4 days per week) [4] and performed a 10 min psychomotor vigilance task (PVT) every ~6 hr during each EDWS [5]. Here we examined PVT attentional failures (count of reaction times > 500 ms, normalized by the Freeman–Tukey transform ) from 295 sessions collected during the second day of each EDWS (500–1900 hr, “post-call”) relative to the overnight (2300–700 hr) cumulative spontaneous sleep duration (binned into 0, >0–1, >1–2, >2–3, >3–4, and >4 hr; Table 1) during the EDWS using a generalized estimating equation (PROC GENMOD, SAS 9.3, Cary, NC). Time of day of PVT administration (early postcall: 500–830 hr versus late postcall: 1000–1900 hr) and study week (week 1, EDWS blocks 1–2; week 2, EDWS blocks 3–4; and week 3, EDWS blocks 5–6) were included as covariates to control for circadian phase and chronic sleep restriction, respectively. p < 0.05 was considered statistically significant.
Table 1.
Odds ratio (95% CI) of attentional failures in PGY-1 resident physicians during Q3 compared with sessions matched for resident, time of day, and time into study during the intervention schedule
| PVT (N)† | Sleep range (hr) | Sleep duration (mean ± SD) | Lapses (mean ± SD) | Odds ratio (95% CI) | ||
|---|---|---|---|---|---|---|
| >1 lapse‡ | >5 lapses | >10 lapses | ||||
| 81 (24) | 0.00–0.00 | 0.00 ± 0.00 | 8.10 ± 9.56 | 3.92 (2.25, 6.84) | 2.80 (1.69, 4.63) | 3.38 (1.84, 6.21) |
| 45 (19) | 0.05–1.00 | 0.74 ± 0.27 | 6.73 ± 8.67 | 1.47 (0.78, 2.76) | 1.73 (0.91, 3.31) | 3.46 (1.64, 7.28) |
| 64 (24) | 1.08–2.00 | 1.60 ± 0.24 | 5.83 ± 7.79 | 2.14 (1.23, 3.74) | 2.02 (1.16, 3.52) | 1.58 (0.73, 3.41) |
| 46 (19) | 2.03–3.00 | 2.53 ± 0.29 | 6.24 ± 7.92 | 2.00 (1.06, 3.78) | 1.83 (0.96, 3.47) | 3.01 (1.41, 6.40) |
| 31 (13) | 3.02–3.96 | 3.43 ± 0.23 | 10.29 ± 11.23 | 2.34 (1.08, 5.06) | 3.60 (1.69, 7.67) | 6.15 (2.75, 13.75) |
| 28 (10) | 4.15–6.33 | 5.04 ± 0.66 | 4.79 ± 5.31 | 2.72 (1.19, 6.20) | 1.68 (0.76, 3.74) | 1.42 (0.46, 4.36) |
†PVT indicates the number of psychomotor vigilance task sessions included in each sleep bin; N indicates the number of resident physicians represented by those PVT sessions.
‡Attentional failure (lapse) cutoffs are based on the median (1 lapse), 75th percentile (5 lapses), and 90th percentile (10 lapses) of all matched sessions during the intervention.
Results
In 202 EDWS included in our analysis, PGY-1 resident physicians obtained 1.6 ± 1.5 hr (mean ± standard deviation) of sleep overnight; total sleep time overnight was <4 hr on 92% of nights. Across 295 PVT sessions, attentional failures were significantly reduced (p = 0.027) following >4 hr of sleep only (all bins referenced to 0 hr of sleep, Figure 1). Attentional failures were higher in study weeks 2 and 3 (p = 0.0008 and p = 0.0006, respectively, referenced to study week 1), as observed previously [5], but no interaction between sleep duration and study week on attentional failures was observed. No significant effect of test time postcall (early postcall versus late postcall) was observed.
Figure 1.
Attentional failures (number of lapses, normalized by ) from all 295 sessions of the PVT included in the current analysis are plotted by sleep duration bin. Individual points within a bin have been offset to show overlapping points. The median and interquartile range (boxplots) and mean (open squares) also are plotted for each sleep duration bin.
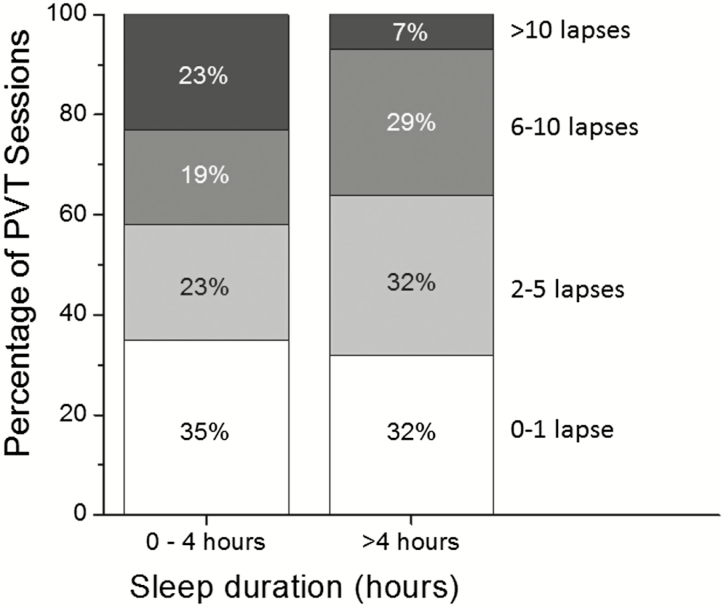
Obtaining >4 hr of sleep overnight during EDWS provides only partial recovery. Although more than 10 attentional failures were observed in only 7% of PVT sessions following >4 hr of sleep overnight (compared with 23% following 0–4 hr of sleep), more than 1 attentional failure was observed in 68% of sessions following >4 hr of sleep (Figure 2). To further examine the impact of EDWS on postcall performance, we compared these 295 PVT sessions with PVT sessions matched for time of day and study week in the same resident physicians during an intervention schedule, which eliminated EDWS, limited continuous duty to no longer than 16 hr per work shift, and allowed resident physicians to sleep at home [4, 6]. Overnight sleep duration prior to these matched PVT sessions was 6.92 ± 1.37 hr (range 3.92–12.77 hr). The odds of incurring more than 1 attentional failure (the median number of attentional failures observed during the non-EDWS sessions) was 2.40 times higher (95% CI: 1.72–3.34) overall (i.e. across all spontaneous overnight sleep durations) on postcall PVT sessions compared with matched sessions completed during non-EDWS, and 2.72 times higher (95% CI: 1.19–6.20) among PGY-1 resident physicians who obtained >4 hr of spontaneous sleep overnight during EDWS (Table 1).
Figure 2.
Stacked bar chart comparing the percentage of PVT sessions following 0–4 hr of spontaneous sleep and >4 hr of sleep in which the number of attentional failures fell between the designated thresholds among PGY-1 resident physicians. White bars: at or below 1 lapses; light gray bars: between 2 and 5 lapses; dark gray bars: between 6 and 10 lapses; black bars: >10 lapses.
Discussion
More than 4 hr of actual sleep was required to decrease significantly the number of attentional failures observed during the second half of an EDWS (“post-call”) when compared with performance following no sleep overnight among PGY-1 resident physicians. Sleeping 4 or fewer hours overnight yielded no significant improvement on any performance measure compared with zero sleep. PGY-1 resident physicians in this study were not provided with protected time for sleep during EDWS. In practice, it is unusual for resident physicians to obtain more than 4 hr of sleep overnight; in our data set, it only occurred in 8% of the EDWS analyzed. In 92% of the EDWS analyzed, resident physicians obtained 4 or fewer hours of total sleep time overnight, including 28% during which no sleep was obtained. Two previous studies of protected sleep time during EDWS have reported only moderate increases in overnight sleep duration compared with no protected time (average increase of 0.43 to 1.00 hr), and in no cases was the average sleep duration more than 4 hr (2.33 to 3.04 hr sleep) [7, 8]. Furthermore, at least one study has reported that 4 hr of protected sleep time led to a slight reduction (of 0.20 hr) in sleep duration [9]. Taken together, these data suggest a net benefit of approximately 10 more min of sleep for each hour of protected time, a proportion much lower than when work hours are reduced to increase resident sleep, where nearly a third (30%) of the extra time available was used for sleep [4].
In the current study, resident physicians remained substantially impaired even after >4 hr of sleep; 50% of PVT sessions within this sleep duration bin had >3.21 attentional failures, a threshold that represents the average number of attentional failures observed among these same resident physicians during an intervention schedule (Figure 1). Thus, despite observing improvements after overnight sleep in this and other studies [7, 8, 10], this improvement is insufficient to restore performance postcall, which remains significantly suboptimal. Importantly, in our recent multicenter cluster-randomized crossover clinical trial of resident physicians (PGY-2 and PGY-3) at six U.S. pediatric intensive care units, we found a significant correlation between attentional failures and resident-physician-related serious medical errors, as assessed from observation and retrospective chart review [11]. Together these findings suggest that the risk of serious medical errors remains high postcall even when residents obtain >4 hr of sleep overnight.
A strength of the study is that it examined spontaneous behavior of resident physicians while working in intensive care units, and therefore, it has high operational validity. It did not examine the role of protected time for sleep overnight, as these policies are difficult to schedule, can affect continuity of care, and have minimal effect on sleep, as outlined above. This approach, however, led to an unequal distribution of data: each resident physician contributed multiple PVT sessions to one or more—but possibly not all—sleep bins. Despite this, an additional strength of the design is that all PVT sessions were matched for time of day and study week in the same resident physician during an intervention schedule, in which no EDWS was scheduled, to compare the relative levels of PVT performance impairment in a within-subject design. EDWS have been shown to reduce clinical performance substantially [12], and both acute and chronic sleep lead to performance equivalent to that while legally drunk [13–15]. In general, we are aware of only a few studies that have shown direct correlations between PVT attentional failures and other operationally relevant outcomes, such as on-the-road driving performance [16], simulated driving [17], and azimuth deviations on an Air Refueling Part Task Trainer [18]. As a sensitive measure of sustained or vigilant attention, the PVT findings from this study suggest that resident physicians working EDWS have an increased risk of committing errors related to impaired attention, a finding that is consistent with a parallel increase in medical error rates and worsening of another objective measure of attention derived from electrooculogram recordings in the same resident physicians on the traditional Q3 schedule compared with the intervention schedule [6].
Our findings are supportive of a growing body of data showing that, even if sleep is obtained, the underlying levels of acute and chronic sleep deprivation make sleep considerably less effective than previously thought in recovering performance [5, 19, 20]. In fact, our recent work indicates that repeated bouts of sleep loss and recovery impair performance more substantially than a shorter average duration of sleep scheduled equally each night [[19, 20]. Although such variable schedules are sanctioned by the ACGME, there are insufficient data on the time course of recovery from such extreme sleep deficiency, and even the dynamics of relatively minor challenges are dramatically underestimated by nearly all current models of sleep–wake regulation [19]. It is not known how sleep and wake are regulated under such extreme conditions. Relying on subjective sleepiness to assess fitness for duty is not tenable [7], as self-ratings of sleepiness are notoriously inconsistent with objective performance measures under conditions of chronic sleep loss [19, 20].
Despite modest improvements in PVT performance and ratings of sleepiness, protected sleep time does not translate into improvements in patient outcomes [7, 8]. Conversely, multiple studies have shown that eliminating EDWS and reducing continuous duty to 16 or fewer hours significantly improve both patient care and resident quality of life, including emotional exhaustion and burnout, and reduce motor vehicle crashes, medical errors, and percutaneous injuries [3, 21, 22]. Our findings demonstrate that PGY-1 resident physicians require more than 4 hr of sleep during overnight on-call shifts to improve next-day PVT performance and, even if they achieve this amount on rare occasions, they remain significantly impaired, providing important evidence for guiding the development of public policies related to the health and safety of resident physicians and their patients.
Funding
The present study was supported by a grant from the National Heart, Lung and Blood Institute (4U01 HL111478). The original study was supported by a grant (R01 HS12032) from the Agency for Healthcare Research and Quality (AHRQ), affording data-confidentiality protection by federal statute (Public Health Service Act 42 U.S.C.); a grant from the National Institute of Occupational Safety and Health (R01 OH07567), which provided a Certificate of Confidentiality for data protection; the Department of Medicine, Brigham and Women’s Hospital; the Division of Sleep Medicine, Harvard Medical School; Brigham and Women’s Hospital; and a General Clinical Research Center grant (M01 RR02635) from the National Center for Research Resources; a traveling fellowship from the Wellcome Trust, United Kingdom (060018/B/99/Z) awarded to Dr. Steven W. Lockley, PhD; an AHRQ career development award (K08 HS13333) to Dr. Christopher P. Landrigan, MD, MPH; an AHRQ National Research Service Award (F32 HS14130) awarded to Dr. John W. Cronin, MD; and a National Heart, Lung, and Blood Institute fellowship in the program of training in Sleep, Circadian, and Respiratory Neurobiology at Brigham and Women’s Hospital (T32 HL079010); Dr. Charles A. Czeisler, PhD, MD, was supported in part by the National Space Biomedical Research Institute, through NASA (NCC 9-58).
Acknowledgments
We acknowledge the Biostatistics Core of the Brigham and Women’s Hospital Center for Clinical Investigation and Bernard Rosner, PhD, for his consultation on the statistical methods employed in the present analysis. We are indebted to the original volunteers, without whom this project could not have been conducted; to the staff of the Coronary Care Unit and Medical Intensive Care Unit, whose cooperation was also vitally important; to the co-authors of the original study, John W. Cronin, MD, Christopher P. Landrigan, MD, MPH., Clark J. Lee, AB, Jeffrey M. Rothschild, MD, MPH, Joel T. Katz, MD, Craig M. Lilly, MD, Peter H. Stone, MD, and Daniel Aeschbach, PhD; to those who helped with the design and complex scheduling of the study, Laura K. Barger, PhD, MPH, DeWitt C. Baldwin, MD, Michael Klompas, MD, Marisa A. Rogers, MD, Jane S. Sillman, MD, Heather L. Gornik, MD, Rainu Kaushal, MD, Orfeu M. Buxton, PhD, and the administrative staff of the Internal Medicine Residency Program; to the Division of Sleep Medicine (DSM) technicians Cathryn Berni, Josephine Golding, Mia Jacobsen, Lynette James, and Marina Tsaoussoglou for their dedication and diligence; to Claude Gronfier, PhD, and the DSM Sleep Core, particularly Alex Cutler, Gregory T. Renchkovsky, and Brandon J. Lockyer, RPSGT., and the DSM Director of Bioinformatics, Joseph M. Ronda, MS, for their expert support; and to Victor J. Dzau, MD, Anthony D. Whittemore, MD, Jeffrey Otten, Matthew Van Vranken, Gary L. Gottlieb, MD, MBA, and Joseph B. Martin, MD, PhD, for their support and encouragement of this work.
Disclosure Statement: All authors report that they have no direct conflicts of interest with this study. In the interest of full disclosure, however, we report the following relationships in the past 3 years: M.A.S.H. has provided paid limited consulting for The MathWorks, Inc. and serves as an adjunct statistics instructor at Merrimack College. C.A. has received a research award/prize from Sanofi-Aventis; contract research support from VicRoads, Rio Tinto Coal Australia, National Transport Commission, Tontine/Pacific Brands; and lecturing fees from Brown Medical School/Rhode Island Hospital, Ausmed, Healthmed, and TEVA Pharmaceuticals; and reimbursements for conference travel expenses from Philips Healthcare. In addition, she has served as a consultant through her institution to the Rail, Bus and Tram Union, the Transport Accident Commission (TAC) and the National Transportation Committee (NTC). She has also served as an expert witness and/or consultant in relation to fatigue and drowsy driving. C.A. is a Theme Leader in the Cooperative Research Centre for Alertness, Safety and Productivity. C.A.C. has received education/research support to BWH from from Cephalon Inc., Mary Ann & Stanley Snider via Combined Jewish Philanthropies, National Football League Charities, Optum, Philips Respironics, Inc., ResMed Foundation, San Francisco Bar Pilots, Schneider Inc., Sysco, Cephalon, Inc., Jazz Pharmaceuticals, Takeda Pharmaceuticals, Teva Pharmaceuticals Industries, Ltd., Sanofi-Aventis, Inc., Sepracor, Inc., and Wake Up Narcolepsy. C.A.C. has received consulting fees and/or served as a paid member of scientific advisory board for Bose Corporation, Boston Celtics, Boston Red Sox, Columbia River Bar Pilots, Institute of Digital Media and Child Development, Klarman Family Foundation, Samsung Electronics, Quest Diagnostics, Inc., Vanda Pharmaceuticals, Zurich Insurance Company, Ltd., Purdue Pharma, LP, McGraw Hill, Houghton Mifflin Harcourt/Penguin, Koninklijke Philips Electronics, N.V., Cephalon, Inc., Washington State Board of Pilotage Commissioners, and Ganésco Inc. C.A.C. owns equity interest in Vanda Pharmaceuticals. C.A.C. holds a number of process patents in the field of sleep/circadian rhythms (e.g. photic resetting of the human circadian pacemaker). These are available from BWH upon request. C.A.C. has also served as an expert on various legal and technical cases related to sleep and/or circadian rhythms including those involving the following commercial entities: Complete General Construction Company, FedEx, Greyhound, HG Energy LLC, South Carolina Central Railroad Co., Steel Warehouse Inc., Stric-Lan Companies LLC, Texas Premier Resource LLC, and United Parcel Service (UPS). C.A.C.’s interests were reviewed and managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies. S.W.L. has received consulting fees from the Atlanta Falcons, Atlanta Hawks, Consumer Sleep Solutions, Noble Insights, OpTerra Energy Services Inc., Pegasus Capital Advisors LP, Serrado Capital, Slingshot Insights, and Team C Racing and has current consulting contracts with Akili Interactive, Apex 2100 Ltd., Delos Living LLC, Headwaters Inc., Hintsa Performance AG, Light Cognitive, Lighting Science Group Corporation, Mental Workout, PlanLED, Six Senses, and Wyle Integrated Science and Engineering. S.W.L. has received unrestricted equipment gifts from Biological Illuminations LLC, Bionetics Corporation, and F.LUX Software LLC; has equity in iSLEEP, Pty; advance author payment and/or royalties from Oxford University Press; honoraria plus travel, accommodation and/or meals for invited seminars, conference presentations or teaching from BHP Billiton, Estee Lauder, Informa Exhibitions (USGBC), and Teague; travel, accommodation and/or meals only (no honoraria) for invited seminars, conference presentations or teaching from Lightfair, USGBC, DIN and SLTBR. S.W.L. has completed investigator-initiated research grants from Biological Illumination LLC and has an ongoing investigator initiated grant from F. Lux Software LLC; S.W.L. holds a process patent for “Systems and methods for determining and/or controlling sleep quality,” which is assigned to the Brigham and Women’s Hospital per Hospital policy. S.W.L. has also served as a paid expert on behalf of several public bodies on arbitrations related to work hours and legal proceedings related to light and health. S.W.L. is also a Program Leader for the CRC for Alertness, Safety and Productivity, Australia.
References
- 1. Accreditation Council for Graduate Medical Education. Common Program Requirements. July 1, 2017. [Google Scholar]
- 2. Bilimoria KY, et al. National cluster-randomized trial of duty-hour flexibility in surgical training. N Engl J Med. 2016;374(8):713–727. [DOI] [PubMed] [Google Scholar]
- 3. Levine AC, et al. Effects of reducing or eliminating resident work shifts over 16 hours: a systematic review. Sleep. 2010;33(8):1043–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lockley SW, et al. ; Harvard Work Hours, Health and Safety Group. Effect of reducing interns’ weekly work hours on sleep and attentional failures. N Engl J Med. 2004;351(18):1829–1837. [DOI] [PubMed] [Google Scholar]
- 5. Anderson C, et al. Deterioration of neurobehavioral performance in resident physicians during repeated exposure to extended duration work shifts. Sleep. 2012;35(8):1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Landrigan CP, et al. Effect of reducing interns’ work hours on serious medical errors in intensive care units. N Engl J Med. 2004;351(18):1838–1848. [DOI] [PubMed] [Google Scholar]
- 7. Volpp KG, et al. Effect of a protected sleep period on hours slept during extended overnight in-hospital duty hours among medical interns: a randomized trial. JAMA. 2012;308(21):2208–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shea JA, et al. A randomized trial of a three-hour protected nap period in a medicine training program: sleep, alertness, and patient outcomes. Acad Med. 2014;89(3): 452–459. [DOI] [PubMed] [Google Scholar]
- 9. Richardson GS, et al. Objective assessment of sleep and alertness in medical house staff and the impact of protected time for sleep. Sleep. 1996;19(9):718–726. [PubMed] [Google Scholar]
- 10. Basner M, et al. Sleep and alertness in medical interns and residents: an observational study on the role of extended shifts. Sleep. 2017;40(4):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rahman SA, et al. Attentional failures are correlated with serious medical errors in resident physicians. Sleep. 2019; 42: Abstract Supplement. [Google Scholar]
- 12. Philibert I. Sleep loss and performance in residents and nonphysicians: a meta-analytic examination. Sleep. 2005;28(11):1392–1402. [DOI] [PubMed] [Google Scholar]
- 13. Dawson D, et al. Fatigue, alcohol and performance impairment. Nature. 1997;388(6639):235. [DOI] [PubMed] [Google Scholar]
- 14. Arnedt JT, et al. Neurobehavioral performance of residents after heavy night call vs after alcohol ingestion. JAMA. 2005;294(9):1025–1033. [DOI] [PubMed] [Google Scholar]
- 15. Huizinga CRH, et al. Evaluating fitness to perform in surgical residents after night shifts and alcohol intoxication: the development of a “fit-to-perform” test. J Surg Educ. 2018;75(4):968–977. [DOI] [PubMed] [Google Scholar]
- 16. Jongen S, et al. Sensitivity and validity of psychometric tests for assessing driving impairment: effects of sleep deprivation. PLoS One. 2015;10(2):e0117045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kosmadopoulos A, et al. The efficacy of objective and subjective predictors of driving performance during sleep restriction and circadian misalignment. Accid Anal Prev. 2017;99(Pt B):445–451. [DOI] [PubMed] [Google Scholar]
- 18. Russo MB, et al. Visual perception, psychomotor performance, and complex motor performance during an overnight air refueling simulated flight. Aviat Space Environ Med. 2005;76 (7 Suppl):C92–103. [PubMed] [Google Scholar]
- 19. Van Dongen HP, et al. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2): 117–126. [DOI] [PubMed] [Google Scholar]
- 20. St Hilaire MA, et al. Modeling neurocognitive decline and recovery during repeated cycles of extended sleep and chronic sleep deficiency. Sleep. 2017;40(1) 1-13. doi: 10.1093/sleep/zsw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reed DA, et al. Systematic review: association of shift length, protected sleep time, and night float with patient care, residents’ health, and education. Ann Intern Med. 2010;153(12):829–842. [DOI] [PubMed] [Google Scholar]
- 22. Busireddy KR, et al. Efficacy of interventions to reduce resident physician burnout: a systematic review. J Grad Med Educ. 2017;9(3):294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]