Abstract
Study Objectives
There are significant discrepancies between the prevalence of snoring and that of objectively defined sleep disordered breathing among pregnant women, suggesting subtle airflow limitations that may not be captured by conventional scoring. This study examined the performance of pulse transit time, an indirect measure of arterial stiffness and sympathetic activation, in pregnancy.
Methods
Pregnant women with obesity and snoring and a group of controls without symptoms of sleep disordered breathing were recruited in the first trimester. Women underwent a level III in-laboratory sleep monitoring study including an electrocardiogram and pulse oximetry, and pulse transit time was measured. Sleep disordered breathing was defined as an apnea–hypopnea index at least five events per hour of sleep. Statistical analysis was performed using Spearman correlation, Fisher’s exact t-test, and univariate analysis.
Results
Of the 222 women, 38 met criteria for sleep disordered breathing. Pulse transit time drops were very prevalent (95% of participants with snoring had > 5 drops per hour). Median apnea–hypopnea index was 0.7 (interquartile range [IQR]: 2.6) events per hour whereas median pulse transit time drop index was 20.70 (IQR: 35.90) events per hour. Pulse transit time index was significantly higher in snorers with apnea–hypopnea index less than five events per hours and participants with apnea–hypopnea index greater than five events per hour compared to controls. Examination of random epochs with pulse transit time drops showed that 95% of pulse transit time drops were associated with airflow limitation.
Conclusions
Pulse transit time ascertains frequent events of sympathetic activation in at-risk women with and without sleep disordered breathing beyond conventional apneas and hypopneas. Pulse transit time may be an important addition to the identification of clinically significant sleep disordered breathing in pregnant women, and may identify more sleep disordered breathing than apnea–hypopnea index.
Keywords: sleep disordered breathing, snoring, pregnancy, sympathetic activation, microarousals
Statement of Significance
Sleep disordered breathing (SDB) commonly impacts pregnant women, and this condition has been associated with adverse perinatal outcomes. As airflow limitations that do not meet criteria for apneas and hypopneas are common in pregnancy, the utility of conventional criteria in the diagnosis of SDB has been questioned. This article highlights the frequency of events of sympathetic activation that occur in women at risk for SDB with and without the condition by apnea–hypopnea index (AHI) criteria, compared to controls. These findings emphasize the important biological changes that occur in pregnant women at risk for SDB, whether or not these women meet criteria for the condition, supporting the claim that pregnancy-specific diagnostic criteria for SDB are needed.
Introduction
Sleep disordered breathing (SDB) in pregnancy, a spectrum of disorders characterized by abnormal breathing during sleep, has gained significant attention in the past decade, especially with respect to its impact on pregnancy outcomes. Both snoring and obstructive sleep apnea (OSA) have been associated with similar adverse perinatal outcomes such as preeclampsia, gestational diabetes, and preterm births [1, 2]. Though therapy is usually offered to patients with OSA, patients with snoring are usually given weight loss and other lifestyle modification advice but are not necessarily “treated.” Although in the general population such an approach is acceptable, this approach may not be ideal in the pregnant population given (1) the lack of safety regarding weight loss; (2) the contracted time between the exposure to SDB and the occurrence of outcomes, and (3) the potential for in utero exposure to SDB to impact the next generation [3–7]. Hence, there may be pregnant women with snoring and airflow limitation who do not meet apnea hypopnea criteria for SDB but remain at risk for perinatal complications, who remain overlooked.
Although snoring is very prevalent in pregnancy [1, 2, 8] and may impact up to a third of all pregnant women, polysomnographically identified SDB is less common in the general pregnant population [9]. Furthermore, data from prior studies that assessed women suspected to have SDB in mid-to-late pregnancy using in-laboratory full polysomnography showed that only about 40%–50% had OSA as defined by an apnea–hypopnea index (AHI) of five events per hour or greater [10]. Thus the criteria used for identifying clinically significant SDB may be different in pregnant patients than in nonpregnant patients. Recent data by the investigators have shown that pregnant women suspected of SDB were significantly more likely to have airflow limitations that do not meet criteria for apnea or hypopnea compared to nonpregnant women matched for age, body mass index (BMI), and AHI [11]. In addition, more than 70% of breaths were found to have inspiratory flow limitation during sleep in women with preeclampsia [12]. These data suggest that events related to subtle airflow limitation are prevalent in the pregnant population, are associated with adverse outcomes, and are not taken into account during conventional scoring of respiratory events during sleep. As consensus agreement on the definition and identification of airflow limitation [13] does not currently exist, and available software detection is not always reliable, alternative measures may be helpful in detecting and identifying more subtle obstructive events.
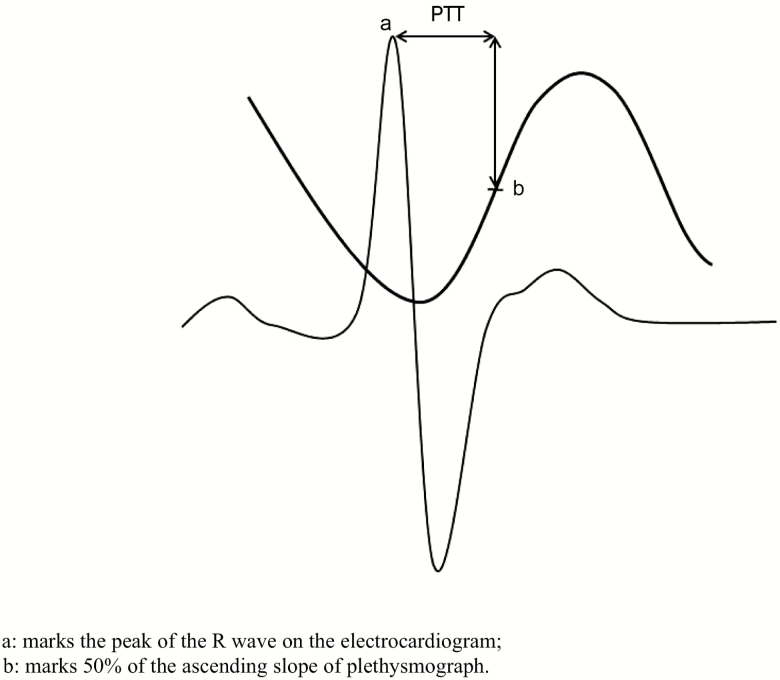
Esophageal pressure monitoring is the gold standard measure of airway resistance and airflow limitation, as esophageal pressure correlates with the negative pleural pressure exerted to overcome an upper airway obstructive event [14]. The use of esophageal monitoring in clinical laboratories has been limited by its invasive nature and the need for an in-laboratory stay. Pulse transit time (PTT) has been proposed as an alternative to esophageal pressure monitoring [15–17] and validated for use in children and adults [17, 18]. PTT has also been validated against intra-arterial blood pressure measurements [19]. PTT is the length of time it takes a pulse pressure to travel from the left ventricle to a peripheral arterial site, typically a fingertip or a foot, and is inversely proportional to arterial stiffness [20]. PTT is measured between the peak of the R wave on electrocardiographic tracing and a constant in the plethysmography tracing of oxygen saturation (see Figure 1). PTT measures the change in arterial stiffness caused by sympathetic activation [21] and is associated with arousals that occur during sleep [22, 23]. In addition, the amplitude of the oscillations in PTT during inspiration correlates with the degree of inspiratory effort in awake healthy individuals breathing through an added inspiratory load [17]. Although arousals at the end of an obstructive event lead to a rise in blood pressure, decreasing PTT [24], a pulsus paradoxus effect results in a steep decline in blood pressure [25], leading to an increase in PTT.
Figure 1.
Pulse transit time (PTT) measurement; a marks the peak of the R wave on the electrocardiogram and b marks 50% of the ascending slope of plethysmograph.
The goals of this study were to (1) examine whether PTT measures correlated with measures of SDB severity in pregnancy and (2) assess if PTT drops occur outside of apnea and hypopnea events. We hypothesized that more PTT drops will occur outside of the traditionally defined apneas and hypopneas in pregnant women with snoring or AHI greater than five events per hour than in controls and that the drops are related to subtle, non-apnea, or hypopnea obstructive events.
Methods
Patients were recruited from community- and hospital-based obstetric practices. Inclusion criteria included a singleton pregnancy, less than 14 weeks of gestation, and risk factors for OSA such as habitual snoring and a BMI that falls in the overweight or obesity range. Women with atrial fibrillation and other heart disease, and those on cardiac or antihypertensive medications were excluded.
In addition, we have recruited a total of 21 normotensive women with normal pregnancy and no symptoms of SDB, to establish PTT data in normal pregnant women. We followed the same protocol for these women and our study sample, as described later.
Demographics were collected at baseline and medical records reviewed for medical history. At enrollment, women answered questionnaires administered by study staff to evaluate for the presence of restless leg syndrome [26] and the risk of SDB [27]. Medication use and social history were also collected. The study was approved by the institutional review board, and all participants signed an informed consent prior to participating.
In-home sleep apnea monitor
All participants underwent testing for SDB using an in-home level III recording device, Nox T3 (CareFusion, San Diego, CA). This device utilizes built-in sensors to include a pressure transducer allowing recording of nasal pressure and snoring, a three-dimensional acceleration sensor for measuring body position and activity, and a microphone for true audio-recording capabilities. The external sensor options used included electrocardiography and dual abdominal/thoracic respiratory inductance plethysmography belts, the latter being the preferred noninvasive technology in the measurement of respiratory effort. The T3 device also supports wireless Bluetooth connectivity, allowing it to record signal from a Bluetooth pulse oximeter. Nox T3 auto-score has been validated against in-laboratory polysomnography as it correctly identifies 100% of OSA-negative participants and 88% of OSA-positive participants [28], and auto-score-derived AHI strongly correlates with polysomnography-derived AHI (r = 0.93). SDB was defined as AHI at least five events per hour, and severity defined per standard AHI criteria (5–14.9: mild; 15–29.9: moderate; ≥ 30: severe). Hypopnea was defined based on the recommended American Academy of Sleep Medicine (AASM) rule [29]. As this was a level III device that did not utilize electroencephalography channels, hypopnea was defined solely on the basis of oxygen desaturation of 3%. Studies were scored by an experienced, polysomnography technician, and supervised by the investigative team. The presence of airflow limitation was examined in a subset of random epochs similarly to previously described methods [11, 12], and breaths were defined as airflow limited if they met criteria for airflow limitation per Aittokallio et al. [30].
PTT measurements
Standard electrocardiogram electrodes were placed on the right shoulder at the right midclavicular line immediately below the clavicle, right waist area over the right iliac crest, and at the lowest left rib over the left midclavicular line. Finger pulse oximetry was recorded using plethysmography, with a sampling rate of 75 Hz. As the exact moment where the aortic valve opens is difficult to detect, PTT is set to measure from a more easily detectable start point, the peak of the R wave on the electrocardiogram. PTT was set to measure the duration from the peak of the R wave on the electrocardiogram to the pulse wave rising 50% from nadir to its peak by plethysmograph (Figure 1). Minimum PTT was set at 0 milliseconds (ms), maximum PTT at 800 ms, and maximum PTT change set at 500 ms. A PTT drop was defined as at least 15 ms decrease in PTT from the average PTT in the 60 s immediately prior, and lasting for at least 5 s [22, 31]. The PTT drop index was defined as the number of drops in PTT that meet the previously defined criteria that occur per hour of sleep. Visual inspection of recordings was performed and periods of artifact removed. Nox software calculates the number of PTT drops that occur with apneas and hypopneas; these data were used to calculate sensitivity of PTT in detecting obstructive events such as apneas and hypopneas. In addition, all individual obstructive events were reviewed for PTT drops that met the aforementioned criteria.
A total of 228 events were randomly selected from 30 patients with either snoring and AHI less than 5 events per hour or AHI at least 5 events per hour. The events were examined for the presence of associated airflow limitation by visual inspection by a single experienced investigator (G.B.).
Statistical analysis
Statistical analyses were performed using SPSS version 20. Descriptive statistics were conducted to calculate percentages, mean, median and standard deviation data. Spearman or Pearson correlations were performed as appropriate to analyze the relationship between continuous measures of PTT and AHI. PTT data were log-transformed and we conducted t-tests and analysis of variance (adjusting for height and body position) to compare the PTT differences in pregnant women with and without SDB. P less than 0.05 was considered to be significant. Sensitivity and specificity were also calculated for the association of pulse transit time index (PTTi) with various obstructive events.
Results
Sample demographics and characteristics
A total of 222 pregnant women agreed to participate and were recruited; a total of 190 had complete PTT and sleep data for analysis. Mean age was 29.8 ± 5.6 years and mean BMI was 34.4 ± 7.9 kg/m2. Mean gestational age at the time of enrollment was 10.6 ± 2.1 weeks. A total of 27% of women were primigravid (see Table 1) and 8.5% had chronic hypertension. Eight women met criteria for restless legs syndrome.
Table 1.
Demographics and baseline characteristics of the cohort
| Whole sample | AHI > 5 events per hour | Snorers AHI < 5 events per hour | Controls | |
|---|---|---|---|---|
| Mean ± SD (N = 190) | Mean ± SD (N = 35) | Mean ± SD (N = 134) | Mean ± SD (N = 21) | |
| Age (years) | 29.8 ± 5.6 | 33.1 ± 4.9 | 29.0 ± 5.6 | 30.3 ± 4.2 |
| Race (%) | ||||
| Caucasian | 55.7% | 57% | 52.7% | 76.2% |
| Black | 17.7% | 17% | 18.2% | 14.3% |
| Asian | 3.9% | 5.8% | 3.4% | 4.8 |
| Native American | 4.9% | 5.8% | 5.4% | 0 |
| Native Hawaiian | 0.5% | 0% | 0.7% | 0 |
| Multiracial | 11.8% | 11.4% | 12.8% | 4.8 |
| Unknown/not reported | 5.4% | 2.8% | 6.8% | 0 |
| Height (inches) | 63.9 ± 3.0 | 64.4 ± 2.2 | 63.7 ± 3.3 | 64.6 ± 2.2 |
| Weight (lbs) | 199.5 ± 46.0 | 230.8 ± 41.0 | 193.5 ± 45.5 | 188.4 ± 36.8 |
| BMI (kg/m2) | 34.4 ± 7.9 | 39.2 ± 7.2 | 33.6 ± 7.8 | 31.8 + 6.15 |
| AHI (events per hour of sleep) Median (IQR) | 0.7 (2.6) | 7.8 (4.8) | 0.4 (1.2) | 0.6 (1.5) |
| Gestational age at study visit (weeks) | 10.6 ± 2.1 | 10.6 ± 2.3 | 10.6 ± 2.0 | 10.4 + 2.3 |
| % of primigravida | 27.1 % | 11.7% | 39% | 38.1% |
| % of women with chronic hypertension | 8.5 % | 15.2% | 8.2% | 0% |
| % of women with pre-gestational diabetes | 3.2 % | 0% | 4.5% | 0% |
Respiratory parameters during sleep
A total of 35 women had an AHI at least five events per hour of sleep. The majority of participants with SDB had mild disease (86.8%). Median AHI was 0.7 (interquartile range [IQR]: 2.6) events per hour. Median apnea index was 0.00 (IQR: 0.20) and median hypopnea index was 0.50 (IQR: 2.40). Median AHI in the group of snorers with AHI less than 5 events per hour was 0.40 (IQR: 1.2) compared to the median AHI in the AHI greater than 5 group of 7.80 (IQR: 4.80). When an AHI at least one event per hour cutoff for the definition and severity of SDB was utilized, a total of 83 women met criteria for SDB.
PTT measurements
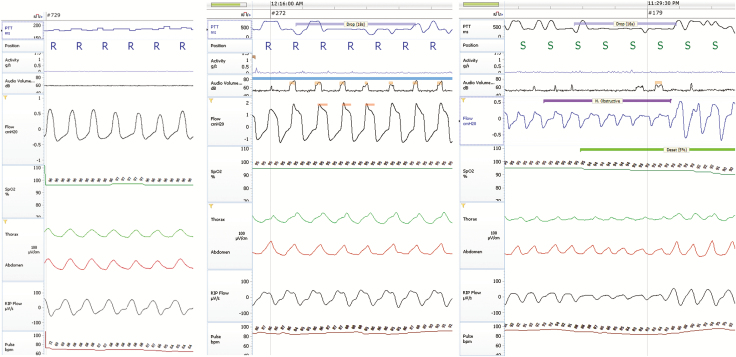
Examples of (PTT drops) measurements with normal breathing, airflow limitation, and a hypopnea are shown in Figure 2A–C, respectively. These figures show a flat PTT in association with nonobstructive breathing (Figure 2A), drops in PTT lasting 18 s in association with airflow limitation (Figure 2B) and a PTT drop pasting for 16 seconds in response to a hypopnea event (Figure 2C).
Figure 2.
(A) Normal breathing; minimal variations in pulse transit time (PTT). (B) Airflow limitation and PTT. (C) Obstructive event and drops in PTT.
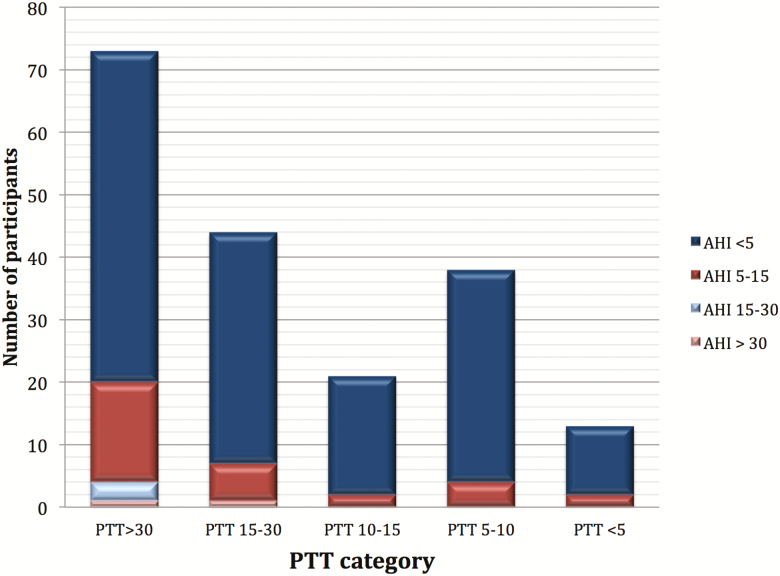
Median PTTi was 20.70 (IQR: 35.90) per hour of sleep, and more than 95% of participants with snoring and obesity (with AHI < or ≥ 5 events per hour) had a PTTi at least 5 events per hour. There were significantly more PTT drops than there were apneas and hypopneas combined. Figure 3 shows the distribution of AHI categories (AHI ≥ 5 events per hour) by PTT drops per hour (PTTi) cutoffs (PTTi cutoffs: ≥ 5, ≥10, ≥15, and ≥30 drops per hour). The vast majority (77%) of participants in categories of PTTi 15–30 and greater than 30 have an AHI less than 5 events per hour.
Figure 3.
Apnea–hypopnea index (AHI) distribution across pulse transit time (PTT) categories.
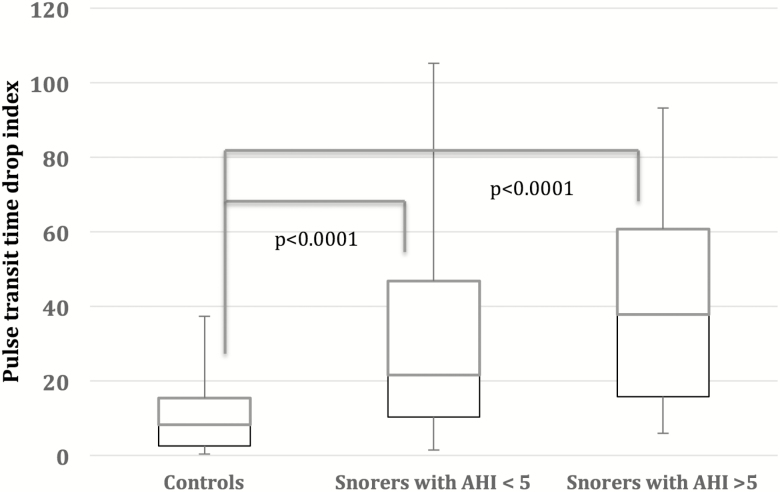
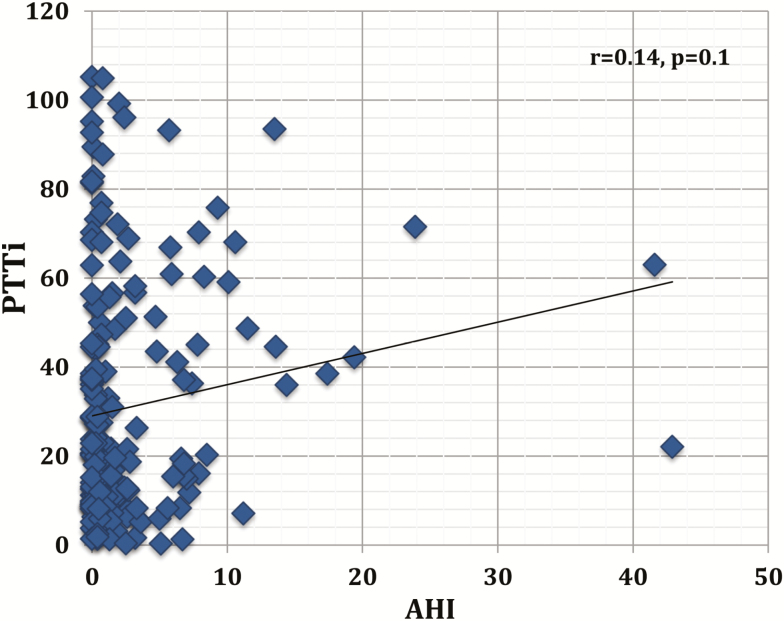
There was a significantly higher PTT drop index in women with AHI greater than 5 events per hour compared to normal controls (median 37.8, IQR (45.0), and 8,2 (12.7), P < 0.00001). Similarly, PTT drop index was higher in snorers with AHI less than 5 events per hour compared to normal controls (21.5 [36.5] vs. 8.2 [12.7], P less than 0.00001), and a significant trend when all three categories were compared, P = 0.02; see Figure 4. In addition, there was a trend toward a higher PTTi when women in the moderated/severe category were compared to women in the mild category (P = 0.09). However, there were no significant correlations between PTTi and AHI in the whole sample of pregnant women (r = 0.12, P = 0.11), Figure 5.
Figure 4.
Pulse transit time drop index in controls, snorers with apnea–hypopnea index (AHI) less than five events per hour, and AHI at least five events per hour, p-value = 0.02 for three group comparison.
Figure 5.
Correlation of pulse transit time (PTT) drop index with apnea–hypopnea index (AHI).
All apneic and hypopneic events were examined for drops in PTT both by the software and individually via visual examination. Overall sensitivity of PTT for all events was 47% by software and 54% by visual analysis. Sensitivity for apneas was marginally higher (63%) compared to sensitivity for hypopneas (52%). Nonetheless, in order to further evaluate PTT drops in relation to SDB, close individual drop examination was performed in 228 random epochs with PTT drops for the association with airflow limitation. PTT drop events were selected if they were not associated with a hypopnea or an apnea. A total of 95.1% of all PTT drops were associated with evidence of airflow limitation.
Discussion
In a cohort of pregnant women with obesity and/or snoring, our main findings were that (1) the PTT drop index is significantly higher in pregnant women with snoring and pregnant women with AHI at least five events per hour compared with pregnant controls without SDB; (2) PTT drop index may identify a significantly higher number of women with SDB than conventional scoring, whereby a significant proportion of women who are “ruled out” for clinically significant SDB based on AHI may have subtle events characterized by arterial stiffening and sympathetic activation that are undetected by conventional scoring; and (3) the vast majority (95%) of randomly selected PTT drops outside of apneas and hypopneas were associated with airflow limitations that did not meet criteria of apneas or hypopneas. Though PTTi did not correlate with AHI, PTTi progressively increased between the three groups of women (controls, snorers with AHI less than five events per hours, and snorers with AHI ≥ five events per hour), and PTTi tended to be higher in participants with AHI in the moderate/severe category compared to those in the mild AHI category. PTT and other measures of arterial stiffness have been shown to correlate with AHI in nonpregnant patients [21, 32]; however, the lack of an association in gravidas can be explained in different ways: (1) a large number of participants with AHI less than five events had a large number of PTT drops; (2) our sample of women with AHI at least five events per hour consisted mainly of women with mild disease, limiting the distribution of AHI; (3) PTT drops occurred in only 52%–63% of hypopneas and apneas. The latter may relate to our definition of PTT drops, which has been extrapolated from the nonpregnant population, and is not necessarily similarly applicable in both sexes. There are sex differences in sympathetic activation, with women being more “sympathetic” in response to sleep disturbances [33], and potential differences being found to be related to sex hormones such as estrogens and progesterone in premenopausal women [34]. These sex and hormonal differences may influence the intensity and duration of sympathetic activation. Therefore, it is possible that PTT drops could have occurred with these apneas and hypopneas but were not recorded by the software if they did not last for more than 5 s. It is possible that ventilatory control and apnea duration are different in pregnancy due to hormonal influences; therefore, it is not clear if duration and intensity of sympathetic activation would be the same in this population and this possibility may impact the definition of a “significant” PTT drop.
PTT may constitute a welcome addition to the diagnostic armamentarium of SDB in pregnancy. Airflow limitation in the form of reported snoring [1, 2] and polysomnographic evidence of airflow limited breaths that do not meet criteria for apnea or hypopnea [11, 12, 35] are both highly prevalent in this population and have, together with conventional apneas and hypopneas [9, 36], been associated with adverse perinatal outcomes. Hence, the definition of clinically significant SDB in pregnancy should likely extend beyond the conventional definition used in the general population. Airflow limitation has been the subject of interesting studies showing sex differences in the prevalence of various classes of airflow limitation [30] and establishing an airflow limitation cutoff in cohort studies [37]. However, as the definition of airflow limitations lacks consensus [13] and physiologic and anatomical changes of pregnancy may impact the shape of airflow limitation, alternative measures that detect more subtle events than apneas and hypopneas can prove highly useful. On the other hand, significant variations exist in the prevalence of polysomnographically defined SDB in pregnancy and stem, at least in part, from the variability in the definition of hypopnea. For instance, definitions have varied from recommended [9] versus alternative definition according to AASM [10], airflow limitation without desaturation [11, 35] to airflow limitation with arousals [38]. The technology used for diagnosis and its ability to detect arousals also plays an important role in explaining the discrepancies [9, 38, 39]. Studies with the highest prevalence of SDB were those where arousals were included in the definition, suggesting that airflow limitations [11] associated with arousals rather than significant desaturations [35] may constitute the crux of the disease in this population.
Our study shows that PTT drop index is significantly higher than AHI, suggesting that PTT is detecting events that do not meet criteria for apnea or hypopnea. Previous literature has shown that arousals occurring at the termination of an obstructive event lead to a transient but significant rise in blood pressure [40]. In addition, autonomic events have been described, whereby autonomic activation manifests as a change in blood pressure and heart rate, and may occur in the absence of obvious electroencephalographic arousal [18, 22]. Furthermore, PTT drops in the pediatric population have been shown to correlate with drops in esophageal pressure, even in the absence of detectable airflow limitation during polysomnography [31]. Given that the vast majority (95.1%) of randomly selected PTT drops in this study are preceded by airflow limitation and that PTTi increases progressively between normal non-snoring controls, snorers with AHI less than five events per hour, and snorers with AHI greater than five events per hour, the high number of PTT drops in our study is likely a reflection of events of increased inspiratory effort [17] and suggest the presence of autonomic activation and alterations in blood pressure in association with increased respiratory effort and respiratory arousals from sleep. Though the depth of PTT oscillation has been proposed as a measure with high inter-reader agreement to distinguish central from obstructive events [41], this application is not very relevant to the pregnant population. There are physiologic changes in pregnancy that may predispose to central respiratory instability and ventilatory overshoot [42]; nonetheless, central events are extremely rare in the pregnant population [9, 42]. Recurrent sympathetic activation in this population may be associated with disturbance in organ perfusion. For instance, a potential important target organ that may explain the association of SDB with adverse perinatal outcomes is the placenta. Placental tissue hypoxia [43] and secretory function alterations [44, 45] have been linked to SDB. Furthermore, a recent meta-analysis has shown that measures of arterial stiffness correlate with future development of placenta-mediated diseases such as preeclampsia and small for gestational age [46]. Future studies should assess the link between the autonomic activation associated with SDB and placental biology and function, and ultimately with adverse pregnancy outcomes.
Strengths of this study include its prospective design, the objective evaluation of SDB, and the continuous, all-night measurement of PTT, as well as the fact that all women were measured at the same stage of pregnancy. However, although PTT has many merits, it is not without drawbacks. PTT measurements are considered semiquantitative and limited in terms of accurately measuring the actual PTT; however, this does not impact its ability to examine quantitative changes in value or drops associated with respiratory effort and autonomic arousals. In addition, measurements are subject to artifact, which could be incorrectly interpreted as a shift in inspiratory effort, or a change in the baseline PTT [47]. These potential errors were minimized in our study by both automatic and manual scoring with removal of artifact associated with movement or poor oxygen saturation signal. Scoring of PTT is also difficult during rapid eye movement sleep given the large variation in respiratory drive that occurs during this sleep stage, making the distinction between normal breathing variations and obstructive events more problematic [47]. PTT is ineffective in patients with existing cardiovascular disease such as arrhythmias or cardiac conduction defects, pacemakers, or in those using vasoactive medication. Such patients were excluded from our study. Finally, another limitation is the use of level III in-home sleep studies, rather than in-laboratory sleep studies, which are limited by the inability to record sleep and measure arousals. It is possible that arousals—not detected with technology used with this device—may correlate with the PTT drop. However, previous studies have shown that although pregnant women may have more arousals (mainly spontaneous) than nonpregnant women, this occurs mainly in the third rather than the first trimester, which does not apply to our study sample [48]. It is possible that respiratory event–related arousals may explain the differences between PTT drops and scored obstructive events. This possibility is not very likely as a similar level III device has been validated by the authors in pregnancy and the correlation between AHI 3% (hypopnea with 3% desaturation and apnea) and respiratory disturbance index was strong (r = 0.90) between the device and simultaneous polysomnography [10]. Performing large-scale pregnancy studies with in-laboratory polysomnography can be quite challenging due to cost as well as family and social constraints [48]. As many patients including pregnant ones are now routinely evaluated with a home sleep monitor, the choice of a level-III device constitutes a real-life scenario in the evaluation of SDB in this population. As women with an AHI at least five events per hour were referred for an intervention following the sleep studies, we were unable to examine maternal outcomes due to the current sample size and the intervention being an important confounder. Future research should focus on the evaluation of PTT during in-laboratory polysomnography to examine the association of this measure with arousals in this population. In addition, the potential impact of PTT measures on perinatal outcomes would be important to examine. Given the influence of sex hormones on sympathetic activation in response to sleep disturbances, studies that incorporate longitudinal pregnant as well as nonpregnant samples and examine circulating hormones would further help answer questions in regard to the effect of sex hormones on the intensity and duration of sympathetic activation and PTT drops in this population.
In conclusion, PTT detects significantly more events of increased inspiratory effort during sleep than conventional scoring of apneas and hypopneas during in-home sleep studies. This technology may prove valuable in evaluating women at risk for SDB and point to the prevalence of clinically relevant subtle disease in this population.
Funding
GB and this work are funded by National Institutes of Health (R01HD078515 and R01HL130702).
Conflict of interest statement: GB has received research equipment from Respironics.
Acknowledgments
We would like to acknowledge all the women who agreed to participate in the study. Ms. Beth Hott for her assistance in manuscript preparation.
Contributor Information
Brittany N Link, Department of Medicine, Brown University, Providence, RI.
Celine Eid, Department of Medicine, Brown University, Providence, RI.
Maggie H Bublitz, Department of Medicine, Warren Alpert Medical School of Brown University, Providence, RI; Department of Psychiatry and Human Behavior, Warren Alpert Medical School of Brown University, Providence, RI.
Martino F Pengo, Sleep Disorder Center, Department of Cardiovascular, Neural, and Metabolic Sciences, IRCCS Istituto Auxologico Italiano, University of Milan, Milan, Italy.
Myriam Salameh, Department of Medicine, The Miriam Hospital, Women’s Medicine Collaborative, Providence, RI.
Karin S Ludwig, Department of Medicine, The Miriam Hospital, Women’s Medicine Collaborative, Providence, RI.
Richard P Millman, Department of Medicine, Warren Alpert Medical School of Brown University, Providence, RI; Division of Pulmonary, Critical Care and Sleep Medicine, Department of Medicine, Rhode Island Hospital, Providence, RI.
Lance Dworkin, Department of Medicine, University of Toledo, Toledo, OH.
Ghada Bourjeily, Department of Medicine, Warren Alpert Medical School of Brown University, Providence, RI; Department of Medicine, The Miriam Hospital, Women’s Medicine Collaborative, Providence, RI; Division of Pulmonary, Critical Care and Sleep Medicine, Department of Medicine, Rhode Island Hospital, Providence, RI.
References
- 1. Bourjeily G, et al. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J. 2010;36(4):849–855. [DOI] [PubMed] [Google Scholar]
- 2. O’Brien LM, et al. Pregnancy-onset habitual snoring, gestational hypertension, and preeclampsia: prospective cohort study. Am J Obstet Gynecol. 2012;207(6):487.e1–487.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salihu HM, et al. Association between maternal symptoms of sleep disordered breathing and fetal telomere length. Sleep. 2015;38(4):559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Louis JM, et al. Obstructive sleep apnea and severe maternal-infant morbidity/mortality in the United States, 1998–2009. Sleep. 2014;37(5):843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cortese R, et al. Epigenomic profiling in visceral white adipose tissue of offspring of mice exposed to late gestational sleep fragmentation. Int J Obes (Lond). 2015;39(9):1432. [DOI] [PubMed] [Google Scholar]
- 6. Khalyfa A, et al. Sex dimorphism in late gestational sleep fragmentation and metabolic dysfunction in offspring mice. Sleep. 2015;38(4):545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khalyfa A, et al. Late gestational intermittent hypoxia induces metabolic and epigenetic changes in male adult offspring mice. J Physiol. 2017;595(8):2551–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Calaora-Tournadre D, et al. [Obstructive Sleep Apnea Syndrom during pregnancy: prevalence of main symptoms and relationship with Pregnancy Induced-Hypertension and Intra-Uterine Growth Retardation]. Rev Med Interne. 2006;27(4):291–295. [DOI] [PubMed] [Google Scholar]
- 9. Facco FL, et al. Association between sleep-disordered breathing and hypertensive disorders of pregnancy and gestational diabetes mellitus. Obstet Gynecol. 2017;129(1):31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sharkey KM, et al. Validation of the Apnea Risk Evaluation System (ARES) device against laboratory polysomnography in pregnant women at risk for obstructive sleep apnea syndrome. J Clin Sleep Med. 2014;10(5):497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bourjeily G, et al. Airflow limitations in pregnant women suspected of sleep-disordered breathing. Sleep Med. 2014;15(5):550–555. [DOI] [PubMed] [Google Scholar]
- 12. Edwards N, et al. Nasal continuous positive airway pressure reduces sleep-induced blood pressure increments in preeclampsia. Am J Respir Crit Care Med. 2000;162(1):252–257. [DOI] [PubMed] [Google Scholar]
- 13. Pamidi S, et al. ; American Thoracic Society Ad Hoc Committee on Inspiratory Flow Limitation. An official American Thoracic Society Workshop Report: noninvasive identification of inspiratory flow limitation in sleep studies. Ann Am Thorac Soc. 2017;14(7):1076–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cherniack RM, et al. A comparison of esophageal and intrapleural pressure in man. J Appl Physiol. 1955;8(2):203–211. [DOI] [PubMed] [Google Scholar]
- 15. Sher AE, et al. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19(2):156–177. [DOI] [PubMed] [Google Scholar]
- 16. Rasheid S, et al. Gastric bypass is an effective treatment for obstructive sleep apnea in patients with clinically significant obesity. Obes Surg. 2003;13(1):58–61. [DOI] [PubMed] [Google Scholar]
- 17. Brock J, et al. Use of pulse transit time as a measure of changes in inspiratory effort. J Ambul Monit. 1993;6(4):295–302. [Google Scholar]
- 18. Pitson DJ, et al. Value of beat-to-beat blood pressure changes, detected by pulse transit time, in the management of the obstructive sleep apnoea/hypopnoea syndrome. Eur Respir J. 1998;12(3):685–692. [DOI] [PubMed] [Google Scholar]
- 19. Patzak A, et al. Continuous blood pressure measurement using the pulse transit time: comparison to intra-arterial measurement. Blood Press. 2015;24(4):217–221. [DOI] [PubMed] [Google Scholar]
- 20. Pollak MH, et al. Aortic-radial pulse transit time and ECG Q-wave to radial pulse wave interval as indices of beat-by-beat blood pressure change. Psychophysiology. 1983;20(1):21–28. [DOI] [PubMed] [Google Scholar]
- 21. Svedmyr S, et al. Vascular stiffness determined from a nocturnal digital pulse wave signal: association with sleep, sleep-disordered breathing, and hypertension. J Hypertens. 2016;34(12):2427–2433. [DOI] [PubMed] [Google Scholar]
- 22. Pitson D, et al. Changes in pulse transit time and pulse rate as markers of arousal from sleep in normal subjects. Clin Sci (Lond). 1994;87(2):269–273. [DOI] [PubMed] [Google Scholar]
- 23. Van de Heyning PH, et al. Implanted upper airway stimulation device for obstructive sleep apnea. Laryngoscope. 2012;122(7):1626–1633. [DOI] [PubMed] [Google Scholar]
- 24. Pitson DJ, et al. Use of pulse transit time as a measure of inspiratory effort in patients with obstructive sleep apnoea. Eur Respir J. 1995;8(10):1669–1674. [DOI] [PubMed] [Google Scholar]
- 25. Parsons GH, et al. Mechanisms of pulsus paradoxus in upper airway obstruction. J Appl Physiol Respir Environ Exerc Physiol. 1978;45(4):598–603. [DOI] [PubMed] [Google Scholar]
- 26. Hening WA. Restless legs syndrome: a sensorimotor disorder of sleep/wake motor regulation. Curr Neurol Neurosci Rep. 2002;2(2):186–196. [DOI] [PubMed] [Google Scholar]
- 27. Netzer NC, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–491. [DOI] [PubMed] [Google Scholar]
- 28. Cairns A, et al. A pilot validation study for the NOX T3™ portable monitor for the detection of OSA. Sleep Breath. 2014;18(3):609–614. [DOI] [PubMed] [Google Scholar]
- 29. Berry RB, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aittokallio T, et al. Analysis of inspiratory flow shapes in patients with partial upper-airway obstruction during sleep. Chest. 2001;119(1):37–44. [DOI] [PubMed] [Google Scholar]
- 31. Katz ES, et al. Pulse transit time as a measure of arousal and respiratory effort in children with sleep-disordered breathing. Pediatr Res. 2003;53(4):580–588. [DOI] [PubMed] [Google Scholar]
- 32. Chami HA, et al. The association between sleep-disordered breathing and aortic stiffness in a community cohort. Sleep Med. 2016;19:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carter JR, et al. Sympathetic neural responses to 24-hour sleep deprivation in humans: sex differences. Am J Physiol Heart Circ Physiol. 2012;302(10):H1991–H1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carter JR, et al. Ovarian cycle and sympathoexcitation in premenopausal women. Hypertension. 2013;61(2):395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Champagne K, et al. Obstructive sleep apnoea and its association with gestational hypertension. Eur Respir J. 2009;33(3):559–565. [DOI] [PubMed] [Google Scholar]
- 36. Louis J, et al. Perinatal outcomes associated with obstructive sleep apnea in obese pregnant women. Obstet Gynecol. 2012;120(5):1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Palombini LO, et al. Inspiratory flow limitation in a normal population of adults in São Paulo, Brazil. Sleep. 2013;36(11):1663–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pamidi S, et al. Maternal sleep-disordered breathing and the risk of delivering small for gestational age infants: a prospective cohort study. Thorax. 2016;71(8):719–725. [DOI] [PubMed] [Google Scholar]
- 39. Reutrakul S, et al. Interactions between pregnancy, obstructive sleep apnea, and gestational diabetes mellitus. J Clin Endocrinol Metab. 2013;98(10):4195–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Somers VK, et al. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Argod J, et al. Differentiating obstructive and central sleep respiratory events through pulse transit time. Am J Respir Crit Care Med. 1998;158(6):1778–1783. [DOI] [PubMed] [Google Scholar]
- 42. Bourjeily G, et al. Central sleep apnea in pregnant women with sleep disordered breathing. Sleep Breath. 2015;19(3):835–840. [DOI] [PubMed] [Google Scholar]
- 43. Ravishankar S, et al. Evidence of placental hypoxia in maternal sleep disordered breathing. Pediatr Dev Pathol. 2015;18(5):380–386. [DOI] [PubMed] [Google Scholar]
- 44. Bourjeily G, et al. Placenta-secreted circulating markers in pregnant women with obstructive sleep apnea. J Perinat Med. 2015;43(1):81–87. [DOI] [PubMed] [Google Scholar]
- 45. Bourjeily G, et al. Obstructive sleep apnea is associated with alterations in markers of fetoplacental wellbeing. J Matern Fetal Neonatal Med. 2015;28(3):262–266. [DOI] [PubMed] [Google Scholar]
- 46. Osman MW, et al. Association between arterial stiffness and wave reflection with subsequent development of placental-mediated diseases during pregnancy: findings of a systematic review and meta-analysis. J Hypertens. 2018;36(5):1005–1014. [DOI] [PubMed] [Google Scholar]
- 47. Smith RP, et al. Pulse transit time: an appraisal of potential clinical applications. Thorax. 1999;54(5):452–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wilson DL, et al. Decreased sleep efficiency, increased wake after sleep onset and increased cortical arousals in late pregnancy. Aust N Z J Obstet Gynaecol. 2011;51(1):38–46. [DOI] [PubMed] [Google Scholar]