Abstract
Study Objectives
Sleep–wake regularity (SWR) is often disrupted in college students and mood disorders are rife at this age. Disrupted SWR can cause repetitive and long-term misalignment between environmental and behavioral cycles and the circadian system which may then have psychological and physical health consequences. We tested whether SWR was independently associated with mood and autonomic function in a healthy adult cohort.
Methods
We studied 42 college students over a 3 week period using daily sleep–wake diaries and continuous electrocardiogram recordings. Weekly SWR was quantified by the interdaily stability of sleep–wake times (ISSW) and mood was assessed weekly using the Beck Depression Inventory-II. To assess autonomic function, we quantified the high-frequency (HF) power of heart rate variability (HRV). Linear mixed effects models were used to assess the relationship between repeated weekly measures of mood, SWR, and HF.
Results
Low weekly ISSW predicted subsequent poor mood and worsening mood independently of age, sex, race, sleep duration, and physical activity. Although no association was found between ISSW and HF, the ISSW-mood association was significantly moderated by nocturnal HF, i.e. reported mood was lowest after a week with low ISSW and high HF. Prior week mood scores did not significantly predict the subsequent week’s ISSW.
Conclusions
Irregular sleep–wake timing appears to precede poor mood in young adults. Further work is needed to understand the implications of high nocturnal HRV in those with low mood and irregular sleep–wake cycles.
Keywords: sleep wake regularity, mood, heart rate variability, autonomic function, circadian misalignment
Statement of Significance
Modern societal constraints can lead to irregular sleep and wake timing in early adulthood. Detrimental effects on psychological wellbeing and physical health are just beginning to emerge. This study shows that irregular sleep–wake cycles independently predict poor mood and change in mood during weeks with high nocturnal heart rate variability in college students. Three-quarters of chronic mental health burden begin before the age of 24; future studies are warranted to test whether increased sleep–wake regularity improves mood and wellbeing at an early stage in adulthood. The observed nocturnal heart rate variability pattern is surprising and may represent maladaptive changes in vagal modulation with unclear long-term health consequences.
Introduction
The sleep–wake cycle displays a ~24 hr rhythm that is regulated in part by the circadian system, but is also influenced by environmental factors such as work and school schedules [1]. Measuring the fluctuations in lifestyle and daily routine, in particular, sleep–wake regularity (SWR), is an emerging field in sleep research [2–5]. We know that the intrinsic circadian system is slow to accommodate sleep schedule changes [6], and misalignments with individuals’ actual sleep–wake cycle, as occasioned by shift work and irregular sleepwake times, have been associated with many adverse health consequences, including poor mood and perceived wellbeing [2, 3, 7–11].
A popular marker of sleep schedule fluctuation has been the concept of social jetlag (SJL), the difference in relative sleep–wake times between weekday and weekend nights [6, 12]. However, SJL uses an arbitrary dichotomization based on the assumption that work-life constraints are greatest during the traditional Monday–Friday paradigm; it is unclear whether these findings generalize to younger adults, such as college students, who are yet to enter the workforce. They may also exhibit rapidly changing daily sleep–wake times, which is believed to have the largest impact on the regulation of the circadian system [2]. One of the most commonly used measures of regularity that can account for day-to-day fluctuations, regardless of the day of the week, is the interdaily stability (IS) [13–15]. Given that occurrences of poor mood alone in earlier life have shown clear associations with later life chronic mental health consequences [16–18], the primary aim of this study was to examine the relationship between environmental-imposed SWR (as measured by the IS of sleep–wake times, ISSW), and mood in healthy, young college students.
In addition, altered functioning of the circadian system may underlie the adverse consequences of irregular sleep–wake and daily activity cycles via circadian influences on the autonomic nervous system (ANS) [19–22]. The ANS system maintains homeostatic control via the sympathetic and parasympathetic systems responding as appropriate during times of stress. Altered autonomic function, as characterized by heart rate variability (HRV) [23], has been linked to premature aging and a host of pathological states, including cardiovascular disease [24–26] and chronic mental health disorders [27–29]. It has been suggested that chronic imbalance between the sympathetic and parasympathetic systems leads to excessive energy demands and partially accounts for the various disease states associated with HRV [23].
However, there have been few studies to date examining the relationship between SWR and autonomic function. Studies involving HRV and circadian/sleep regulation have mainly been in disorders such as insomnia [30], tested sleep deprivation [31], or classified sleep stages [32]. Therefore, the second aim of our study was to examine the effect of SWR on autonomic function. Finally, we also explored the relationship between other potential contributors and circadian misalignment (social jetlag, chronotype, and delayed bedtime) on SWR, mood, and autonomic function.
Methods
Participants
Forty-two students at local colleges completed the study. All participants were between 19 and 29 years old (Mean [SD]: 22.7 [2.7] years old; 22 females and 20 males). Racial make-up included 18 (42%) Caucasians, 18 (45%) Asians, and 5 (12%) African Americans. All participants were healthy, nonsmoking, and drug- and medication free (except oral contraceptives), and denied sleep or psychiatric disorders. Specifically, participants were asked about their habitual sleep–wake times, duration of sleep, problems falling asleep (insomnia), falling asleep at inappropriate times (narcolepsy), and daytime fatigue or sleep apnea diagnosis. Any history of psychiatric care or diagnoses, medications (particularly antidepressants, antianxiety, antipsychotics, substance abuse, or sleeping pills) led to exclusion. The study was approved by the local Institutional Review Board. Participants provided informed consent prior to participation.
Protocol and data collection
The study consisted of an initial in-lab visit and a 3 week ambulatory phase. Participants were screened by phone, seen at an initial visit to the laboratory to determine eligibility, and again at the end of the study period. During the initial visit, body weight, height, body-mass index (BMI), blood pressure (BP), heart rate (HR), and respiratory rate (RR) were recorded. Participants also completed the Morning–Evening Questionnaire (MEQ score) [33] for chronotype assessment, and the Beck Depression Inventory-II (BDI) questionnaire for assessment of mood [34]. During the ambulatory phase, participants wore a Holter monitor (DF-010, DELBio, INC.) for continuous electrocardiogram (ECG) recordings and were asked to complete the sleep–wake diary and physical activity diary each day.
Sixty-two candidates were invited to the lab visit after initial phone screening. Fifty-eight completed the initial visit and were eligible for the study. Sixteen withdrew within the first week (the main issue was the continuous ECG monitor strap on the chest, some moved away from the city, reported that daily diaries were too tedious, or no reason given). Thus, 42 students completed the study and their data entered our analysis.
Sleep–wake regularity
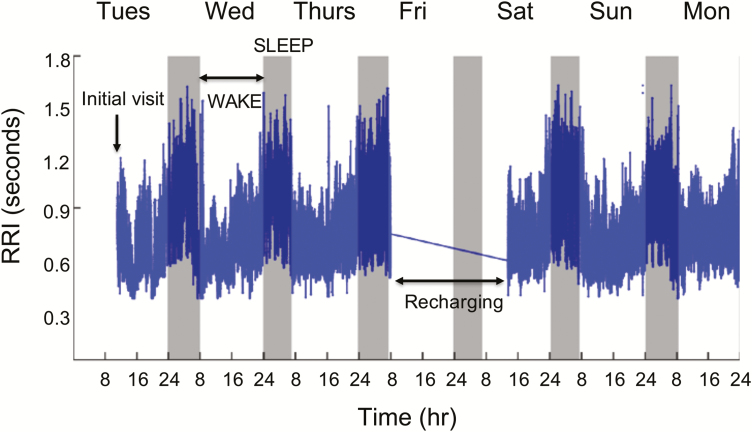
Sleep–wake diaries were reviewed with participants at the final visit to confirm sleep–wake patterns. The sleep–wake cycles were then plotted alongside the ECG-derived R–R intervals (RRI; see Figure 1 for representative participant data for 1 week). We found the majority of sleep schedules matched HR (inverse of RRI) responses consistent with sleep (increased RRI; decreased HR).
Figure 1.
Subject sleep–wake diary and electrocardiogram raw data. Representative subject week 1 data (7 days, 6 nights) for ECG-derived R–R interval values (RRI). Shaded regions represent reported sleep periods from diaries. Note the rise in RRI (fall in heart rate) during sleep periods. Gap in data due to scheduled recharging on the 4th day.
To quantify the regularity of the sleep–wake cycles in each week, we used the interdaily stability (ISSW) analysis. This provides a measure of the extent to which a periodic time series is similar in shape from cycle to cycle, and has been widely used to quantify the stability of daily motor activity rhythms [13, 15, 35]. In this study, we applied the analysis to the time series of sleep–wake cycles and obtained ISSW as a measure of SWR [36]. Ranging from 0% to 100%, larger ISSW values indicate more similar sleep–wake schedules from day to day (e.g. 100% indicates the exactly same sleep–wake timings everyday) (see Supplementary Figure S1).
Finally, we also used the sleep–wake diaries to calculate delayed bedtime as the total time spent (at least 2 hr) past their habitual sleep times per week. Social jetlag was calculated as the mean difference between the midpoint of sleep during weekdays and weekends across the 3 week period [6, 12]. Physical activity (PA) questionnaires were used to generate weekly Metabolic Equivalent of Task (MET)-minutes products as a quantitative marker of physical activity levels [37].
Mood assessment
To assess mood, the BDI questionnaire was administered for all participants at baseline during the initial visit, and at the end of week 3 during the final visit. After an interim analysis of the first 27 participants, we modified the protocol by adding two additional BDI questionnaires to be completed by the final 15 participants at home, immediately after weeks 1 and 2. This enabled us to better examine the temporal relationship between ISSW and BDI without any reported increase in participant task fatigue. In addition to weekly BDI scores, we calculated a change in BDI (ΔBDI) using the participant’s initial visit BDI score as a baseline. In total, 72 end-of-week BDI questionnaires (42 week 3 BDI scores, plus 30 additional BDI scores from the final 15 participants for weeks 1 and 2) were analyzed in this study.
Heart rate and heart rate variability
During the initial in-lab visit, all participants were instructed to wear a Holter monitor continuously for 3 days before taking off and charging the device for 1 day (see Figure 1 for representative participant data for 1 week). The same 4 day cycle was repeated over 21 days before the second in-lab visit. Thus, for each participant, there were up to 16 days of ECG data. The average duration of high-quality, continuous ECG recordings that entered analysis for each participant was ~250 hr (10.5 days), evenly represented across 3 weeks. Heart rate was calculated as the inverse of the RRI and qualitatively used where available to corroborate sleep–wake times. A third of the ECG data were obtained from sleep episodes.
For the analysis of HRV, cardiac interbeat intervals were first obtained using a QRS wave detector based on the amplitude and first derivative of the ECG waveform and were verified by a trained technician to ensure that only “normal-to-normal” R-wave times were included. Power spectra were calculated by fast Fourier transform of 3.41 Hz cubic spline resampled data across nonoverlapped 10 min time windows. The high-frequency (HF) power at 0.15–0.40 Hz was used as the primary measure of autonomic activity. The other frequency- and time-domain HRV indices were also explored: low-frequency power (LF: 0.04–0.15 Hz), ratio of LF and HF powers (LF/HF), the standard deviation (SDNN), square root of the mean of squares (RMSSD), and percentage > 50 ms (pNN50) of differences between adjacent normal-to-normal intervals. Using participants’ sleep–wake diaries, 30 min either side of each sleep–wake transition was excluded. HRV data were obtained in 10 min bins for the entire study period and then separated into sleep (HF sleep) and wake (HF wake) episodes for the 3 week study period, according to participants’ sleep–wake diaries.
Statistical analysis
To estimate the effect of a student’s weekly ISSW on his/her subsequent end-of-week BDI and change in BDI, we used a linear mixed effects model (LMM) with subject as a random factor for intercept, and restricted maximum likelihood to account for the repeated weekly measures across the 3 week study. We constructed models accounting for potential covariates: demographics (age, sex, and race), physical activity, and sleep duration. Similar models were used to explore the effects of chronotype, delayed bedtime and social jetlag, separately, while controlling the effect of ISSW. For the purposes of data presentation, the mean participant ISSW was calculated and grouped into lower and upper half of participant ISSW. Weekly ISSW were also binned into quartiles for data visualization. Partial correlations were used for nonrepeated measures. All analyses were performed using JMP Pro 13 (SAS Institute Inc., Cary, NC). Variables were log-transformed if found not to be normally distributed (e.g. HF and LF). Data are presented as mean (±standard deviation), unless otherwise indicated. Statistical significance was accepted as p < 0.05.
Results
Sleep–wake regularity is independent of sleep duration and physical activity
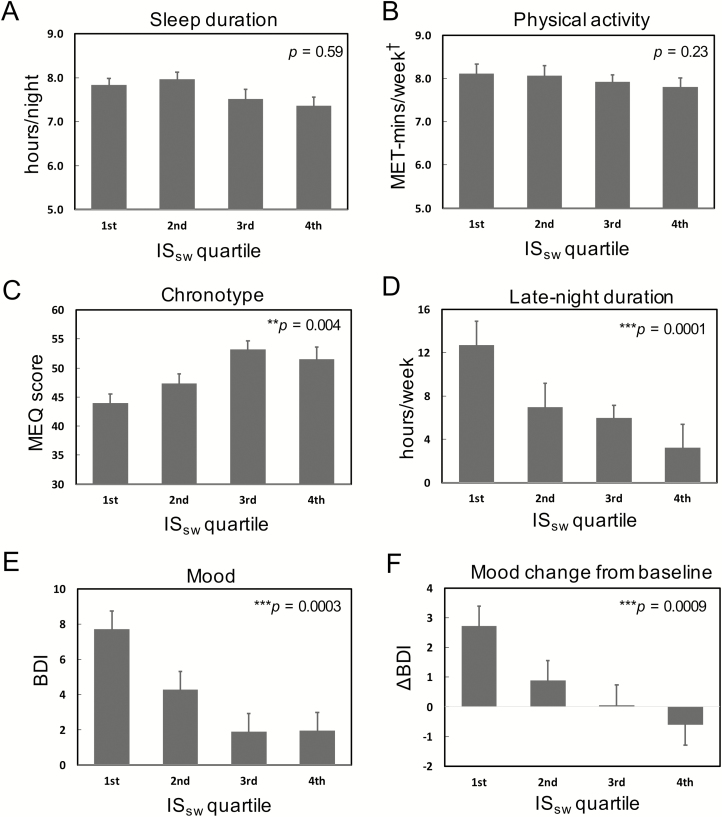
Table 1 summarizes student demographics (age, sex, and race), baseline vital signs, diary-derived results, and HRV results dichotomized by lower and upper half of mean participant ISSW, which was 71.6 (13.4), and ranged from 61.9 (lower half) to 81.4 (upper half). The students slept 7.7 (0.7) hr per night on average. We did not find any significant relationship between weekly ISSW and sleep duration (p = 0.59) or physical activity (p = 0.23) in linear mixed model (LMM) analysis (Figure 2, A and B). Moreover, no significant relationship was observed between demographics, baseline HR, RR, BP, BMI, or number of nights completed and ISSW (all p > 0.05).
Table 1.
Study participant characteristics by sleep–wake regularity (ISsw)
| Lower half of ISsw | Upper half of ISsw | All | |
|---|---|---|---|
| Subjects | 21 | 21 | 42 |
| ISsw | 61.9 (12.1) | 81.4 (4.8) | 71.6 (13.4) |
| Demographics | |||
| Age (years) | 22.2 (2.8) | 23.2 (2.6) | 22.7 (2.7) |
| Female | 52.4% (11) | 52.4% (11) | 52.4% (22) |
| Caucasian | 47.6% (10) | 38.1% (8) | 42.9% (18) |
| Asian | 38.1% (8) | 52.4% (11) | 45.2% (19) |
| Black/African American | 14.3% (3) | 9.5% (2) | 11.9% (5) |
| Baseline vital signs | |||
| HR (bpm) | 69.5 (11.6) | 70.2 (10.6) | 69.8 (11.0) |
| BP (mmHg) | 117/68 (13/10) | 116/67 (10/8) | 116/68 (12/9) |
| BMI (kg/m2) | 24.1 (3.1) | 23.3 (3.9) | 23.7 (3.5) |
| RR (breaths/min) | 17.3 (3.1) | 17.8 (2.4) | 17.6 (2.8) |
| Sleep and activity measures | |||
| Chronotype (MEQ score)—baseline | 45.8 (7.0) | 52.0 (7.3) | 48.9 (7.7) |
| Sleep duration (hr/night) | 7.8 (0.7) | 7.6 (0.8) | 7.7 (0.7) |
| Delayed bedtime (hr/week) | 10.7 (8.5) | 5.0 (6.4) | 7.8 (8.0) |
| Social jetlag (min) | 53.0 (99.4) | 26.9 (51.3) | 40.6 (80.4) |
| Physical activity (MET-min/week)† | 8.0 (0.6) | 7.8 (0.9) | 7.9 (0.7) |
| Mood | |||
| BDI—during study | 6.2 (5.1) | 2.1 (2.6) | 4.2 (4.5) |
| ΔBDI—weekly change | 1.4 (2.7) | −0.2 (1.6) | 0.6 (2.3) |
| Hear rate variability (during study) | |||
| Heart rate (sleep) | 65.3 (10.1) | 62.6 (6.6) | 63.9 (8.5) |
| Heart rate (wake) | 79.5 (7.7) | 83.1 (7.9) | 81.3 (7.9) |
| High-frequency power (sleep)† | 2.91 (0.42) | 3.12 (0.25) | 3.02 (0.36) |
| High-frequency power (wake)† | 2.63 (0.34) | 2.60 (0.25) | 2.62 (0.29) |
Data for 42 participants. Mean (standard deviation) and percentages (number of subjects). Lower half (1st and 2nd quartiles), upper half (3rd and 4th quartiles) for mean participant ISsw across the study.
ISsw = interdaily stability of sleep–wake times; HR = heart rate; bpm = beats per minute; BP = blood pressure; mmHg = millimeters mercury; BMI = body mass index; RR = respiratory rate; MEQ = morning–eveningness questionnaire; min = minutes; MET-min = Metabolic Equivalent of Task x minutes product; BDI = Beck Depression Inventory; ΔBDI = change in BDI.
†log-transformed.
Figure 2.
Effects of sleep–wake regularity on sleep, activity, and mood measures. Data (mean plus standard error bars) binned by weekly ISsw quartiles for illustration purposes; p values represent results from mixed effects models, in which ISsw was included as a continuous variable. (A) Weekly sleep duration. (B) Weekly physical activity assessed by Metabolic Equivalent of Task x minutes product (MET-mins). (C) Chronotype determined by morning–eveningness questionnaire (MEQ). (D) Weekly late-night duration. (E) Weekly mood assessed by Beck Depression Inventory (BDI). (F) Weekly BDI change from baseline (ΔBDI). Weekly sleep–wake regularity was estimated by interdaily stability of sleep–wake times (ISsw). †log transformed. *p < 0.05; **p < 0.01; ***p < 0.001. Error bars indicate standard errors.
Sleep–wake regularity is an independent predictor of mood and change in mood
The mean participant Beck Depression Inventory-II score (BDI) and change in BDI (ΔBDI) were 4.2 (4.5) and 0.6 (2.3), respectively (Table 1). Mean ΔBDI of all participants was not significantly different from zero (p = 0.09). LMM analysis showed that weekly ISSW was negatively associated with end-of-week BDI (b = −0.16, 95% CI: −0.24 to −0.08, p = 0.0003; Figure 2E and Table 2, Model 1a) and its change from baseline value (ΔBDI: b = −0.11, 95% CI: −0.17 to −0.05, p = 0.0009; Figure 2F and Table 2, Model 1a) independently of demographics, sleep duration, and physical activity. We then matched BDI scores with the subsequent week’s ISSW, but surprisingly found no significant relationship (p > 0.05). There were no significant differences in demographics, ISSW or BDI (all p > 0.05) between the first 27 subjects and the final 15 subjects (same protocol, additional home BDI assessment on weeks 1 and 2).
Table 2.
Linear mixed effects models for associations between sleep–wake regularity (ISsw), heart rate variability (HF), and mood (BDI)
| Model | BDI | ΔBDI | |||||
|---|---|---|---|---|---|---|---|
| b | 95% CI | P | b | 95% CI | P | ||
| 1a | ISSW | −0.16 | (−0.24 to −0.08) | 0.0003*** | −0.11 | (−0.17 to −0.05) | 0.0009*** |
| 1b | ISSW | −0.19 | (−0.27 to −0.11) | 0.00002*** | −0.13 | (−0.19 to −0.06) | 0.0002*** |
| HF sleep | 5.87 | (1.77 to 9.98) | 0.006** | 3.03 | (0.16 to 5.91) | 0.039* | |
| 1c | ISSW | −0.21 | (−0.29 to −0.13) | 0.000002*** | −0.14 | (−0.20 to −0.08) | 0.00001*** |
| HF sleep† | 6.46 | (2.68 to 10.2) | 0.002** | 3.08 | (0.44 to 5.72) | 0.023* | |
| ISSW * HF-sleep† | −0.27 | (−0.45 to −0.09) | 0.005** | −0.21 | (−0.34 to −0.08) | 0.003** |
Linear mixed effects models showing the effects of weekly ISsw (Model 1a), with HF sleep (Model 1b), and also with ISsw *HF sleep (Model 1c) on weekly mood (BDI) and change in mood (ΔBDI). Adjusted for demographics, physical activity, mean sleep duration, and heart rate during sleep.
BDI = Beck Depression Inventory; ΔBDI = change in BDI; ISsw = interdaily stability of sleep–wake times; HF sleep = high-frequency power during sleep.
Bold values indicate statistically significant (p < 0.05).
†log-transformed. *p < 0.05; **p < 0.01; ***p < 0.001.
Nocturnal heart rate variability moderates the association between sleep–wake regularity and mood
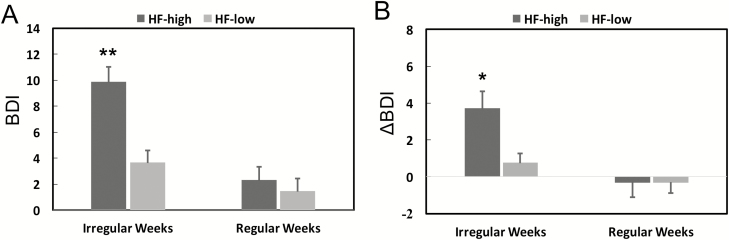
Mean weekly heart rate (HR) was significantly lower (p < 0.0001; Supplementary Figure S2A) and HF power of HRV was significantly higher during sleep compared with wake (p < 0.001; Supplementary Figure S2B) regardless of whether in the first two quartiles (Irregular Weeks) or the upper two quartiles (Regular Weeks). We did not find a significant relationship between ISSW and HF during sleep or wake. However, there was a significant interaction effect for ISSW and HF during sleep (ISSW*HF sleep; Table 2, Model 1c) on both BDI (b = −0.27, 95% CI: −0.45 to −0.09, p = 0.005) and ΔBDI (b = −0.21, 95% CI: −0.34 to −0.08, p = 0.003).
To further explore the interaction effect, we examined the differences in BDI/ΔBDI between “Irregular Weeks” (bottom half of ISSW values) and “Regular Weeks” (top half of ISSW values) by separating the cases with “HF-high” (top half of HF values) and the cases with “HF-low” (bottom half of HF values) during sleep. We found that after Irregular Weeks with HF-high during sleep, students reported significantly higher BDI (Figure 3A) and ΔBDI (Figure 3B) than Regular Weeks. Groups were equally sized and compared using post hoc Student’s t-test for the ISSW*HF sleep interaction term, adjusted for demographics, physical activity, mean sleep duration, and heart rate during sleep. Consistently, similar interaction effects on BDI and ΔBDI were observed for other HRV variables associated with vagal modulation (e.g. SDNN, RMSSD, and pNN50). The interaction effect on BDI (but not on ΔBDI) was borderline significant for LF (p = 0.046 for BDI and p = 0.24) but not for LF/HF (see Supplementary Table S1).
Figure 3.
Interaction effect of sleep–wake regularity and high-frequency power component of heart rate variability during sleep on mood. (A) Weekly mood assessed by Beck Depression Inventory (BDI). (B) Weekly BDI change from baseline (ΔBDI). For illustration, data were divided into four categories base on HF (HF-high: 1st and 2nd quartiles; HF-low: 3rd and 4th quartiles) and weekly ISsw (Irregular weeks: 1st and 2nd quartiles; regular weeks: 3rd and 4th quartiles). Comparison between groups using mixed effects models to account for repeated measures across weeks, and post hoc Student’s t-test for the ISsw*HF sleep interaction term, adjusted for demographics, physical activity, mean sleep duration, and heart rate during sleep. HF was log transformed. *p < 0.05; **p < 0.01 and represents difference from irregular weeks and HF high group. Error bars indicate standard errors.
Sleep–wake regularity and mood are associated with chronotype, delayed bedtime, but not social jetlag
We further explored whether participants with preference towards evening chronotype were more likely to suffer from lifestyle constraints leading to greater delayed bedtime, social jetlag, or lower ISSW, and potentially, poorer mood. Mean MEQ score was 48.9 (7.7) suggestive of “intermediate types” on average. Lower ISSW was associated with preference towards evening (lower MEQ; Figure 2C) and greater delayed bedtime (Figure 2D). Lower MEQ (b = −0.23, CI: −0.41 to −0.05, p = 0.012; Table 3, Model 2a) and delayed bedtime (b = 0.16, CI: 0.03 to 0.28, p = 0.014; Table 3, Model 2b) predicted higher BDI scores, but was not related to ΔBDI. However, neither remained significant once ISSW was introduced into the models (Table 3, Model 2c). Mean social jetlag was 41 ± 52 (SD) min and surprisingly not associated with ISSW (r2 = 0.01, p = 0.16), MEQ (r2 = 0.01, p = 0.57), or mood (r2 = 0.01, p = 0.96). We did not find any differences in HF during sleep or wake related to chronotype, delayed bedtime, or social jetlag.
Table 3.
Linear mixed effects models for associations between sleep–wake regularity (ISsw), chronotype (MEQ), delayed bedtime, and mood (BDI)
| Model | BDI | ΔBDI | |||||
|---|---|---|---|---|---|---|---|
| b | 95% CI | P | b | 95% CI | P | ||
| 2a | Chronotype (MEQ) | −0.23 | (−0.41 to −0.05) | 0.012* | −0.03 | (−0.16 to 0.10) | 0.62 |
| 2b | Delayed-bedtime | 0.16 | (0.03 to 0.28) | 0.014* | 0.02 | (−0.07 to 0.11) | 0.68 |
| 2c | ISSW | −0.10 | (−0.18 to −0.01) | 0.022* | −0.08 | (−0.18 to −0.01) | 0.018* |
| Chronotype (MEQ) | −0.12 | (−0.31 to 0.06) | 0.18 | 0.02 | (−0.12 to 0.16) | 0.77 | |
| Delayed bedtime | 0.05 | (−0.08 to 0.25) | 0.43 | 0.05 | (−0.08 to −0.19) | 0.43 |
Linear mixed effects models showing the effects of chronotype (Model 2a), late-night duration (Model 2b) and both with ISsw included (Model 2d), on mood (BDI), and change in mood (ΔBDI). Adjusted for demographics, physical activity, and mean sleep duration.
ISsw = interdaily stability of sleep–wake times; HF sleep = high-frequency power during sleep; BDI = Beck Depression Inventory; ΔBDI = change in BDI.
*p < 0.05.
Discussion
To the best of our knowledge, this is the first study to examine the relationship between sleep–wake regularity (SWR), mood, and HRV. We measured the HF power component, a marker of vagal modulation, in healthy young college students free from sleep and mood disorders, and medications, over an extended period of time. We identified a strong temporal association for low weekly ISSW preceding poor mood, and worsening mood, independent of age, sex, physical activity, and sleep duration in this cohort. There was a significant interaction effect between ISSW and nocturnal HF on mood such that significantly worse mood and worsening mood was reported by students after irregular weeks with high HF during sleep. Irregular sleep–wake cycles were more likely in individuals of evening types and were associated with greater delayed bedtime, whereas social jetlag in these students was unrelated.
Directional effect of sleep–wake regularity on mood
The observed effect of sleep–wake irregularity (ISSW) on mood is in keeping with recent studies linking regularity to happiness [38], wellbeing [11, 37], and even academic performance [2, 39]. A recent study also showed that irregular sleepers aged 50 and over-reported higher rates of depression symptoms [3]. Although studies have shown short-sleep duration to be associated with poor health outcomes [40] including depression [41], there was no relationship here between weekly ISSW and mean sleep duration. Interestingly, we found that prior BDI did not predict the next week’s ISSW. Change in BDI ranged from −4 to 9 but was not significantly different from zero, implying stable group mood with some degree of intraparticipant variability during the 3 week study period, which is typical for a healthy cohort [42]. That ISSW was able to predict intraparticipant changes in mood in our LMMs, however, lends further evidence that ISSW may be tightly coupled to weekly individual changes in mood.
To put some context on these findings, moving a student from the lower quartile (mean ISSW = 52) to the upper quartile (mean ISSW = 88) for a given week would translate into a decrease of 7.5 points (95% CI: 4.7–10.4; using our fully adjusted model in Table 2, Model 2b) for BDI. Given that moving from “mild mood disturbance” (BDI 11–16) to “moderate depression” (BDI 21–30) may require as little as a 5-point change, this is potentially a clinically significant effect size. Moreover, although the BDI-II is a robust assessment of mood with a retest interval mean of 2 weeks (ranging from 1 week to 6 months in 82% of studies) [43], there is evidence that testing at 1 week intervals (as we did in this study) leads to an underestimation of true depression symptoms [42]. Thus, the measured BDI scores in our study are unlikely to be overestimations of mood severity.
Despite the strong association and effect size, we remain cautious regarding interpretation of these findings. It is likely that many confounders exist, including timing of meals, personal or academic stressors, and type of physical activity. In addition, some have argued that mood may cyclically worsen during the luteal phase of the menstrual cycle [44, 45]. Although this study was not set up to address this, it is reassuring that no sex differences were found, and the study duration (21 days) encompassed three-quarters of a typical 28 day cycle. Finally, the study was designed to incorporate a full week for SWR/mood assessment; it could well be that the association between irregularity and poor mood is stronger for a different time lag (e.g. less than 7 days). Future studies are warranted to clarify the temporal relationship between SWR and mood, before causality or potential interventions can be considered.
Heart rate variability during irregular weeks and low mood
Preserved HRV, a marker of ANS control, has been thought to reflect physiological wellbeing, a healthy degree of social engagement and flexible adjustment to environmental demands [46]. Surprisingly, we found that participants who experienced weeks with irregular sleep–wake cycles (low ISSW) and nocturnally high vagal tone (indicated by HF) reported the highest BDI and increases in BDI. The interaction was independent of sleep duration and persisted after inclusion of chronotype and delayed bedtime. In addition, we replicated the same finding using other HRV markers of vagal modulation (e.g. SDNN, RMSSD, and pNN50; Supplementary Table S1).
Many possible mechanisms may underlie the effect of higher HF during sleep on the SWR-mood association. (1) One possibility is that those with low SWR and poor mood exhibited greater sleepiness or sleep propensity; this has been shown to increase cardiac vagal modulation in the first cycle of nonrapid eye movement (NREM) sleep [47]. A prospective study of 13 medical interns also showed increased HF during their first sleep episode after 33.5 hr shifts that corresponded to sleepiness scores [48]. (2) Activities close to sleep could have led to HRV changes during sleep. For example, moderate, habitual drinking of alcohol was suggestive of higher HF in healthy young adults of similar age (mean 20.5) to our participants [49], and higher SDNN in women with cardiovascular disease [50]. (3) The circadian rhythm of HRV and its relation to sleep stages has been noted by others [19, 20, 51, 52] such that the relative time spent in different sleep stages or shifted circadian phases may partially explain our nocturnal HF findings. It is possible that the higher nocturnal HF may be a reflection of stage-specific alterations in the intensity of cardiac vagal modulation secondary to irregular sleep–wake cycles and poor mood. The potential ramifications are unclear, but markers of vagal modulation such as HF during sleep have already been shown to play a role in human memory consolidation [53] and warrant follow-up studies using polysomnography.
We were able to demonstrate the well-documented increase in vagal modulation (lower HR and higher HF) during sleep versus wake, in keeping with prior studies [32, 51, 54]. However, it was surprising that only HRV during sleep (not during wakefulness) was a significant moderator and certainly needs replicating in future studies. Reassuringly, there was an appropriate representation of sleep (one third of the ECG data) compared with wake episodes, which was equally represented across weeks. We speculate that the observed HRV during sleep episodes was least likely to be affected by the unmeasured confounders mentioned above, and thus, closest to in-lab conditions.
Finally, it is worth noting that persistently high HRV may not always be protective. For instance, increased HRV during sleep was associated with cardiovascular events in diabetics [55] and predicted mortality in the elderly [56]. Persistent vagal rebound after repetitive psychological stressors is an adaptation noted in rodents [57] and humans [58] and has been linked to abnormal mood repair [59], recurrent depression [60], and even mood-disordered suicidal ideation [61]. High-nocturnal vagal modulation during young adulthood, together with poor mood and irregular sleep–wake cycles, has unknown impacts on long-term cardiovascular health. There could feasibly be burnout of a protective vagal system, where it is unable to keep check on sympathetic surges associated with cardiac events in later adult life [57, 58, 62], a potential mechanism for the coexistence of mood disorders and cardiovascular diseases [63, 64].
The role of chronotype, delayed bedtime, and social jetlag
SWR and mood are likely affected by a mixture of inherent and external lifestyle-related factors. Prior studies have linked evening chronotype to depression [65–67]. However, the underlying mechanism is not clear. In this study, we confirmed that students with preference towards evening chronotype exhibited greater delayed bedtime and irregular sleep–wake times (lower ISsw). Although chronotype and delayed bedtime were associated with poor mood, they were not related to change in mood. In addition, their associations with mood disappeared when adjusting for the effect of ISsw. These results suggest that SWR likely explains a significant amount of the effects of chronotype and delayed bedtime on mood, but also has independent predictive value for change in mood.
Despite recent studies linking social jetlag and health outcomes [12], we did not find any links for social jetlag with mood or other sleep variables in this cohort. This is somewhat surprising given that ISsw, to some extent, is supposed to incorporate social jetlag. One explanation is the small sample size and large variability, i.e. irregular students did report on average double the weekday–weekend shift, but also double the standard deviation (53 [99.4] vs 26.9 [51.3] min, Table 1). This is perhaps not surprising given that students are not yet constrained to a typical work week schedule and is in keeping with prior findings [68]. ISsw likely better represents daily fluctuation in college students’ lifestyle than social jetlag, since the weekday–weekend shift may not be pronounced in these individuals; thus, controlling for the weekday–weekend shift had no effect on our final model.
Overall, our findings point to a strong link between SWR and mood, which is significantly moderated by nocturnal HRV, as measured by HF. Irregular sleep–wake cycles has already been shown to disrupt circadian timing [2]; together with these findings, we postulate that SWR represents a common endpoint between evening chronotype and delayed bedtime, acting as a marker of habitual disruption to the circadian system.
Strengths, limitations, and future directions
Strengths of this study include good non-Caucasian representation, control for physical activity levels, unique extent of ECG recordings, and weekly study design for temporal assessment of regularity, mood, and autonomic signals. However, these findings should be interpreted with caution. This is a healthy cohort with subclinical BDI scores that limit generalizability. Mechanisms linking SWR and depressive disorder itself may differ and need to be further clarified. Although the study aimed to recruit healthy participants with normal BMI and vital signs, we were unable to definitively exclude disorders that are undiagnosed, nondisclosed, or typically not self-reported (e.g. sleep apnea or periodic limb movement disorder).
One potential difficulty in both the interpretation of the nocturnal HRV results and in SWR assessment lies in the fact that sleep–wake classification itself is known to be challenging regardless of methodology [69, 70]. Unfortunately, polysomnography is not feasible in field studies of this type and duration. Although we did review diaries with participants, alongside heart rate tracings, where available, it is conceivable that those who slept at irregular times (low SWR) and reported poor mood, either overestimated their sleep duration, or had unreported awakenings/activity at night, leading to an overestimation of nocturnal HRV, particularly since vagal modulation predominates before each transition from wake to sleep [71]. However, when we excluded 30 min either side of reported sleep and wake times, our results remained the same. Others have begun implementing nonlinear ECG and/or respiratory signals to detect sleep–wake states; this may best serve as an aid to sleep diaries since they remain inaccurate 1 in 5 times [72–74]. However, given our findings, this is certainly a direction for future analysis with ECG data, where available, to supplement sleep diaries, not only for state classification, but also for potential markers of sleep quality.
Taken together, further studies may be able to combine continuous cardiac and sleep monitoring with accurate recording of timing of activities, food, light exposure, and daily life stressors to delineate potential stage-specific vagal modulation differences in irregular sleepers with poor mood; this may represent an opportunity for identifying important factors affecting mood, performance, and lifelong productivity at an early stage in adulthood.
Funding
This work was supported by National Institutes of Health (NIH) grants T32GM007592 (to L.G.), R01AG048108, and RF1AG059867 (to K.H.). P.L. was supported by the International Postdoctoral Exchange Fellowship (20150042) from the China Postdoctoral Council. F.A.J.L.S. was supported in part by NIH grants R01HL118601, R01DK099512, R01DK102696, R01DK105072, and R01HL140574.
Conflict of interest statement. F.A.J.L.S. has received lecture fees from Bayer HealthCare, Sentara HealthCare, Philips, Vanda Pharmaceuticals, and Pfizer Pharmaceuticals.
Supplementary Material
References
- 1. Czeisler CA, et al. Sleep and circadian rhythms in humans. Cold Spring Harb Symp Quant Biol. 2007;72:579–597. [DOI] [PubMed] [Google Scholar]
- 2. Phillips AJK, et al. Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing. Sci Rep. 2017;7(1):3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lunsford-Avery JR, et al. Validation of the sleep regularity index in older adults and associations with cardiometabolic risk. Sci Rep. 2018;8(1):14158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Monk TH, et al. Circadian type and bed-timing regularity in 654 retired seniors: correlations with subjective sleep measures. Sleep. 2011;34(2):235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Soehner AM, et al. Circadian preference and sleep-wake regularity: associations with self-report sleep parameters in daytime-working adults. Chronobiol Int. 2011;28(9):802–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wittmann M, et al. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23(1–2):497–509. [DOI] [PubMed] [Google Scholar]
- 7. Sano A, et al. 0182 Influence of weekly sleep regularity on self-reported wellbeing. Sleep. 2017;40 (suppl_1):A67–A68. [Google Scholar]
- 8. Lemola S, et al. Variability of sleep duration is related to subjective sleep quality and subjective well-being: an actigraphy study. PLoS One. 2013;8(8):e71292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prigerson HG, et al. Lifestyle regularity and activity level as protective factors against bereavement-related depression in late-life. Depression. 1995;3(6):297–302. doi: 10.1002/depr.3050030607 [DOI] [Google Scholar]
- 10. Taub JM. Behavioral and psychophysiological correlates of irregularity in chronic sleep routines. Biol Psychol. 1978;7(1):37–53. doi:10.1016/0301-0511(78)90041–8 [DOI] [PubMed] [Google Scholar]
- 11. Wong M, et al. 1091 youth’s bedtime regularity mediates the association of depression and anxiety with negative attention bias. Sleep. 2017;40 (suppl_1):A407. [Google Scholar]
- 12. Wong PM, et al. Social jetlag, chronotype, and cardiometabolic risk. J Clin Endocrinol Metab. 2015;100(12):4612–4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Witting W, et al. Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol Psychiatry. 1990;27(6):563–572. [DOI] [PubMed] [Google Scholar]
- 14. Zuurbier LA, et al. Fragmentation and stability of circadian activity rhythms predict mortality: the Rotterdam study. Am J Epidemiol. 2015;181(1):54–63. [DOI] [PubMed] [Google Scholar]
- 15. Van Someren EJ, et al. Bright light therapy: improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol Int. 1999;16(4):505–518. [DOI] [PubMed] [Google Scholar]
- 16. Kaplan GA, et al. Psychosocial predictors of depression. Prospective evidence from the human population laboratory studies. Am J Epidemiol. 1987;125(2):206–220. [DOI] [PubMed] [Google Scholar]
- 17. Syed SA, et al. Early life stress, mood, and anxiety disorders. Chronic Stress Thousand Oaks Calif. 2017;1 doi: 10.1177/2470547017694461https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5482282/. Accessed December 4, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mental Health By the Numbers | NAMI: National Alliance on Mental Illness https://www.nami.org/learn-more/mental-health-by-the-numbers. Accessed September 7, 2018.
- 19. Buijs RM, et al. The circadian system and the balance of the autonomic nervous system. Handb Clin Neurol. 2013;117:173–191. [DOI] [PubMed] [Google Scholar]
- 20. Burgess HJ, et al. Sleep and circadian influences on cardiac autonomic nervous system activity. Am J Physiol. 1997;273(4 Pt 2):H1761–H1768. [DOI] [PubMed] [Google Scholar]
- 21. Calandra-Buonaura G, et al. Cardiovascular autonomic dysfunctions and sleep disorders. Sleep Med Rev. 2016;26:43–56. [DOI] [PubMed] [Google Scholar]
- 22. Hilton MF, et al. Endogenous circadian control of the human autonomic nervous system. Comput Cardiol. 2000;27:197–200. [PubMed] [Google Scholar]
- 23. Thayer JF, et al. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141(2):122–131. [DOI] [PubMed] [Google Scholar]
- 24. Hillebrand S, et al. Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: meta-analysis and dose-response meta-regression. Europace. 2013;15(5):742–749. [DOI] [PubMed] [Google Scholar]
- 25. Dekker JM, et al. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Atherosclerosis risk in communities. Circulation. 2000;102(11):1239–1244. [DOI] [PubMed] [Google Scholar]
- 26. Colhoun HM, et al. The association of heart-rate variability with cardiovascular risk factors and coronary artery calcification: a study in type 1 diabetic patients and the general population. Diabetes Care. 2001;24(6):1108–1114. [DOI] [PubMed] [Google Scholar]
- 27. Licht CMM, et al. Association between major depressive disorder and heart rate variability in the Netherlands Study of Depression and Anxiety (NESDA). Arch Gen Psychiatry. 2008;65(12):1358–1367. [DOI] [PubMed] [Google Scholar]
- 28. Brunoni AR, et al. Heart rate variability is a trait marker of major depressive disorder: evidence from the sertraline vs. electric current therapy to treat depression clinical study. Int J Neuropsychopharmacol. 2013;16(9):1937–1949. [DOI] [PubMed] [Google Scholar]
- 29. Gorman JM, et al. Heart rate variability in depressive and anxiety disorders. Am Heart J. 2000;140 (4 Suppl):77–83. [DOI] [PubMed] [Google Scholar]
- 30. Bonnet MH, et al. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med. 1998;60(5):610–615. [DOI] [PubMed] [Google Scholar]
- 31. Henelius A, et al. Heart rate variability for evaluating vigilant attention in partial chronic sleep restriction. Sleep. 2014;37(7):1257–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bonnet MH, et al. Heart rate variability: sleep stage, time of night, and arousal influences. Electroencephalogr Clin Neurophysiol. 1997;102(5):390–396. [DOI] [PubMed] [Google Scholar]
- 33. Horne JA, et al. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 34. Beck AT, Steer RA, Brown GK. BDI-II: Beck Depression Inventory Manual. 2nd edn. San Antonio, TX: Psychological Corporation, 1996. [Google Scholar]
- 35. Sokolove PG, et al. The chi square periodogram: its utility for analysis of circadian rhythms. J Theor Biol. 1978;72(1):131–160. [DOI] [PubMed] [Google Scholar]
- 36. Wu JQ, et al. Circadian rest-activity rhythms predict cognitive function in early Parkinson’s disease independently of sleep. Mov Disord Clin Pract. 2018;5(6):614–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Craig CL, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. [DOI] [PubMed] [Google Scholar]
- 38. Sano A, et al. Prediction of happy-sad mood from daily behaviors and previous sleep history. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:6796–6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Medeiros ALD, et al. The relationships between sleep-wake cycle and academic performance in medical students. Biol Rhythm Res. 2001;32(2):263–270. [Google Scholar]
- 40. Wong PM, et al. Shorter sleep duration is associated with decreased insulin sensitivity in healthy white men. Sleep. 2015;38(2):223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhai L, et al. Sleep duration and depression among adults: a meta-analysis of prospective studies. Depress Anxiety. 2015;32(9):664–670. [DOI] [PubMed] [Google Scholar]
- 42. Longwell BT, et al. The differential effects of weekly, monthly, and bimonthly administrations of the beck Depression Inventory-II: psychometric properties and clinical implications. Behav Ther. 2005;36(3):265–275. [Google Scholar]
- 43. Wang Y-P, et al. Psychometric properties of the Beck Depression Inventory-II: a comprehensive review. Rev Bras Psiquiatr Sao Paulo Braz 1999. 2013;35(4):416–431. [DOI] [PubMed] [Google Scholar]
- 44. Sanders D, et al. Mood, sexuality, hormones and the menstrual cycle. I. Changes in mood and physical state: description of subjects and method. Psychosom Med. 1983;45(6):487–501. [DOI] [PubMed] [Google Scholar]
- 45. Romans S, et al. Mood and the menstrual cycle: a review of prospective data studies. Gend Med. 2012;9(5):361–384. [DOI] [PubMed] [Google Scholar]
- 46. Rottenberg J. Cardiac vagal control in depression: a critical analysis. Biol Psychol. 2007;74(2):200–211. [DOI] [PubMed] [Google Scholar]
- 47. Delamont RS, et al. Sleep deprivation and its effect on an index of cardiac parasympathetic activity in early nonREM sleep in normal and epileptic subjects. Sleep. 1998;21(5):493–498. [DOI] [PubMed] [Google Scholar]
- 48. Lin YH, et al. Physiological and psychological impacts on male medical interns during on-call duty. Stress. 2012;15(1):21–30. [DOI] [PubMed] [Google Scholar]
- 49. Quintana DS, et al. Moderate alcohol intake is related to increased heart rate variability in young adults: implications for health and well-being. Psychophysiology. 2013;50(12):1202–1208. [DOI] [PubMed] [Google Scholar]
- 50. Janszky I, et al. Wine drinking is associated with increased heart rate variability in women with coronary heart disease. Heart. 2005;91(3):314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Boudreau P, et al. Circadian variation of heart rate variability across sleep stages. Sleep. 2013;36(12):1919–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Trinder J, et al. Autonomic activity during human sleep as a function of time and sleep stage. J Sleep Res. 2001;10(4):253–264. [DOI] [PubMed] [Google Scholar]
- 53. Whitehurst LN, et al. Autonomic activity during sleep predicts memory consolidation in humans. Proc Natl Acad Sci USA. 2016;113(26):7272–7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cabiddu R, et al. Modulation of the sympatho-vagal balance during sleep: frequency domain study of heart rate variability and respiration. Front Physiol. 2012;3:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Eguchi K, et al. Increased heart rate variability during sleep is a predictor for future cardiovascular events in patients with type 2 diabetes. Hypertens Res. 2010;33(7):737–742. [DOI] [PubMed] [Google Scholar]
- 56. Stein PK, et al. Sometimes higher heart rate variability is not better heart rate variability: results of graphical and nonlinear analyses. J Cardiovasc Electrophysiol. 2005;16(9):954–959. [DOI] [PubMed] [Google Scholar]
- 57. Carnevali L, et al. Vagal modulation of resting heart rate in rats: the role of stress, psychosocial factors, and physical exercise. Front Physiol. 2014;5:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mezzacappa ES, et al. Vagal rebound and recovery from psychological stress. Psychosom Med. 2001;63(4):650–657. [DOI] [PubMed] [Google Scholar]
- 59. Yaroslavsky I, et al. Combinations of resting RSA and RSA reactivity impact maladaptive mood repair and depression symptoms. Biol Psychol. 2013;94(2):272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kovacs M, et al. Maladaptive mood repair, atypical respiratory sinus arrhythmia, and risk of a recurrent major depressive episode among adolescents with prior major depression. Psychol Med. 2016;46(10):2109–2119. [DOI] [PubMed] [Google Scholar]
- 61. Lin Y, et al. Resting respiratory sinus arrhythmia is related to longer hospitalization in mood-disordered repetitive suicide attempters. World J Biol Psychiatry Off J World Fed Soc Biol Psychiatry. 2015;16(5):323–333. [DOI] [PubMed] [Google Scholar]
- 62. Lambert EA, et al. Morning surge in blood pressure is associated with reactivity of the sympathetic nervous system. Am J Hypertens. 2014;27(6):783–792. [DOI] [PubMed] [Google Scholar]
- 63. Musselman DL, et al. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55(7):580–592. [DOI] [PubMed] [Google Scholar]
- 64. Hare DL, et al. Depression and cardiovascular disease: a clinical review. Eur Heart J. 2014;35(21):1365–1372. [DOI] [PubMed] [Google Scholar]
- 65. Au J, et al. The relationship between chronotype and depressive symptoms: a meta-analysis. J Affect Disord. 2017;218:93–104. [DOI] [PubMed] [Google Scholar]
- 66. Vetter C, et al. Prospective study of chronotype and incident depression among middle- and older-aged women in the Nurses’ Health Study II. J Psychiatr Res. 2018;103:156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Antypa N, et al. Chronotype associations with depression and anxiety disorders in a large cohort study. Depress Anxiety. 2016;33(1):75–83. [DOI] [PubMed] [Google Scholar]
- 68. Knapen SE, et al. Social jetlag and depression status: results obtained from the Netherlands Study of Depression and Anxiety. Chronobiol Int. 2018;35(1):1–7. [DOI] [PubMed] [Google Scholar]
- 69. Paquet J, et al. Wake detection capacity of actigraphy during sleep. Sleep. 2007;30(10):1362–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ancoli-Israel S, et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. [DOI] [PubMed] [Google Scholar]
- 71. Kuo TB, et al. Asymmetry in sympathetic and vagal activities during sleep-wake transitions. Sleep. 2008;31(3):311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Adnane M, Jiang Z. Automatic sleep-wake stages classifier based on ECG. In: 2009 ICCAS-SICE. Fukuoka, Japan; 2009:493–498. https://ieeexplore.ieee.org/abstract/document/5334769 [Google Scholar]
- 73. Adnane M, et al. Sleep–wake stages classification and sleep efficiency estimation using single-lead electrocardiogram. Expert Syst Appl. 2012;39(1):1401–1413. [Google Scholar]
- 74. Karlen W, et al. Sleep and wake classification with ECG and respiratory effort signals. IEEE Trans Biomed Circuits Syst. 2009;3(2):71–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.