Abstract
Study Objectives
Sleep inertia, subjectively experienced as grogginess felt upon awakening, causes cognitive performance impairments that can require up to 1.5 hr to dissipate. It is unknown, however, how chronic sleep restriction (CSR) influences the magnitude and duration of sleep inertia–related performance deficits.
Methods
Twenty-six healthy participants were enrolled in one of two in-laboratory sleep restriction protocols (one 32 day randomized control and one 38 day protocol) that separated the influence of sleep and circadian effects on performance using different “day”-lengths (20 and 42.85 hr day-lengths, respectively). The sleep opportunity per 24 hr day was the equivalent of 5.6 hr for each CSR condition and 8 hr for the Control condition. Participant’s performance and subjective sleepiness were assessed within ~2 min after electroencephalogram-verified awakening and every 10 min thereafter for 70 min to evaluate performance and subjective sleepiness during sleep inertia.
Results
Performance within 2 min of awakening was ~10% worse in CSR conditions compared with Control and remained impaired across the dissipation of sleep inertia in the CSR conditions when compared with Control. These impairments in performance during sleep inertia occurred after only chronic exposure to sleep restriction and were even worse after awakenings during the biological nighttime. Interestingly, despite differences in objective performance, there were no significant differences between groups in subjective levels of sleepiness during sleep inertia.
Conclusions
CSR worsens sleep inertia, especially for awakenings during the biological night. These findings are important for individuals needing to perform tasks quickly upon awakening, particularly those who obtain less than 6 hr of sleep on a nightly basis.
Clinical Trial
The study “Sleep Duration Required to Restore Performance During Chronic Sleep Restriction” was registered as a clinical trial (#NCT01581125) at clinicaltrials.gov (https://clinicaltrials.gov/ct2/show/NCT01581125?term=NCT01581125.&rank=1).
Keywords: sleep inertia, forced desynchrony, insufficient sleep, circadian, subjective sleepiness
Statement of Significance
The grogginess we experience upon awakening, or sleep inertia, results in substantial impairment in cognitive performance. Although this impairment dissipates over time, it is unknown how chronic insufficient sleep influences sleep inertia. We investigated how chronic sleep restriction, equivalent to that of ~5.6 hr of sleep opportunity per night, affects performance upon awakening compared with the equivalent of ~8 hr of sleep opportunity per night. Cognitive performance in sleep-restricted individuals was worse within 2 min of awakening, remained worse across the dissipation of sleep inertia, was worse during the biological night, and worsened as days of insufficient sleep increased. These findings have implications for all individuals needing to perform tasks quickly after awakening, especially those who do not regularly obtain sufficient sleep.
Introduction
Millions of individuals routinely sleep less than 6 hr per night on work days [1], an amount insufficient for optimal physiological [2] and cognitive functioning. Chronically obtaining insufficient sleep [3–5], the circadian time at which performance is assessed [6], and sleep inertia [7, 8] all cause drastic cognitive performance decrements. Although these performance decrements often do not have severe immediate consequences, in some specific situations this amount of insufficient sleep could have far-reaching implications. For example, over 65% of medical residents, who often need to respond rapidly after awakening, report routinely sleeping less than 6 hr per night [9]. In resident physicians working extended-shift schedules, performance immediately upon awakening plays a role in reducing alertness in on-call performance [10] and has been hypothesized to contribute to increased levels of percutaneous injuries after a nap [11]. In military personnel, abruptly waking from sleep greatly impairs tactical planning [12] and in nonmedical professionals, individuals often must perform tasks shortly after awakening (e.g. driving to work or school, responding to an emergency) that may put them at risk for accidents due to impaired alertness and performance [13]. Additionally, as sleep restriction has been shown to dissociate objective performance from subjective feelings of alertness [3, 14, 15], a mismatch during sleep inertia when cognitive performance is greatly impaired, as described above, could have disastrous consequences. Although there have been numerous reports of acute sleep deprivation (i.e. one extended wake episode) leading to worsened performance during sleep inertia [16–19], the effects of chronic sleep restriction (i.e. multiple nights of insufficient sleep) on objective performance and subjective sleepiness immediately upon awakening, which may affect a larger proportion of people daily, is unknown.
We investigated the impact of acute and chronic sleep restriction (CSR) with sleep inertia (i.e. subjective and objective cognitive deficits experienced immediately after awakening) on human performance and subjective sleepiness using two separate circadian-forced desynchrony protocols (Supplementary Figure S1). In a forced desynchrony protocol, sleep and wakefulness activities are evenly distributed relative to circadian timing by using non-24 hr “days,” enabling performance to be examined at different combinations of time awake and circadian phases [20]. In the current study, the forced desynchrony protocol allowed for examination of performance during sleep inertia throughout CSR not only in the morning hours when day-working individuals would normally awaken, but also during the nighttime hours when shiftworkers or those needing to awaken quickly from sleep to respond to an emergency may awaken. We hypothesized that individuals experiencing CSR (i.e. a 1:3.3 sleep:wake ratio as opposed to a more traditional 1:2 sleep:wake ratio) would have impaired performance immediately upon awakening and across the dissipation of sleep inertia when compared with a Control condition that underwent the same protocol with the traditional sleep:wake ratio.
Methods
Participants
Twenty-six healthy participants (aged 26.5 ± 4.4 years, 14 female) partook in the study procedures; 17 (10 female) in the 20 hr “day” and 9 (4 female) in the 42.85 hr “day” protocol. Participants were deemed medically healthy based on self-report, physical examination by a physician, laboratory testing of metabolic and hematological levels, a clinical interview with a psychologist, and overnight clinical sleep screening. Exclusion criteria consisted of a body mass index <18 or >29.9, history of night-shift work or transmeridian travel <3 months prior to study, self-reported habitual sleep duration <7 or >9 hr averaged across the week, pregnancy, and use of any prescription medication. During the 3 weeks of at-home monitoring and throughout the protocol, participants abstained from any drug or over-the-counter medication use, caffeine, alcohol, nicotine, or other foreign substances as verified via urine toxicology before and at admission to the laboratory. All participants provided written informed consent and all study procedures were approved by the Partners Healthcare Institutional Review Board. All methods were performed in accordance with HIPAA regulations and the Declaration of Helsinki.
Experimental design
For at least 3 weeks prior to the inpatient protocol, participants maintained an approximate 10 hr per night sleep schedule at their self-reported habitual timing that was verified by wrist actigraphy (Actiwatch-L Mini Mitter/Respironics), sleep logs, and call-ins to a time-stamped voicemail recording system immediately prior to going to bed and upon waking.
For the inpatient portion of the study, participants were studied in a sound-attenuated, dimly-lit (<4 lux) suite free from external time cues, and were not informed of the specifics of the protocol (e.g. cycle-length, time, date, and condition). All events were scheduled related to the participant’s habitual timing, as determined from the 3 weeks of home monitoring. Upon admission to the laboratory, participants were taught the Digit Symbol Substitution Task (DSST), a task that measures working memory and processing speed by requiring the participant to match symbols with numbers on a keyboard [21], by a study investigator and instructed to perform the DSST several times with the study investigator to attenuate any learning effects of the DSST. Participants were allowed to practice the DSST as many times as needed. Participants were also instructed how to complete the Karolinska Sleepiness Scale (KSS), a questionnaire asking participants to identify on a 1 (most alert) to 9 (most sleepy) scale how sleepy they feel in that instant [22].
Participants were first scheduled to 3 days of 12 hr overnight sleep opportunities and 4 hr daytime nap opportunities to diminish any potential residual sleep loss upon entering each protocol. During wakefulness of these 3 days, participants were given the DSST several times. The steepest portion of the learning curve for this task was therefore expected to be completed before the data reported here. These “Sleep Satiation” days were followed by three Baseline nights of a 10 hr sleep opportunity at habitual times. After the three Baseline nights, participants were scheduled to sleep and wakefulness on non-24 hr cycles (“forced desynchrony”).
During scheduled wakefulness, participants were allowed to engage in sedentary activities (e.g. read, watch movies, talk, or play board games with a researcher) and wakefulness was verified by continuous monitoring by research staff and continuous polysomnographic (PSG) recordings.
At each scheduled awakening, the participant’s bed was elevated to a semirecumbent posture; performance on the DSST and subjective sleepiness from the KSS began within 2 min and continued every 10 min thereafter for 1.5 hr. Only tests occurring after awakening from PSG recorded sleep at scheduled awakening were used for these analyses (Supplementary Table S1).
On the first day of the inpatient portion of the protocol, participants in the 20 hr “day” protocol were randomized (Parallel Assignment, separately for each sex) to condition by the study investigator randomly selecting either Control or CSR conditions blindly from a concealed envelope (Supplementary Figure S2); for the 42.85 hr “day” protocol, all participants were scheduled to a CSR condition [5]. Under 20 hr “day” Control conditions, all behaviors and activities were scheduled to be similar to the CSR conditions with the exception that participants were provided the equivalent of 8 hr scheduled sleep per 24 hr (twenty-four 20 hr cycles, 6.67 hr sleep:13.33 hr wake, n = 8). Under CSR conditions, they were restricted to the equivalent of only 5.6 hr scheduled sleep per 24 hr [CSRShort (twenty-four 20 hr cycles, 4.67 hr sleep:15.33 hr wake, n = 9) [15] and CSRLong (twelve 42.85 hr cycles, 10 hr sleep:32.85 hr wake, n = 9)] (Supplementary Figure S1) [5]. Use of a Control condition and these two protocols enabled quantification of the influence of CSR on sleep inertia performance independent of both other behaviors and of the length of the immediately preceding sleep and wakefulness episodes (Supplementary Table S2).
Statistical analysis
Nonorthogonal spectral analysis of hourly serum melatonin from the forced desynchrony portion of each protocol was used to estimate intrinsic circadian period and circadian phase for each individual [20]. Circadian phase was not available for two participants due to blood drawing complications: one in each of the Control and CSRshort conditions. Thus, these two individuals were excluded from analyses related to circadian day and night.
Baseline values during the three habitual baseline days before the forced desynchrony condition and postsleep inertia values during the forced desynchrony condition were calculated using tests occurring 1.5–14 hr after awakening to account for differing maximum wakefulness lengths between conditions. Thus, a total of 6 tests per day were used in analysis for each condition. Independent t-tests were used to determine differences in a priori planned comparisons in performance immediately upon awakening and postsleep inertia (>120 min after awakening). As we did not have specific a priori hypotheses for differences at each time point across the dissipation of sleep inertia, we did not perform separate comparisons for each individual time point. To compare performance and subjective sleepiness across the dissipation of sleep inertia (10–70 min after awakening), the number of correct responses during a 2 min DSST and choice on the KSS was analyzed using mixed-effects models with condition, time from awakening, and their interaction as fixed factors and participant as a random factor to account for interparticipant differences. SAS 9.4 PROC TTEST was used for planned comparisons upon awakening and postsleep inertia and statistical analyses for comparisons across the dissipation of sleep inertia were performed using PROC MIXED (variance components).
Results
Sleep restriction and performance upon awakening throughout the forced desynchrony protocol
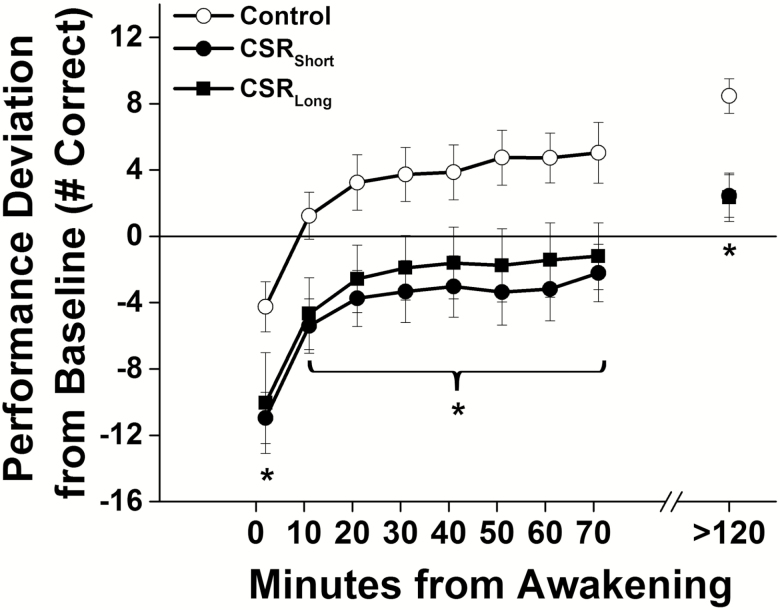
When compared with Baseline levels, performance on the first test (i.e. 2 min after awakening) averaged throughout the forced desynchrony was lower by 6.8% (SD 6.4%) in the Control condition, and by 18.5% (7.6%) and 16.4% (14.7%) in the CSRShort and CSRLong conditions, respectively (Figure 1, Supplementary Figure S3). The two CSR conditions did not differ [t(23) = 0.42, p=0.68] and their data were combined. The ~10% of additional performance impairment upon awakening in the CSR(Short and Long combined) conditions when compared with the Control condition was significantly lower [t(24) = −2.89, p = 0.008]. Performance across the dissipation of sleep inertia (10–70 min after awakening) was similar in both CSR conditions (F1,2044 = 0.2, p = 0.66), and significantly impaired in the combined CSR conditions compared with the Control condition (Figure 1, F1,2964 = 8.5, p = 0.0082) with no significant interaction effect for condition by time from awakening (F7,2964 = 0.55, p = 0.80). On average, individuals in the CSR conditions did not reach Baseline levels of performance for approximately 70 min, which is ~7 times longer than the Control condition values that returned to Baseline levels only ~10 min after awakening. Post-sleep-inertia test levels were higher than Baseline levels, which was expected from known learning effects using this metric [23], and significantly higher (by 10 responses in 2 min) for Control than for combined CSR conditions {Figure 1, [t(24) = −3.85, p = 0.0008]}.
Figure 1.
Performance after awakening under different sleep conditions throughout the forced desynchrony protocol. Number of correct responses on the Digit Symbol Substitution Task (DSST) under Control conditions (equivalent of 8 hr scheduled sleep per 24 hr, open symbols) and under both chronic sleep restriction (CSR) conditions (equivalent of 5.6 hr scheduled sleep per 24 hr, closed symbols) are plotted as average (± St. Err; error bars) deviation from Baseline for each individual (solid horizontal line) on y-axis and by minutes since awakening from sleep on x-axis. Data plotted below average Baseline performance levels indicate performance impairment. Asterisks indicate a significant difference using mixed model techniques and t-tests (p < 0.05) between the combined CSR conditions and Controls. Data are from n = 26 participants.
Acute and chronic impact of sleep restriction
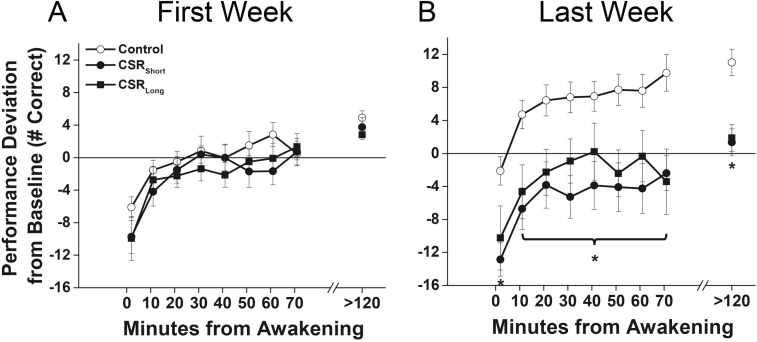
We next examined whether acute and chronic effects of sleep restriction differed by comparing the conditions during the first and last week of each protocol. During the first week of each protocol, there were no significant differences between the CSR(Short and Long combined) and Control conditions in the test immediately upon awakening [t(24) = −1.42, p = 0.17], across the dissipation of sleep inertia (F1,764 = 1.10, p = 0.29), or in postsleep inertia testing [t(24) = −1.44, p = 0.16] (Figure 2A). During the last week of each protocol, however, the test immediately upon awakening was 15.9% lower in CSR(Short and Long combined) condition compared with Control condition [t(24) = −2.74, p = 0.01], performance across the dissipation of sleep inertia was worse (F1,771 = 10.75, p = 0.001), and postsleep inertia tests were lower [t(24) = −3.86, p = 0.0007] (Figure 2B). There were no significant interaction effects of condition by time from awakening for tests occurring either during the first (F7,764 = 1.98, p = 0.06) or last week (F7,771 = 0.36, p = 0.93).
Figure 2.
Performance after awakening under different sleep conditions in the (A) first and (B) last week of each forced desynchrony protocol. Number of correct responses on the Digit Symbol Substitution Task (DSST) under Control conditions (equivalent of 8 hr scheduled sleep per 24 hr, open symbols) and under both chronic sleep restriction (CSR) conditions (equivalent of 5.6 hr scheduled sleep per 24 hr, closed symbols) are plotted as average (± St. Err; error bars) deviation from Baseline for each individual (solid horizontal line) on y-axis and by minutes since awakening from sleep on x-axis. Data plotted below average Baseline performance levels indicate performance impairment. Asterisks indicate a significant difference using mixed model techniques and t-tests (p < 0.05) between the combined CSR conditions and Controls. Data are from n = 26 participants.
Influence of circadian timing on performance upon awakening
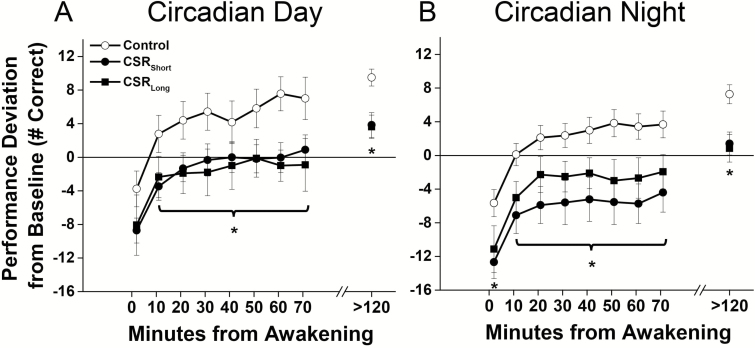
To determine the impact of circadian timing, we separated the tests into those that occurred only during the circadian day (150–270 circadian degrees relative to fit melatonin maximum) or circadian night (330–90 circadian degrees) [5]. When individuals awoke during the circadian day (i.e. habitual times), there was no difference between CSR and Control conditions immediately upon awakening [t(22) = −1.37, p = 0.19], although values postawakening were lower across the 1.5 hr of dissipation of sleep inertia (F1,884 = 5.6, p = 0.02), and during postsleep inertia testing [t(23) = −3.63, p = 0.001] in the CSR(Short and Long combined) compared with Control condition (Figure 3A). Awakening during the circadian night was associated with worse performance immediately after awakening {~10%, [t(23) = −2.28, p = 0.03]}, across the dissipation of sleep inertia (F1,1433 = 7.82, p = 0.005), and in postsleep inertia testing [t(23) = −3.57, p = 0.001] in the CSR(Short and Long combined) condition compared with Control condition (Figure 3B). There were no significant interaction effects of condition by time from awakening for tests occurring either during the circadian day (F7,884 = 0.86, p = 0.54) or night (F7,1433 = 0.45, p = 0.87).
Figure 3.
Performance after awakening under different sleep conditions during (A) circadian day and (B) circadian night phases during each forced desynchrony protocol. Number of correct responses on the Digit Symbol Substitution Task (DSST) under Control conditions (equivalent of 8 hr scheduled sleep per 24 hr, open symbols) and under both chronic sleep restriction (CSR) conditions (equivalent of 5.6 hr scheduled sleep per 24 hr, closed symbols) are plotted as average (± St. Err; error bars) deviation from Baseline for each individual (solid horizontal line) on y-axis and by minutes since awakening from sleep on x-axis. Data plotted below average Baseline performance levels indicate performance impairment. Asterisks indicate a significant difference using mixed model techniques and t-tests (p < 0.05) between the combined CSR conditions and Controls. Data are from n = 24 participants.
Sleep restriction and subjective sleepiness upon awakening
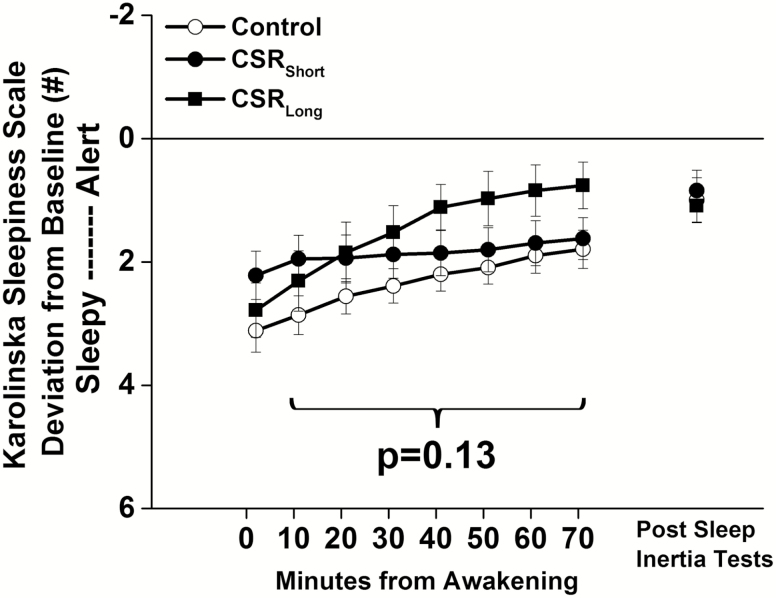
There were no significant differences between the two CSR conditions in subjective sleepiness (F1,2195 = 0.43, p = 0.51) throughout the forced desynchrony protocol and the two conditions were again combined. Unlike the DSST performance, there were no significant condition (F1,3115 = 2.33, p = 0.13) or condition by time from awakening interaction effects (F7,3115 = 0.34, p = 0.93) for subjective sleepiness between the CSR(Short and Long combined) condition and the Control condition throughout the forced desynchrony protocol (Figure 4). This was also true during the first week (condition, F1,815 = 0.06, p = 0.81; condition by time awake, F7,815 = 0.70, p = 0.67) and last week (condition, F1,815 = 3.16, p = 0.08; condition by time awake, F7,815 = 0.12, p = 0.99) of the protocol and for tests occurring during the circadian day (condition, F1,951 = 2.03, p = 0.16; condition by time awake, F7,951 = 0.44, p = 0.88) or circadian night (condition, F1,1643 = 0.62, p = 0.43; condition by time awake, F7,1643 = 0.36, p = 0.93).
Figure 4.
Subjective sleepiness after awakening under different sleep conditions. Number selected on the Karolinska Sleepiness Scale (KSS) under Control conditions (equivalent of 8 hr scheduled sleep per 24 hr, open symbols) and under both chronic sleep restriction (CSR) conditions (equivalent of 5.6 hr scheduled sleep per 24 hr, closed symbols) are plotted as average (± St. Err; error bars) deviation from Baseline for each individual (solid horizontal line) on y-axis and by minutes since awakening from sleep on x-axis. Higher KSS scores indicate higher subjective sleepiness; note the inverted axis. Data are from n = 26 participants.
Discussion
The chronic insufficient sleep often experienced by millions of individuals, including health care, emergency, security, and military professionals who may need to act at high levels of performance rapidly upon awakening, can greatly negatively affect that performance. Notably, the combined effects of sleep inertia and chronic insufficient sleep display similar levels of performance impairment to that of the neurobehavioral-performance decrements induced by alcohol intoxication [24]. Strikingly, performance levels on average did not return to baseline levels until ~70 min after awakening on this simple test under CSR conditions and the CSR conditions did not subjectively feel sleepier than the Control condition, signifying a dissociation between objective performance and subjective recognition. These findings therefore potentially have implications for the time shortly after awakening when many individuals may be performing important duties (e.g. driving to work or school) and they do not feel sleepy even though their performance is impaired. If individuals do not feel sleepy, they many not take appropriate countermeasures.
The use of two forced desynchrony protocols of differing absolute cycle lengths, but the same sleep:wake ratios, allows us to draw conclusions about the acute and chronic impact of sleep restriction and sleep inertia on cognitive performance, independent of absolute sleep duration. Since we found no difference between the two CSR conditions in any point of our analysis, we can conclude that it is the history of sleep restriction, rather than immediate prior sleep or wake duration, which causes performance decrements during sleep inertia.
Sleep inertia, assessed using reaction time, is worse after awakening from a sleep episode following extended wakefulness without chronic sleep restriction (i.e. in sleep deprivation or acute sleep restriction conditions) [16–19]. We have previously found that restricting sleep affects overall cognitive performance both acutely and chronically [5, 15]; however, it was unknown how sleep inertia may be affected by a chronic exposure to sleep restriction. The absence of differences in the first week of each protocol was unexpected; however, our amounts of acute sleep restriction may not have been drastic enough, or our measure of performance may not be sensitive enough, to see immediate differences. Previous reports of sleep loss used ≤3 hr of scheduled sleep per 24 hr [16, 19], whereas the current protocol used 5.6 hr of scheduled sleep per 24 hr day. Our choice of CSR sleep duration may be more translatable to real-world conditions, as many American’s report sleeping less than 6 hr on a nightly basis [1].
The results also suggest that individuals cannot “learn” to live with less sleep over ~30 days; instead, cognitive performance declines. This point may be of particular importance as we found a disassociation between the objective performance of our participants and how they rated their subjective sleepiness. Although this has been shown previously for testing occurring throughout the wake episode during sleep restriction [3, 14, 15], our findings of this disassociation during sleep inertia highlight that potential subjective misperception of performance level can occur very early in the waking day and may have serious implications for those who need to perform a task quickly upon awakening and do not realize that they are impaired. Future work is needed to examine how extended wake before the sleep episode (i.e. sleep deprivation) may interact with chronic insufficient sleep to affect performance during sleep inertia; this situation may occur in a person working an extended duration work shift or pulling an “all-nighter” and needing to take a nap prior to performing a task.
We also investigated how circadian timing interacts with chronic sleep restriction in affecting sleep inertia, as may occur in an individual working an overnight or early morning shift. It has been well established that circadian timing plays an important role in cognitive performance, and sleep inertia has been shown to be worse during the circadian nighttime hours [21, 25–27]. We found that during CSR, tests immediately upon awakening were not significantly different between conditions during the circadian day, but performance across the dissipation of sleep inertia and postsleep inertia testing was poorer. Tests occurring during the circadian night, however, had even worse outcomes in the combined CSR groups as compared to the Control group upon awakening and across the dissipation of sleep inertia. This is relevant for people who may be awoken from sleep at night to work (e.g. medical professionals and safety personnel who are allowed to sleep during night-work shifts). It is unclear why only the test upon awakening was not different during the circadian day, but one hypothesis may be that participants in the CSR conditions awoke from a “deeper” sleep than Controls during this time; this requires further investigation using spectral analysis as the percent of time participants were awoken from slow-wave sleep was not significantly different in the current study. Performance during sleep inertia has been found to be more impaired if individuals are awoken from slow-wave sleep [16, 17, 28], though this has been contested during simulated extended nighttime work schedules [29]. As we did not see any statistical difference between conditions in the percent of awakenings out of slow wave sleep, future work will need to further tease apart sleep and circadian dynamics to determine why only the test upon awakening was not different.
Our current study did have several limitations. The age range (20–34 years) of our participants was somewhat limited and may restrict the generalizability of the findings. Performance during sleep inertia is worse in older individuals [21] and later chronotypes [30], with later chronotype being commonplace in adolescent populations [31]; thus, we anticipate our findings would extend to older and younger populations and shift-working populations who often have sleep disturbances and are at increased risk for poorer health [2]. Additionally, although we analyzed the same number of tests in all conditions in postsleep inertia testing, the use of different waking durations meant that the Control and CSRLong conditions had more tests after sleep inertia testing ended than the CSRShort condition. This extra testing may have allowed for added learning in those conditions [23]; however, this was not observed between the CSRLong and CSRShort conditions at any point in the protocol suggesting that no additional learning occurred due to extra testing. Furthermore, our in-laboratory procedures may not depict how an individual may cope with sleep inertia in real-world situations. For example, caffeine is commonly used to promote alertness and can improve performance during sleep inertia after a nap [32]. We did not allow individuals any caffeine use for ~3 weeks prior to entering the study or during study procedures; thus, it is unknown how caffeine or other alerting factors known to improve sleep inertia [33] may interact with chronic insufficient sleep. We also studied participants in dimly-lit environment (<4 lux), which may have allowed for greater impairments in performance to manifest than would be observed in real-world settings where bright light use is common [34] and has an alerting effect [35, 36]. Future work should examine how chronic short sleep may interact with these potential countermeasures in different populations.
In summary, our findings highlight that chronic short sleep of durations that are common in modern society can greatly magnify performance impairments immediately upon awakening and across the dissipation of sleep inertia. These findings may have relevance for all individuals obtaining short sleep schedules including medical professionals that make clinically important decisions or perform procedures after sleeping on-call, parents that must wake suddenly to attend to a screaming child, security or military personnel that might need to respond to a threat that awakens them, or high school students that drive to school in a rush within an hour after awakening.
Funding
This work was supported by National Institutes of Health (NIH) (F32DK107146, T32HL007901, KL2TR002370, K24HL105664, R01HL114088, R01GM105018, R01HL128538, P01AG009975, R21HD086392), Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541), and financial contributions from Harvard University and its affiliated academic healthcare centers and National Space Biomedical Research Institute (HFP02802, HFP04201, HDP0006). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, The Brigham and Women’s Hospital, Harvard University and its affiliated academic health care centers, or the NIH.
Conflict of interest statement. A.W.M. and W.W. have nothing to disclose; J.T.H. is currently employed by Supernus Pharmaceuticals Inc.; D.A.C. has served on the Merck Speaker’s bureau from 2015–2017; and C.A.C. has received consulting fees from or served as a paid member of scientific advisory boards for Columbia River Bar Pilots, Ganésco Inc., Institute of Digital Media and Child Development, Klarman Family Foundation, Samsung Electronics, Vanda Pharmaceuticals, Washington State Board of Pilotage Commissioners, and Zurich Insurance Company, Ltd. C.A.C. has also received education/research support from Optum, Philips Respironics, Inc., San Francisco Bar Pilots, Schneider Inc., Sysco, and Vanda Pharmaceuticals. The Sleep and Health Education Program of the Harvard Medical School Division of Sleep Medicine, and the Sleep Matters Initiative (which C.A.C. directs) have received funding for educational activities from Cephalon, Inc., Jazz Pharmaceuticals, ResMed, Takeda Pharmaceuticals, Teva Pharmaceuticals Industries Ltd., Sanofi-Aventis, Inc., Sepracor, Inc., Wake Up Narcolepsy, and Mary Ann & Stanley Snider via Combined Jewish Philanthropies. C.A.C. is the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc. and holds a number of process patents in the field of sleep/circadian rhythms (e.g. photic resetting of the human circadian pacemaker). Since 1985, C.A.C. has also served as an expert on various legal and technical cases related to sleep and/or circadian rhythms including those involving the following commercial entities: Complete General Construction Company, FedEx, Greyhound, HG Energy LLC, South Carolina Central Railroad Co., Stric-Lan Companies LLC, and United Parcel Service (UPS). C.A.C. owns or owned an equity interest in Vanda Pharmaceuticals. He received royalties from Houghton Mifflin Harcourt/Penguin, McGraw Hill, and Koninklijke Philips Electronics, N.V. for the Actiwatch-2 and Actiwatch-Spectrum devices. Dr. Czeisler’s interests were reviewed and managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies; E.B.K. has received travel reimbursement from the Associated Professional Sleep Society and National Sleep Foundation and has served as consultant for Pfizer Inc and in cases involving transportation safety and sleep deprivation.
Funding
This work was supported by National Institutes of Health (NIH) (F32DK107146, T32HL007901, KL2TR002370, K24HL105664, R01HL114088, R01GM105018, R01HL128538, P01AG009975, R21HD086392), Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541), and financial contributions from Harvard University and its affiliated academic healthcare centers and National Space Biomedical Research Institute (HFP02802, HFP04201, HDP0006). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, The Brigham and Women’s Hospital, Harvard University and its affiliated academic health care centers, or the NIH.
Conflict of interest statement. A.W.M. and W.W. have nothing to disclose; J.T.H. is currently employed by Supernus Pharmaceuticals Inc.; D.A.C. has served on the Merck Speaker’s bureau from 2015–2017; and C.A.C. has received consulting fees from or served as a paid member of scientific advisory boards for Columbia River Bar Pilots, Ganésco Inc., Institute of Digital Media and Child Development, Klarman Family Foundation, Samsung Electronics, Vanda Pharmaceuticals, Washington State Board of Pilotage Commissioners, and Zurich Insurance Company, Ltd. C.A.C. has also received education/research support from Optum, Philips Respironics, Inc., San Francisco Bar Pilots, Schneider Inc., Sysco, and Vanda Pharmaceuticals. The Sleep and Health Education Program of the Harvard Medical School Division of Sleep Medicine, and the Sleep Matters Initiative (which C.A.C. directs) have received funding for educational activities from Cephalon, Inc., Jazz Pharmaceuticals, ResMed, Takeda Pharmaceuticals, Teva Pharmaceuticals Industries Ltd., Sanofi-Aventis, Inc., Sepracor, Inc., Wake Up Narcolepsy, and Mary Ann & Stanley Snider via Combined Jewish Philanthropies. C.A.C. is the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc. and holds a number of process patents in the field of sleep/circadian rhythms (e.g. photic resetting of the human circadian pacemaker). Since 1985, C.A.C. has also served as an expert on various legal and technical cases related to sleep and/or circadian rhythms including those involving the following commercial entities: Complete General Construction Company, FedEx, Greyhound, HG Energy LLC, South Carolina Central Railroad Co., Stric-Lan Companies LLC, and United Parcel Service (UPS). C.A.C. owns or owned an equity interest in Vanda Pharmaceuticals. He received royalties from Houghton Mifflin Harcourt/Penguin, McGraw Hill, and Koninklijke Philips Electronics, N.V. for the Actiwatch-2 and Actiwatch-Spectrum devices. Dr. Czeisler’s interests were reviewed and managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies; E.B.K. has received travel reimbursement from the Associated Professional Sleep Society and National Sleep Foundation and has served as consultant for Pfizer Inc and in cases involving transportation safety and sleep deprivation.
Supplementary Material
Acknowledgments
The authors thank the participants and Center for Clinical Investigation staff and their support in conducting these studies.
References
- 1. Foundation NS. Executive summary of the 2005 “Sleep in America” poll. 2005; 8–13. Retrieved athttps://www.sleepfoundation.org/professionals/sleep-america-polls/2005-adult-sleep-habits-and-styles.
- 2. McHill AW, et al. Role of sleep and circadian disruption on energy expenditure and in metabolic predisposition to human obesity and metabolic disease. Obes Rev. 2017;18 Suppl 1:15–24. [DOI] [PubMed] [Google Scholar]
- 3. Van Dongen HP, et al. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2):117–126. [DOI] [PubMed] [Google Scholar]
- 4. Belenky G, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12(1):1–12. [DOI] [PubMed] [Google Scholar]
- 5. Cohen DA, et al. Uncovering residual effects of chronic sleep loss on human performance. Sci Transl Med. 2010;2(14):14ra3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wright KP, Jr., et al. Relationship between alertness, performance, and body temperature in humans. Am J Physiol Regul Integr Comp Physiol. 2002;283(6):R1370–R1377. [DOI] [PubMed] [Google Scholar]
- 7. Wertz AT, et al. Effects of sleep inertia on cognition. JAMA. 2006;295(2):163–164. [DOI] [PubMed] [Google Scholar]
- 8. Jewett ME, et al. Time course of sleep inertia dissipation in human performance and alertness. J Sleep Res. 1999;8(1):1–8. [DOI] [PubMed] [Google Scholar]
- 9. Baldwin DC Jr, et al. Sleep deprivation and fatigue in residency training: results of a national survey of first- and second-year residents. Sleep. 2004;27(2):217–223. [DOI] [PubMed] [Google Scholar]
- 10. Basner M, et al. Sleep and alertness in medical interns and residents: An observational study on the role of extended shifts. Sleep. 2017;40:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ayas NT, et al. Extended work duration and the risk of self-reported percutaneous injuries in interns. JAMA. 2006;296(9):1055–1062. [DOI] [PubMed] [Google Scholar]
- 12. Horne J, et al. Sudden early-morning awakening impairs immediate tactical planning in a changing ‘emergency’ scenario. J Sleep Res. 2011;20(2):275–278. [DOI] [PubMed] [Google Scholar]
- 13. Santhi N, et al. Morning sleep inertia in alertness and performance: effect of cognitive domain and white light conditions. PLoS One. 2013;8(11):e79688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bermudez EB, et al. Prediction of Vigilant Attention and Cognitive Performance Using Self-Reported Alertness, Circadian Phase, Hours since Awakening, and Accumulated Sleep Loss. PLoS One. 2016;11(3):e0151770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McHill AW, et al. Chronic sleep curtailment, even without extended (>16-h) wakefulness, degrades human vigilance performance. Proc Natl Acad Sci U S A. 2018;115(23):6070–6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dinges DF, et al. Assessing performance upon abrupt awakening from naps during quasi-continuous operations. Behav Res Meth Instr & Comp. 1985;17:37–45. [Google Scholar]
- 17. Rosa RR, et al. Sleep stages, auditory arousal threshold, and body temperature as predictors of behavior upon awakening. Int J Neurosci. 1985;27(1-2):73–83. [DOI] [PubMed] [Google Scholar]
- 18. Balkin TJ, et al. Relationship between sleep inertia and sleepiness: cumulative effects of four nights of sleep disruption/restriction on performance following abrupt nocturnal awakenings. Biol Psychol. 1988;27(3):245–258. [DOI] [PubMed] [Google Scholar]
- 19. Miccoli L, et al. Comparing sleep-loss sleepiness and sleep inertia: lapses make the difference. Chronobiol Int. 2008;25(5):725–744. [DOI] [PubMed] [Google Scholar]
- 20. Czeisler CA, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284(5423):2177–2181. [DOI] [PubMed] [Google Scholar]
- 21. Silva EJ, et al. Sleep inertia varies with circadian phase and sleep stage in older adults. Behav Neurosci. 2008;122(4):928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Akerstedt T, et al. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52(1-2):29–37. [DOI] [PubMed] [Google Scholar]
- 23. Wright KP, Jr., et al. Sleep and wakefulness out of phase with internal biological time impairs learning in humans. J Cogn Neurosci. 2006;18(4):508–521. [DOI] [PubMed] [Google Scholar]
- 24. Dawson D, et al. Fatigue, alcohol and performance impairment. Nature. 1997;388(6639):235. [DOI] [PubMed] [Google Scholar]
- 25. Burke TM, et al. Sleep inertia, sleep homeostatic and circadian influences on higher-order cognitive functions. J Sleep Res. 2015;24(4):364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scheer FA, et al. An endogenous circadian rhythm in sleep inertia results in greatest cognitive impairment upon awakening during the biological night. J Biol Rhythms. 2008;23(4):353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hilditch CJ, et al. Sleep inertia during a simulated 6-h on/6-h off fixed split duty schedule. Chronobiol Int. 2016;33(6):685–696. [DOI] [PubMed] [Google Scholar]
- 28. Tassi P, et al. EEG spectral power and cognitive performance during sleep inertia: the effect of normal sleep duration and partial sleep deprivation. Physiol Behav. 2006;87(1):177–184. [DOI] [PubMed] [Google Scholar]
- 29. Signal TL, et al. Duration of sleep inertia after napping during simulated night work and in extended operations. Chronobiol Int. 2012;29(6):769–779. [DOI] [PubMed] [Google Scholar]
- 30. Ritchie HK, et al. Impact of sleep inertia on visual selective attention for rare targets and the influence of chronotype. J Sleep Res. 2017;26(5):551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roenneberg T, et al. A marker for the end of adolescence. Curr Biol. 2004;14(24):R1038–R1039. [DOI] [PubMed] [Google Scholar]
- 32. Van Dongen HP, et al. Caffeine eliminates psychomotor vigilance deficits from sleep inertia. Sleep. 2001;24(7):813–819. [DOI] [PubMed] [Google Scholar]
- 33. Hayashi M, et al. The alerting effects of caffeine, bright light and face washing after a short daytime nap. Clin Neurophysiol. 2003;114(12):2268–2278. [DOI] [PubMed] [Google Scholar]
- 34. Kyba CCM, et al. Artificially lit surface of Earth at night increasing in radiance and extent. Sci Adv. 2017;3(11):e1701528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vandewalle G, et al. Light as a modulator of cognitive brain function. Trends Cogn Sci. 2009;13(10):429–438. [DOI] [PubMed] [Google Scholar]
- 36. Badia P, et al. Bright light effects on body temperature, alertness, EEG and behavior. Physiol Behav. 1991;50(3):583–588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.