Abstract
Background
The White House, the American Heart Association, the Agency for Healthcare Research and Quality, and the National Heart, Lung and Blood Institute have all recently acknowledged the need to disaggregate Asian American subgroups to better understand this heterogeneous racial group. This study aims to assess racial/ethnic differences in relative contribution of risk factors of gestational diabetes mellitus (GDM) among Asian subgroups (Asian Indian, Chinese, Filipino, Japanese, Korean, and Vietnamese), Hispanics, non-Hispanic blacks, and nonHispanic whites.
Methods:
Pregnant women in 2007–2012 were identified through California state birth certificate records and linked to the electronic health records in a large mixed-payer ambulatory care organisation in Northern California (n = 24 195). Relative risk and population attributable fraction (PAF) for specific racial/ethnic groups were calculated to assess the contributions of advanced maternal age, overweight/obesity (Centers for Disease Control and Prevention (CDC) standards and World Health Organization (WHO)/American Diabetes Association (ADA) body mass index cut-offs for Asians), family history of type 2 diabetes, and foreign-born status.
Results:
GDM was most prevalent among Asian Indians (19.3%). Relative risks were similar across all race/ethnic groups. Advanced maternal age had higher PAFs in non-Hispanic whites (22.5%) and Hispanics (22.7%). Meanwhile family history (Asian Indians 22.6%, Chinese 22.9%) and foreign-borne status (Chinese 40.2%, Filipinos 30.2%) had higher PAFs in Asian subgroups. Overweight/obesity was the most important GDM risk factor for non-Hispanic whites, Hispanics, Asian Indians, and Filipinos when the WHO/ADA cut-off points were applied. Advanced maternal age was the only risk factor studied that was modified by race/ethnicity, with non-Hispanic white and Hispanic women being more adversely affected than other racial/ethnic groups.
Conclusions:
Overweight/obesity, advanced maternal age, family history of type 2 diabetes, and foreign-borne status are important risk factors for GDM. The relative contributions of these risk factors differ by race/ethnicity, mainly due to differences in population prevalence of these risk factors.
Gestational diabetes mellitus (GDM) complicates 9.2% of pregnancies in the US in 2010, and the prevalence of GDM has increased dramatically in the past 20 years.1,2 GDM is a significant risk factor for type 2 diabetes, and up to 70% of women with a history of GDM will develop type 2 diabetes in their lifetime.3–5 Risk factors for GDM, such as overweight and obesity, family history of type 2 diabetes, and advanced maternal age, are well established in the literature and recognised by national organisations such as the Centers for Disease Control and Prevention (CDC) and American Diabetes Association (ADA).6–9
It is also known that the burden of GDM differs by race/ethnicity, with Asian Americans and Hispanics having significantly higher prevalence of GDM compared with non-Hispanic whites (NHWs).2,10,11 Risk factors of GDM, however, appear to have different contributions to the disease across racial/ethnic groups. For example, Asian Americans have been shown to have the highest prevalence of GDM, despite lower pre-pregnancy body mass index (BMI).12,13 Immigrants may also experience a different burden of disease from US-borne individuals because of multidimensional factors such as socioeconomic status, lifestyle, and acculturation. Previous research has found a higher prevalence of GDM among foreign-borne individuals compared with US-borne individuals, even after taking into account maternal age, parity, educational attainment, and weight during pregnancy.14 No existing studies have comprehensively studied the relative contribution of each risk factor to GDM (assessed using the population attributable fraction, PAF) among racial/ethnic groups, in particular across Asian subgroups, along with foreign-borne status.
The WHO and the ADA have recommended the use of lower BMI cut-off points for Asians (<23 kg/m2 for normal weight) as the lower BMI cut-offs better identify health risks for type 2 diabetes and cardiovascular disease among Asians.15,16 However, very few studies have examined the utilisation of lower BMI cut-off points for GDM screening in Asian subgroups.12,13
The purpose of this study was to (i) examine the prevalence of GDM and its risk factors across the six largest Asian American subgroups, NHWs, nonHispanic blacks (NHBs), and Hispanics; and (ii) assess the relative contributions of well-established GDM risk factors (advanced maternal age, pre-pregnancy overweight/obesity using both CDC standards and WHO/ADA BMI cut-offs for Asians, family history of type 2 diabetes) to GDM for specific racial/ethnic groups. We also explored maternal birth place (foreign-borne status) as an independent and novel risk factor for GDM.
Methods
This is a retrospective cohort study. The study population consisted of women who received prenatal care between 2007 and 2012 at a mixed-payer, outpatient health care organisation that annually serves approximately 1 000 000 patients in northern California. Data from pregnant patients during the study period were extracted from California state birth certificate records and linked to the electronic health records (EHR) of the study health care organisation using mother’s name, child’s name, delivery date, mother’s date of birth, and first six characters of street address. A total of 98% of women with delivery dates in the EHR were successfully matched with the state birth certificate records based on this linking algorithm. All data sets analysed by the research team were de-identified according to the Health Insurance Portability and Accountability Act standard; the study was approved by the study health care organisation’s Institutional Review Board.
Ascertainment of GDM status
Information from both California state birth certificate records and the EHR was used complementarily to identify patients with GDM. On the state birth certificate, GDM was captured using the pregnancy complication indicator for the diagnosis of GDM. Patients meeting one or more of the following criteria in the EHR were also identified as patients with GDM: International Classification of Diseases (ICD)-9 coding for GDM (648.8, 648.80–648.84), abnormal laboratory test results as defined by the California Diabetes and Pregnancy Program SweetSuccess 2008 [100 g oral glucose tolerance test (OGTT): 2 out of 4 abnormal values (fasting: 95 mg/dL, 1 h; 180 mg/dL, 2 h; 155 mg/dL, 3 h; 140 mg/dL); 75 g OGTT: 1 out of 3 abnormal values (fasting: 95 mg/dL, 1 h; 180 mg/dL, 2 h; 155 mg/dL)], or antidiabetic medication utilisation during pregnancy.6,17–19
Ascertainment of GDM risk factors
Maternal race/ethnicity was extracted from the state birth certificate records (92%), supplemented by selfreported maternal race/ethnicity from EHR (8%). Selfreported race/ethnicity information was collected at the study health care organisation using a questionnaire described previously.20,21 The concordance between the two data sources was high (89%). Race/ethnicity information from the state birth certificate was used in case of discordance. Other maternal demographic and clinical information, such as educational attainment, parity, and smoking status during pregnancy, was obtained from the state birth certificate records. Smoking status (smoker or not) was defined by any cigarette use from 3 months before pregnancy to delivery. Primary insurance type was extracted from the EHR billing records.
GDM risk factors were identified using information from both data sources complementarily. Advanced maternal age was extracted from the EHR and defined as ≥35 years.22 BMI was calculated by information extracted from the state birth certificate. During the data collection process for the state birth certificate, information is largely abstracted from mother’s medical records/EHR. If not available, information may be obtained and recorded on the state birth records using self-reported height and pre-pregnancy weight.23 Thus BMI was calculated by information extracted from the state birth certificate and BMI was further classified as follows based on standard cut-off points recommended by the CDC: normal weight <25 kg/m2, overweight 25–29.9 kg/m2, and obese ≥30 kg/m2.24 For Asians, alternative BMI cut-offs recommended by WHO and ADA (overweight/obesity ≥23 kg/m2) were also used to explore whether the lower cut-off points better capture GDM risk among Asian Americans.15,16 Family history of type 2 diabetes was extracted from the EHR and defined as the presence of type 2 diabetes in parents and siblings. Foreign-borne status was identified through maternal place of birth information on the state birth certificate.
Statistical analysis
The prevalence of GDM risk factors in Asian American subgroups, NHBs, and Hispanics was assessed and compared with NHWs using pairwise chi-square test for categorical variables and pairwise Wilcoxon test for continuous variables. Age-adjusted prevalence of GDM for each racial/ethnic group was estimated using direct standardisation to NHW population at the study health care organisation with 5- to 7-year age categories (18–24, 25–29, 30–34, 35–39, 40–45 years). To evaluate the relationships between the risk factors of interest and GDM, multivariable-adjusted relative risks with 95% confidence intervals of GDM for each racial/ethnic group, compared with NHW, were calculated based on a log-binomial model adjusting for additional clinical and demographic characteristics including maternal educational attainment, parity, smoking status, and insurance type.
For each risk factor, the PAF of GDM was calculated for the five largest racial/ethnic groups (n > 1000), including NHW, Hispanic, Asian Indian, Chinese, and Filipino. The PAF for individual risk factors quantifies relative contribution of the risk factor to the population prevalence of GDM. It serves as an estimation of the proportion of GDM that can be reduced if exposure to the risk factor can be reduced or eliminated. The magnitude of the PAF depends on both the population prevalence of the risk factor and the relative risk of the risk factor.25 We also calculated the total PAF for all risk factors as an estimation of the proportion of GDM that can be reduced if exposure to all the studied risk factors can be eliminated together as a group. We calculated 95% confidence intervals. Statistical analyses were performed using Stata 13 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP).
Results
Women aged 18–45 years with a singleton live birth were identified from the state birth certificate records and were included if they had one or more prenatal visits at the study health care organisation (n = 26 397). Women with type 1 or type 2 diabetes before the studied pregnancy (n = 899) were excluded if identified in the EHR using previously developed EHR protocols, or if indicated on the state birth certificate.26 Women were also excluded if they did not belong to the nine studied racial/ethnic groups (n = 1303). Thus, we identified a total of 24 195 eligible pregnant women (37.2% NHW, 1.8% NHB, 15.6% Hispanics, 21.0% Asian Indian, 13.3% Chinese, 4.5% Filipino, 2.8% Japanese, 1.9% Korean, and 1.9% Vietnamese). For women with multiple pregnancies (n = 5383) during this 6-year study period, only the first pregnancy episode was selected for inclusion in this study.
Prevalence of GDM and its risk factors
Patient characteristics are described in Table 1. There were significant racial/ethnic differences in the prevalence of GDM risk factors, as shown in Table 1. Proportions of patients with advanced maternal age (≥35 years) ranged from 18.0% in Asian Indians to 51.9% in Japanese. Compared with NHWs, NHBs, Hispanics, Asian Indians, and Filipinos had lower proportions of mothers aged 35 or older, whereas Chinese, Japanese, and Vietnamese had higher proportions of mothers with advanced maternal age (≥35 years).
Table 1.
Patient characteristics, across race/ethnicity
| Patient characteristics | Non-Hispanic white | Non-Hispanic black | Hispanic | Asian Indian |
Chinese | Filipino | Japanese | Korean | Vietnamese |
|---|---|---|---|---|---|---|---|---|---|
| Total number | 9011 | 432 | 3777 | 5069 | 3206 | 1096 | 682 | 462 | 460 |
| Age at delivery (years) | 32.9 | 30.8 | 29.6 | 31.3 | 34.3 | 32.6 | 34.8 | 33.9 | 33.9 |
| ≥35years (%) | 38.1 | 27.8 | 21.6 | 18.0 | 46.9 | 35.0 | 51.9 | 42.2 | 43.7 |
| Education (%) | |||||||||
| High school or less | 10.7 | 24.4 | 49.1 | 1.2 | 1.6 | 7.4 | 7.0 | 2.8 | 8.8 |
| Some college | 16.2 | 30.6 | 25.5 | 1.4 | 4.3 | 25.5 | 14.7 | 7.7 | 10.3 |
| College degree or above | 73.1 | 45.0 | 25.4 | 97.4 | 94.1 | 67.1 | 78.3 | 89.5 | 80.9 |
| Parity (%) | |||||||||
| 0 | 57.1 | 53.5 | 44.6 | 55.8 | 60.7 | 49.5 | 58.2 | 56.7 | 53.9 |
| 1 | 30.4 | 28.2 | 31.8 | 41.4 | 33.4 | 32.0 | 34.2 | 35.3 | 34.8 |
| 2 or more | 12.5 | 18.3 | 23.6 | 2.8 | 5.9 | 18.5 | 7.6 | 8.0 | 11.3 |
| Smoking (%) | 1.6 | 2.6 | 0.9 | 0.1 | 0.1 | 0.8 | 0.3 | 0.4 | 0.7 |
| Primary Insurance (%) | |||||||||
| Medi-Cal | 4.7 | 13.9 | 28.1 | 0.3 | 0.8 | 1.6 | 0.3 | 0.9 | 2.0 |
| HMO | 35.0 | 35.4 | 30.5 | 27.6 | 38.7 | 43.6 | 22.4 | 30.7 | 39.8 |
| PPO | 58.8 | 44.4 | 38.3 | 71.8 | 59.8 | 53.3 | 74.6 | 67.5 | 57.2 |
| Other | 1.5 | 6.3 | 3.1 | 0.3 | 0.7 | 1.5 | 2.7 | 0.9 | 1.0 |
| Pre-pregnancy BMI (%) | |||||||||
| Normal weight (BMI < 25) | 70.1 | 48.4 | 49.8 | 71.9 | 91.1 | 69.9 | 92.5 | 87.5 | 88.9 |
| Overweight (25 ≤ BMI < 30) | 20.3 | 29.2 | 30.0 | 23.4 | 7.8 | 20.9 | 6.5 | 10.0 | 9.6 |
| Obese (BMI ≥ 30) | 9.6 | 22.4 | 20.2 | 4.7 | 1.1 | 9.2 | 1.0 | 2.5 | 1.5 |
| Pre-pregnancy BMI (WHO/ADA) (%) | |||||||||
| Normal weight (BMI < 23) | 49.5 | 76.7 | 46.4 | 81.7 | 71.2 | 75.7 | |||
| Overweight (23 ≤ BMI < 27.5) | 39.0 | 20.5 | 37.2 | 15.5 | 24.0 | 19.3 | |||
| Obese (BMI ≥ 27.5) | 11.5 | 2.8 | 16.4 | 2.8 | 4.8 | 5.0 | |||
| Family history of T2DM (%) | 36.0 | 47.6 | 57.1 | 58.4 | 39.7 | 58.1 | 31.3 | 37.4 | 46.9 |
| Nativity (%) | |||||||||
| Foreign-borne | 21.2 | 27.6 | 50.0 | 97.0 | 85.8 | 68.6 | 85.9 | 85.5 | 90.4 |
| GDM (%) | 7.0 | 4.9 | 10.8 | 17.8 | 16.4 | 18.8 | 8.5 | 12.1 | 18.7 |
| Age-adjusted GDM (%) | 7.0 | 4.9 | 13.3 | 19.3 | 15.3 | 19.0 | 9.7 | 12.9 | 18.8 |
PPO, preferred provider organisation; HMO, health maintenance organisation.
Normal weight (BMI < 25 kg/m2) patients ranged from 48.4% in NHBs to 92.5% in Japanese according to the CDC standards. Asian subgroups, except for Filipinos, had higher proportions of mothers with normal weight prior to pregnancy compared with NHWs, whereas NHBs and Hispanics had higher proportions of mothers overweight or obese. Using the WHO/ADA BMI cut-off points for Asians greatly increased the prevalence of overweight and obesity (BMI ≥ 23 kg/m2) among Asian American subgroups, in particular among Asian Indians and Filipinos, whose prevalence increased by 22.4% and 23.5%, respectively.
Racial/ethnic minority groups, except for Japanese and Koreans, had significantly higher prevalence of family history of type 2 diabetes than NHWs. Foreignborne mothers ranged from 21.2% in NHWs to 97.0% in Asian Indians.
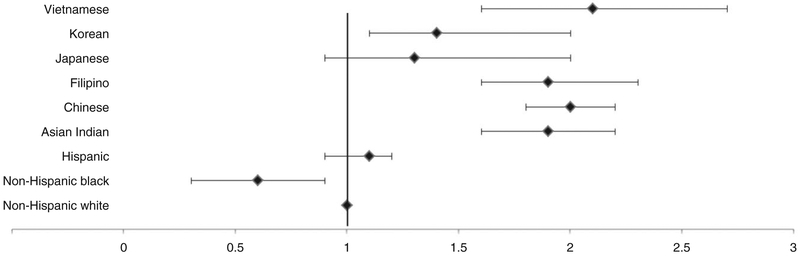
Age-adjusted prevalence of GDM was significantly higher among Hispanics (13.3%) and all Asian American subgroups (Asian Indian: 19.3%, Chinese: 15.3%, Filipino: 19.0%, Korean: 12.9%, Vietnamese: 18.8%), except for Japanese (9.7%), compared with NHWs (7.0%) (Table 1). The racial/ethnic differences between Asian subgroups and NHWs persisted after adjusting for maternal education, parity, smoking, insurance, and the studied GDM risk factors (Figure 1). There were no significant differences in adjusted GDM risk between Hispanics, Japanese and NHWs. NHBs had a significantly lower risk of GDM compared with NHWs.
Figure 1.
Adjusted relative risk (95% CI) of GDM by race/ethnicity. Adjusted for maternal education, parity, smoking, insurance type, with NHWs as reference group.
Relative contributions of risk factors to GDM
We then examined the adjusted relative risks and adjusted PAFs of GDM by risk factors (Table 2) in the five largest racial/ethnic groups (NHW, Hispanic, Asian Indian, Chinese, and Filipino). Interaction terms were tested to explore potential racial/ethnic differences in the studied GDM risk factors. There was a significant interaction between race/ethnicity and advanced maternal age (P < 0.05). Specifically advanced maternal age had a smaller but still significant impact on GDM among Asian Indians [relative risk (RR) 1.3, 95% CI 1.1, 1.5] and Chinese [RR 1.3, 95% CI 1.1, 1.5] compared with NHWs [RR 1.8, 95% CI 1.5, 2.2] and Hispanics [RR 2.0, 95% CI 1.6, 2.6].
Table 2.
Adjusted relative risk and population attributable fraction of gestational diabetes mellitus for risk factors, across major racial/ethnic groups
| Non-Hispanic white | Hispanic | Asian Indian |
Chinese | Filipino | |
|---|---|---|---|---|---|
| RR (95% CI) | |||||
| Maternal age ≥35 | 1.8 (1.5, 2.2) | 2.0 (1.6, 2.6) | 1.3 (1.1, 1.5) | 1.3 (1.1, 1.5) | 1.6 (1.2, 2.1) |
| Overweight or obese | 2.0 (1.7, 2.4) | 2.2 (1.7, 2.8) | 1.7 (1.5, 2.0) | 1.5 (1.2, 1.9) | 1.6 (1.2, 2.1) |
| WHO/ADA overweight or obese | 1.9 (1.7, 2.2) | 1.6 (1.3, 1.9) | 1.9 (1.4, 2.5) | ||
| Family history of diabetes | 1.7 (1.4, 2.0) | 1.6 (1.2, 2.1) | 1.8 (1.5, 2.3) | 1.6 (1.4, 1.9) | 1.4 (1.1, 1.8) |
| Foreign-borne | 1.3 (1.1, 1.6) | 1.4 (1.1, 1.7) | 1.3 (0.8, 2.1) | 1.5 (1.1, 2.0) | 1.5 (1.1, 1.8) |
| PAF (95% CI) | |||||
| Maternal age ≥35 | 22.5 (14.1, 30.5) | 22.7 (13.4, 31.5) | 8.5 (2.5, 14.4) | 12.3 (1.4, 22.9) | 18.9 (2.3, 34.4) |
| Overweight or obese | 28.9 (22.4, 35.1) | 42.3 (29.6, 53.5) | 25.5 (17.4, 33.3) | 7.9 (3.4, 12.3) | 23.3 (11.1, 34.9) |
| WHO/ADA overweight or obese | 39.0 (29.7, 47.6) | 15.9 (10.8, 20.9) | 38.2 (21.2, 53.0) | ||
| Family history of diabetes | 18.5 (11.6, 25.3) | 18.0 (7.9, 27.8) | 22.6 (12.8, 32.0) | 22.9 (13.1, 32.2) | 17.9 (0, 34.5) |
| Foreign-borne | 5.4 (1.2, 9.6) | 17.2 (3.5, 30.3) | 22.0 (0, 49.3) | 40.2 (20.7, 56.6) | 30.2 (7.4, 50.0) |
| Total PAF | 57.2 (41.3, 69.7) | 69.5 (46.5, 83.7) | 65.9 (22.4, 87.5)a | 65.8 (36.2, 83.3)a | 71.1 (30.7, 89.8)a |
Calculated using WHO/ADA BMI cut-off points for Asians.
CI, confidence interval (adjusted for maternal education, parity, smoking, insurance status, and all risk factors).
The adjusted PAFs of maternal age ≥35 years ranged from 8.5% among Asian Indians to 22.7% among Hispanics (Table 2). When the CDC standard cut-off points were used, the adjusted PAFs of overweight/obesity ranged from 7.9% among Chinese to 42.3% among Hispanics. When the WHO/ADA BMI cut-off points for Asians were applied, PAFs increased for all studied Asian American subgroups (Asian Indian: 39.0%, Chinese: 15.9%, Filipinos: 38.2%). The PAFs of family history of type 2 diabetes ranged from 17.9% among Filipinos to 22.9% among Chinese. The estimated PAF of foreign-borne status was highest among racial/ethnic minorities such as Chinese (40.2%) and Filipinos (30.2%), and lowest among NHWs (5.4%) who were mostly US borne. Collectively, the studied risk factors altogether contribute to 57.2% of GDM among NHWs, 69.5% among Hispanics, 65.9% among Asian Indians, 65.8% among Chinese, and 71.1% among Filipinos.
Comment
Our study demonstrates substantial variation of prevalence and risk factors of GDM across racial/ethnic groups. Advanced maternal age, overweight/obesity, family history of type 2 diabetes, and foreignborne status are shown to be significant contributors to GDM in our study population, which has diverse racial/ethnic and cultural backgrounds. Together, the studied risk factors accounted for almost two thirds of the risk for GDM, ranging from 57.2% among NHWs to 71.1% among Filipinos. The observed variation in PAF can be attributable to racial/ethnic differences in relative risks and prevalence rates of these risk factors.
From a public health perspective, overweight/obesity is considered the most important risk factor for GDM because it is the most modifiable among the four studied risk factors. Our findings provide quantitative evidence to support this statement. Overweight/obesity was associated with the largest adjusted PAF for GDM across all racial/ethnic groups studied, except for Chinese. When CDC standard BMI cut-off points were used, overweight/obesity was a more important risk factor for NHWs and Hispanics than for Asian Americans because of its higher relative risk and higher prevalence among NHWs and Hispanics. The use of WHO/ADA BMI cut-off points for Asians increased the prevalence of overweight/obesity and strengthened the association between overweight/obesity and GDM risk for Asians. When using WHO/ADA cut-off points for Asians, the overweight/obesity PAF suggests that in this cohort, up to 39.0 % of GDM among Asian Indians, 15.9% among Chinese and 38.2% among Filipinos could be attributed to pre-pregnancy overweight/obesity. This finding supports the use of WHO/ADA BMI cut-off points for Asian Americans because the standard BMI cut-off points may misclassify overweight/obesity status among Asian Americans and underestimate the impact of being overweight/obesity on GDM. Consistent with the recent ADA position statement, our study suggests that lower BMI cut-offs (≥23 kg/m2) should be applied to Asian Americans when screening for GDM, in addition to type 2 diabetes.
Advanced maternal age and family history of type 2 diabetes are also important risk factors for GDM. Advanced maternal age is particularly important for NHWs and Hispanics because of its stronger association with GDM risk in these populations compared with Asian Americans. We speculate that these differences represent the ways the pathophysiology of GDM may differ by race/ethnicity. Replication in future studies is needed. Similarly, although family history of type 2 diabetes is associated with increased GDM risk among all studied racial/ethnic groups, it is more powerful for Asian Indians and Chinese. Both family history and maternal age are generally considered non-modifiable; however, public health and medical care approaches can be employed to reduce their impact on GDM. For example, information about GDM risk associated with advanced maternal age can help women make an informed decision about pregnancy timing. In addition, special care and patient education such as culturally sensitive family-based interventions can be provided to women with a family history of type 2 diabetes to help prevent GDM and type 2 diabetes because they are at elevated risk for both conditions.27
Also consistent with our hypothesis, we found that foreign-borne NHWs, Hispanics, Chinese, and Filipinos had a significantly higher risk of GDM compared with their US-borne counterparts. This corroborates findings from another study that evaluated GDM by race/ethnicity and country of birth.14 Findings from our study further demonstrate that disparities in GDM risk exist in foreign-borne women even after controlling for other GDM risk factors. Although it remains unclear why the foreign-borne population has a significantly higher risk for GDM, health care organisations and policymakers should consider providing culturally tailored prevention and intervention to immigrant populations to help them maintain a healthy lifestyle and to buffer the negative effects of potential risk exposure. In addition, based on our findings, WHO/ADA BMI cut-off points for Asians should be applied to the Asian immigrants considering their particularly high risk for GDM. In addition, future studies are needed to more fully explore the impact of acculturation on GDM among foreign-borne mothers, as length of time in the US is likely a better marker of acculturation than simply place of birth, which was the only variable available to us for analysis on the current birth records.
The main strengths of this study include using information from a large health care organisation with a uniquely diverse population enriched with Asian Americans. Although the majority of patients in the study cohort had health insurance and thus medically underserved populations were under-represented, this geographic and socioeconomic limitation also provides better internal validity for racial/ethnic group comparison by minimising potential unmeasured socioeconomic confounders. In addition, information from both EHR and state birth certificate were used complementarily to better capture mothers’ characteristics and their GDM outcome. Furthermore, by using the PAF we took both relative risk and population prevalence of these risk factors into account when assessing their contribution to GDM in this population.
The findings from this study should be interpreted with caution. First, the prevalence of GDM may be underestimated because our study included all pregnancies, not just pregnancies that were screened for GDM at the study organisation. However, we used GDM status from the state birth certificate to augment GDM test results and diagnoses in the EHR. In fact, very few GDM cases were reported additionally on the state birth certificate. Our estimates of GDM prevalence are comparable with findings from prior studies.12,13 Second, PAF assumes a causal association between a risk factor and outcome. Thus, we have focused on well-documented risk factors for GDM. The study design and exposure ascertainment methods ensured that these risk factors preceded the outcome. Confounders of the risk factor–GDM association were controlled for in the multivariable regression models, and adjusted RRs were used for computing PAF. Third, because PAF can be influenced by the prevalence of exposure to the risk factors in the population, results in our study population may not apply to another population with a different GDM risk factor profile. However, we found comparable PAF estimates for overweight/obesity with previous findings from the state of Florida, suggesting some robustness of our estimates.28 Fourth, we used prepregnancy BMI information from the state birth records. Although this is the best available information on pre-pregnancy BMI, we should be aware that some of this information is self-reported and thus subject to self-report bias. Finally, we identified mothers with GDM using information from EHR and the state birth certificate. Two thirds (66%) of GDM cases were identified through EHR only, 3% through state birth certificate records only, and 31% through both data sources. Regarding the disagreement of GDM cases between EHR and state birth certificate records in our study, we conducted further analysis and found no significant difference in maternal characteristics (race/ethnicity, maternal age, BMI, family history of type 2 diabetes, foreign-borne status) between GDM cases identified by EHR and state birth certificate records. Previous national studies have indicated that state birth certificate may underestimate GDM prevalence when comparing with other data source such as the Pregnancy Risk Assessment Monitoring System Questionnaire.1 Our study suggests that the use of EHR may provide important complementary information to state birth certificate.
Conclusions
Asian Americans are one of the fastest growing racial/ethnic minority groups and have been shown to have the highest rates of GDM among all racial/ethnic groups. In order to reduce the overall burden of GDM, future studies should focus on tailored lifestyle modification and weight control to reduce the prevalence of risk factors in these populations. For example, the WHO/ADA BMI cut-off points for Asians should be applied to Asian mothers for screening and intervention purposes.
Acknowledgement
This study was supported by a grant from the American Diabetes Association (7–12-CT-55). The authors report no conflict of interest.
Footnotes
This study has been presented as a poster at the American Diabetes Association’s 74th Scientific Sessions, 13–17 June 2014. San Francisco, CA.
References
- 1.DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007–2010. Preventing Chronic Disease 2014; 11:130415. Available at http://www.cdc.gov/pcd/issues/2014/13_0415.htm, [last accessed July 2015]. [DOI] [PMC free article] [PubMed]
- 2.Ferrara A Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care 2007; 30 (Suppl. 2):S141–S146. [DOI] [PubMed] [Google Scholar]
- 3.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 2009; 373:1773–1779. [DOI] [PubMed] [Google Scholar]
- 4.Lee AJ, Hiscock RJ, Wein P, Walker SP, Permezel M. Gestational diabetes mellitus: clinical predictors and long-term risk of developing type 2 diabetes: a retrospective cohort study using survival analysis. Diabetes Care 2007; 30:878–883. [DOI] [PubMed] [Google Scholar]
- 5.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002; 25:1862–1868. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Position statement. Diabetes Care 2011; 34:S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King H Epidemiology of glucose intolerance and gestational diabetes in women of childbearing age. Diabetes Care 1998; 21 (Suppl. 2):B9–B13. [PubMed] [Google Scholar]
- 8.Solomon CG, Willett WC, Carey VJ, Rich-Edwards J, Hunter DJ, Colditz GA, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA: The Journal of the American Medical Association 1997; 278:1078–1083. [PubMed] [Google Scholar]
- 9.Hunsberger M, Rosenberg KD, Donatelle RJ. Racial/ethnic disparities in gestational diabetes mellitus: findings from a population-based survey. Women’s Health Issues 2010; 20:323–328. [DOI] [PubMed] [Google Scholar]
- 10.Dabelea D, Snell-Bergeon JK, Hartsfield CL, Bischoff KJ, Hamman RF, McDuffie RS. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care 2005; 28:579–584. [DOI] [PubMed] [Google Scholar]
- 11.Thorpe LE, Berger D, Ellis JA, Bettegowda VR, Brown G, Matte T, et al. Trends and racial/ethnic disparities in gestational diabetes among pregnant women in New York City, 1990–2001. American Journal of Public Health 2005; 95:1536–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SY, Saraiva C, Curtis M, Wilson HG, Troyan J, England L, et al. Fraction of gestational diabetes mellitus attributable to overweight and obesity by race/ethnicity, California, 2007–2009. American Journal of Public Health 2013; 103:e65–e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hedderson M, Ehrlich S, Sridhar S, Darbinian J, Moore S, Ferrara A. Racial/ethnic disparities in the prevalence of gestational diabetes mellitus by BMI. Diabetes Care 2012; 35:1492–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hedderson MM, Darbinian JA, Ferrara A. Disparities in the risk of gestational diabetes by race-ethnicity and country of birth. Paediatric and Perinatal Epidemiology 2010; 24:441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO expert consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363:157–163. [DOI] [PubMed] [Google Scholar]
- 16.Hsu WC, Araneta MR, Kanaya AM, Chiang JL, Fujimoto W. BMI cut points to identify at-risk Asian Americans for type 2 diabetes screening. Diabetes Care 2015; 38:150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diabetes California and Program Pregnancy. Guidelines for diagnosis of hyperglycemia in pregnancy 2012; Available at: http://www.cdph.ca.gov/programs/cdapp/Pages/default.aspx [last accessed August 2014].
- 18.International Association of Diabetes and Pregnancy Study Group Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coustan DR, Lowe LP, Metzger BE, Dyer AR. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: paving the way for new diagnostic criteria for gestational diabetes mellitus. American Journal of Obstetrics and Gynecology 2010; 202:654.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palaniappan LP, Wong EC, Shin JJ, Moreno MR, Otero-Sabogal R. Collecting patient race/ethnicity and primary language data in ambulatory care settings: a case study in methodology. Health Services Research 2009; 44 (5 Pt 1):1750–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azar KM, Moreno MR, Wong EC, Shin JJ, Soto C, Palaniappan LP. Accuracy of data entry of patient race/ethnicity/ancestry and preferred spoken language in an ambulatory care setting. Health Services Research 2012; 47 (1 Pt 1):228–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Usta IM, Nassar AH. Advanced maternal age. Part I: obstetric complications. American Journal of Perinatology 2008; 25:521–534. [DOI] [PubMed] [Google Scholar]
- 23.Khan H Developing standards for linking electronic health records and vital records systems. 2012 National Conference on Health Statistics. http://www.cdc.gov/nchs/ppt/nchs2012/SS-34_KHAN.pdf. [last accessed March 2015]. [Google Scholar]
- 24.Centers for Disease Control and Prevention (2009a). Adult BMI. Available at http://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html. [last accessed August 2014].
- 25.Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes and Control 2007; 18:571–579. [DOI] [PubMed] [Google Scholar]
- 26.Bhalla V, Zhao B, Azar KM, Wang EJ, Choi S, Wong EC, et al. Racial/ethnic differences in the prevalence of proteinuric and nonproteinuric diabetic kidney disease. Diabetes Care 2013; 36:1215–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.García-Huidobro D, Bittner M, Brahm P, Puschel K. Family intervention to control type 2 diabetes: a controlled clinical trial. Farm Pract. 2011; 28:4–11. [DOI] [PubMed] [Google Scholar]
- 28.Kim SY, England L, Sappenfield W, Wilson HG, Bish CL, Salihu HM, et al. Racial/ethnic differences in the percentage of gestational diabetes mellitus cases attributable to overweight an obesity, Florida, 2004–2007. Preventing Chronic Disease 2012; 9:110249 Available at http://www.cdc.gov/pcd/issues/2012/11_0249.htm, [last accessed July 2015]. [DOI] [PMC free article] [PubMed] [Google Scholar]