Abstract
Background
The APC trial showed that cyclooxygenase-2 (Cox-2) inhibitor, celecoxib, decreased adenoma development in patients at high risk for colorectal cancer. A prospectively planned analysis of the APC trial tested the hypothesis that expression of target enzymes in adenomas removed before beginning study treatment would identify individuals at high risk of adenoma development, and/or predict response to Cox-2 inhibition.
Methods
Pre-treatment adenomas were examined using immunohistochemistry (IHC) to assess expression of Cox-2 (high vs. low) and 15-prostaglandin dehydrogenase (15-PGDH, presence vs. loss). Mantel-Cox test evaluated whether these markers predicted benefit from celecoxib for reduction of adenoma detection.
Results
Patients whose pre-treatment adenomas demonstrated elevated Cox-2 achieved the greatest adenoma reduction with celecoxib treatment (RR 0.37; 95% confidence interval (CI) 0.22–0.61; p=0.0001). This reduction was less in the low Cox-2 category (RR 0.64, 95% CI 0.56–0.73). Patients whose pre-treatment adenomas showed 15-PGDH loss had a similar treatment-associated reduction in adenoma detection (RR 0.60; 95% CI 0.52–0.69; p<0.0001). In contrast, patients with intact tumor 15-PGDH expression did not significantly benefit from celecoxib (RR 0.73; 95% CI 0.47–1.12; p=0.15). However, subset analysis suggested that this lack of response to celecoxib was confined to those patients with 15-PGDH intact tumors who were also using cardioprotective aspirin.
Conclusions
The expression of Cox-2 and 15-PGDH in pre-treatment adenomas provides predictive information in patients treated with celecoxib for prevention of colorectal adenomas.
Impact
The results of this study show that Cox-2 and 15-PGDH are characteristics of colorectal adenomas that may be used to predict NSAID chemoprevention efficacy.
Introduction
Colorectal cancer (CRC) is a common cause of morbidity and death, yet it is also a disease for which effective prevention is possible. For decades, the removal of pre-malignant adenomas by endoscopic polypectomy has been the mainstay of preventive treatment (1). More recently, it has become clear that regular aspirin use also decreases CRC incidence and mortality (2,3). Despite this, 135,400 new CRC cases were identified in the US in 2016 (4). Because CRC is often diagnosed at a late stage, this disease also continues to claim the lives of 50,000 Americans per year (4).
Increased tissue prostaglandin synthesis, a feature of chronic inflammation, is a proposed mechanism of tumor promotion in the colorectum. Prostaglandin E2 (PGE2), a key mucosal inflammatory mediator, is produced when membrane phospholipids are metabolized by cyclooxygenases. PGE2 rapidly exerts its signaling effects via receptor binding and is then rapidly degraded by another tissue-specific enzyme, 15-prostaglandin dehydrogenase (15-PGDH), thereby limiting inflammatory signaling under normal circumstances. Tumors of the colorectum demonstrate increased PGE2 levels, and this condition occurs in association with elevated expression of the pro-inflammatory inducible cyclooxygenase-2 (Cox-2) enzyme, as well as loss of 15-PGDH (5–7).
The role of prostaglandin synthesis in development of colorectal neoplasia was dramatically illustrated by results from randomized clinical trials of the selective Cox-2 inhibitor, celecoxib, for prevention of pre-malignant adenomas (8–10). These trials studied colorectal adenoma formation in patients at high risk for CRC, and showed treatment-associated reductions in newly detected adenomas ranging from 13.7–31.8%. The drug also reduced by 41.3–54.7% the detection of higher risk lesions, which included advanced adenomas that were large (≥ 1 cm diameter) and/or demonstrated villous histology or high grade dysplasia. Unfortunately, the larger of the trials also identified a small but significant association between Cox-2 inhibitor use and occurrence of serious cardiovascular events (11). Although subsequent secondary analyses indicated that this risk may have been restricted to patients with cardiovascular risk factors prior to drug treatment, this toxicity was considered serious enough to prevent use of selective Cox-2 inhibitors for routine prevention of CRC (10). Adenoma trials were not specifically designed to test cardiovascular toxicity, and emerging data is now helping to better define the risk of Cox-2 inhibitor treatment. A post-marketing trial randomized 24,222 arthritis patients to receive celecoxib 100 mg bid, ibuprofen 600 mg tid, or naproxen 375 mg bid (12). These patients all had either an established history of cardiovascular disease or an increased risk for cardiovascular disease. The median treatment duration was 20.3 months and mean follow-up period was 34.1 months. During this time, serious cardiovascular events were experienced by 2.3% of patients receiving celecoxib, and 2.7% and 2.5% in the ibuprofen and naproxen groups, respectively. In addition, celecoxib users experienced significantly fewer gastrointestinal or renal adverse events. Given this new understanding of the risks associated with celecoxib, we used resources from the APC trial to more accurately identify patients most likely to have benefited from Cox-2 inhibitor chemoprevention.
Here, we report the results of a prospectively planned study of the relationship between target tissue prostaglandin activity and response to the selective Cox-2 inhibitor, celecoxib. The Adenoma Prevention with Celecoxib (APC) trial showed that celecoxib administration prevented colorectal adenomas, even in patients who developed adenomas while using aspirin on a regular basis (9). In a preliminary analysis of patients treated on the APC trial, those with low levels of 15-PGDH in normal colon samples demonstrated resistance to celecoxib treatment for adenoma prevention (6). We conducted a prospectively planned analysis of the APC trial to test the hypothesis that expression of target enzymes in adenomas removed before beginning study treatment would identify individuals at high risk of adenoma development, and/or predict response to Cox-2 inhibition.
Materials and Methods
Study Design
The APC Trial tested whether celecoxib would reduce the occurrence of endoscopically-detected colorectal adenomas. Details of participant recruitment and randomization for the APC trial cohort have been published previously (9). Exclusion criteria included a history of FAP, hereditary nonpolyposis colon cancer, inflammatory bowel disease, or large bowel resection other than appendectomy. Treatment consisted of either placebo, celecoxib 200mg bid, or celecoxib 400mg bid, with randomization stratified based upon pre-randomization low dose aspirin use (defined as doses of 325 mg po qod or 162.5 mg po qd) and clinical site. A study investigator performed a complete colonoscopy with visualization of the cecum and endoscopic removal of all polyps at 1 and 3 years after randomization. All polyps removed during these colonoscopies were reviewed by a central study pathologist. Investigator-reported adverse events were classified according to MedRA 8.1 criteria. Patients then returned to usual care, which entailed colonoscopic surveillance without chemoprevention. The trial involved 91 clinical sites; 72 in the United States, 8 in Australia, 10 in Canada, and 1 in Great Britain. Each site received human subjects committee approval of the study protocol, and all patients provided written consent prior to study enrollment. Study medication use was discontinued for all APC Trial participants on December 17, 2004. Median duration of study medication use was 2.95 years for the placebo group, and 2.94 and 2.99 years for the 200 mg bid and 400 mg bid celecoxib groups, respectively (10). All authors had access to the study data and reviewed and approved the final manuscript.
Immunohistochemistry for measurement of adenoma 15-PGDH and Cox-2 levels
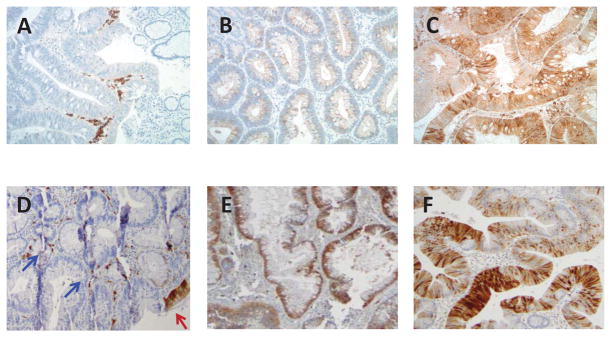
Of the 1822 patients with at least one on study colonoscopy, 1295 (71.1%) with available biospecimens for Cox-2 and 15-PGDH immunohistochemistry (IHC) evaluation were included in this study (Figure 1). Formalin-fixed, paraffin-embedded 4 μm sections of adenomas removed during pre-treatment and on-study colonoscopies were stained with antibodies to detect 15-PGDH (5) and Cox-2 (13), using standard IHC techniques (Figure 2 a–f). A single pathologist, who was blinded to study treatment scored all specimens for Cox-2 (DD), and 15-PGDH (AS).
Figure 1.
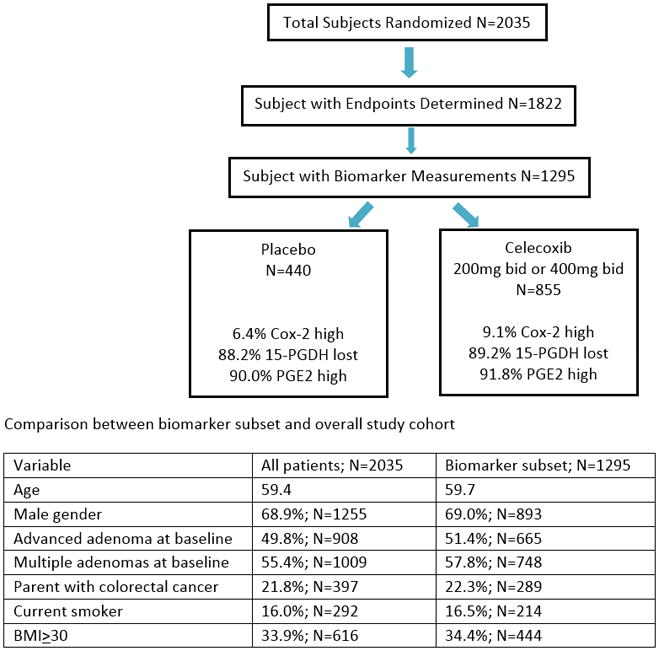
contains an image demonstrating the patient population and characteristics
Figure 2.
Cox 2 immunohistochemical staining
Figure contains immunohistochemical staining for Cox2 showed a wide spectrum of cytoplasmic reactivity ranging from no staining (A), weak staining (B) to diffuse strong staining (C) in the lesional adenoma cells. 15-PDGH immunostain was scored as loss of staining when cytoplasmic positivity was present only in the surface epithelium (D; red arrow) with no staining in the adenoma crypt epithelium (D; blue arrows). Note the positive staining in the stromal inflammatory cells (D) that served as an internal positive control. 15-PGDH was scored as positive when there was staining observed in >1% of cells in both surface and crypt epithelium and the intensity in these cases ranged from moderate (E) to strong (F).
Tissue Cox-2 expression was quantified according to both cytoplasmic staining intensity and relative percent staining (14). Adenomatous epithelium and stromal cells within the adenoma were scored according to the following scheme: 0- no staining, 1- weak, 2- moderate and 3- strong, intense expression. Relative percent staining was graded 0–3 using the following scheme: 0 or <1 percent = 0, 1–30% = 1, 31–74%=2, 75% or greater = 3. Sections of an adenoma were stained in parallel and served as a reference positive control to standardize scoring. Final scores equaled (stroma area × stroma intensity) + (epithelium area × epithelium intensity). Scores ranged from 0–18. To test the hypothesis that Cox-2 overexpression was associated with greater celecoxib efficacy, we chose the 90% level (score=12) as the cut-off for a dichotomized variable of high versus low Cox-2 expression. Expression of adenoma 15-PGDH was scored as being present when there was staining observed in >1% of cells in both surface and crypt epithelium. Cases with staining of surface epithelium only were scored as having 15-PGDH loss. Expression of adenoma 15-PGDH was quantified by relative percent staining of both surface and crypt epithelium. Any case where both crypt and surface epithelium demonstrated >1% staining were considered 15-PGDH present. All other cases were categorized as 15-PGDH loss. The PGE2 level was estimated to be low if Cox-2 level was low and 15-PGDH was present. Otherwise, PGE2 level was estimated to be high. Multiple pre-treatment adenomas were available for study in 41% of cases. In this situation, the patient’s condition was classified as Cox-2 high if any individual adenoma had Cox-2 score >=12 and 15-PGDH present if any individual adenoma met the 15-PGDH scoring criteria described above.
Statistical Analysis
All primary efficacy analyses were performed on an intention-to-treat basis, with primary endpoints determined for all patients with follow-up colonoscopies regardless of whether the patient complied with study drug use. The primary efficacy endpoint was detection of an adenoma during a post-randomization colonoscopy. In addition, the study plan included analysis of Cox-2 expression in adenomas removed from participants at baseline (pre-treatment) and during each study-related colonoscopy. Measurement of tumor 15-PGDH expression was added later, as the relevance of this enzyme to celecoxib chemoprevention was not recognized until after the trial was completed. Figure 1 provides data according to reporting recommendations for tumor marker prognostic studies (REMARK) guidelines. Analysis of the subset of participants for whom biomarker analysis was achieved showed consistency with respect to the overall study population for key variables associated with adenoma risk, including gender, presence of advanced or multiple adenomas at baseline, cancer family history, tobacco use, and BMI.
The associations of biomarkers’ expression in baseline adenomas with demographic characteristics and CRC risk factors were compared with Chi-square tests. The comparisons of treatment effects on cumulative incidence of adenoma within each category of biomarkers, or the comparisons of biomarkers’ prognostic performance within placebo group were performed with Mantel-Cox test (15). The summary risk ratio (RR), the weighted average relative risk over the time intervals, and corresponding 95% confidence interval (95% CI) were estimated. A similar test was performed according to one of the stratification factors, aspirin usage such that an exact two-sided p-value of the summary RR was computed due to the small number of events. For 444 participants who had adenoma recurrence, McNemar’s test was used to evaluate the changes in biomarker status before and after treatment. The Chi-square test was used to evaluate whether the change in biomarkers differed between celecoxib and placebo. All statistical analyses were performed with SAS software and all reported P values were two-sided with no adjustment for multiple comparisons.
Results
Loss of adenoma 15-PGDH expression is common and presence of 15-PGDH expression was associated with more advanced and multifocal disease
This study confirmed earlier work showing that loss of 15-PGDH expression is a common feature of early colorectal tumor formation. 15-PGDH was lost (i.e. lost in all pre-treatment adenomas tested in an individual subject) in 89.2% of participants, and this incidence was not affected by concurrent use of cardioprotective aspirin (Table 1). Previous analysis of the APC Trial showed that the presence of multiple or large adenomas at baseline predicted greater risk of post-polypectomy adenoma development (9). These well-known prognostic indicators are currently used in clinical practice to guide the timing of post-polypectomy surveillance colonoscopy (16). Interestingly, among those minority of patients (10.7%) whose adenomas retained 15-PGDH, most (70%) had advanced adenomas; whereas, advanced adenomas were present in only 49% of the larger group of 15-PGDH negative lesions (p<0.0001). Patients whose index adenoma retained 15-PGDH were also more likely to have multiple adenomas at baseline (33% vs. 25%; p=0.05). Examination of CRC family history showed that patients whose adenomas retained 15-PGDH were slightly less likely to have a parent with CRC than those whose tumors lost 15-PGDH (16% vs. 23%; p=0.05). High adenoma Cox-2 expression was relatively uncommon; it was detected in only 8.2% of cases. High Cox-2 expression was not associated with any of the risk variables studied (Table 1).
TABLE 1.
Association of biomarkers with demographic characteristics and CRC risk factors
| Characteristic | Strata | 15-PGDH absent (N=1156; 89.2%) | 15-PGDH present (N=139; 10.8%) | p-value | Cox-2 high (N=106; 8.2%) | Cox-2 low (N=1189; 91.8%) | p-value |
|---|---|---|---|---|---|---|---|
| Age (years) | 0.34 | 0.06 | |||||
| <65 | 786 (68%) | 100 (72%) | 64 (60%) | 822 (69%) | |||
| ≥65 | 370 (32%) | 39 (28%) | 42 (40%) | 367 (31%) | |||
| Gender | 0.87 | 0.81 | |||||
| Female | 358 (31%) | 44 (32%) | 34 (32%) | 368 (31%) | |||
| Male | 798 (69%) | 95 (68%) | 72 (68%) | 821 (69%) | |||
| Race | 0.96 | 0.99 | |||||
| Caucasian | 1080 (93%) | 130 (94%) | 99 (93%) | 1111 (93%) | |||
| Other | 76 (7%) | 9 (6%) | 7 (7%) | 78 (7%) | |||
| CRC family history1 | 0.05 | 0.42 | |||||
| Yes | 267 (23%) | 22 (16%) | 27 (25%) | 262 (22%) | |||
| No | 889 (77%) | 117 (84%) | 79 (75%) | 927 (78%) | |||
| Smoking History | 0.13 | 0.10 | |||||
| Current smoker | 183 (16%) | 31 (22%) | 11 (10%) | 203 (17%) | |||
| Former smoker | 532 (46%) | 62 (45%) | 47 (44%) | 547 (46%) | |||
| Never smoked | 440 (38%) | 46 (33%) | 48 (45%) | 438 (37%) | |||
| BMI | 0.27 | 0.49 | |||||
| ≥30 | 402 (35%) | 42 (30%) | 39 (38%) | 405 (34%) | |||
| <30 | 748 (65%) | 97 (70%) | 65 (63%) | 780 (66%) | |||
| History of diabetes | 0.26 | 0.96 | |||||
| Yes | 107 (9%) | 17 (12%) | 10 (9%) | 114 (10%) | |||
| No | 1049 (91%) | 122 (88%) | 96 (91%) | 1075 (90%) | |||
| History of cardiovascular disease1 | 0.84 | 0.92 | |||||
| Yes | 157 (14%) | 18 (13%) | 14 (13%) | 161 (14%) | |||
| No | 999 (86%) | 121 (87%) | 92 (87%) | 1028 (86%) | |||
| Advanced adenoma at baseline3 | <.0001 | 0.47 | |||||
| Yes | 568 (49%) | 97 (70%) | 58 (55%) | 607 (51%) | |||
| No | 588 (51%) | 42 (30%) | 48 (45%) | 582 (49%) | |||
| 3 or more adenomas at baseline | 0.05 | 0.44 | |||||
| Yes | 292 (25%) | 46 (33%) | 31 (29%) | 307 (26%) | |||
| No | 864 (75%) | 93 (67%) | 75 (71%) | 882 (74%) | |||
| Aspirin Use4 | 0.83 | 0.91 | |||||
| Yes | 364 (31%) | 45 (32%) | 34 (32%) | 375 (32%) | |||
| No | 792 (69%) | 94 (68%) | 72 (68%) | 814 (68%) | |||
| Study treatment | 0.66 | 0.23 | |||||
| Placebo | 388 (34%) | 52 (37%) | 28 (26%) | 412 (35%) | |||
| Celecoxib 200mg bid | 392 (34%) | 44 (32%) | 39 (37%) | 397 (33%) | |||
”yes” indicates history of parent with CRC
investigator reported, treatment emergent cardiovascular and thrombotic adverse events, including all events defined as myocardial infarction, cardiovascular therapeutic procedure, cerebrovascular disease, peripheral vascular disease, peripheral vascular therapeutic procedure, venous thrombosis or thromboembolism, and death or circulatory collapse due to cardiovascular cause
advanced adenoma = ≥ 1 cm diameter, villous or tubulovillous histology or high grade dysplasia
”yes” indicates using aspirin at a dose of ≤325 mg po qod or 162.5 mg po qd at study entry and continuing during study treatment
High Cox-2 expression in pre-treatment adenomas predicted greater response to celecoxib chemoprevention
The main purpose of this biomarker analysis was to determine whether markers of PGE2 expression in target lesions predicted either higher risk of recurrent adenoma formation, or benefit from celecoxib treatment for the primary study outcome of adenoma detection. Study of patients treated on the placebo arm showed that neither high Cox-2 expression nor 15-PGDH loss in pre-treatment adenomas was associated with increased post-polypectomy adenoma recurrence (Table 2). However, expression of Cox-2 and 15-PGDH did predict celecoxib response. Patients whose baseline adenomas demonstrated elevated Cox-2 achieved the greatest overall reduction in adenoma detection when treated with celecoxib (Table 2; RR 0.37,95% CI 0.22–0.61; p=0.0001). These patients constituted 8.2% of the participants for which biomarker determination was possible, and the 63% reduction in overall adenoma detection achieved with celecoxib treatment in this group was almost double that of patients in the low Cox-2 category (RR 0.64, 95% CI 0.56–0.73; p<0.0001). Patients with loss of 15-PGDH in baseline adenomas responded to celecoxib with a 40% risk reduction in adenoma detection (RR=0.60, 95% CI 0.52–0.69; p<0.0001), whereas patients whose tumors retained 15-PGDH demonstrated a nonsignificant 27% risk reduction (RR 0.73; 95% CI 0.47–1.12; p=0.15; Table 2).
TABLE 2.
Prognostic and predictive biomarker analysis for pretreatment adenoma Cox-2 and 15-PGDH determination
| Prognostic biomarker assessment: Patients receiving placebo | ||||
|---|---|---|---|---|
| Biomarker result | No. at risk | 3-year cumulative adenoma incidence (SE) | Risk Ratio (95% confidence interval) | p-value |
| 15-PGDH present | 52 | 51.5% (7.2) | 1.20 (0.87, 1.65) | 0.25 |
| 15-PGDH absent | 388 | 63.3% (2.5) | ||
| Cox-2 high | 28 | 70.5% (9.2) | 0.78 (0.57, 1.08) | 0.18 |
| Cox-2 low | 412 | 61.4% (2.5) | ||
| Predictive biomarker assessment: All patients | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Biomarker result | No. at risk | 3-year cumulative adenoma incidence %(SE) | Risk Ratio (95% confidence interval) | p-value | No. at risk | 3-year cumulative adenoma incidence %(SE) | Risk Ratio (95% confidence interval) | p-value | |
| 15-PGDH present | 15-PGDH absent | ||||||||
| Placebo | 52 | 51.5 (7.2) | 0.73 (0.47, 1.12) | 0.15 | Placebo | 388 | 63.3 (2.5) | 0.60 (0.52,0.69) | <0.0001 |
| Celecoxib | 87 | 42.4 (5.8) | Celecoxib | 768 | 41.9 (1.9) | ||||
| Cox-2 high | Cox-2 low | ||||||||
| Placebo | 28 | 70.5 (9.2) | 0.37 (0.22,0.61) | 0.0001 | Placebo | 412 | 61.4 (2.5) | 0.64 (0.56,0.73) | <0.0001 |
| Celecoxib | 78 | 34.1 (5.8) | Celecoxib | 777 | 42.7 (1.9) | ||||
For patients using aspirin, those whose baseline adenomas lacked 15-PGDH had a high risk of disease recurrence that was significantly reduced by celecoxib treatment
Approximately one-third of patients enrolled on the APC trial were chronic users of aspirin at cardioprotective doses (10). Because removal of adenomas at baseline was a study enrollment criterion, these patients were identified as being resistant to aspirin as a chemopreventive agent. The primary study analysis found that celecoxib use achieved equal chemopreventive efficacy among aspirin users and non-users (10). In this study, a total of 126 participants who used aspirin also showed loss of 15-PGDH in their pre-treatment adenomas. The 3-year cumulative recurrence rate on placebo for these patients was not significantly different from that of 262 aspirin non-users (63.0% vs. 63.4%; RR 1.01, 95%CI 0.82–1.24; p=0.95; Table 3). The RR associated with celecoxib use was also similar for aspirin users and non-users with 15-PGDH loss (RR 0.60 vs. 0.60 and 95% CI 0.47–0.77 vs. 0.51–0.71 respectively; Table 3).
TABLE 3.
Association of biomarkers and study treatment outcome with pre-treatment and on study aspirin use
| Aspirin users | ||||
|---|---|---|---|---|
| Biomarker result, patients receiving placebo | No. at risk | 3-year cumulative adenoma incidence (SE) | Risk Ratio (95% confidence interval) | p-value |
| 15-PGDH present | 16 | 32.5% (12.1) | 2.10 (0.95, 4.65) | 0.05 |
| 15-PGDH absent | 126 | 63.0% (4.5) | ||
| Cox-2 high | 12 | 83.3% (13.6) | 0.62 (0.41,0.96) | 0.09 |
| Cox-2 low | 130 | 57.6% (4.5) | ||
| No Aspirin use | ||||
|---|---|---|---|---|
| Biomarker result, patients receiving placebo | No. at risk | 3-year cumulative adenoma incidence (SE) | Risk Ratio (95% confidence interval) | p-value |
| 15-PGDH present | 36 | 60.0% (8.5) | 0.98(0.70, 1.38) | 0.99 |
| 15-PGDH absent | 262 | 63.4% (3.1) | ||
| Cox-2 high | 16 | 63.5% (12.3) | 0.92(0.58,1.46) | 0.82 |
| Cox-2 low | 282 | 63.1% (3.0) | ||
| Predictive biomarker assessment: All patients | |||
|---|---|---|---|
| Risk Ratio for on-study adenoma detection (95% confidence interval) | |||
| All patients | ASA users | No ASA use | |
| 15-PGDH present | 0.73 (0.47,1.12) N=139 |
1.16 (0.45,2.96) N=45 |
0.60 (0.36,0.99) N=94 |
| 15-PGDH absent | 0.60 (0.52,0.69) N=1156 |
0.60 (0.47,0.77) N=364 |
0.60 (0.51,0.71) N=792 |
| Cox-2 high | 0.37 (0.22,0.61) N=106 |
0.16 (0.05,0.50) N=34 |
0.50 (0.27,0.93) N=72 |
| Cox-2 low | 0.64 (0.56,0.73) N=1189 |
0.71 (0.55,0.91) N=375 |
0.61 (0.52,0.72) N=814 |
Exploratory comparisons were made for the subset of patients using aspirin whose baseline adenomas retained 15-PGDH expression. In these aspirin-using individuals, those treated with placebo (i.e. not celecoxib) had a low rate of disease recurrence of 32.5%, compared to 63.0% for those with 15-PGDH loss (RR 2.1; 95% CI 0.95–4.65; p=0.05; Table 3). These aspirin-using patients with 15-PGDH-positive index adenomas did not show further reduction in new adenoma detection with celecoxib treatment (RR 1.16; 95% CI 0.45–2.96). In contrast, for patients not using aspirin, equally high levels of adenoma recurrence were seen in the placebo arm for those either with or without 15-PGDH loss (RR 0.98, 95%CI (0.70–1.38); p=0.99). Further subset analysis showed that individuals not using aspirin but whose adenomas retained 15-PGDH did demonstrate a significant chemopreventive response to celecoxib (RR 0.60; 95% CI 0.36–0.99). Thus, the apparent resistance to celecoxib in individuals whose adenomas retained 15-PGDH appears due to the already strong reduction of recurrence risk among aspirin users in this group.
Celecoxib use did not alter Cox-2 expression in adenomas that developed during treatment
Analysis of biomarker expression in pre-treatment adenomas found no association between biomarker prevalence and aspirin use (Table 1). We also examined adenomas removed during on-study colonoscopies to determine if celecoxib use altered biomarker expression in these target lesions. In 200 patients treated with placebo and 244 patients receiving celecoxib, adenomas removed during on-treatment colonoscopies were available for biomarker determination. Biomarker expression was remarkably stable throughout study treatment. For Cox-2 determination, 88.0% of those receiving placebo and 87.7% of those treated with celecoxib retained baseline adenoma biomarker status in recurrent adenomas (Table 4). These figures were 84.5% and 85.6%, respectively, for 15-PGDH detection. For patients treated with celecoxib, 18 (7.8%) changed adenoma status from Cox-2 low to Cox-2 high upon disease recurrence, and 12 (85.7%) changed adenoma status from Cox-2 high to Cox-2 low (Table 4). For patients treated with placebo, 15-PGDH expression changed from present to lost in 13 (6.5%) and from lost to present in 18 (9.0%). For celecoxib-treated patients, these values were 24 (9.8% and 11 (4.5%), respectively (McNemar’s test p=0.03). These numbers showed that the overall prevalence of recurrent disease with respect to Cox-2 expression roughly matched the prevalence of the biomarker at baseline, and this did not differ by treatment (p=0.99). Upon disease recurrence, there was a significant change in 15-PGDH expression for patients receiving celecoxib, with more loss of expression than gain of expression. In patients receiving placebo, there was more gain of 15-PGDH expression than loss, but this difference between treatments was not significant (p=0.09).
TABLE 4.
Change in biomarkers following celecoxib treatment
| Patients treated with Celecoxib (N=244) | |||||||
|---|---|---|---|---|---|---|---|
| Cox-2 at baseline | 15-PGDH at baseline | ||||||
| high | low | present | absent | ||||
| Cox-2 at recurrence | high | 2 (0.8%) | 18 (7.4%) | 15-PGDH at recurrence | present | 4 (1.6%) | 11 (4.5%) |
| low | 12 (4.9%) | 212 (86.9%) | absent | 24 (9.8%) | 205 (84.0%) | ||
| P= 0.27 | P= 0.03 | ||||||
| Patients treated with Placebo (N=200) | |||||||
|---|---|---|---|---|---|---|---|
| Cox-2 at baseline | 15-PGDH at baseline | ||||||
| high | low | present | absent | ||||
| Cox-2 at recurrence | high | 1 (0.5%) | 14 (7.0%) | 15-PGDH at recurrence | present | 4 (2.0%) | 18 (9.0%) |
| low | 10 (4.9%) | 175 (87.3%) | absent | 13 (6.5%) | 165 (82.5%) | ||
| P=0.41 | P=0.37 | ||||||
Tumor PGE2 estimation provided no additional prognostic or predictive information
To account for the roles of Cox-2 and 15-PGDH in synthesis and degradation of PGE2, respectively, a combined variable was developed. High PGE2 cases were defined as those demonstrating high Cox-2 expression, 15-PGDH loss, or both conditions. Low PGE2 cases were those with both low Cox-2 levels and intact 15-PGDH expression. Patients with low estimated tumor PGE2 accounted for 8.8% of the participants studied. Results of prognostic and predictive studies showed that estimated PGE2 results were similar to those obtained from the 15-PGDH analysis alone (supplementary table 1). Compared to cases with high estimated PGE2 levels, patients with adenomas with reduced PGE2 tended to have lower recurrence on the placebo arm (49.4% vs. 63.3%; RR 0.78; 95% CI 0.54, 1.11; p=0.15); and this characteristic was confined to the subset who used aspirin (28.0% vs. 63.3%; RR 0.40; 95% CI 0.16,0.98; p=0.02). Cases with low estimated PGE2 levels also did not benefit from celecoxib for adenoma prevention (RR 0.95; 95% CI 0.60, 1.49; p=0.83). This is compared to a 34.9% reduction in adenoma detection by 3 years for patients with high PGE2 (RR 0.59; 95% CI 0.51,0.68; p<0.0001). This lack of response also appeared to be confined to the subset using aspirin (RR 1.78; 95% CI 0.63–5.01).
Of the seven patients who developed CRC while on the APC trial, three received placebo and four were treated with celecoxib. Pre-treatment biomarker determinations were possible for six of these patients (Table 5). For Cox-2, one patient had high levels and 5 had low expression. 15-PGDH was lost in all six patients.
TABLE 5.
Colorectal cancers identified during APC trial
| Patient | Study treatment | Time from randomization to detection of CRC (months) | Post-treatment adenoma available for staining? | Biomarker status at baseline (Pre-treatment) | |
|---|---|---|---|---|---|
| Cox-2 | 15-PGDH | ||||
| …688 | placebo | 6 | Yes | high | absent |
| …168 | placebo | 36 | Yes | low | absent |
| …192 | placebo | 36 | Yes | low | absent |
| …691 | celecoxib | 12 | No | N/D | N/D |
| …623 | celecoxib | 36 | Yes | low | absent |
| …036 | celecoxib | 36 | Yes | low | absent |
| …293 | celecoxib | 36 | Yes | low | absent |
Discussion
Selective Cox-2 inhibitors are powerful agents for chemoprevention of colorectal adenomas, and as a result have great potential to reduce mortality from CRC. Unfortunately, because selective Cox-2 inhibitors have been associated with a risk of cardiovascular toxicity, their use for chemoprevention is currently recommended only for patients with extremely high CRC risk due to a diagnosis of Familial Adenomatous Polyposis (17). Long-term aspirin use is associated with an approximately 50% reduction in CRC incidence and CRC deaths. Although aspirin is not tolerated by some individuals due to gastrointestinal toxicities and bleeding complications, its wide availability at low cost and additional benefits for prevention of cardiovascular disease leads to its acceptance for general use to prevent sporadic CRC (18). To date, no study of adenoma or CRC prevention has directly compared the efficacy and safety of aspirin and celecoxib. However, randomized trials showed that Cox-2 inhibitors achieved equivalent adenoma prevention in patients who developed multiple or large adenomas despite routinely using aspirin, compared to those who did not, indicating that this can be an effective option in patients for whom aspirin chemoprevention fails (8,10).
Data from a variety of sources are helping to inform the use of selective Cox-2 inhibitor use for prevention of sporadic CRC. The APC Trial tested two doses of celecoxib, and showed that although an 800 mg daily dose produced slightly greater adenoma prevention, this dose was also associated with substantially higher cardiovascular toxicity (11). This dose is no longer recommended for any indication. In the APC trial, the risk of celecoxib-associated cardiovascular complications was related to pre-existing cardiovascular risk factors, defined as a history of atherosclerotic heart disease, age > 65 years, smoking, hypertension, or hyperlipidemia (10). New data from a large trial of arthritis patients that was specifically designed to assess cardiovascular risk showed that individuals using a low dose of celecoxib (100mg) had equivalent rates of serious cardiovascular toxicities and fewer renal and gastrointestinal events than those using naproxen or ibuprofen (12). The rate of cardiovascular toxicity of 2.3% for a cohort receiving a median of 20 months of celecoxib seems modest when accounting for the fact that this cohort also had pre-existing cardiovascular disease and/or high risk of cardiovascular complications. Still, the lack of a placebo comparator limits our ability to apply these data directly to CRC chemoprevention in the general population.
The results shown here provide data suggesting new areas for study, and in some cases may even guide treatment. Although neither biomarker tested was associated with a statistically significant difference in adenoma recurrence, patients whose tumors expressed high levels of Cox-2 tended to have greater adenoma recurrence and this risk was reduced by 63% with celecoxib use. This suggests that this subset of patients may benefit from celecoxib, particularly if they have failed aspirin chemoprevention and have no cardiovascular risk factors. For those whose pre-treatment lesions retained 15-PGDH expression, celecoxib did not produce a significant anti-tumor response, but this lack of celecoxib benefit appears to be confined only to those also taking aspirin, suggesting that these patients should not be additionally treated with a Cox-2 inhibitor. Several factors lend credibility to these data. The analysis utilized a large randomized trial that prospectively collected tissue for analysis and included as a prospective secondary endpoint the study of high adenoma Cox-2 levels as a predictive biomarker for celecoxib response. Limitations of this analysis include the relatively small number of patients in the highest risk categories. In addition, the contribution of 15-PGDH to tissue PGE2 levels was not understood at the onset of the APC trial, and plans to study this important biomarker were added after study initiation.
An uncommon but intriguing subset of participants demonstrated adenomas that retained 15-PGDH expression. There were 139 individuals in this category. These patients had more advanced adenomas (70% vs. 49%), and a slightly higher percentage had multiple adenomas at study entry (33% vs. 25%). In the absence of NSAID use, the risk of adenoma development in individuals with 15-PGDH positive adenomas appeared to be the same as those whose index adenomas had lost 15-PGDH, because among those neither using aspirin or treated with celecoxib, the likelihood of developing new adenomas was nearly equal (60% versus 63.4%, respectively). In contrast to patients whose adenomas lacked 15-PGDH, those whose index adenomas retained 15-PGDH did not show a reduction in adenoma detection with celecoxib treatment (RR 0.73; 95% CI 0.47,1.12; p=0.15). Further subset analysis, limited by small numbers, suggested that this lack of response was confined to the subset of patients using aspirin. Among those who did not use aspirin, celecoxib reduced new adenoma detection (RR 0.60; 95% CI 0.36,0.99), however, among aspirin users, no celecoxib effect was observed (RR 1.16; 95% CI 0.45, 2.96). Further analysis showed that individuals whose adenomas retained 15-PGDH who were using aspirin had an already low recurrence risk of 32.5%, compared to a 60% recurrence risk for those with 15-PGDH positive adenomas who were not using aspirin (p=0.07), and as compared to a 63.0% disease recurrence in those taking aspirin and not administered celecoxib, but whose index adenomas showed 15-PGDH loss (p=0.05).
The association of aspirin use with reduced adenoma recurrence among those with 15-PGDH positive index adenomas in part recapitulates findings of a previous study that examined effects of 15-PGDH expression in normal colon mucosa. In that study, a marked association of aspirin use with lower colon cancer risk was observed in individuals with above, but not below, average 15-PGDH expression in normal colon mucosa (19). These two studies may reflect the same underlying biology, particularly if we make the presumption that 15-PGDH positive adenomas most likely arose in individuals with already high 15-PGDH expression in the normal colon. This presumption is supported by data showing that in normal human colon mucosa, 15-PGDH levels are highly reproducible in biopsies throughout the colon and when repeated after a 4-month interval (20).
In a previous study, we also measured 15-PGDH expression in normal colonic mucosa and studied the relationship between this variable and celecoxib effect in adenoma prevention. In a substudy of the APC trial, RT-PCR was used to quantify 15-PGDH in normal rectal mucosa biopsies from 16 patients treated with celecoxib but not using aspirin (6). Of the 5 individuals who developed new adenomas during celecoxib treatment, all had 15-PGDH values that were lower than the median for the group. The present study examined 15-PGDH expression in adenomas rather than in normal colon mucosa, and found that celecoxib was effective in reducing new adenomas in individuals in which an index adenoma had lost 15-PGDH expression. Future studies of the relationship between Cox-2 and 15-PGDH gene expression in an individual’s colonic mucosa and in colorectal adenomas, as well as corresponding protein expression will be of value in further testing this model.
Our analysis showed that administering celecoxib did not alter the distribution of Cox-2 and expression in recurrent versus pre-treatment adenomas, although compared to placebo, patients treated with celecoxib were slightly more likely to develop disease with 15-PGDH loss. Previous work found that aspirin administration did not alter 15-PGDH expression in normal colon mucosa (20). These results are also potentially at variance with previous work showing that high Cox-2 expression was less likely in colon cancers that developed in aspirin users compared to those in non-aspirin users, suggesting that this is an area that will also benefit from additional clinical studies.
Consistent with previous reports, and we found that 15-PGDH expression was downregulated in 89.3% of adenoma cases. Loss of 15-PGDH expression in colon neoplasia has been shown to reflect inactivation of TGF-β signaling, upregulation of EGFR signaling, and also activation of signaling via Wnt pathway/β-catenin-TCF4 (21). This suggests that the minority of colon adenomas that retain 15-PGDH expression may arise by an alternative pathway that does not involve activation of Wnt signaling and loss of TGF-β signaling that is typical of the classical pathway of colorectal tumorigenesis (22). This hypothesis is further supported by our observation that adenomas retaining 15-PGDH expression demonstrated 15-PGDH expression in crypt cells, a location of active Wnt signaling in which 15-PGDH is absent in normal colonic mucosa. Further investigation of these unusual 15-PGDH containing adenomas is currently underway.
There are a few limitations in this study. It should be emphasized that the prognostic influence of aspirin use among 15-PGDH containing adenoma participants should be interpreted with caution due to the exploratory nature and small sample size. The specific underlying mechanism needs to be investigated in future studies. In addition, the study method involved centrally evaluated biomarkers by a single pathologist, and this could introduce bias. Finally, the observed imbalance of family history and the presence of advanced adenoma between two 15-PGDH groups at baseline has little potential confounding impact on the negative findings between 15-PGDH expression and adenoma recurrence risk. Nevertheless, an independent study to validate both biomarkers’ performance is needed before using Cox-2 and 15-PGDH to guide clinical chemoprevention.
In summary, our results demonstrate the importance of 15-PGDH loss to early intestinal tumorigenesis, and suggest differences in response to NSAID chemoprevention based upon expression of both Cox-2 and 15-PGDH expression in pre-malignant adenomas. Achieving safe and effective CRC prevention requires a two-part approach. First, we must optimize current methods of prevention by identifying individuals who are most likely to benefit from their use. For example, it is reasonable to postulate that a subset of patients at particularly high risk for CRC development, yet resistant to aspirin chemoprevention, would benefit from celecoxib use, particularly if the patient has no cardiovascular risk factors. Second, we must continue to study the molecular biology of early colorectal neoplasia and its transition to invasive disease so that new agents with optimal safety and efficacy can be developed.
Supplementary Material
Acknowledgments
grant support: This work was supported by the US National Cancer Institute, N01-CN-95015 (MMB).
abbreviations
- APC Trial
Adenoma Prevention with Celecoxib Trial
- Cox-2
cyclooxygenase-2
- CRC
colorectal cancer
- IHC
immunohistochemistry
- 15-PGDH
15-prostaglandin dehydrogenase
- PGE2
prostaglandin E2
Footnotes
There are no conflicts of interest to report for any of the authors of this manuscript except Sanford Markowitz.
References
- 1.Elmunzer BJ, Hayward RA, Schoenfeld PS, et al. Effect of flexible sigmoidoscopy-based screening on incidence and mortality of colorectal cancer: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2012;9:e1001352. doi: 10.1371/journal.pmed.1001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369:1603–1613. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 3.Latchford AR, Maeda Y, Clark SK. Non-steroidal antiinflammatory Drugs (NSAIDs) and aspirin for presenting recurrence and metachronous colorectal carcinomas in patients previously treated for colorectal cancer. Cochrane Database of Systematic Reviews. 2013;1:CD010325. [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 5.Myung SJ, Rerko RM, Yan M, et al. 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12098–12102. doi: 10.1073/pnas.0603235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan M, Myung SJ, Fink SP, et al. 15-Hydroxyprostaglandin dehydrogenase inactivation as a mechanism of resistance to celecoxib chemoprevention of colon tumors. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9409–9413. doi: 10.1073/pnas.0902367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan M, Rerko RM, Platzer P, et al. 12-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist, is a TGF-beta-induced suppressor of human gastrointestinal cancers. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17468–17473. doi: 10.1073/pnas.0406142101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arber N, Eagle CJ, Spicak J, et al. Celecoxib for prevention of colorectal adenomatous polyps. New England Journal of Medicine. 2006;355:885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 9.Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. New England Journal of Medicine. 2006;355:873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 10.Bertagnolli MM, Eagle CJ, Zauber AG, et al. Five-year efficacy and safety analysius of the adenoma prevention with celecoxib trial. Cancer Prevention Research. 2009;2:310–321. doi: 10.1158/1940-6207.CAPR-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. New England Journal of Medicine. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 12.Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. New England Journal of Medicine. 2016;375:2519–2529. doi: 10.1056/NEJMoa1611593. [DOI] [PubMed] [Google Scholar]
- 13.Soslow RA, Dannenberg AJ, Rush D, et al. Cox-2 is expressed in human pulmonary, colonic, and mammary epithelium. Cancer. 2000;89:2637–2645. doi: 10.1002/1097-0142(20001215)89:12<2637::aid-cncr17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Aberrant crypt foci in the Adenoma Prevention with Celecoxib Trial. Cho NL, Redston M, Zauber AG, Carothers AM, et al. Cancer Prev Res. 2008;1:21–31. doi: 10.1158/1940-6207.CAPR-07-0011. [DOI] [PubMed] [Google Scholar]
- 15.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemotherapy Reports. 1966;50:163–170. [PubMed] [Google Scholar]
- 16.US Preventive Services Task Force. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for colorectal cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2006;315:2564–2575. doi: 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- 17.Kim B, Giardiello FM. Chemoprevention in familial adenomatous polyposis. Best Pract Res Clin Gastroenterol. 2011;25:607–622. doi: 10.1016/j.bpg.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan AT, Ladabaum U. Where do we stand with aspirin for the prevention of colorectal cancer? The USPSTF recommendations Gastroenterology. 2016;150:14–18. doi: 10.1053/j.gastro.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 19.Fink SP, Yamauchi M, Nishihara R, et al. Aspirin and the risk of colorectal cancer in relation to the expression of 15-hydroxyprostaglandin dehydrogenase (15-PGDH, HPGD) Sci Transl Med. 2014;6:233re2. doi: 10.1126/scitranslmed.3008481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fink SP, Yang D-H, Barnholtz-Sloan JS, et al. Colonic 15-PGDH levels are stable across distance and time, and are not perturbed by aspirin intervention. Dig Dis Sci. 2013;58:2615–2622. doi: 10.1007/s10620-013-2670-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smartt HJM, Greenhough A, Moronez-Moran P, et al. Beta-catenin represses expression of the tumour suppressor 15-prostablandin dehydrogenase in the normal intestinal epithelium and colorectal tumour cells. Gut. 2012;61:1306–1314. doi: 10.1136/gutjnl-2011-300817. [DOI] [PubMed] [Google Scholar]
- 22.Markowitz SD, Bertagnolli MM. Molecular Basis of Colorectal Cancer. New England Journal of Medicine. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.