Abstract
Study Objectives
Sleep disturbances increase vulnerability for depression, but the mechanisms underlying this relationship are not well known. We investigated the effects of experimental sleep disruption on response bias (RB), a measure of reward learning previously linked to depression, and the moderating role of positive affect responses.
Methods
Participants (N = 42) were healthy adults enrolled in a within-subject crossover sleep disruption experiment that incorporated one night of uninterrupted sleep (US) and one night of forced awakenings (FA) in random order. On the day following each experimental sleep night, participants completed a probabilistic reward task to assess RB, and the Positive and Negative Affect Schedule-X. Participants were subgrouped according to positive affect responses: Preserved Positive Affect (i.e. positive affect scores maintained or increased; n = 15) or Reduced Positive Affect (i.e. positive affect scores decreased; n = 27) following FA.
Results
Contrary to our hypotheses, across participants, RB did not significantly differ between the US and FA sleep conditions (p = .67). However, the effect of sleep condition on RB was moderated by positive affect response (p = .01); those with preserved positive affect showed heightened RB following FA, whereas those with reduced positive affect showed diminished RB following FA. Changes in negative affect between US and FA did not moderate RB.
Conclusion
The inability to preserve positive affect through periods of sleep disruption may be a marker of diminished reward learning capability. Understanding how sleep disruption impacts positive affect responses and reward learning identifies a pathway by which sleep disturbances may confer risk for depression.
Keywords: sleep, sleep disruption, reward, reward learning, positive affect
Statement of Significance:
This work is significant because it provides novel data revealing that positive affective responses to sleep disruption are variable across individuals and that these responses are associated with distinct patterns of reward learning. By showing that reward learning deficits are concentrated among individuals reporting a loss of positive affect to sleep disruption, this study identifies multiple putative targets to prevent or treat major depression that may be the focus of future research efforts.
Introduction
Sleep is increasingly recognized as a critical regulator of mental health [1–3]. Sleep disturbances are highly prevalent, have steadily increased in prevalence over time [4], and are comorbid with several psychiatric disorders, particularly major depressive disorder (MDD) [5, 6]. For example, longitudinal evidence has revealed a prospective association whereby insomnia symptoms predict future depression incidence and severity [7–11]. The mechanisms accounting for this temporal relationship have not yet been elucidated. Although experimental sleep deprivation paradigms are not proxies for specific sleep disorders, they help explicate, in a controlled setting, the acute associations between sleep loss and cognitive/behavioral outcomes of interest, and therefore, provide a useful starting point when attempting to unravel complex clinical and behavioral relationships [12]. We undertook this study to investigate the extent to which experimental sleep disruption—a novel paradigm in which sleep is disrupted by repeated forced awakenings (FA)—alters reward learning, a reliable indicator of depression risk [13–15].
Patients with MDD demonstrate abnormal affect regulation [16–18], including difficulty generating and maintaining positive affect [19]. Performance on the probabilistic reward task (PRT), a signal detection measure of reward learning, has been validated as a sensitive assay of anhedonia [20–22], a clinical phenomenon characterized by the inability to derive pleasure from naturally rewarding events or other positive stimuli. The PRT discriminates depressed individuals from healthy controls [21] and is associated with deficits in multiple dimensions of depression, including both behavioral and neurobiological measures of positive valence systems [15, 23]. To our knowledge, no study has evaluated the effects of sleep loss (through either experimental or observational methods) on PRT performance. However, prior studies have shown that circadian misalignment [24], evening chronotype [25], and the interaction of a circadian clock gene (PER2) and sleep midpoint [26], are all associated with deficits in behavioral and neural responses to rewards, leading us to hypothesize that experimental sleep disruption would be associated with diminished PRT performance.
Sleep has long been known to modulate affective function, but recent research has shown that self-reported poor sleep quality [27–29], experimental sleep deprivation [30], and experimental sleep disruption [31, 32] all appear to impair positive affective function to a greater degree than negative affective function (for a review, see Palmer and Alfano [33]). For example, in a population-based study of women in Belgium, lower levels of sleep continuity and quality prospectively predicted clinical depressive symptomatology over the course of a year [29]. A finer-grained analysis of daily sleep and affect relationships in that sample revealed that nights of especially poor sleep continuity and quality robustly predicted diminished next-day positive affect to a greater extent than negative affect, suggesting that sleep deprivation-induced decreases in positive affect may serve as a risk factor for depression [29].
This study evaluated the role of experimental sleep disruption and subsequent positive affective responses on reward learning, assessed via the PRT, in healthy adults with normal sleep. Using a within-subject crossover design involving one night of uninterrupted sleep (US) and one night of experimental sleep disruption via FA, in random order, this study aimed to test whether (1) a night of experimental sleep disruption attenuates reward learning on the PRT (Hypothesis 1), and/or (2) if this effect is magnified among those who experience reduced positive affect (i.e. decrease in positive affective scores) as a result of the sleep disruption procedure (Hypothesis 2). Changes in negative affect in relation to reward learning were also explored.
Methods and Materials
Participants
The initial sample comprised 52 healthy adults. However, PRT data for eight participants failed quality control analysis (described later), and two participants did not complete the protocol, resulting in a final sample of N = 42 (27 females and 15 males). Of them, 48% were white (n = 20), 38% were African American (n = 16), 7% were Asian (n = 3), and 7% were of unknown race (n = 3). The average age was 26.92 years (SD = 6.65). Participants were all enrolled in a parent study [R01 DA032922 (MTS; MRI)] that involved two nights of each sleep condition; the measures reported here were all administered after the first night of each sleep condition. We have previously reported that positive affect was attenuated in a sample of participants that included 20 of the 42 participants contributing data to this study [31]. The aims of that project were centered around main effects of sleep disruption on positive affect and related measures, and therefore do not overlap with those of this article, which is principally focused on the effect of sleep disruption on PRT performance and the extent to which positive affective response moderates that effect.
Study eligibility
Eligibility was determined through a phone screen and three subsequent screening visits. The first screening visit included questionnaires related to participants’ medical history, mental health, and sleep quality. Inclusion and exclusion criteria are summarized in Table 1. Participants were required to be low caffeine users (self-reported ≤2 cups of coffee, or the equivalent, per day), nonsmokers, free of medical and psychiatric comorbidities, and “normal” sleepers. A trained professional conducted a Structured Interview for Sleep Disorders with each participant to rule out insomnia and verify “normal” sleep according to research diagnostic criteria [34] as follows: (1) individual has no complaints of sleep disturbance or daytime symptoms attributable to unsatisfactory sleep; (2) individual has a routine standard sleep/wake schedule characterized by regular bedtimes and rising times; (3) there is no evidence of a sleep-disruptive medical or mental disorder; (4) there is no evidence of sleep disruption due to a substance exposure, use, abuse, or withdrawal; and (5) there is no evidence of a primary sleep disorder.
Table 1.
General inclusion and exclusion criteria
| Inclusion criteria |
| • Healthy, 18–48 year olds meeting Research Diagnostic Criteria for Normal Sleepers • Nonsmoker/nicotine user • Low caffeine users (≤ 2 cups of coffee or equivalent per day) • Stable sleep phase within 09:00 pm and 10:00 am • Pittsburgh Sleep Quality Index Total Score < 5 • Total sleep time between 6.5 and 8.5 hours/night; sleep efficiency ≥85% (confirmed with averages of 1 week of sleep diary and actigraphy monitoring); Epworth Sleepiness Scale <10 |
| Exclusion criteria |
| • BMI ≥35 • History of chronic pain (lifetime history of pain persisting for ≥6 months) • Acute pain (measured via McGill Pain Questionnaire and 2 weeks of baseline sleep diaries) • Significant medical/psychiatric morbidity within 6 months or lifetime history of: bipolar disorder, psychotic disorder, recurrent major depression, posttraumatic stress disorder, or seizures • Significant symptoms of psychological distress (T-scores >64 on the Brief Symptom Inventory global scales) • Respiratory, hepatic, renal, or cardiac conditions that would contraindicate opioid administration • Lifetime history of substance abuse or dependence, including: alcohol; opioid use >36 doses or >7 days consecutive use • Prior adverse reactions to general anesthetics/opioids or capsaicin • Clinically significant abnormal complete blood count or comprehensive metabolic profile • Positive toxicology screen for recreational drugs (THC), stimulants, opioids, or benzodiazepines • Pregnant or lactating women • Polysomnography-confirmed apnea–hypopnea index < 10 • Significant lifetime history of serious head injury that is judged to influence pain processing or sleep systems |
Participants then completed a week of sleep diaries to verify that sleep was stable within the appropriate window (between 09:00 pm and 10:00 am on the majority of days, verified with a 7-day sleep diary). Diaries were the primary measure used to verify eligibility at the second screening visit, which also included blood work and a history and physical by a physician or nurse practitioner. Participants who remained eligible returned for an overnight PSG in the clinical research unit (CRU), which ruled out occult sleep disorders (e.g. obstructive sleep apnea, and periodic limb movement disorder). Standard urine toxicology screens were administered before the start of each inpatient stay to test for the presence of recreational drugs and opioid medications. Participants who remained eligible stayed in the CRU for the experimental phase.
The protocol was approved by the Johns Hopkins University and UCLA Institutional Review Boards, and all participants completed informed consent before participation.
Study design
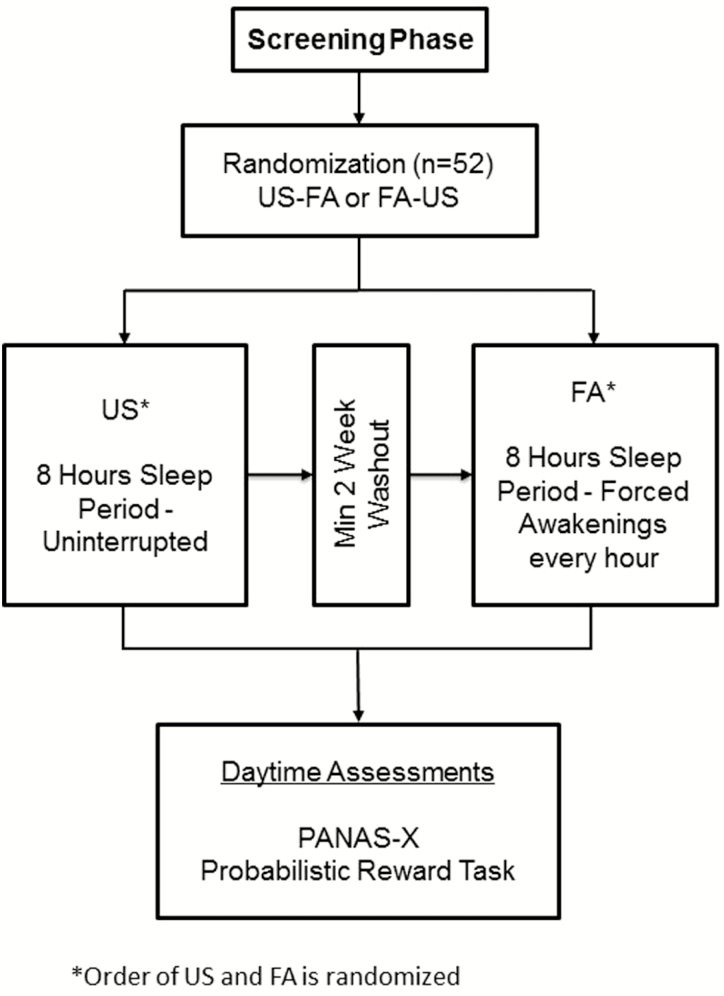
Figure 1 graphically displays the study design, which was a within-person crossover experiment in which participants underwent separate conditions of US and FA. Sleep condition order was randomized and stratified by sex, body mass index (>25 vs. ≤25), age (18–32 vs. 33–48 years), and Chinese ethnicity (due to ethnic difference in morphine metabolism relevant to parent study aims) [35]. At initial screening, participants underwent diagnostic psychological testing; a history and physical examination; and laboratory tests for infection, renal and hepatic function, and the presence of recreational drugs, as described earlier.
Figure 1.
Study design (adapted from Finan et al. [31]).
Eligible participants were then scheduled for an inpatient stay at the Johns Hopkins Bayview Medical Center Clinical Research Unit. Following an adaptation night, participants underwent either one night of US or one night of FA. On the day following the first experimental sleep night, participants completed questionnaires, the PRT, and other tasks described elsewhere [31]. PRT administration generally occurred before lunch between 10:30 am and 11:30 am. After a minimum 2-week washout period (to control for possible residual effects of the sleep manipulation), participants returned for the opposite sleep condition.
While inpatient for the study, participants consumed a heart-healthy diet free of fried, high fat, and high sodium foods. Breakfast was served around 7:30 am, lunch around 12 pm, and dinner by 5:30 pm. Caffeine, nicotine, alcohol, and other recreational drugs were prohibited during the inpatient stay at the clinical research unit. Naps were not permitted while on the unit and participants were monitored by nursing staff to verify compliance. Participants were outfitted with full polysomnography before the 11:00 pm Lights Out time. Polysomnography data have been reported elsewhere [31] and were not a focus of the present investigation.
Sleep manipulation
Uninterrupted sleep
Participants slept undisturbed during an 8-hour (480 minutes) sleep opportunity.
Forced awakenings.
The night was divided into eight 1-hour intervals, of which seven included a 20-minute FA period and one included a full 60-minute awakening, resulting in a 280-minute sleep opportunity period, as previously detailed [36]. Awakening periods were randomly determined. Participants were asked to sit upright in bed with the lights on to prevent microsleep and were monitored during these periods. Participants were not permitted to the leave the inpatient unit during this time.
Measures
Probabilistic reward task
The PRT is a reward-learning task grounded within signal detection theory [21, 37]. Participants were presented with three 100-trial blocks. Each trial consisted of the presentation of two perceptually similar stimuli that the participant was required to differentiate. For this study, a schematic face (diameter: 25 mm; eyes: 7 mm) was presented in the center of a 15-inch monitor. At the start of the trial, the face was presented without a mouth. After a brief delay (500 ms), a straight line appeared as the mouth. The line varied between 10.0 mm (little mouth) and 11.0 mm (big mouth) across trials. Participants were instructed to press the “v” or “m” key to indicate mouth line length (mouth/key associations varied randomly across participants and were counterbalanced across administrations). A total of 40 correct trials per block were followed by a monetary reward (“Correct!! You won 20 cents”). Although long and short mouths were presented at equal frequency, correct identification of one of the mouth lengths (the “rich” stimulus) was rewarded three times more frequently than correct identification of the other “lean” stimulus (30 vs. 10 per block). Participants were not aware of this asymmetrical reinforcement schedule. To avoid practice effects between the two separate administrations, two versions of the PRT were administered and counterbalanced between sessions: one in which the length of the mouth varied and another in which the length of the nose varied (which varied between 5.0 and 5.31 mm).
The extent to which participants biased their responding toward the rich stimulus relative to the lean stimulus provides an index of reward learning called response bias (RB). RB is calculated as follows:
To compute response bias for cases that had a zero in the formula, 0.5 was added to every cell in the matrix [38].
In addition to RB, discriminability—an index of more general task difficulty—was calculated as follows:
Participants’ accuracy, as a function of stimulus (rich vs. lean), block, and condition (US vs. FA) is provided in Supplementary Table 1.
Before analyses, PRT data were subject to a quality control assessment. Briefly, trials with reaction times shorter than 150 ms or longer than 1500 ms were excluded, as were trials with reaction times falling outside the range of mean +/– 3SD. Data regarding the number of trials excluded for these reasons, by block and condition, are provided in Supplementary Table 2. In addition, to ensure adequate exposure to the asymmetrical reinforcement schedule, participants displaying below chance (<55%) accuracy and/or more than 10% outlier trials (n = 8) were excluded from analysis.
Positive and Negative Affect Schedule-X
The Positive and Negative Affect Schedule-X (PANAS-X) [39] is a 60-item self-report instrument that assesses the degree to which participants indicate that they have felt a particular emotion on a 5-point Likert scale, where 1 = “Very Slightly or Not at All” and 5 = “Extremely”. Participants were asked to use “Today” as a temporal frame of reference for rating emotional states. The PANAS-X yields two general subscales for positive and negative affect, respectively, along with discreet emotion subscales. The general positive and negative affect scales were used for the present analyses. The measure is well validated and performs strongly in within-person designs [39].
Data analytic strategy
The study yielded a nested data structure (observations nested within people), which is effectively addressed with mixed-effects modeling. Models tested the within-subject effect of sleep condition on RB, both aggregated across blocks, and in a block-by-block time series. All models included a random intercept and were fit with restricted maximum likelihood.
Eight participants were missing PRT data from one of the two sleep conditions. Mixed-effects models are capable of handling missing data under the assumption that data were missing at random. Data inspection, however, indicated that 6 of the 8 missing PRT data points were associated with FA. This suggests that FA may have contributed to missingness. Therefore, we elected to include for analysis only those participants who provided complete data (N = 42).
Initial models tested the main effect of Sleep Condition on RB across the three blocks of the PRT. Subsequently, we fit a Sleep Condition × Block interaction to determine the rate of change in RB across blocks. We then included the Positive Affect Response grouping indicator (Preserved Positive Affect vs. Reduced Positive Affect; defined in the Results section) as a Level 2 interaction term. First, we fit a Sleep Condition × Positive Affect Response interaction and next a Sleep Condition × Block × Positive Affect Response interaction. The analytic strategy to examine Block effects is consistent with the original PRT study by Pizzagalli et al. [37]. and numerous subsequent studies examining response bias (e.g. [14,15,21]). Models described earlier were fit with age and sex as covariates.
Finally, we re-ran primary models in two ways to gauge the specificity of results. First, we substituted a Negative Affect Response indicator for Positive Affective Response to verify the extent to which results were specific to affective valence. Second, we exchanged RB for discriminability as the dependent variable to evaluate the extent to which results were reward-specific or instead associated with more general aspects of task performance, such as time-on-task. Analyses were conducted using SPSS MIXED (IBM SPSS Statistics, v24).
Results
Manipulation checks
As part of the screening process, participants completed sleep diaries during the week before first admission. Participants’ average bedtime was 11:44 pm and average wake time was 7:49 am (as a comparison, participants’ fixed bedtime and wake time in the experimental phase were 11:00 pm and 7:00 am, respectively). A scatterplot of subject-by-subject bedtimes and wake times is presented in Supplementary Figure 1.
The FA procedure successfully increased sleepiness and fatigue. Following FA relative to US, participants reported greater sleepiness at the approximate time of testing (11:00 am) (US sleepiness: M = 1.68, SD = 0.85; FA sleepiness: M = 2.86, SD = 1.18; p < .001) and fatigue (US fatigue: M = 4.98, SD = 1.97; FA fatigue: M = 10.52, SD = 3.27; p < .001). Polysomnography-derived estimates of sleep architecture and performance on the psychomotor vigilance task as a function of sleep condition are summarized in Supplementary Table 3. In addition, psychomotor vigilance measures were compared between Positive Affect Response groups and are summarized in Supplementary Table 4. The Sleep Condition × Positive Affect Response interaction tests for lapses and reciprocal response time were not statistically significant (p’s > .40), suggesting that Positive Affect Response groups did not differ in alertness following FA.
Tests of sleep disruption effects on reward learning
Contrary to Hypothesis 1, there was no evidence for a main effect of Sleep Condition (p = .67) or Sleep Condition × Block interaction (p = .86). Together, these findings suggest that, across participants, neither overall RB nor change in RB across blocks of the PRT was affected by sleep condition. The main effect of Block was significant (B = .05, t = 3.36, p = .001), revealing a significant increase in RB across blocks, on average across sleep conditions.
Identification of Positive Affect Response groups
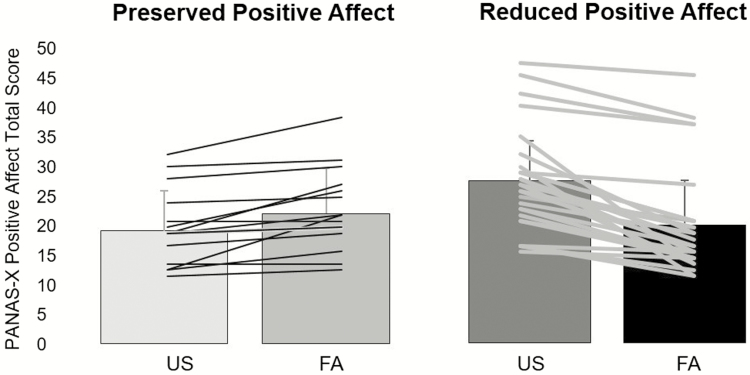
There was variability across participants in the effect of sleep disruption on positive affect (Figure 2). Decreases in positive affect occurred following FA in 27 participants, whereas positive affect was maintained or increased following FA in 15 participants (Figure 2). Thus, the value of zero was the cut point for the preserved positive affect (i.e. “Preserved Positive Affect Group”), whereas those who had a reduction in positive affect following FA relative to US of 1 point or greater were classified as “Reduced Positive Affect group.” This cut point allowed us to examine whether individual differences in the ability to preserve positive affect following sleep disruption influenced PRT performance, a key aim of the study.
Figure 2.
Changes in positive affect characterizing Preserved and Reduced Positive Affect Responses.
Positive affect scale scores as a function of sleep condition and Positive Affect Response group are summarized in Table 2. Notably, after US, the Reduced Positive Affect group had significantly higher positive affect compared with the Preserved Positive Affect group (F = 10.73, p < .002.), although after FA these two groups did not differ in positive affect levels (p = .47).
Table 2.
Positive affect scale scores as a function of sleep condition and Positive Affect Response groups
| US | FA | |||
|---|---|---|---|---|
| Preserved Positive Affect group | Reduced Positive Affect group | Preserved Positive Affect group | Preserved Positive Affect group | |
| Positive Affect Scores | 18.47 (6.61) | 26.63 (8.28) | 21.33 (7.39) | 19.37 (8.76) |
| Negative Affect Scores | 10.73 (2.05) | 10.85 (2.07) | 11.20 (2.10) | 10.85 (1.66) |
Means (and SDs) are provided in each cell.
Positive Affect Response moderates sleep disruption effects on reward learning
Because Positive Affect Response groups significantly differed in positive affect after US, we first ran a correlation analysis to assess the degree of correlation between US positive affect scores and reward learning after FA. US positive affect scores did not significantly correlate with RB after FA (r = .009, p = .96) or the difference in RB between US and FA (r = –.25, p = .10). Similarly, US positive affect scores did not significantly correlate with the ΔRB (i.e. the difference in RB between Block 1 and Block 3), following FA (r = –.08, p = .61) or the difference in ΔRB between US and FA (r = –.20, p = .20). Still, given the potential for US positive affect scores to influence Positive Affect Response group-based results, we elected to covary mean positive affect and person-centered positive affect (i.e. the within-subject deviation of positive affect from one’s mean) in the primary model testing whether Positive Affect Response moderated the effect of Sleep Condition on RB. These variables were weakly correlated (r = –.06, p = .71), supporting their use as independent regressors.
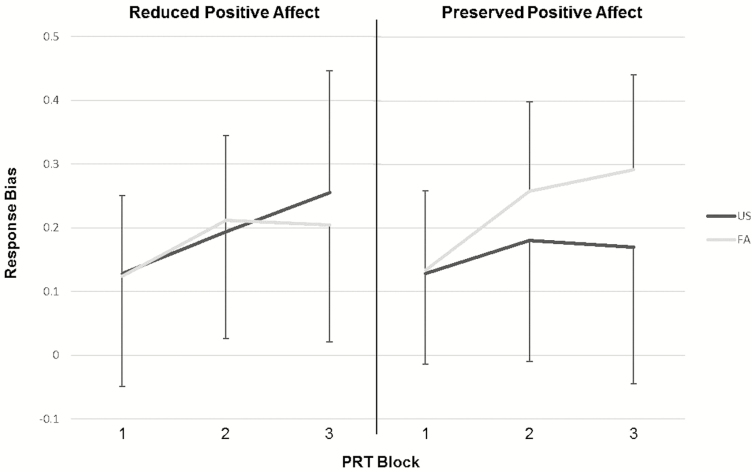
Consistent with Hypothesis 2, there was a significant Sleep Condition × Positive Affect Response interaction (B = .16, t = 2.50, p = .01). The full model parameters are displayed in Table 3. The source of the interaction is displayed in Figure 3 and shows that the Reduced Positive Affect group evidenced diminished RB following FA, whereas the Preserved Positive Affect group evidenced increased RB following FA. A follow-up analysis entering Block as a factor supported the primary analysis and revealed a Sleep Condition × Positive Affect Response × Block interaction that approached statistical significance (B = .08, t = 1.88, p = .06).
Table 3.
Mixed-effects model parameters
| Estimates of fixed fffects | |||||
|---|---|---|---|---|---|
| Parameter | B | SE | df | t | P |
| Intercept | .08 | .09 | 37.69 | .84 | .41 |
| age | .004 | .003 | 35.13 | 1.33 | .19 |
| sex | .04 | .04 | 35.61 | 1.01 | .32 |
| Sleep condition | –.09 | .04 | 200.56 | –2.53 | .01 |
| Positive affect response | –.06 | .05 | 90.91 | –.134 | .18 |
| Mean positive affect | .001 | .002 | 35.34 | .60 | .55 |
| State positive affect | –.006 | .004 | 204.55 | –1.38 | .17 |
| Sleep condition × positive affect response | .16 | .06 | 206.15 | 2.50 | .01 |
| Random effects | |||||
| Parameter | B | B | Wald Z | P | |
| Residual | .02 | .002 | 10.00 | <.001 | |
| Intercept | .007 | .003 | 2.75 | .006 |
Fixed and random effects from the primary mixed effects model are presented.
Figure 3.
Differential effect of sleep condition on response bias as a function of positive affect response.
Exploratory post hoc analyses
Because the Sleep Condition × Positive Affect Response × Block interaction approached significance, exploratory post hoc analyses were conducted to examine the simple effects of Sleep Condition and Block on RB separately within each Positive Affect Response group.
Simple effects within the Reduced Positive Affect group.
The Reduced Positive Affect group showed a significant total effect of Block following US (B = .06, t = 3.72, p < .001), within which there were marginally significant increases in RB from Block 1 to Block 2 (p = .06) and from Block 2 to Block 3 (p = .07). There was also a main effect of Block following FA (B = .04, t = 2.02, p = .05), within which there was a significant increase in RB from Block 1 to Block 2 (p = .04) but not from Block 2 to Block 3 (p = .85). Together, these data suggest that despite showing intact ability to learn from reward following US, the Reduced Positive Affect group evidenced impaired reward learning following FA.
Simple effects within the Preserved Positive Affect group.
The Preserved Positive Affect group did not show a significant effect of Block following US (p = .34), but did following FA (B = .08, t = 4.02, p < .001). Specifically, a significant increase in RB from Block 1 to Block 2 was observed following FA (p = .008), but not US (p = .26). This suggests that reward learning acquisition was augmented following FA in Preserved Positive Affect group. In contrast, the change in RB from Block 2 to Block 3 was not significant for either sleep condition (p’s > .23) in the Preserved Positive Affect group. Both Block 2 (B = .08, t = 2.28, p = .04) and Block 3 (B = 12, t = 2.33, p = .04) RB were significantly higher in FA relative to US among the Preserved Positive Affect group. Together, these effects suggest that reward learning acquisition was enhanced and maintained by sleep disruption in those who preserved positive affect.
Test of positive affect specificity.
To verify whether individual differences in the effects of sleep disruption on RB were moderated specifically by positive affective response, we constructed a similar factor differentiating participants on the basis of negative affective response to the FA condition. In contrast to findings using positive affect to subgroup participants, no Sleep Condition × Negative Affective Response interaction emerged in the prediction of RB (p = .36).
Test of reward learning specificity.
In addition, we investigated whether the Sleep Condition × Positive Affect Response interaction was specific to RB, and not reflective of general task difficulty. To do this, we re-ran the primary model substituting RB for discriminability as the dependent variable. Results showed that there was not a significant Sleep Condition main effect (p = .15) or Sleep Condition × Positive Affect Response interaction in the prediction of discriminability (p = .12).
Discussion
This study shows that the effects of experimental sleep disruption on reward learning depend on one’s positive affective response to sleep disruption. A main effect analysis failed to reveal differences in reward learning between US and sleep disruption conditions. However, splitting the sample into those who reported reduced positive affect following sleep disruption versus those who maintained or even gained positive affect following sleep disruption revealed opposing effects of sleep disruption on reward learning: the Reduced Positive Affect group evidenced attenuated reward learning whereas the Preserved Positive Affect group evidenced enhanced reward learning after sleep disruption. We previously showed that experimental sleep disruption significantly diminishes levels of positive affect [31, 40] as well as the propensity for evoked positive emotions to inhibit pain, without increasing the propensity for evoked negative emotions to facilitate pain [31]. The present findings extend this work by showing that sleep disruption-induced deficits in positive affect are related to deficits in reward learning. These moderating effects were also stronger for positive affect than negative affect. Furthermore, the fact that the group-based differences were observed while controlling for mean and person-centered positive affect levels supports the interpretation that it is one’s positive affective response to sleep disruption, rather than the trait or state-based positive affect level that dictates changes in reward learning.
Reward learning, as assessed through the PRT, is a robust indicator of risk for depression [20–22, 41]. The finding that RB is attenuated following sleep disruption, but only among those experiencing reduced positive affect, not negative affect, follows prior studies that have shown reward learning to be impaired among individuals with clinical symptoms of anhedonia. Deficits in reward learning are correlated with anhedonic symptoms among patients with MDD [14], lower self-reported hedonic capacity in healthy participants [14], and attenuated activation in the nucleus accumbens [42] and pregenual anterior cingulate cortex [43], mesocorticolimbic brain regions implicated in the experience of positive affect [44–46]. To evaluate the clinical significance of the present findings, similar hypotheses should be tested with clinical populations and directly evaluate the extent to which behavioral findings correlate with alterations in neurobiological function (e.g. via fMRI).
Although we expected individuals with reduced positive affective responses to sleep disruption to evidence greater reward learning deficits than those with preserved positive affective responses, we had not hypothesized that the latter group would show enhanced reward learning. A growing body of literature supports the broad notion that sleep loss augments reward system function, but these studies have primarily focused on risk-taking and explicit reward-related decision making. For example, studies have shown that total sleep deprivation increases activation in key nodes of the corticostriatal circuits (e.g. ventral striatum and ventral medial prefrontal cortex) during recognition of positively valenced affective stimuli [47] and both reward anticipation and receipt [48–50]. Similarly, poor sleep quality and evening chronotype, which are common characteristics of insomnia [51, 52], are correlated with risky decision making [53] and increased ventral striatal activity during monetary reward receipt [54], respectively. Our behavioral findings complement this body of research and support the broad interpretation that the effect of sleep loss on positive valence systems is nuanced and may be influenced by a variety of factors related to both sleep and reward-related measurement.
Notably, our results show that the propensity to gain versus maintain/lose negative affect following sleep disruption did not moderate the effects of sleep disruption on reward learning. This suggests that the present findings are specific to positively valenced emotional responses, and therefore reflect individual differences in positive affective response to a greater degree than general affective function. This finding further supports prior work with the PRT showing that reward learning deficits are more strongly associated with anhedonic characteristics of depression than negatively valenced depressive symptoms [14]. Taken together with our prior finding that sleep disruption more strongly impaired positive than negative affect [34], and evidence that MDD is substantially more likely than anxiety to develop secondary to insomnia [12], the present experimental findings suggest that diminished positive emotionality and reward learning may be interdependent mechanisms linking insomnia to depression.
This study had some limitations that deserve mention. First, it was a generally young sample, so we were unable to probe differences in reward learning across the developmental spectrum. Second, although we have found sex differences in other effects of sleep disruption, such as pain sensitivity [55], we did not have a large enough sample to examine whether sex moderated the interaction of sleep condition and positive affective response. Third, we only assessed changes in reward learning after a single night of sleep disruption and were, therefore, unable to characterize dose–response effects or the possible reversibility of reward learning changes with recovery sleep. Fourth, the Positive Affect Response groups were unbalanced in this sample, with the Preserved Positive Affect group at approximately half the size of the Reduced Positive Affect group. Future studies evaluating the reproducibility of these findings should recruit larger samples to determine if the distribution we observed here is representative of the population and if the reward-learning differences are statistically stable with larger cell sizes.
Summary and future directions
In this study, we show that the effects of sleep disruption on reward learning are contingent on one’s positive affective responses to acute sleep disruption. The present findings help to unify a complex literature on sleep and positive valence systems, which has shown that sleep loss both blunts positive affective function [34] and augments risk-taking behaviors [53] and neural responses to rewards [48–50]. Here, we show that a behavioral index of reward learning is attenuated in individuals who experience reductions in positive affect following sleep disruption but is enhanced in those whose positive affect is more resilient to the effects of sleep disruption.
The present results lay the groundwork for several future research directions. First, future experimental studies should directly compare reward learning changes in the context of total sleep deprivation versus sleep disruption experimental paradigms to determine if qualitative differences in sleep loss influence reward learning. Second, studies should evaluate if our results, observed via acute experimental sleep disruption, can be extended in patients with chronic sleep problems, such as those with sleep maintenance insomnia. Because our experimental model is qualitatively different than clinical insomnia, a logical next step will be to test whether there is a dose–response effect of insomnia symptom severity on reward learning, and the extent to which such an effect is moderated by naturally occurring positive affect assessed in the course of daily life. Finally, longitudinal studies are needed to evaluate when depressive symptoms develop in patients with primary insomnia relative to changes in positive affect and reward learning. Positive clinical findings, then, could warrant translational clinical intervention research to evaluate if mitigating positive affect reductions in response to sleep loss can reverse reward learning deficits and, consequently, diminish insomnia-related risk for depression.
Funding
The authors acknowledge funding for the present work from the National Institute of Health/National Institute on Drug Abuse: K23DA03915 (P.H.F.); R01DA032922 (M.T.S./M.R.I.). D.A.P. was partially supported by R37MH068376.
Disclosures
Over the past 3 years, Dr. Pizzagalli received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehreinger Ingelheim, Posit Science and Takeda Pharmaceuticals for activities unrelated to the present study. Dr. Pizzagalli has a financial interest in BlackThorn Therapeutics, which has licensed the copyright to the Probabilistic Reward Task through Harvard University. Dr. Pizzagalli’s interests were reviewed and are managed by McLean Hospital and Partners HealthCare in accordance with their conflict of interest policies. No funding from these entities was used to support the current work, and all views expressed are solely those of the authors.
Conflict of interest statement. The other authors declare no conflicts of interest with the present work.
Supplementary Material
References
- 1. Petrov ME, et al. Prevalence of sleep disorders by sex and ethnicity among older adolescents and emerging adults: relations to daytime functioning, working memory and mental health. J Adolesc. 2014;37(5):587–597. [DOI] [PubMed] [Google Scholar]
- 2. Reid KJ, et al. Sleep: a marker of physical and mental health in the elderly. Am J Geriatr Psychiatry. 2006;14(10):860–866. [DOI] [PubMed] [Google Scholar]
- 3. Sivertsen B, et al. Mental health problems in adolescents with delayed sleep phase: results from a large population-based study in Norway. J Sleep Res. 2015;24(1):11–18. [DOI] [PubMed] [Google Scholar]
- 4. Kronholm E, et al. Prevalence of insomnia-related symptoms continues to increase in the Finnish working-age population. J Sleep Res. 2016;25(4):454–457. [DOI] [PubMed] [Google Scholar]
- 5. Breslau N, et al. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39(6):411–418. [DOI] [PubMed] [Google Scholar]
- 6. Ford DE, et al. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479–1484. [DOI] [PubMed] [Google Scholar]
- 7. Buysse DJ, et al. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31(4):473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baglioni C, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135(1–3):10–19. [DOI] [PubMed] [Google Scholar]
- 9. Sarsour K, et al. Association of insomnia severity and comorbid medical and psychiatric disorders in a health plan-based sample: insomnia severity and comorbidities. Sleep Med. 2010;11(1):69–74. [DOI] [PubMed] [Google Scholar]
- 10. Cho HJ, et al. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165(12):1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee E, et al. Persistent sleep disturbance: a risk factor for recurrent depression in community-dwelling older adults. Sleep. 2013;36(11):1685–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lim J, et al. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. 2010;136(3):375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumar P, et al. Abnormal temporal difference reward-learning signals in major depression. Brain. 2008;131(Pt 8):2084–2093. [DOI] [PubMed] [Google Scholar]
- 14. Vrieze E, et al. Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiatry. 2013;73(7):639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaiser RH, et al. Frontostriatal and dopamine markers of individual differences in reinforcement learning: a multi-modal investigation. Cereb Cortex. 2017;28:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ballmaier M, et al. Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: an MRI-based parcellation of the prefrontal cortex. Am J Psychiatry. 2004;161(1):99–108. [DOI] [PubMed] [Google Scholar]
- 17. Cheng W, et al. Medial reward and lateral non-reward orbitofrontal cortex circuits change in opposite directions in depression. Brain. 2016;139(Pt 12):3296–3309. [DOI] [PubMed] [Google Scholar]
- 18. Knutson B, et al. Neural responses to monetary incentives in major depression. Biol Psychiatry. 2008;63(7):686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heller AS, et al. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc Natl Acad Sci USA. 2009;106(52):22445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morris BH, et al. Reward learning in pediatric depression and anxiety: preliminary findings in a high-risk sample. Depress Anxiety. 2015;32(5):373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pizzagalli DA, et al. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2008;43(1):76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu WH, et al. Deficits in sustaining reward responses in subsyndromal and syndromal major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(4):1045–1052. [DOI] [PubMed] [Google Scholar]
- 23. Vrieze E, et al. Measuring extrastriatal dopamine release during a reward learning task. Hum Brain Mapp. 2013;34(3):575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hasler BP, et al. Weekend-weekday advances in sleep timing are associated with altered reward-related brain function in healthy adolescents. Biol Psychol. 2012;91(3):334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hasler BP, et al. An altered neural response to reward may contribute to alcohol problems among late adolescents with an evening chronotype. Psychiatry Res. 2013;214(3):357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Forbes EE, et al. PER2 rs2304672 polymorphism moderates circadian-relevant reward circuitry activity in adolescents. Biol Psychiatry. 2012;71(5):451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bower B, et al. Poor reported sleep quality predicts low positive affect in daily life among healthy and mood-disordered persons. J Sleep Res. 2010;19(2):323–332. [DOI] [PubMed] [Google Scholar]
- 28. Kalmbach DA, et al. The interplay between daily affect and sleep: a 2-week study of young women. J Sleep Res. 2014;23(6):636–645. [DOI] [PubMed] [Google Scholar]
- 29. de Wild-Hartmann JA, et al. Day-to-day associations between subjective sleep and affect in regard to future depression in a female population-based sample. Br J Psychiatry. 2013;202:407–412. [DOI] [PubMed] [Google Scholar]
- 30. Talbot LS, et al. Sleep deprivation in adolescents and adults: changes in affect. Emotion. 2010;10(6):831–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Finan PH, et al. Partial sleep deprivation attenuates the positive affective system: effects across multiple measurement modalities. Sleep. 2017;40(1):zsw017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Finan PH, et al. The effects of sleep continuity disruption on positive mood and sleep architecture in healthy adults. Sleep. 2015;38(11):1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palmer CA, Alfano CA. Sleep and emotion regulation: an organizing, integrative review. Sleep Med Rev. 2017; 31:6–61. [DOI] [PubMed] [Google Scholar]
- 34. Edinger JD, et al. ; American Academy of Sleep Medicine Work Group Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27(8):1567–1596. [DOI] [PubMed] [Google Scholar]
- 35. Zhou HH, et al. Ethnic differences in response to morphine. Clin Pharmacol Ther. 1993;54(5):507–513. [DOI] [PubMed] [Google Scholar]
- 36. Smith MT, et al. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30(4):494–505. [DOI] [PubMed] [Google Scholar]
- 37. Pizzagalli DA, et al. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57(4):319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hautus MJ. Corrections for extreme proportions and their biasing effects on estimated values ofd′. Behav Res Methods Instrum Comput. 1995;27(1):46–51. [Google Scholar]
- 39. Watson D, Clark LA.. The PANAS-X: Manual for the Positive and Negative Affect Schedule-Expanded Form. 1999. [Google Scholar]
- 40. Finan PH, et al. Validation of a wireless, self-application, ambulatory electroencephalographic sleep monitoring device in healthy volunteers. J Clin Sleep Med. 2016; 12(11):1443–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol. 2014;10:393–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pizzagalli DA, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166(6):702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Whitton AE, et al. Blunted neural responses to reward in remitted major depression: a high-density event-related potential study. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1(1):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Caruana F, et al. Mirth and laughter elicited by electrical stimulation of the human anterior cingulate cortex. Cortex. 2015;71:323–331. [DOI] [PubMed] [Google Scholar]
- 45. Wacker J, et al. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. Neuroimage. 2009;46(1):327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wager TD, et al. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59(6):1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gujar N, et al. Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. J Neurosci. 2011;31(12):4466–4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Libedinsky C, et al. Sleep deprivation alters valuation signals in the ventromedial prefrontal cortex. Front Behav Neurosci. 2011;5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mullin BC, et al. Sleep deprivation amplifies striatal activation to monetary reward. Psychol Med. 2013;43(10):2215–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Venkatraman V, et al. Sleep deprivation biases the neural mechanisms underlying economic preferences. J Neurosci. 2011;31(10):3712–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chan JW, et al. Eveningness and insomnia: independent risk factors of nonremission in major depressive disorder. Sleep. 2014;37(5):911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ong JC, et al. Characteristics of insomniacs with self-reported morning and evening chronotypes. J Clin Sleep Med. 2007;3(3):289–294. [PMC free article] [PubMed] [Google Scholar]
- 53. Telzer EH, et al. The effects of poor quality sleep on brain function and risk taking in adolescence. Neuroimage. 2013;71:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hasler BP, et al. Eveningness among late adolescent males predicts neural reactivity to reward and alcohol dependence 2 years later. Behav Brain Res. 2017;327:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Smith MT, et al. Sex Differences in Measures of Central Sensitization and Pain Sensitivity to Experimental Sleep Disruption: Implications for sex Differences in Chronic Pain. Sleep. 2019;42(2):zsy209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.